Abstract
Background
BDNF Val66Met by chronic stress interaction has been studied using childhood stress as a moderator, but has not been widely studied using chronic stress in adulthood.
Methods
Two independent samples were used: Duke-CG (238 Caucasians) and MESA (5524 Caucasians, African Americans and Hispanics). Chronic stress in Duke-CG was operationalized as having primary caregiving responsibility for a spouse or relative with diagnosed Alzheimer's disease or other major dementia; chronic stress in MESA was defined using chronic burden score constructed from self-reported problems of health (self and someone close), job, finance and relationships. CES-D scale was the measure of depression in both samples. The BDNF Val66Met by adulthood chronic stress interaction predicting CES-D was examined using linear regression, adjusted for covariates.
Results
The main effect of BDNF Val66Met genotype on CES-D scores was non-significant (ps > 0.607) but the adulthood chronic stress indicator was significant (ps < 0.001) in both samples. The BDNF Val66Met genotype by adulthood chronic stress interaction was also significant (ps < 0.039) in both samples. The impact of chronic stress in adulthood on CES-D scores was significantly larger in Val/Val genotype individuals than Met carriers.
Conclusion
We found in two independent samples that depression levels increased significantly more as a function of adulthood chronic stress Val/Val genotype carriers than Met carriers. Individuals with the Val/Val genotype and chronic stress exposure could be targeted for interventions designed to reduce risk of depression if this finding is confirmed in future studies.
Keywords: BDNF Val66Met, Depression, Chronic stress, Rs6265, CES-D
1. Introduction
Brain-derived neurotrophic factor (BDNF) is one of the mammalian neurotrophin-family proteins that supports the survival of existing neurons, contributes to the growth and differentiation of new neurons and synapses (Huang and Reichardt, 2001), and protects against stress-induced neuronal damage (Radecki et al., 2005). In the brain, it is active in the hippocampus, cortex, and basal forebrain – areas vital to learning, memory, and higher thinking (Yamada and Nabeshima, 2003). Studies have reported that BDNF may be involved in the pathophysiology of depression and/or the effective treatment of depression. The role of BDNF in depression is also supported by studies that indicate the variation in the human BDNF gene associated with depression, although the findings are mixed.
BDNF protein is encoded by the BDNF gene in humans on chromosome 11. BDNF Val66Met (rs6265) – is a SNP with a G/A allele polymorphism, resulting in variation between valine and methionine at codon66 (Bath and Lee, 2006; Egan et al., 2003). The Val/Val genotype has been reported to be associated with increased CNS gene expression relative to Val/Met and Met/Met (McHughen et al., 2010). Findings from studies that examine the association between this functional BDNF Val66Met and depression are mixed. Some studies have found such an association (Czira et al., 2011; Duncan et al., 2009; Hwang et al., 2006; Licinio et al., 2009; Ribeiro et al., 2007; Sen et al., 2003; Taylor et al., 2007; Verhagen et al., 2010), others have not (Chen et al., 2008; Gratacos et al., 2007; Hong et al., 2003; Schumacher et al., 2005; Surtees et al., 2007). Even among the studies finding an association between BDNF Val66Met and depression, the risk conferred by the genotype is inconsistent. Some studies showed that individuals with the Val/Val (G/G) genotype had an increased depressive trait, such as major depression disorder (Licinio et al., 2009; Ribeiro et al., 2007), scores on Beck Depression Inventory – II (BDI – II) (Duncan et al., 2009), and depression facet scores in neuroticism (Sen et al., 2003), whereas others report that Met/Met (A/A) subjects have more severe symptoms of depression (Czira et al., 2011; Hwang et al., 2006; Taylor et al., 2007; Verhagen et al., 2010). These reported associations of both alleles with increased levels of depression indicate that the reported association of the Val/Val genotype with increased CNS gene expression (McHughen et al., 2010) does not result in a straightforward association of that genotype with resistance to depression. Instead, they raise possibility that environmental factors moderate the influence of Val66Met on BDNF expression and hence the association of BNDF genotype with indices of depression.
Chronic stress is one such environmental factor that is a trigger for several psychiatric disorders, including depression (Kendler et al., 1999). Chronic stress has neurotoxic effects, including damage to hippocampal cells (Sapolsky, 2000) that may underlie symptoms of depression. Existing evidence supports the involvement of BDNF Val66Met in stress-sensitivity, depressive states and the development of brain structures related to emotional processing and depression, like the hippocampus and amygdala (Gatt et al., 2007; Joffe et al., 2009; Shirayama et al., 2002; van Wingen et al., 2010). The association between BDNF Val66Met and intermediate traits or depressive symptoms may be moderated, therefore, by chronic stress. The effect of this interaction on depression remains unclear, but a few studies have found that childhood stress moderates BDNF Val66Met effects on depressive symptoms. Gatt et al. (Gatt et al., 2009) reported a significant interaction of BDNF Val66Met and early life stress predicting hippocampal and amygdala volumes and working memory among Europeans. Consistent with the reported increased gene expression associated with the Val/Val genotype, the combination of BDNF Met carriers and exposure to early life stress predicted reduced gray matter in hippocampus and poorer working memory. Another recent study found that childhood stressful life events were associated with an affective memory bias (a potential cognitive intermediate phenotype for depression) but only in European young men carrying the BNDF Met allele (van Oostrom et al., 2012). One study showed that childhood sexual abuse had a greater impact on depressive symptoms in Met carriers of BDNF gene than in Val/Val group among Spanish young adults (Aguilera et al., 2009). These findings suggest that effects of childhood stress on depression and related phenotypes are more pronounced in BNDF Met carriers.
