Abstract
Purpose
This study aimed to assess the effect of the HEALTHY intervention on the metabolic syndrome (Met-S), fitness, and physical activity levels of US middle-school students.
Methods
Cluster randomized controlled trial conducted in 42 (21 intervention) US middle schools. Participants were recruited at the start of sixth grade (2006) when baseline assessments were made, with post-assessments made 2.5 yr later at the end of eighth grade (2009). The HEALTHY intervention had four components: 1) improved school food environment, 2) physical activity and eating educational sessions, 3) social marketing, and 4) revised physical education curriculum. Met-S risk factors, 20-m shuttle run (fitness), and self-reported moderate to vigorous physical activity (MVPA) were assessed at each time point. Ethnicity and gender were self-reported. Obesity status (normal weight, overweight, or obese) was also assessed.
Results
At baseline, 5% of the participants were classified with Met-S, with two-thirds of the males and one-third of the females recording below average baseline fitness levels. Control group participants reported 96 min of MVPA at baseline with 103 min reported by the intervention group. There were no statistically significant (P < 0.05) differences in Met-S, fitness, or MVPA levels at the end of the study after adjustment for baseline values and confounders. There were no differences in any ethnic, obesity, or ethnic × obesity subgroups for either gender.
Conclusions
The HEALTHY intervention had no effect on the Met-S, fitness, or physical activity levels. Approaches that focus on how to change physical activity, fitness, and Met-S using nonschool or perhaps in addition to school based components need to be developed.
Keywords: SCHOOL-BASED INTERVENTION, PREVENTION, ADOLESCENTS, MULTICOMPONENT
The metabolic syndrome (Met-S) has been defined as a clustering of risk factors for cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM) and includes glucose intolerance, hypertension, dyslipidemia, and abdominal obesity (37). Cardiovascular fitness (fitness) and physical activity are two variables that are strongly associated with Met-S, CVD, and T2DM. Low aerobic fitness has been associated with increased prevalence of the Met-S (6), whereas the maintenance of fitness has been associated with a reduced risk of CVD mortality (1). Physical activity is associated with a reduction in the risk of CVD (31) and T2DM among adults (11). Fitness and physical activity levels are therefore essential for the prevention of Met-S, CVD, and T2DM.
Childhood is a key period in the development of fitness, physical activity behaviors, and Met-S risk factors. Data from the National Health and Nutrition Examination survey (NHANES) showed that Met-S prevalence among US adolescents increased from 9.2% in 1988–1994 to 12.7% in 1999–2000 (4). Our own data from 2003 showed that 9.5% of an ethnically diverse sample of US eighth-grade youth (i.e., 13–14 yr olds) were classified with Met-S (30). NHANES data have shown that many children and adolescents do not meet physical activity recommendations (33). NHANES data (1999–2002) also indicate that approximately a third of US adolescents (12–19 yr) did not meet recommended fitness levels (25). Both fitness and physical activity have been shown to moderately track from childhood into adolescence and onto adulthood (20). Thus, there is urgent need to develop interventions to prevent Met-S by increasing adolescent physical activity and fitness.
Higher body mass is associated with higher cholesterol, glucose, blood pressures, and waist circumference among youth (13). Higher body mass has also been associated with lower physical activity (24) and fitness levels (25) among youth. Met-S prevalence also differs by ethnicity and gender among youth (13). Thus, when assessing the effect of any intervention on Met-S prevalence, fitness, or activity, it is important to assess whether effects differ by adiposity, gender, or ethnicity.
In this article, we report on the effect of the HEALTHY intervention, a multicomponent, school-based, cluster randomized controlled trial on the physical activity, fitness, and Met-S prevalence. The specific research question being addressed is whether participation in the 3-yr HEALTHY intervention yielded higher fitness and physical activity levels or lower Met-S prevalence in the intervention group when compared with the control group. We also examined whether the effect of the intervention differed by baseline adiposity status, gender, or ethnic group.
METHODS
Details of the HEALTHY intervention have been presented in detail elsewhere (10). Briefly, however, the HEALTHY intervention was a cluster randomized controlled trial conducted in 42 (21 intervention) middle schools across the United States. Data were collected at seven field centers (Baylor College of Medicine, Houston, TX; Oregon Health & Science University, Portland, OR; University of California at Irvine, Irvine, CA; Temple University, Philadelphia, PA; University of North Carolina at Chapel Hill, Chapel Hill, NC; University of Pittsburgh, Pittsburgh, PA; and University of Texas Health Science Center at San Antonio, San Antonio, TX), with a coordinating center in Washington, DC (George Washington University Biostatistics Center). Schools were required to have at least 50% of students eligible for free or reduced-price lunch or belonging to an ethnic minority group, as well as having annual student dropout rate from all causes ≤25%. Participants were recruited at the start of sixth grade when baseline assessments were made, with post-assessments made 2.5 yr later at the end of eighth grade. Participants in the intervention schools received the HEALTHY multicomponent intervention. Control group activities were limited to recruitment and data collection only. Students were given a $50 incentive for participating in the baseline data collection and $60 for the end of study data collection. This study was approved by an institutional review board at each field center, and written informed parental consent and child assent were obtained for all participants.
The overall focus of the intervention was on helping students to consume a healthier diet and engage in increased physical activity. The intervention had four integrated components. The first component was a change in the total school food environment, with the nutritional quality of food and beverages provided during school breakfast and lunch periods improved. The second component was a program of peer-led, teacher-facilitated learning activities known as FLASH (Fun Learning Activities for Student Health). Five FLASH modules were implemented over five semesters of the HEALTHY study. Each module contained sessions that were designed to be delivered on a weekly basis to foster self-awareness, knowledge, decision-making skills, and peer involvement for health behavior change. The third component was a social marketing campaign that had a different theme for each semester of the intervention. The five themes were water consumption, encouraging physical activity instead of sedentary time, high-quality versus low-quality food, energy balance, and life choices. Each theme was supported by branding, posters, and messaging that was prominently displayed and reinforced across the school. The fourth element was a revised, more active, physical education (PE) curriculum. The PE curriculum was designed to facilitate higher student participation in the lessons and spend more time engaged in moderate to vigorous physical activity (MVPA) during PE lessons. PE teachers were trained in how to deliver the new program by an expert teacher. Schools also received around $10,000 of equipment and a teacher assistant to facilitate small group activities that were intended to increase activity time during the sessions. The primary outcome variables for the study were body mass index (BMI), fasting insulin, and fasting glucose. The results for these three outcomes have been reported elsewhere (32). This article reports the effect of the intervention on Met-S prevalence, fitness, and physical activity.