It is possible, however, that the importance and the effects of chronic stress that occurs during childhood on adult depressive symptoms may be quite different from the effects of chronic stress in adulthood. Jaffee et al. (Jaffee et al., 2002) have shown that risk factors experienced during childhood (e.g., perinatal insults, caretaker instability) were associated with juvenile-onset depression, whereas, these risk factors were less related to adult-onset depression. BDNF levels fluctuate (lower in childhood and rise in early adulthood) and have different functions throughout development, suggesting that effects on gene expression vary during development and may have different effects on behavioral outcomes at different life stages (Perea et al., 2012). Therefore, the interaction of BDNF Val66Met and adulthood chronic stress may exhibit a different impact on depression compared with its interaction with childhood stress. Perea et. al (Perea et al., 2012) recently reported that among Spanish undergraduate students the chronic stress during college years (an adulthood stress) was associated with increased negative affectivity (an underlying construct for both depressive and anxiety disorders) regardless of the BDNF Val66Met genotype, while, in this same sample, greater stress during childhood was associated with greater negative affectivity only in Met carriers. In contrast to these studies showing a larger impact of childhood stress on depression among Met carriers, Perroud et al. (Perroud et al., 2008) reported a larger impact of childhood trauma on risk of violent suicide attempt in adulthood among persons with the Val/Val genotype.
Taken altogether, the findings reviewed above suggest that the BNDF Met allele might confer increased vulnerability to depression after chronic stress exposure during childhood, but the interaction between BDNF Val66Met and chronic stress in adulthood has received much less attention. Moreover, several studies (Duncan et al., 2009; Licinio et al., 2009; Ribeiro et al., 2007; Sen et al., 2003) that have shown that the Val/Val genotype per se was associated with increased depressive traits or symptoms as a main effect (not moderated by childhood stress exposure). In the present paper, therefore, we evaluate the impact of the interaction of BDNF Val66Met and chronic stress in adulthood on depressive symptoms, first, in a U.S. sample where chronic stress is defined as caregiving for a relative with Alzheimer's disease or other dementia; and, second, in a public-access database – the Multi-Ethnic Study of Atherosclerosis (MESA) – in which chronic stress is evaluated using a measure of chronic burden, i.e., health problems (self and someone close), job, finance and relationship problems.
2. Methods
2.1. Participants
2.1.1. Duke CG
The Duke Caregiving (Duke-CG) participants were enrolled in 2001–2004 and detailed study procedures are described elsewhere (Kring et al., 2010). Briefly, caregivers, defined as having the primary responsibility for care of a spouse or relative with diagnosed Alzheimer's disease or other major dementia, were recruited using flyers, advertisements in the local media, and community outreach efforts. Controls were identified by asking caregivers to nominate two to five friends with similar demographic factors (e.g., gender, age, and race) who lived in their neighborhood. Informed written consent forms approved by the Duke University Medical Center Institutional review board were obtained from all subjects. A questionnaire battery was given to participants during the home visit by a nurse and returned on their visit to the General Clinical Research Center at Duke University Medical Center. The clinic visit was scheduled during the same week as the in-home visit, and consisted of a general physical examination, and a blood sample was drawn for genotyping. The frequency of the Met allele in African Americans was insufficient (0 Met/Met and 6 Val/Met) for statistical analyses; therefore, African-Americans were excluded in the present study The 238 Caucasians with non-missing values on all measures of interest were included in the present study, consisting of 122 caregivers and 116 controls.
2.1.2. Mesa
MESA is a federally funded multi-center, longitudinal cohort study of the factors that influence the progression of mild subclinical cardiovascular disease (CVD) to severe subclinical and clinical CVD in a multi-ethnic group of subjects (Bild et al., 2002). Information on the sampling frame and study design has been previously reported (Bild et al., 2002). Briefly, subjects without a history of clinical CVD, were recruited from six U.S. communities. Institutional review board approval was obtained at all MESA sites. Adults weighing > 300 pounds were not eligible for participation. MESA data included four ethnic groups: Caucasian, Chinese, African-American and Hispanic. The frequency of the Met allele was much higher in an Asian sample (47%) than the frequencies of 17–21% that have been observed in western samples (Kim et al., 2007), therefore, we excluded Chinese samples in this study. The data used in the present study includes the first examination between July 2000 and August 2002 for self-reported ethnic groups Caucasian, African-American, and Hispanic. This resulted in 5524 MESA participants for the current study.
2.2. Depression measure
The Center for Epidemiologic Studies of Depression (CES-D) scale was used to measure depressive symptoms in both samples. CES-D is a widely used 20-item self-report scale designed to measure depressive symptoms in a general population (Radloff, 1977). Items were scored on a 4-point scale (0–3) with the total CES-D score ranging 0 to 60. CES-D scores were transformed using the square root value in both samples to better meet the distributional assumptions of the statistical model.
2.3. Genotyping
The genotyping of BDNF Val66Met (rs6265) for the Duke-CG sample was conducted using ABI 7900 Taqman system (Applied Biosystems, Carlsbad, California, USA) using standard genotyping protocols implemented at the Center of Human Genetics at Duke University Medical Center. Genotyping of BDNF Val66Met for the MESA sample was from genome-wide genotyping results performed at the Broad Institute of Harvard and MIT (Boston, Massachusetts, USA) and at Affymetrix (Santa Clara, California, USA) using the Affymetric Genome-Wide Human SNP Array 6.0. Both assays met quality control standards established in both labs.
2.4. Chronic stress measurement
In the Duke-CG sample, caregiving stress was used as the chronic stress for adults. Caregivers and controls were based on the sampling design of the study. Caregivers were coded 1 and controls were coded 2.
In the MESA sample, chronic burden was assessed using a measure of adulthood chronic stress based on a 5-item self-report questionnaire related to health and life: “Please tell us whether any of the following has been a problem for you – serious ongoing health problem (yourself); serious ongoing health problem (someone close to you); ongoing difficulties with your job or ability to work; ongoing financial strain; and ongoing difficulties in a relationship with someone close to you. The response of “yes” to any of 5 questions was scored as 1 and 0 if response was “no”. The total score was calculated by summing the item responses; scores this ranged from 0 = lowest stress to 5 = highest stress. If a response was missing on any of the five items, no total score was coded.
2.5. Covariates
Covariates were selected a priori based on substantive knowledge. Use of medication commonly prescribed to treat depression was collected by self-report in both samples. This medication included tricyclic anti-depressants, tricyclic anti-depressant/anti-psychotic combinations, MAO inhibitors, and non-tricyclic antidepressants other than MAO inhibitors (SSRIs) in both Duke-CG and MESA samples. Medication use was coded 1 if any of these medications were used, and 0 for none used. MESA data was collected from 6 sites which coded as 3 – Forsyth County, North Carolina; 4 – New York, New York; 5 – Baltimore City and Baltimore County, Maryland; 6 – St. Paul, Minnesota; 7 – Chicago, Illinois; and 8 – Los Angeles County, California.
2.6. Statistical analysis
The primary analysis was carried out using multiple linear regression models. In a preliminary phase of analysis, we evaluated non-additivity by testing effects on depressive symptom levels of three-way interactions of BDNF Val66Met by caregiving status/chronic burden by each of the three demographic variables, race, gender, and age. If the three-way terms were not significant, it indicated that gene effects on depressive symptoms were not moderated by race, gender or age and the three-way interactions can be removed from the model. Analyses then tested a model predicting CES-D scores, with main effects terms for BDNF Val66-Met genotype and chronic stress, the BDNF Val66Met × chronic stress interaction term. In the MESA sample, the model also included the adjustment covariates gender, age, BMI, antidepressant use, race, and site. Race and site were not included in the model for the Duke-CG sample, as the Duke-CG sample is comprised of only white participants and was obtained at a single site. Due to the low frequency of Met/Met carriers, Met/Met and Val/Met were collapsed into a single group, which we labeled as Met carriers. Therefore the analysis compares Val/Val with the combined group of Met/Met and Val/Met genotypes. Regression analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, North Carolina). In a supplementary analysis to rule out gene/environment associations, we also examined the association between BDNF Val66Met genotype and caregiving status or chronic burden. A significance level of 0.05 was used for all analyses.
3. Results
3.1. Participant characteristics
Table 1 shows the demographic characteristics, genotype frequencies and chronic stress distributions for the two samples. There were more women than men in the Duke-CG sample, whereas in MESA gender was relatively balanced. The mean age was early sixties and non-significantly different (p = 0.490) between the Duke-CG and MESA samples. The mean BMI was larger (p < 0.001) in the MESA sample than Duke-CG sample. A greater (p < 0.001) percentage of subjects in the Duke-CG sample used anti-depressants, as compared to the MESA sample. In the Duke-CG sample the number of caregivers was similar to the number of controls. In MESA, about one third of subjects reported no chronic burden. CES-D scores were significantly higher (p < 0.001) in the Duke-CG sample, as compared to in the MESA sample. The frequencies of BDNF Val66Met did not differ between Duke-CG and MESA samples. The overall genotype distribution met Hardy– Weinberg equilibrium (HWE) in the Duke-CG sample (p = 0.52) but not in the MESA sample (p < 0.0001). The deviation in the MESA sample may be due to genotyping error or sample stratification. The BDNF genotype was obtained from public dbGap data which had been examined for genotyping error by published criteria. We do not have access to the raw data to further evaluate genotyping error as the source of HWE disequilibrium. The genotype distribution in Table 1 included Caucasians, African Americans and Hispanics. We tested HWE stratified by ethnic group and found that the deviation from HWE was only seen in the Caucasians (p = 0.001), which seems to be driving the deviation from HWE in the overall dataset. We further examined HWE in Caucasians by collection site. Site 3 and Site 4 showed a deviation from HWE suggesting that HWE could also be due to differences in the sites. If we excluded data at these two sites, and there was no longer a deviation from HWE (p = 0.204). Our further analyses were adjusted for site to control for these potential differences.
Table 1. Study sample characteristics.