Procedures
Fitness was assessed by the 20-m shuttle test (20–MST) using standard procedures (18). As we were interested in whether the intervention effect differed by baseline adiposity status (normal weight vs obese), it was important to have a measure of fitness that was not expressed in relation to body mass (i.e., liters of oxygen per kilogram of body mass). Consequently, we used the directly recorded number of shuttle run laps completed in all analyses. For descriptive purposes, participant fitness levels were also classified as below average, average, or above average using the FITNESSGRAM, laps completed criteria (36). The primary focus of this study was the prevention of obesity and reduction of the risk factors for T2DM and associated cardiometabolic risk factors at the end of middle school, i.e., eighth grade. Thus, because of the complexity of the study, the large study size and the cost implications for all measurements, we focused our limited resources toward assessments at the sixth and eighth grades only.
As piloting of the PE component had shown that the revised curriculum yielded classes in which more than 50% of the lesson duration was spent engaged in MVPA (14) and we were interested in the effect of all elements of the intervention (revised PE classes, social marketing campaign, and behavioral component) on physical activity, we opted for an assessment of habitual physical activity rather on physical during PE classes. Because of the cost issues discussed above, a self-reported physical activity instrument was selected. Ideally, we would have used an objective assessment such as accelerometers on the entire cohort, but this would have been prohibitively expensive. We considered using accelerometers on a subsample of the participants but obtaining data that captured the physical activity levels of boys and girls from different ethnic groups, with a range of baseline activity and adiposity indicators from each school and each of the seven sites would also have necessitated a large sample that was beyond the budget for this intervention. We therefore used the 2-d version of the Self-Administered Physical Activity Checklist (SAPAC) to estimate physical activity levels and mean minutes of MVPA per day (28) at baseline (sixth grade) and at the end of study (eighth grade). The SAPAC was chosen because a study with 205 female and 116 male US adolescents, which compared the SAPAC with the previous-day physical activity recall (21), showed that the test–retest reliabilities, validity, and reliabilities of the two measures were comparable. The SAPAC, however, allowed the respondents to record short bouts of activity whereas the previous-day physical activity recall recorded activity in 30-min blocks. Because the comparison study also reported that adolescents were unable to accurately recall activity for more than 2 d, we opted to use the SAPAC as a 2-d physical activity recall.
To ensure that the SAPAC provided data on two weekdays, the instrument was completed during school hours on any day between Wednesday and Friday. Responses for time of day (before, during, or after school) were Winsorized (34) or transformed to replace extreme values of the distribution with defined cutoff values. The Winsorization cutoff values for each period were determined based on the distribution of the data at baseline and set at or near the 95th percentile for activities of at least 4 METs, thus removing time for low-level activities. The maximum values were 90 min before school, 120 min during school, and 240 min after school or a total of 450 min·d−1. MVPA was determined based on activities of at least 4.6 METs (9).
Participants were called the night before data collection to remind them not to consume anything but water after midnight. Experienced pediatric phlebotomists obtained fasting blood samples. Serum samples were shipped to the central blood laboratory at the University of Washington Northwest Lipid Research Laboratories. Analyses of glucose were performed on a Roche P module autoanalyzer by the hexokinase method using reagent from Roche Diagnostics, Indianapolis, IN. Insulin was measured by a two-site immunoenzymometric assay performed using a Tosoh 1800 autoanalyzer (South San Francisco, CA). Measurements of total cholesterol, cholesterol in the lipoprotein fractions, and triglyceride (TG) concentrations were performed enzymatically on the Roche Modular-P autoanalyzer using methods standardized to the Centers for Disease Control and Prevention Reference Methods. Determination of HDL-C was performed after precipitation of apolipoprotein B–containing particles by dextran sulfate Mg2+. LDL-C was calculated using the Friedewald equation (7).
Anthropometric measurements were conducted by personnel who were trained and certified in recording height, body weight, and waist circumference. Height and body weight were measured without shoes using the Prospective Enterprises PE-AIM-101 stadiometer (Portage, MI) and the Alpha 882 electronic scale (SECA Corporation, Hanover, MD). BMI (kg·m−2) was calculated and converted to an age- and gender-specific BMI percentile using the Centers for Disease Control and Prevention 2000 criteria (3). Youth with BMI ≥85th but <95th percentile were classified as overweight and those ≥95th percentile were classified as obese. Waist circumference was taken using a Gulick tape measure (G-tape) with a tension device on bare skin measured just above the iliac crest with the average of the first two measures that were within 1 cm of each other used in all analyses. Waist circumferences were converted to age-, sex-, and race-specific percentiles (5). Blood pressure was recorded three times using an automated blood pressure monitor (HEM- 907XL; Omron, Vernon Hills, IL). The initial value was recorded after the participant had been seated quietly for 5 min, with each subsequent value recorded 1 min after the preceding recording. The mean of the second and third recordings was used in all subsequent analyses.
Ethnicity and race were collected by student self-report: anyone checking “Hispanic or Latino” ethnicity was classified as Hispanic, non-Hispanics choosing only “Black or African American” race were classified as black, non- Hispanics choosing only “White” race were classified as white, and all other response categories were combined into “Other.” A parent or guardian completed a questionnaire providing highest level of education in the household, which was classified as low (less than high school or some high school), middle (high school graduate or some college or specialized training), or high (college or university graduate or postgraduate training or degree). Pubertal status was self-reported in private using the Pubertal Development Scale (27) and converted to five pubertal stage groups.
Analyses
Met-S was defined according to the International Diabetes Federation criteria whereby an adolescent has Met-S if he/she has abdominal obesity (waist circumference ≥90th percentile) and two of the following: fasting blood TG levels ≥1.7 mmol·L−1, HDL-C <1.03 mmol·L−1, blood pressure (BP) ≥130 mm Hg systolic or ≥85 mm Hg diastolic, or fasting glucose ≥5.6 mmol·L−1 (37).