| Duke-CG | MESA | |
|---|---|---|
| Number of samples | 238 | 5524 |
| Age (years, mean ± SD) | 61.7 ± 13.6 | 62.2 ± 10.2 |
| BMI (kg/m2, mean ± SD)a | 26.5 ± 5.1 | 28.9 ± 5.4 |
| Anti-depressant Medication use (%)a | 43 (18.1) | 459 (8.3) |
| CES-D (mean ± SD)a | 10.0 ± 9.5 | 7.7 ± 7.7 |
| Gender | ||
| Men (%) | 65 (27.3) | 2618 (47.4) |
| Women (%) | 173 (72.7) | 2906 (52.6) |
| BDNF Val66Met genotype | ||
| Val/Val (%) | 162 (68.1) | 4117 (74.5) |
| Val/Met (%) | 67 (28.1) | 1246 (22.6) |
| Met/Met (%) | 9 (3.8) | 161 (2.9) |
| Caregiving stress | ||
| Caregiver (%) | 122 (51.3) | |
| Control (%) | 116 (48.7) | |
| Chronic burden | ||
| 0 (%) | 1753 (31.7) | |
| 1 (%) | 1782 (32.3) | |
| 2 (%) | 1149 (20.8) | |
| 3 (%) | 516 (9.4) | |
| 4 (%) | 240 (4.3) | |
| 5 (%) | 84 (1.5) |
The variable was significant different between Duke-CG and MESA samples
3.2. Main and interaction effects of BDNF Val66Met and adulthood chronic stress
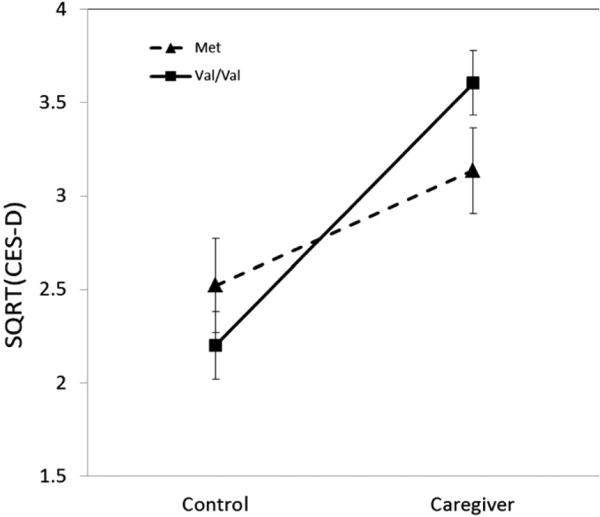
In the Duke-CG sample, none of the three way interactions were significant (ps > 0.1). The BDNF Val66Met genotype was not associated with caregiving status (p = 0.241) indicating at best a very modest correlation between BDNF Val66Met genotype and caregiving status. The main effect of caregiving stress group was statistically significantly associated with CES-D scores (p < 0.001), but the main effect of BDNF Val66Met genotype was not significant (p > 0.607). The interaction of BDNF Val66Met × caregiving stress status was significantly associated with CES-D scores (p = 0.039, Table 2). This interaction is illustrated in Fig. 1, where it can be seen that the increase in CES-D scores as a function of caregiving stress was larger in participants with the Val/Val genotype (p < 0.001, Cohen's d = 0.72) than those carrying the Met allele (p = 0.045, Cohen's d = 0.49).
Table 2. Model estimates, standard errors (SE) and p-values on square root of CES-D scores in both samples.
| Effect | Duke-CG | MESA | ||||
|---|---|---|---|---|---|---|
| Estimate | SE | P | Estimate | SE | P | |
| Intercept | 1.854 | 0.675 | 0.007 | 1.887 | 0.170 | <0.0001 |
| Age | −0.008 | 0.007 | 0.289 | 0.003 | 0.002 | 0.045 |
| Gender (female as reference) | −0.039 | 0.223 | 0.860 | −0.243 | 0.035 | <0.0001 |
| Anti-depressant mediaction | 0.329 | 0.231 | 0.156 | 0.409 | 0.063 | <0.0001 |
| BMI | 0.038 | 0.017 | 0.027 | 0.004 | 0.003 | 0.167 |
| Race = Caucasian | −0.319 | 0.051 | <0.0001 | |||
| Race = African American | −0.313 | 0.057 | <0.0001 | |||
| Site = 3 | −0.061 | 0.068 | 0.376 | |||
| Site = 4 | 0.347 | 0.061 | <0.0001 | |||
| Site = 5 | 0.078 | 0.068 | 0.255 | |||
| Site = 6 | 0.191 | 0.062 | 0.002 | |||
| Site = 7 | 0.084 | 0.072 | 0.243 | |||
| BDNF Val66Met | 0.320 | 0.277 | 0.250 | 0.097 | 0.057 | 0.088 |
| Chronic Stress | 1.402 | 0.217 | <0.0001 | 0.430 | 0.017 | <0.0001 |
| BDNF Val66Met × Chronic Stress | −0.788 | 0.379 | 0.039 | −0.091 | 0.032 | 0.005 |
Reference group: no anti-depressant medication use, Val/Val genotype, no chronic stress exposure and females in Duke-CG;no anti-depressant medication use, Val/Val genotype, no chronic stress exposure, female Hispanic at site = 8 in MESA.
Fig.1.
The mean of SQRT (CES-D) by BNDF Val66Met genotype and caregiving status in Duke-CG, interaction p = 0.039.
In MESA, the three-way interactions again were not significant (ps > 0.194). The correlation between BDNF Val66Met genotype and chronic burden in MESA also was non-significant (p = 0.523).
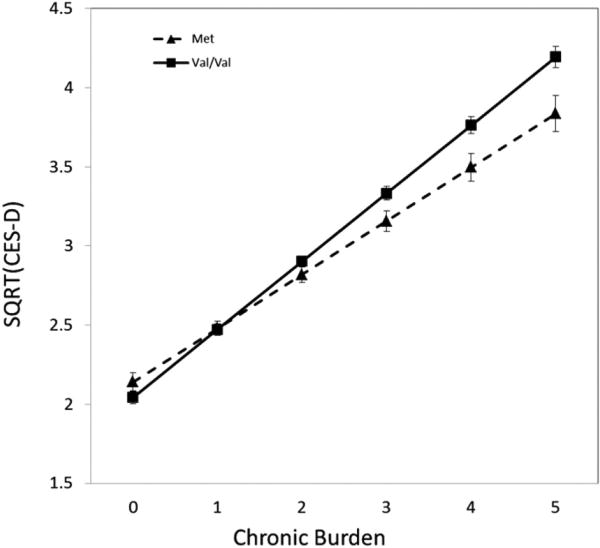
Similar to the findings in the Duke-CG sample, the main effect of chronic burden on CES-D scores was statistically significant (p < 0.001) in MESA, and the main effect of BDNF Val66Met polymorphism was not significant (p = 0.680). Again, as in the Duke-CG sample, the interaction of BDNF Val66Met × chronic burden was significantly associated with CES-D scores (p = 0.005, Table 2). Although there is a deviation from HWE in the MESA sample probably driven by the differences at site 3 and site 4 in Caucasians, we adjusted for site in the analyses to control these differences. We also performed the analyses excluding data at these two sites and the interaction remained significant, suggesting that the lack of HWE is not driving the significant result. As shown in Fig. 2, in subjects with lower chronic burden scores, CES-D scores did not differ between BDNF Val66Met genotype, but as chronic burden increased, CES-D scores increased, and the increase in CES-D scores as a function of chronic burden was larger in participants with the Val/Val genotype than those carrying the Met allele. Among individuals with high chronic burden = 5, the predicted mean CES-D scores for Val/Val carriers was higher than Met carriers (p = 0.005, Cohen's d = 0.60), while among subjects with low chronic burden = 0, mean CES-D scores did not differ significantly between Val/Val carriers and Met carriers (p = 0.090, Cohen's d = 0.16). The mean CES-D score of 17.6 among those with chronic burden = 5 and the Val/Val genotype is in the clinically significant range (>16) for this scale (Radloff, 1977), thereby highlighting the potential clinical significance of the larger impact of Val/Val genotype in persons exposed to high chronic stress levels.
Fig. 2.
The mean of SQRT (CES-D) by BNDF Val66Met genotype and chronic burden in MESA, interaction p = 0.005.
4. Discussion
Our results show a consistent pattern of moderation of the association between chronic stress in adulthood and depressive symptom levels by BDNF Val66Met genotype in two independent samples. In contrast to the pattern observed for childhood stress in other studies, where persons carrying the Met allele have exhibited impaired memory (Gatt et al., 2009), decreased recall of positive words (van Oostrom et al., 2012) and increased depressive symptoms (Aguilera et al., 2009), we found in two samples that depressive symptom levels increased significantly more as a function of chronic stress among those with the Val/Val genotype than those carrying the Met allele. Observing a similar pattern in two independent samples makes a strong case that the association between BDNF Val66Met genotype and depressive symptoms is moderated by adulthood chronic stress in a way that differs from the moderation of BDNF Val66Met by childhood stress.
In addition to differential moderation of gene effects by chronic stress occurring in childhood vs. adulthood, the present findings also may explain the inconsistent main effects of BDNF Val66Met genotype on depressive symptom levels in prior research with some studies showing higher depression levels in Met carriers (Czira et al., 2011; Hwang et al., 2006; Taylor et al., 2007; Verhagen et al., 2010). Other studies, however, found the main effect of Val/Val genotype associated with higher levels of depressive symptoms or disorders. One used a sample of 189 cigarette smokers who smoked an average of 16 cigarettes per day (Duncan et al., 2009), one used a family sample of subjects with a systolic blood pressure in the upper 15% of blood pressure distribution at earlier examination (Sen et al., 2003), and the samples in two others (Licinio et al., 2009; Ribeiro et al., 2007) consisted of Mexican-Americans living in the Los Angeles area. Each of these samples probably could be considered a group exposed to chronic stress, whether that accompanying the habit of cigarette smoking or having high blood pressure or of being a member of a minority group in a large metropolitan area (Farley et al., 2005; Finch and Vega, 2003). The present study's findings of increased impact of chronic stress on depressive symptoms in persons with the Val/Val genotype in two independent samples could account for the finding of increased depressive symptoms/disorders in these four studies (Duncan et al., 2009; Licinio et al., 2009; Ribeiro et al., 2007; Sen et al., 2003) using samples that likely to be characterized as exposed to increased levels of chronic stress in adulthood. In contrast, studies finding no effect of Val/Val genotype, or even an effect of the Met allele, to increase depressive symptoms were conducted in samples of older adults not likely to be selected for exposure to chronic stress (Czira et al., 2011).
There are additional prior studies that found significant BDNF Val66Met genotype × childhood adversity effects on risk of violent suicide attempt or depressive symptoms in adulthood (Aguilera et al., 2009; Gatt et al., 2009; Perroud et al., 2008; van Oostrom et al., 2012; Wichers et al., 2008). Similar to our findings, one study found increased risk of violent suicide attempts in persons with the Val/Val genotype and exposure to sexual abuse in childhood, but no effect of childhood trauma in Met carriers (Perroud et al., 2008). However, our finding that Val/Val conferred higher risk (bigger effect size) for depression among groups chronically stressed in adulthood was inconsistent with the findings in other studies that found a larger impact of childhood sexual abuse on adult depressive symptoms in Met carriers than in Val/Val group (Aguilera et al., 2009; Gatt et al., 2009; van Oostrom et al., 2012; Wichers et al., 2008). This inconsistency could stem from the difference in the importance and effects of chronic stress between childhood and adulthood. As previously noted, significant childhood adversity has been differentially related to juvenile vs. adultonset depression (Jaffee et al., 2002). In addition, most individuals who experience depression in adulthood do not report depression during childhood (Klein et al., 1999) although a significant proportion of depressed children become depressed adults (Harrington et al., 1990; Lewinsohn et al., 2000); and children and adults responded differently to anti-depressant drugs indicating a difference in their biological response to chronic stress (Bylund and Reed, 2007) Also, BDNF levels fluctuate through the lifespan (lower in childhood and rise in early adulthood) and have different functions throughout development, particularly during development, that could result in varying behavioral outcomes (Perea et al., 2012). Czira et al. (2011) reported the association between BDNF Val66Met genotype and depression symptoms was restricted to older individuals (≥74 years) and men, suggesting age and gender effects in this association. Retrospective reports of childhood stress are subject to recall bias that was associated with genotype in the studies that found Met carriers more sensitive to effects of childhood stress (Wichers et al., 2008). In our study the BDNF Val66Met genotype was not associated with chronic stress in adulthood (ps > 0.241) in both Duke-CG and MESA samples. The stronger association between stressful life events and prevalence of depression in Met carriers than those with the Val/Val genotype in an elderly Korean sample (Kim et al., 2007) is at variance with our findings in the present study. The frequency of the Met allele was much higher in this Korean sample (47%) than the frequencies of 17–21% that have been observed in western samples (Kim et al., 2007), suggesting the possibility that effects of the BDNF Val66-Met variants on gene function could be different in Asian populations.