A total of 4063 students provided complete data in both sixth and eighth grades for at least one outcome of interest, and all analyses were limited to this data set. The number of students with complete data for each analysis is provided in the tables. Descriptive statistics with means and SD for continuous measures and percentages for categorical variables are presented. General linear mixed models were used to analyze differences between intervention and control schools (22) with the covariance structure appropriately adjusted for variability both between cluster (school) and within cluster. This was accomplished by using the PROC MIXED procedure for continuous data and the PROC GLIMMIX procedure for categorical data incorporating a random effect group assignment within school in the model. Because of the skewness and zero values, the fitness and physical activity variables were square root–transformed. Models were adjusted for family history of diabetes, parental education, and pubertal status. All analyses were then repeated in ethnic, gender, and obesity subgroups. As previously reported (32), the power calculation for this study was based on detecting change in the prevalence of overweight and obesity. As such, the P values reported within this article represent findings associated with secondary outcomes and are provided to help facilitate the interpretation of the data only with α set at 0.05. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
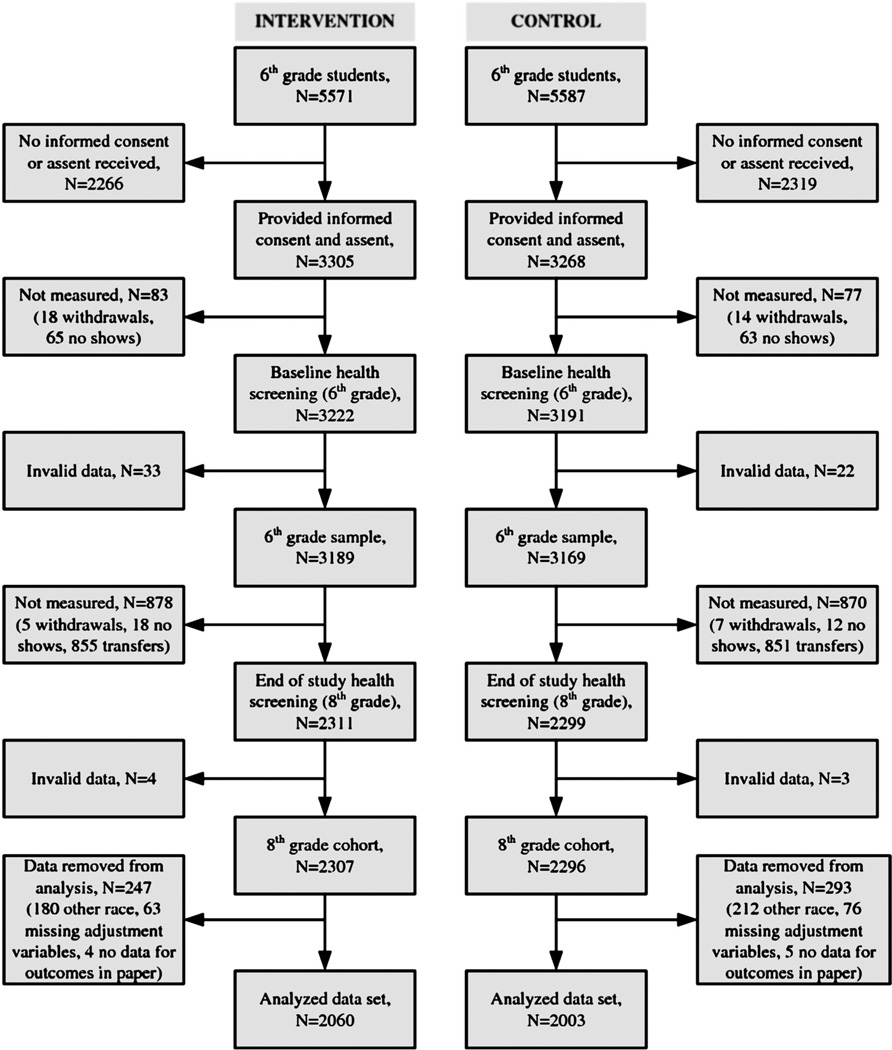
Participant characteristics are presented in Table 1, and a CONSORT-style flowchart of participant progress is presented in Figure 1. There were 5571 and 5587 potential intervention and control group participants with baseline data collected on 3222 (57.8%) and 3191 (57.1%) of these participants. Overall, 57.6% of the sixth-grade students were recruited to participate in the study, and of these, 73.4% (4603) were assessed for the primary outcome in the eighth grade. The final analyzed data set comprised 2060 intervention and 2003 control group participants thereby representing 63.9% and 62.8% of the participants who provided baseline data. The analyzed sample was composed of 59.0% Hispanic, 19.8% black, and 21.3% white, with 50.3% of the participants being either overweight (19.5%) or obese (30.8%). χ2 tests indicated that there were no statistically significant differences in the baseline demographic characteristics of the intervention and control groups.
TABLE 1.
Participant characteristics presented overall and by intervention arm (n = 4063).
| Overall | Control | Intervention | P a | |
|---|---|---|---|---|
| Age (yr), mean ± SD | 11.3 ± 0.6 | 11.3 ± 0.6 | 11.2 ± 0.5 | 0.1955 |
| Female (%) | 52.4 | 52.2 | 52.5 | 0.8508 |
| Race/ethnicity (%) | ||||
| Hispanic | 59.0 | 58.9 | 59.1 | 0.1626 |
| Black | 19.8 | 17.1 | 22.3 | |
| White | 21.3 | 24.0 | 18.6 | |
| Positive reported family history of diabetes (%) | 13.0 | 13.5 | 12.6 | 0.4085 |
| Highest household education (%) | ||||
| No high school diploma | 28.1 | 28.0 | 28.1 | 0.5447 |
| Some college | 53.4 | 52.7 | 54.2 | |
| College grad or higher | 18.5 | 19.3 | 17.7 | |
| BMI percentile (%) | 73.3 ± 27.8 | 73.2 ± 28.1 | 73.4 ± 27.6 | 0.8642 |
| <85 | 49.7 | 49.8 | 49.5 | 0.6130 |
| 85–94 | 19.5 | 19.1 | 19.9 | |
| ≥95 | 30.8 | 31.1 | 30.6 |
Comparison between intervention and control groups.
FIGURE 1.
CONSORT-style flowchart.
The prevalence of the International Diabetes Federation (IDF)-defined Met-S is presented by intervention group in Table 2, with the prevalence of each Met-S component presented for all participants and just those with the Met-S at each time point presented in Table 3. Five percent of the participants had Met-S at both time points, with the highest prevalence rates among the Hispanic group. All of the 210 participants with Met-S at baseline were classified as overweight or obese. One of the 209 participants who had Met-S at follow-up was classified as normal weight.
TABLE 2.