The present study had certain limitations. Depressive symptoms were assessed using the CES-D scores, rather than a clinical diagnosis and thus the results may not reflect observations in clinical samples. The measure of chronic stress was different across the two samples and the stress indexed by the measure of chronic burden in MESA was likely to be less severe than that of being a caregiver for a relative with major dementia. Although we adjusted for the use of anti-depressant medications in both samples, it is still possible that the use of anti-depressants may have resulted in an underestimate of depressive symptoms. The evaluation of gene–environment interactions is not a simple matter (Munafo and Flint, 2009; Zammit, Owen, & Lewis, 2010a; Zammit, Wiles, & Lewis, 2010b), and we cannot be certain what mechanism is responsible for our finding that Val/Val genotype is associated with a larger impact of chronic stress on depressive symptoms in our two samples. One possibility is that the effect of Val/Val genotype on BDNF expression is different in older persons exposed to current stress than in younger persons exposed to stress in childhood. It is also possible that chronic stress exerts epigenetic effects that modify the influence of Val/Val genotype on BDNF expression. More research will be required to identify the responsible mechanism(s). Further research is needed to better understand the biological mechanisms of BDNF polymorphisms in brain functioning and in response to chronic stress, and may help us explain the inconsistency of the risk alleles of BDNF Val66Met on depressive symptoms.
In conclusion, this study's findings in two independent samples of increased depressive symptoms in persons with the Val/Val genotype who are exposed to chronic stress in adulthood make a strong case for moderation of the association between BDNF Val66Met genotype and depression by exposure to chronic stress in adulthood. The fact that both samples in the present study were older also suggests the importance of this gene with regard to symptoms of depression in late life. More research will be required to determine which BDNF Val66Met variants are associated with increased depression risk in persons exposed to childhood trauma and whether there are population differences in not only allele frequency but also allele effects on gene function. If the present findings are confirmed in further research, intervention studies could be undertaken to determine whether behavioral interventions that have been found effective in reducing depression symptom levels in persons exposed to chronic stress (Bishop et al., 2005; Williams et al., 2010) will be effective in preventing depression in persons with the Val/Val genotype who are living under conditions of chronic stress. Table 2
Acknowledgments
We thank Professor Elizabeth R. Hauser and Dr. Abanish Singh who applied for the authorized access to dbGap to use MESA data.
Role of the funding source: This research was supported by the National Heart, Lung, and Blood Institutes grant P01 HL036587; the National Institute on Aging grant R01AG19605, with co-funding by National Institute of Environmental Health Sciences; the Duke Clinical Research Unit grant M01 RR000030; and the Duke Behavioral Medicine Research Center. MESA and the MESA SHARe project are conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with MESA investigators. Support for MESA is provided by contracts N01-HC-95159 through N01-HC-95169 and RR-024156. Funding for genotyping was provided by NHLBI Contract N02-HL-6-4278 and N01-HC-65226. This manuscript was not prepared in collaboration with MESA investigators and does not necessarily reflect the opinions or views of MESA, or the NHLBI.
Footnotes
Contributors: RJ and BHB came up with the specific hypothesis of this study; ICS and RBW designed the study and collected data; RJ managed the literature searches and analyses, and undertook the statistical analyses; RJ, BHB, and MAB discussed and interpreted the data and results; RJ wrote the first draft of the manuscript; BHB, MAB, ICS and RBW edited the manuscript and gave suggestions. All authors contributed to and have approved the final manuscript.
Conflict of interest: Redford B. Williams is a founder and major stockholder in Williams LifeSkills, Inc.
References
- Aguilera M, Arias B, Wichers M, Barrantes-Vidal N, Moya J, Villa H, et al. Early adversity and 5-HTT/BDNF genes: new evidence of gene-environment interactions on depressive symptoms in a general population. Psychological Medicine. 2009;39:1425–32. doi: 10.1017/S0033291709005248. [DOI] [PubMed] [Google Scholar]
- Bath KG, Lee FS. Variant BDNF (Val66Met) impact on brain structure and function. Cognitive, Affective, and Behavioral Neuroscience. 2006;6:79–85. doi: 10.3758/cabn.6.1.79. [DOI] [PubMed] [Google Scholar]
- Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multiethnic study of atherosclerosis: objectives and design. American Journal of Epidemiology. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- Bishop GD, Kaur D, Tan VL, Chua YL, Liew SM, Mak KH. Effects of a psychosocial skills training workshop on psychophysiological and psychosocial risk in patients undergoing coronary artery bypass grafting. American Heart Journal. 2005;150:602–9. doi: 10.1016/j.ahj.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Bylund DB, Reed AL. Childhood and adolescent depression: why do children and adults respond differently to antidepressant drugs? Neurochemistry International. 2007;51:246–53. doi: 10.1016/j.neuint.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Lawlor DA, Lewis SJ, Yuan W, Abdollahi MR, Timpson NJ, et al. Genetic association study of BDNF in depression: finding from two cohort studies and a meta-analysis. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147B:814–21. doi: 10.1002/ajmg.b.30686. [DOI] [PubMed] [Google Scholar]
- Czira ME, Wersching H, Baune BT, Berger K. Brain-derived neurotrophic factor gene polymorphisms, neurotransmitter levels, and depressive symptoms in an elderly population. Age (Dordrecht) 2011 doi: 10.1007/s11357-011-9313-6. http://dx.doi.org/10.1007/s11357-011-9313-6 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Duncan LE, Hutchison KE, Carey G, Craighead WE. Variation in brain-derived neurotrophic factor (BDNF) gene is associated with symptoms of depression. Journal of Affective Disorders. 2009;115:215–9. doi: 10.1016/j.jad.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Farley T, Galves A, Dickinson LM, Perez Mde J. Stress, coping, and health: a comparison of Mexican immigrants, Mexican-Americans, and non-Hispanic whites. Journal of Immigrant Health. 2005;7:213–20. doi: 10.1007/s10903-005-3678-5. [DOI] [PubMed] [Google Scholar]
- Finch BK, Vega WA. Acculturation stress, social support, and self-rated health among Latinos in California. Journal of Immigrant Health. 2003;5:109–17. doi: 10.1023/a:1023987717921. [DOI] [PubMed] [Google Scholar]
- Gatt JM, Clark CR, Kemp AH, Liddell BJ, Dobson-Stone C, Kuan SA, et al. A genotype-endophenotype-phenotype path model of depressed mood: integrating cognitive and emotional markers. Journal of Integrative Neuroscience. 2007;6:75–104. doi: 10.1142/s0219635207001398. [DOI] [PubMed] [Google Scholar]
- Gatt JM, Nemeroff CB, Dobson-Stone C, Paul RH, Bryant RA, Schofield PR, et al. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Molecular Psychiatry. 2009;14:681–95. doi: 10.1038/mp.2008.143. [DOI] [PubMed] [Google Scholar]
- Gratacos M, Gonzalez JR, Mercader JM, de Cid R, Urretavizcaya M, Estivill X. Brain-derived neurotrophic factor Val66Met and psychiatric disorders: meta-analysis of case-control studies confirm association to substance-related disorders, eating disorders, and schizophrenia. Biological Psychiatry. 2007;61:911–22. doi: 10.1016/j.biopsych.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Harrington R, Fudge H, Rutter M, Pickles A, Hill J. Adult outcomes of childhood and adolescent depression. I. Psychiatric status. Archives of General Psychiatry. 1990;47:465–73. doi: 10.1001/archpsyc.1990.01810170065010. [DOI] [PubMed] [Google Scholar]
- Hong CJ, Huo SJ, Yen FC, Tung CL, Pan GM, Tsai SJ. Association study of a brain-derived neurotrophic-factor genetic polymorphism and mood disorders, age of onset and suicidal behavior. Neuropsychobiology. 2003;48:186–9. doi: 10.1159/000074636. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annual Review of Neuroscience. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JP, Tsai SJ, Hong CJ, Yang CH, Lirng JF, Yang YM. The Val66Met polymorphism of the brain-derived neurotrophic-factor gene is associated with geriatric depression. Neurobiology of Aging. 2006;27:1834–7. doi: 10.1016/j.neurobiolaging.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Moffitt TE, Caspi A, Fombonne E, Poulton R, Martin J. Differences in early childhood risk factors for juvenile-onset and adult-onset depression. Archives of General Psychiatry. 2002;59:215–22. doi: 10.1001/archpsyc.59.3.215. [DOI] [PubMed] [Google Scholar]
- Joffe RT, Gatt JM, Kemp AH, Grieve S, Dobson-Stone C, Kuan SA, et al. Brain derived neurotrophic factor Val66Met polymorphism, the five factor model of personality and hippocampal volume: Implications for depressive illness. Human Brain Mapping. 2009;30:1246–56. doi: 10.1002/hbm.20592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. American Journal of Psychiatry. 1999;156:837–41. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Kim YH, et al. Interactions between life stressors and susceptibility genes (5-HTTLPR and BDNF) on depression in Korean elders. Biological Psychiatry. 2007;62:423–8. doi: 10.1016/j.biopsych.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Klein DN, Schatzberg AF, McCullough JP, Dowling F, Goodman D, Howland RH, et al. Age of onset in chronic major depression: relation to demographic and clinical variables, family history, and treatment response. Journal of Affective Disorders. 1999;55:149–57. doi: 10.1016/s0165-0327(99)00020-8. [DOI] [PubMed] [Google Scholar]