Preassessment and postassessment prevalence of the IDF-defined Met-S (n = 3859).a
| Control (n= 1907) |
Intervention (n= 1953) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence of Met-S at Sixth Grade |
Prevalence of Met-S at Eighth Grade |
Prevalence of Met-S at Sixth Grade |
Prevalence of Met-S at Eighth Grade |
|||||||
| n | Pct | n | Pct | n | Pct | n | Pct | P | ||
| Overall | 99 | 5.2 | 110 | 5.8 | 111 | 5.7 | 99 | 5.1 | 0.3443 | |
| Male | All | 44 | 4.8 | 73 | 8.0 | 49 | 5.3 | 68 | 7.3 | 0.5124 |
| Hispanic | 33 | 6.0 | 53 | 9.7 | 35 | 6.5 | 55 | 10.2 | 0.8857 | |
| Black | 1 | 0.7 | 4 | 2.9 | 3 | 1.5 | 5 | 2.5 | 0.7647 | |
| White | 10 | 4.5 | 16 | 7.1 | 11 | 5.7 | 8 | 4.1 | N/A | |
| Female | All | 55 | 5.5 | 37 | 3.7 | 62 | 6.1 | 31 | 3.0 | N/A |
| Hispanic | 36 | 6.2 | 25 | 4.3 | 45 | 7.2 | 23 | 3.7 | N/A | |
| Black | 6 | 3.4 | 4 | 2.3 | 6 | 2.6 | 3 | 1.3 | N/A | |
| White | 13 | 5.4 | 8 | 3.3 | 11 | 6.6 | 5 | 3.0 | N/A | |
| BMI <85th percentile | 0 | 0 | 1 | 0.1 | 0 | 0 | 0 | 0 | N/A | |
| Male | All | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | N/A |
| Hispanic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | N/A | |
| Black | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | N/A | |
| White | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | N/A | |
| Female | All | 0 | 0 | 1 | 0.2 | 0 | 0 | 0 | 0 | N/A |
| Hispanic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | N/A | |
| Black | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | N/A | |
| White | 0 | 0 | 1 | 0.7 | 0 | 0 | 0 | 0 | N/A | |
| BMI ≥85th percentile | 99 | 10.4 | 109 | 11.5 | 111 | 11.3 | 99 | 10.1 | 0.3340 | |
| Male | All | 44 | 9.1 | 73 | 15.1 | 49 | 10.0 | 68 | 13.9 | 0.5104 |
| Hispanic | 33 | 10.9 | 53 | 17.4 | 35 | 11.0 | 55 | 17.4 | 0.9289 | |
| Black | 1 | 1.4 | 4 | 5.4 | 3 | 3.6 | 5 | 6.0 | 0.8174 | |
| White | 10 | 9.4 | 16 | 15.1 | 11 | 12.4 | 8 | 9.0 | N/A | |
| Female | All | 55 | 11.8 | 36 | 7.7 | 62 | 12.6 | 31 | 6.3 | N/A |
| Hispanic | 36 | 12.7 | 25 | 8.8 | 45 | 14.8 | 23 | 7.6 | N/A | |
| Black | 6 | 6.6 | 4 | 4.4 | 6 | 5.1 | 3 | 2.5 | N/A | |
| White | 13 | 14.0 | 7 | 7.5 | 11 | 15.3 | 5 | 6.9 | N/A | |
P values represent comparisons between intervention and control groups with the model adjusted for baseline values, family history of diabetes, year 8 Tanner stage, and parental education.
N/A, not applicable.
TABLE 3.
Prevalence of each component of Met-S for all participants and those with IDF-defined Met-S (n = 3860).
| All Participants |
Participants with Met-S |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total |
Control |
Intervention |
Total |
Control |
Intervention |
|||||||
| n | Pct | n | Pct | n | Pct | n | Pct | n | Pct | n | Pct | |
| Sixth grade | ||||||||||||
| Waist ≥90th percentile | 1140 | 29.5 | 558 | 29.3 | 582 | 29.8 | 210 | 100.0 | 99 | 100.0 | 111 | 100.0 |
| TG ≥150 mg·dL−1 | 388 | 10.1 | 204 | 10.7 | 184 | 9.4 | 146 | 69.5 | 65 | 65.7 | 81 | 73.0 |
| HDL <40 mg·dL−1 | 518 | 13.4 | 245 | 12.8 | 273 | 14.0 | 172 | 81.9 | 80 | 80.8 | 92 | 82.9 |
| SBP ≥130 or DBP ≥85 mm Hg | 132 | 3.4 | 73 | 3.8 | 59 | 3.0 | 43 | 20.5 | 27 | 27.3 | 16 | 14.4 |
| Fasting glucose ≥100 µU·mL−1 | 621 | 16.1 | 311 | 16.3 | 310 | 15.9 | 93 | 44.3 | 47 | 47.5 | 46 | 41.4 |
| Eighth grade | ||||||||||||
| Waist ≥90th percentile | 861 | 22.3 | 439 | 23.0 | 422 | 21.6 | 209 | 100.0 | 110 | 100.0 | 99 | 100.0 |
| TG ≥150 mg·dL−1 | 263 | 6.8 | 129 | 6.8 | 134 | 6.9 | 129 | 61.7 | 66 | 60.0 | 63 | 63.6 |
| HDL <40 mg·dL−1 | 608 | 15.8 | 307 | 16.1 | 301 | 15.4 | 152 | 72.7 | 79 | 71.8 | 73 | 73.7 |
| SBP ≥130 or DBP ≥85 mm Hg | 191 | 4.9 | 109 | 5.7 | 82 | 4.2 | 60 | 28.7 | 32 | 29.1 | 28 | 28.3 |
| Fasting glucose ≥100 µU·mL−1 | 859 | 22.3 | 450 | 23.6 | 409 | 21.0 | 134 | 64.1 | 71 | 64.5 | 63 | 63.6 |
There were 83 (40 control school, 43 intervention) who had the Met-S in the sixth grade, which tracked into the eighth grade. There were no significant differences in Met-S prevalence at the follow-up assessment between the intervention and control groups when all participants were included or when any of the subgroups were compared. Inspection of Table 3 indicates that, of those participants who had the Met-S at sixth grade, 69.5% had high TG and 81.9% had low HDL-C with similar proportions evident at the eighth-grade assessment. It is, however, noticeable that the proportion of participants with the Met-S who had high fasting glucose levels increased from 44.3% at sixth grade to 64.1% at eighth grade.