- Kring SI, Brummett BH, Barefoot J, Garrett ME, Ashley-Koch AE, Boyle SH, et al. Impact of psychological stress on the associations between apolipoprotein E variants and metabolic traits: findings in an American sample of caregivers and controls. Psychosomatic Medicine. 2010;72:427–33. doi: 10.1097/PSY.0b013e3181de30ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Rohde P, Seeley JR, Klein DN, Gotlib IH. Natural course of adolescent major depressive disorder in a community sample: predictors of recurrence in young adults. American Journal of Psychiatry. 2000;157:1584–91. doi: 10.1176/appi.ajp.157.10.1584. [DOI] [PubMed] [Google Scholar]
- Licinio J, Dong C, Wong ML. Novel sequence variations in the brain-derived neurotrophic factor gene and association with major depression and antidepressant treatment response. Archives of General Psychiatry. 2009;66:488–97. doi: 10.1001/archgenpsychiatry.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHughen SA, Rodriguez PF, Kleim JA, Kleim ED, Marchal Crespo L, Procaccio V, et al. BDNF val66met polymorphism influences motor system function in the human brain. Cerebral Cortex. 2010;20:1254–62. doi: 10.1093/cercor/bhp189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Flint J. Replication and heterogeneity in gene × environment interaction studies. International Journal of Neuropsychopharmacology. 2009;12:727–9. doi: 10.1017/S1461145709000479. [DOI] [PubMed] [Google Scholar]
- Perea CS, Paternina AC, Gomez Y, Lattig MC. Negative affectivity moderated by BDNF and stress response. Journal of Affective Disorders. 2012;136:767–74. doi: 10.1016/j.jad.2011.09.043. [DOI] [PubMed] [Google Scholar]
- Perroud N, Courtet P, Vincze I, Jaussent I, Jollant F, Bellivier F, et al. Interaction between BDNF Val66Met and childhood trauma on adult's violent suicide attempt. Genes, Brain and Behavior. 2008;7:314–22. doi: 10.1111/j.1601-183X.2007.00354.x. [DOI] [PubMed] [Google Scholar]
- Radecki DT, Brown LM, Martinez J, Teyler TJ. BDNF protects against stress-induced impairments in spatial learning and memory and LTP. Hippocampus. 2005;15:246–53. doi: 10.1002/hipo.20048. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Ribeiro L, Busnello JV, Cantor RM, Whelan F, Whittaker P, Deloukas P, et al. The brain-derived neurotrophic factor rs6265 (Val66Met) polymorphism and depression in Mexican-Americans. Neuroreport. 2007;18:1291–3. doi: 10.1097/WNR.0b013e328273bcb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biological Psychiatry. 2000;48:755–65. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- Schumacher J, Jamra RA, Becker T, Ohlraun S, Klopp N, Binder EB, et al. Evidence for a relationship between genetic variants at the brain-derived neurotrophic factor (BDNF) locus and major depression. Biological Psychiatry. 2005;58:307–14. doi: 10.1016/j.biopsych.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Sen S, Nesse RM, Stoltenberg SF, Li S, Gleiberman L, Chakravarti A, et al. BDNF coding variant is associated with the NEO personality inventory domain neuroticism, a risk factor for depression. Neuropsychopharmacology. 2003;28:397–401. doi: 10.1038/sj.npp.1300053. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. Journal of Neuroscience. 2002;22:3251–61. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surtees PG, Wainwright NW, Willis-Owen SA, Sandhu MS, Luben R, Day NE, et al. No association between the BDNF Val66Met polymorphism and mood status in a non-clinical community sample of 7389 older adults. Journal of Psychiatric Research. 2007;41:404–9. doi: 10.1016/j.jpsychires.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Zuchner S, McQuoid DR, Steffens DC, Speer MC, Krishnan KR. Allelic differences in the brain-derived neurotrophic factor Val66Met polymorphism in late-life depression. American Journal of Geriatric Psychiatry. 2007;15:850–7. doi: 10.1097/JGP.0b013e318050c9d5. [DOI] [PubMed] [Google Scholar]
- van Oostrom I, Franke B, Rijpkema M, Gerritsen L, Arias-Vasquez A, Fernandez G, et al. Interaction between BDNF Val66Met and childhood stressful life events is associated to affective memory bias in men but not women. Biological Psychology. 2012;89:214–9. doi: 10.1016/j.biopsycho.2011.10.012. [DOI] [PubMed] [Google Scholar]
- van Wingen G, Rijpkema M, Franke B, van Eijndhoven P, Tendolkar I, Verkes RJ, et al. The brain-derived neurotrophic factor Val66Met polymorphism affects memory formation and retrieval of biologically salient stimuli. Neuroimage. 2010;50:1212–8. doi: 10.1016/j.neuroimage.2010.01.058. [DOI] [PubMed] [Google Scholar]
- Verhagen M, van der Meij A, van Deurzen PA, Janzing JG, Arias-Vasquez A, Buitelaar JK, et al. Meta-analysis of the BDNF Val66Met polymorphism in major depressive disorder: effects of gender and ethnicity. Molecular Psychiatry. 2010;15:260e–71. doi: 10.1038/mp.2008.109. [DOI] [PubMed] [Google Scholar]
- Wichers M, Kenis G, Jacobs N, Mengelers R, Derom C, Vlietinck R, et al. The BDNF Val(66)Met × 5-HTTLPR × child adversity interaction and depressive symptoms: an attempt at replication. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147B:120–3. doi: 10.1002/ajmg.b.30576. [DOI] [PubMed] [Google Scholar]
- Williams VP, Bishop-Fitzpatrick L, Lane JD, Gwyther LP, Ballard EL, Vendittelli AP, et al. Video-based coping skills to reduce health risk and improve psychological and physical well-being in Alzheimer's disease family caregivers. Psychosomatic Medicine. 2010;72:897–904. doi: 10.1097/PSY.0b013e3181fc2d09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Nabeshima T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. Journal of Pharmacological Sciences. 2003;91:267–70. doi: 10.1254/jphs.91.267. [DOI] [PubMed] [Google Scholar]
- Zammit S, Owen MJ, Lewis G. Misconceptions about gene-environment interactions in psychiatry. Evidence-Based Mental Health. 2010a;13:65–8. doi: 10.1136/ebmh.13.3.65. [DOI] [PubMed] [Google Scholar]
- Zammit S, Wiles N, Lewis G. The study of gene–environment interactions in psychiatry: limited gains at a substantial cost? Psychological Medicine. 2010b;40:711–6. [Google Scholar]