The number of shuttle run laps recorded at baseline (sixth grade) and follow-up (eighth grade) is presented in Table 4. For both the intervention and control groups, the mean number of laps increased from 21 at baseline to 27 at follow-up, but there were no statistically significant differences between the two groups at the follow-up assessment. Moreover, when the models were rerun by ethnic and gender subgroups, there were no statistically significant differences. When the FITNESSGRAM criteria were applied to the baseline data, a larger proportion of males had “below-average” aerobic fitness levels (64.4% control group, 60.5% intervention group) compared with females (38.9% control group, 37.9% intervention group). In contrast, after assessment, the percent of males with “below-average” aerobic fitness (64.8% control and 64.5% intervention) was largely unchanged; however, there was a clear deterioration in the relative fitness levels of the girls with 65.9% of the control and 65.5% of the intervention girls being classified as having below-average fitness levels. Further stratification by baseline gender, ethnicity, and baseline obesity status (normal weight or overweight/obese) did not yield differences between the two intervention groups.
TABLE 4.
Preassessment and postassessment of fitness via 20-MST (laps) (n = 3709).a
| Control (n= 1839) |
Intervention (n= 1871) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sixth-Grade Laps |
Eighth-Grade Laps |
Sixth-Grade Laps |
Eighth-Grade Laps |
|||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | P | ||
| Overall | 21.3 | 12.4 | 27.3 | 17.5 | 21.1 | 12.0 | 27.3 | 17.1 | 0.7182 | |
| Male | All | 23.5 | 14.0 | 34.7 | 19.7 | 23.8 | 14.2 | 34.5 | 19.3 | 0.9895 |
| Hispanic | 22.7 | 14.0 | 35.6 | 20.2 | 23.7 | 14.7 | 35.6 | 19.9 | 0.5552 | |
| Black | 24.0 | 13.5 | 31.9 | 18.6 | 23.9 | 13.5 | 32.9 | 19.1 | 0.8166 | |
| White | 25.2 | 14.1 | 34.1 | 18.7 | 23.8 | 13.2 | 33.1 | 17.7 | 0.8511 | |
| Female | All | 19.2 | 10.4 | 20.5 | 11.6 | 18.7 | 9.0 | 20.9 | 11.3 | 0.6925 |
| Hispanic | 18.9 | 10.3 | 20.9 | 11.6 | 18.5 | 8.3 | 22.1 | 11.7 | 0.9999 | |
| Black | 17.1 | 10.1 | 17.7 | 10.9 | 18.9 | 10.0 | 17.8 | 10.0 | 0.8851 | |
| White | 21.6 | 10.5 | 21.7 | 11.7 | 19.3 | 10.1 | 20.5 | 10.7 | 0.6761 | |
| BMI <85th percentile | 26.5 | 13.5 | 32.1 | 18.5 | 26.0 | 12.7 | 31.0 | 17.8 | 0.8598 | |
| Male | All | 30.5 | 15.0 | 42.0 | 20.2 | 30.9 | 14.3 | 40.4 | 19.2 | 0.5943 |
| Hispanic | 29.7 | 16.0 | 43.8 | 20.9 | 32.4 | 15.3 | 43.5 | 19.8 | 0.4008 | |
| Black | 30.1 | 13.4 | 37.0 | 19.7 | 28.9 | 13.1 | 37.7 | 18.1 | 0.6797 | |
| White | 32.3 | 13.7 | 41.4 | 18.5 | 30.0 | 12.9 | 37.0 | 18.1 | 0.3617 | |
| Female | All | 23.3 | 11.1 | 24.2 | 12.4 | 22.1 | 9.6 | 23.2 | 12.1 | 0.9373 |
| Hispanic | 23.1 | 11.3 | 24.8 | 12.7 | 21.5 | 8.9 | 24.2 | 12.7 | 0.6350 | |
| Black | 21.9 | 10.3 | 22.1 | 11.7 | 23.1 | 10.6 | 20.3 | 11.0 | 0.5533 | |
| White | 24.6 | 10.9 | 24.3 | 12.0 | 23.0 | 10.6 | 23.3 | 10.8 | 0.8873 | |
| BMI ≥85th percentile | 16.0 | 8.5 | 22.5 | 14.9 | 16.3 | 9.0 | 23.8 | 15.4 | 0.4857 | |
| Male | All | 17.4 | 9.4 | 28.4 | 16.8 | 17.5 | 10.7 | 29.3 | 17.9 | 0.5998 |
| Hispanic | 17.3 | 9.1 | 29.4 | 17.3 | 17.9 | 11.0 | 30.3 | 18.2 | 0.9626 | |
| Black | 18.3 | 10.9 | 27.0 | 16.3 | 17.2 | 11.0 | 26.2 | 18.5 | 0.7857 | |
| White | 17.0 | 9.1 | 26.3 | 15.5 | 16.6 | 9.4 | 28.6 | 16.3 | 0.2179 | |
| Female | All | 14.5 | 7.1 | 16.2 | 8.9 | 15.1 | 6.6 | 18.3 | 9.8 | 0.4465 |
| Hispanic | 14.5 | 6.8 | 16.8 | 8.6 | 15.4 | 6.4 | 19.8 | 10.2 | 0.3602 | |
| Black | 12.6 | 7.5 | 13.4 | 8.2 | 14.5 | 6.9 | 15.4 | 8.2 | 0.9709 | |
| White | 16.5 | 7.5 | 17.4 | 10.0 | 14.8 | 7.2 | 16.7 | 9.3 | 0.6823 | |
P values represent comparisons between intervention and control groups with the model adjusted for baseline values, family history of diabetes, year 8 Tanner stage, and parental education. Square root transformation of laps was used for analysis.
Mean minutes of MVPA per day at baseline (sixth grade) and follow-up (eighth grade) are presented by intervention arm in Table 5. There was a 7.4-min decline in the control group’s minutes of MVPA per day from baseline to follow-up, with a comparable 7.9-min decline among the intervention group. There were no statistically significant differences between the two groups at follow-up or for any of the gender, ethnic, or obesity status subgroup.
TABLE 5.
Preassessment and postassessment of MVPA minutes (n = 3686).a
| Control (n= 1800) |
Intervention (n= 1886) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Minutes of MVPA at Sixth Grade |
Minutes of MVPA at Eighth Grade |
Minutes of MVPA at Sixth Grade |
Minutes of MVPA at Eighth Grade |
|||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | P | ||
| Overall | 94.4 | 73.1 | 87.0 | 72.0 | 101.2 | 74.3 | 93.3 | 74.9 | 0.2520 | |
| Male | All | 114.8 | 79.6 | 114.1 | 78.3 | 119.1 | 80.4 | 123.4 | 80.9 | 0.2107 |
| Hispanic | 123.7 | 78.5 | 117.3 | 81.8 | 125.8 | 81.1 | 125.0 | 83.0 | 0.3066 | |
| Black | 111.0 | 85.9 | 107.7 | 74.4 | 117.9 | 80.9 | 123.3 | 77.1 | 0.1344 | |
| White | 95.6 | 75.0 | 110.4 | 71.4 | 102.0 | 75.8 | 118.9 | 79.2 | 0.7161 | |
| Female | All | 75.9 | 61.1 | 62.3 | 55.1 | 84.8 | 64.1 | 65.9 | 56.3 | 0.4949 |
| Hispanic | 79.5 | 61.2 | 61.3 | 54.3 | 86.9 | 63.7 | 65.2 | 57.3 | 0.8219 | |
| Black | 70.9 | 64.9 | 56.3 | 55.6 | 86.4 | 66.8 | 66.0 | 53.5 | 0.2822 | |
| White | 71.1 | 57.6 | 68.6 | 56.2 | 74.7 | 61.1 | 68.6 | 56.7 | 0.6194 | |
| BMI <85th percentile | 93.3 | 73.8 | 86.5 | 72.4 | 99.2 | 75.0 | 92.5 | 76.6 | 0.3609 | |
| Male | All | 115.1 | 80.6 | 116.3 | 78.1 | 121.9 | 82.0 | 125.3 | 82.8 | 0.4322 |
| Hispanic | 124.4 | 81.1 | 123.8 | 80.4 | 127.9 | 84.7 | 126.0 | 83.6 | 0.8185 | |
| Black | 106.5 | 82.5 | 101.6 | 76.0 | 121.1 | 82.7 | 121.9 | 80.6 | 0.2114 | |
| White | 100.8 | 76.5 | 109.1 | 73.2 | 110.0 | 74.5 | 127.9 | 84.1 | 0.3405 | |
| Female | All | 76.1 | 62.8 | 63.1 | 57.7 | 81.0 | 63.3 | 66.0 | 59.1 | 0.5311 |
| Hispanic | 78.4 | 62.7 | 60.0 | 56.9 | 84.9 | 63.0 | 63.4 | 57.8 | 0.6057 | |
| Black | 77.0 | 68.3 | 58.1 | 55.9 | 80.2 | 68.0 | 70.9 | 60.9 | 0.2012 | |
| White | 70.9 | 60.0 | 71.9 | 59.6 | 69.0 | 57.4 | 68.5 | 61.3 | 0.4241 | |
| BMI ≥85th percentile | 95.6 | 72.5 | 87.4 | 71.5 | 103.1 | 73.7 | 94.2 | 73.2 | 0.2417 | |
| Male | All | 114.7 | 78.9 | 112.3 | 78.5 | 116.8 | 79.1 | 121.7 | 79.4 | 0.1637 |
| Hispanic | 123.2 | 76.7 | 112.3 | 82.6 | 124.3 | 78.7 | 124.4 | 82.8 | 0.1058 | |
| Black | 115.3 | 89.4 | 113.5 | 73.0 | 113.6 | 78.7 | 125.1 | 72.6 | 0.3181 | |
| White | 90.1 | 73.4 | 111.8 | 69.8 | 92.9 | 76.6 | 108.8 | 72.4 | 0.8872 | |
| Female | All | 75.7 | 59.1 | 61.3 | 52.1 | 89.0 | 64.8 | 65.9 | 53.2 | 0.6858 |
| Hispanic | 80.6 | 59.8 | 62.8 | 51.6 | 89.1 | 64.4 | 67.0 | 56.9 | 0.9773 | |
| Black | 65.1 | 61.5 | 54.5 | 55.6 | 92.6 | 65.4 | 61.1 | 44.7 | 0.9717 | |
| White | 71.4 | 54.0 | 63.5 | 50.4 | 82.6 | 65.6 | 68.8 | 50.0 | 0.9693 | |
P values represent comparisons between intervention and control groups with the model adjusted for baseline values, family history of diabetes, year 8 Tanner stage, and parental education. Square root transformation of minutes was used for analysis.
DISCUSSION
The data presented in this study have shown that 5% of an ethnically diverse sample of early adolescents had Met-S, but a 3-yr multicomponent intervention had no effect on Met-S prevalence when compared with a control group. Further examination indicated that the baseline Met-S prevalence differed by ethnicity, with the highest prevalence among the Hispanic participants (6.5% vs 2.1% for black and 5.5% for white). The baseline Met-S prevalence was higher when the sample was limited to just overweight males (9.6%) and females (12.2%). There was no intervention effect on Met-S prevalence within these subgroups or within the ethnic and obesity status subgroups. Met-S has been shown to track from adolescence to adulthood (23), and adult Met-S is associated with an increased risk of developing CVD and T2DM (17). Because there are serious health implications for youth who possess Met-S, the failure to affect Met-S prevalence in this study indicates a need to identify more effective intervention approaches.
There was no difference in the number of laps completed by the intervention and control groups at the end of the study. Because the 20-MST has been shown to be sensitive to change in youth interventions (16), the lack of an effect on fitness is unlikely to be a function of measurement. The absence of an effect could, however, be a function of variation in the dose of the PE (min·wk−1) offered across the seven field centers. Because exposure to the PE classes at intervention and control schools was broadly comparable with mean PE class lengths of 57 and 55 min in control and intervention schools at baseline and 54 and 55 min at the eighth grade, there does not seem to be an evidence of difference between intervention and control schools. It is therefore reasonable to conclude that the intervention had no effect on the participant’s fitness.
There was no difference in the mean minutes of daily (in and out of school) MVPA between the intervention and control groups at the end of the intervention. Although a large proportion of the physical activity intervention efforts were targeted toward the PE provision during curriculum time, the PE lessons, the behavioral lessons, and the social marketing campaign were also designed to encourage additional habitual physical activity. The SAPAC instrument was therefore chosen as the physical activity measure to capture the intended change in habitual physical activity. Thus, although it might be the case that the SAPAC was not sufficiently sensitive to capture change during PE lessons, the primary focus was on the change in overall physical activity of which physical education was an important component. We may have been able to detect a change in physical activity if we had used an objective measure such as using accelerometers. For example, accelerometers would have facilitated a segmented analysis (12) in which it would have been possible to examine if a change in physical activity occurred during school hours. Equally, it may have been the case that activity levels in the intervention group changed during the early phases of the intervention, but these changes were not maintained. As such, it is possible to argue that the lack of objective, sensitive assessments during the intervention period may also have hindered our ability to capture key changes. However, because the primary aim of the study was reduction in modifiable risk factors for T2DM at the end of eighth grade, we directed our limited resources toward assessing physical activity at the end of the study.
A closer examination of the physical activity, fitness, and Met-S data reveals an interesting trend. Overall, minutes of MVPA declined by approximately 8% during the 2.5-yr period, whereas the number of laps completed in the 20-MST increased by approximately 30% overall, with the majority of the increase occurring in the boys and little or no change in the girls. In comparison, the proportion of the overall sample exhibiting Met-S was stable during the 2-yr period. However, the Met-S data (Table 2) show that the proportion of boys with Met-S increased by ~51%, whereas the proportion of girls decreased by ~42%. Intriguingly, physical activity and fitness levels of the boys are basically unchanged and the proportion of boys with Met-S is increasing, whereas the physical activity and fitness of the girls are declining and the proportion of girls exhibiting Met-S is declining. Because both physical activity and fitness have been associated with Met-S, these findings suggest that the amount of change in both variables may not have been sufficient to significantly affect Met-S. Potentially, greater changes in physical activity could have influenced changes in body fat or fitness, which would have a direct effect on the Met-S.
The lack of success in achieving change in fitness, physical activity, and Met-S is consistent with the broader literature in which the majority of school-based interventions designed to increase physical activity or prevent weight gain have either reported no effect or very small effects in subgroups (35). Several studies have reported that short-term physical education interventions (≤6 months) (26,29) have yielded positive effects on fitness levels, whereas longer-term studies have yielded no effect (2). It is also important to note that a 3-yr intensive activity program that was delivered after school in US elementary schools yielded a positive change in fitness at the end of each year, but the positive change was lost over the summer vacation (8). It therefore seems plausible that intervention effects could have been ameliorated over the summer months, but the absence of interim measures precluded the detection on such effects on fitness, physical activity, and Met-S. It is, however, important to remember that the primary aim of this study was to assess the longer-term effect of the intervention on fitness, physical activity, and Met-S. As such, although it may have been scientifically interesting to know if the effect had shorter-tem effects on these variables, the more important public health question is whether the intervention had a longer-term effect on these outcomes and it was the key public health question that was the focus of our research.
The failure to affect physical activity, fitness, or Met-S suggests that alternative intervention approaches are needed. Participation in PE and structured group-based PA programs declines throughout adolescence (15), suggesting that future interventions may need to address individual differences in lifestyle activity habits outside of school. We may therefore need to consider alternative approaches to changing youth physical activity, fitness, and ultimately Met-S. Social ecological models (19) suggest that there are multiple levels of influence on youth behavior, and although we made changes to the in-school PA environment, we did not address familial, peer, or wider environmental approaches to increasing physical activity such as active travel to school, which are likely to be important influences on youth physical activity and fitness. Future interventions should consider these wider social and environmental factors on youth physical activity and fitness.
Strengths and limitations
The data presented here are from a large cluster-designed randomized controlled trial with the majority of the participants (>70%) coming from ethnic minority groups that are at an increased risk of T2DM. The study also included participants from across the United States, and the geographical diversity in sites mimics the US school system. Moreover, this was a multicomponent intervention that was based on the best available evidence with further development during 4 yr of formative work in which the intervention elements were refined (10). As such, the intervention represents a well-thought-out, well-delivered intervention in a high-risk group. There are, however, many limitations that need to be recognized. First, we used the 2-d version of the SAPAC to estimate physical activity levels and sedentary behaviors. Although this is a valid instrument, the wide variance in student response may have affected our ability to detect change. Second, the 20-MST is a field test that has been widely used and validated, but we do not have any information about the consistency in student effort, i.e., did all students work as hard in the test. Third, we used the IDF criteria to define Met-S, and although these criteria were developed to provide a universally accepted criteria, it is plausible that other findings may have resulted if other Met-S definitions had been used. Finally, because the focus of this study was to evaluate a difference between the intervention and control groups at the end of eighth grade, we directed our resources toward assessments at the baseline (sixth grade) and postassessment (eighth grade) periods, which prevented interim assessments of change in outcomes.
CONCLUSIONS
The HEALTHY intervention, a complex school-based intervention, had no effect on the Met-S, fitness, or physical activity levels of youth at risk of developing T2DM. The study suggests that school-based behavioral interventions may not be sufficiently intense to facilitate change in these variables. Alternative approaches that focus on how to change physical activity, fitness, and ultimately Met-S in all relevant childhood environments need to be developed. It is unlikely that school-based programming alone can increase activity sufficiently to produce the desired changes.
Acknowledgments
This work was completed with funding from the National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health grants U01-DK61230, U01 DK61249, U01-DK61231, and U01-DK61223 to the STOPP-T2D collaborative group.
This report is also research arising from a Career Development Fellowship (to Dr. Jago) supported by the National Institute for Health Research. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health.
The authors thank the administration, faculty, staff, students, and their families at the middle schools and school districts who participated in the HEALTHY study.
The results of the current study do not constitute endorsement by the American College of Sports Medicine.
The following individuals and institutions constitute the HEALTHY Study Group (* indicates principal investigator or director):
Study Chair
Children’s Hospital Los Angeles: F.R. Kaufman
Field Centers
Baylor College of Medicine: T. Baranowski,* L. Adams, J. Baranowski, A. Canada, K.T. Carter, K.W. Cullen, M.H. Dobbins, R. Jago, A. Oceguera, A.X. Rodriguez, C. Speich, L.T. Tatum, D. Thompson,M.A. White, C.G. Williams
Oregon Health & Science University: L. Goldberg,* D. Cusimano, L. DeBar, D. Elliot, H.M. Grund, S. McCormick, E. Moe, J.B. Roullet, D. Stadler
Temple University: G. Foster* (Steering Committee Chair), J. Brown, B. Creighton,M. Faith, E.G. Ford, H. Glick, S. Kumanyika, J. Nachmani, L. Rosen, S. Sherman, S. Solomon, A. Virus, S. Volpe, S. Willi
University of California at Irvine: D. Cooper,* S. Bassin, S. Bruecker, D. Ford, P. Galassetti, S. Greenfield, J. Hartstein, M. Krause, N. Opgrand, Y. Rodriguez, M. Schneider
University of North Carolina at Chapel Hill: J. Harrell,* A. Anderson, T. Blackshear, J. Buse, J. Caveness, A. Gerstel, C. Giles, A. Jessup, P. Kennel, R. McMurray, A.-M. Siega-Riz, M. Smith, A. Steckler, A. Zeveloff
University of Pittsburgh: M.D. Marcus,* M. Carter, S. Clayton, B. Gillis, K. Hindes, J. Jakicic, R. Meehan, R. Noll, J. Vanucci, E. Venditti
University of Texas Health Science Center at San Antonio: R. Treviño,* A. Garcia, D. Hale, A. Hernandez, I. Hernandez, C. Mobley, T. Murray, J. Stavinoha, K. Surapiboonchai, Z. Yin
Coordinating Center
George Washington University: K. Hirst,* K. Drews, S. Edelstein, L. El ghormli, S. Firrell, M. Huang, P. Kolinjivadi, S. Mazzuto, T. Pham, A. Wheeler
Project Office
National Institute of Diabetes and Digestive and Kidney Diseases: B. Linder,* C. Hunter, M. Staten
Central Biochemistry Laboratory
University of Washington Northwest Lipid Metabolism and Diabetes Research Laboratories: S.M. Marcovina*
HEALTHY intervention materials are available for download at http://www.healthystudy.org/
Clinical trial registration information: NCT00458029
References
- 1.Blair SN, Kohl HW, 3rd, Barlow CE, Paffenbarger RS, Jr, Gibbons LW, Macera CA. Changes in physical fitness and allcause mortality: a prospective study of healthy and unhealthy men. JAMA. 1995;273(14):1093–1098. [PubMed] [Google Scholar]
- 2.Boyle-Holmes T, Grost L, Russell L, et al. Promoting elementary physical education: results of a school-based evaluation study. Health Educ Behav. 2010;37(3):377–389. doi: 10.1177/1090198109343895. [DOI] [PubMed] [Google Scholar]
- 3.2000 CDC Growth Charts for the United States. Atlanta (GA): Centers for Disease Control; 2009. [cited 2009 Jun 1]. Centers for Disease Control National Center for Health Statistics. Available from: http://www.cdc.gov/growthcharts. [Google Scholar]
- 4.de Ferranti SD, Gauvreau K, Ludwig DS, Newburger JW, Rifai N. Inflammation and changes in metabolic syndrome abnormalities in US adolescents: findings from the 1988–1994 and 1999–2000 National Health and Nutrition Examination Surveys. Clin Chem. 2006;52(7):1325–1330. doi: 10.1373/clinchem.2006.067181. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American and Mexican-American children and adolescents. J Pediatr. 2004;145:439–444. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 6.Finley CE, LaMonte MJ, Waslien CI, Barlow CE, Blair SN, Nichaman MZ. Cardiorespiratory fitness, macronutrient intake, and the metabolic syndrome: the Aerobics Center Longitudinal Study. J Am Diet Assoc. 2006;106(5):673–679. doi: 10.1016/j.jada.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Fridewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 8.Gutin B, Yin Z, Johnson M, Barbeau P. Preliminary findings of the effect of a 3-year after-school physical activity intervention on fitness and body fat: the Medical College of Georgia FitKid Project. Int J Pediatr Obes. 2008;3(1 suppl):3–9. doi: 10.1080/17477160801896457. [DOI] [PubMed] [Google Scholar]
- 9.Harrell JS, McMurray RG, Bangdiwala SI, Baggett C, Pearce PF, Pennel M. Determining MET values in children and adolescents. Med Sci Sports Exerc. 2003;35(suppl 5):S342. doi: 10.1249/01.mss.0000153115.33762.3f. [DOI] [PubMed] [Google Scholar]
- 10.Hirst K, Baranowski T, DeBar L, et al. HEALTHY study rationale, design and methods: moderating risk of type 2 diabetes in multi-ethnic middle school students. Int J Obes (Lond) 2009;33(suppl 4):S4–S20. doi: 10.1038/ijo.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm E. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med. 2001;161(12):1542–1548. doi: 10.1001/archinte.161.12.1542. [DOI] [PubMed] [Google Scholar]
- 12.Jago R, Fox KR, Page AS, Brockman R, Thompson JL. Physical activity and sedentary behaviour typologies of 10–11 year olds. Int J Behav Nutr Phys Act. 2010;7:59. doi: 10.1186/1479-5868-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jago R, Harrell JS, McMurray RG, Edelstein S, El Ghormli L, Bassin S. Prevalence of abnormal lipid and blood pressure values among an ethnically diverse population of eighth-grade adolescents and screening implications. Pediatrics. 2006;117(6):2065–2073. doi: 10.1542/peds.2005-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jago R, McMurray RG, Bassin S, et al. Modifying middle school physical education: piloting strategies to increase physical activity. Pediatr Exerc Sci. 2009;21(2):171–185. doi: 10.1123/pes.21.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston LD, Delva J, O’Malley PM. Sports participation and physical education in American secondary schools: current levels and racial/ethnic and socioeconomic disparities. Am J Prev Med. 2007;33(suppl 4):S195–S208. doi: 10.1016/j.amepre.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Kriemler S, Zahner L, Schindler C, et al. Effect of school based physical activity programme (KISS) on fitness and adiposity in primary schoolchildren: cluster randomized controlled trial. BMJ. 2010;340:c785. doi: 10.1136/bmj.c785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laaksonen DE, Lakka HM, Niskanen LK, Kaplan GA, Salonen JT, Lakka TA. Metabolic syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. Am J Epidemiol. 2002;156(11):1070–1077. doi: 10.1093/aje/kwf145. [DOI] [PubMed] [Google Scholar]
- 18.Leger LA, Lambert J. A maximal multistage 20-m shuttle run test to predict V̇O2max. Eur J Appl Physiol Occup Physiol. 1982;49(1):1–12. doi: 10.1007/BF00428958. [DOI] [PubMed] [Google Scholar]
- 19.McLeroy KR, Bibeau D, Steckler A, Glanz K. An ecological perspective on health promotion programs. Health Educ Q. 1988;15(4):351–377. doi: 10.1177/109019818801500401. [DOI] [PubMed] [Google Scholar]
- 20.McMurray RG, Harrell JS, Bangdiwala SI, Hu J. Tracking of physical activity and aerobic power from childhood through adolescence. Med Sci Sports Exerc. 2003;35(11):1914–1922. doi: 10.1249/01.MSS.0000093612.59984.0E. [DOI] [PubMed] [Google Scholar]
- 21.McMurray RG, Ring KB, Treuth MS, et al. Comparison of two approaches to structured physical activity surveys for adolescents. Med Sci Sports Exerc. 2004;36(12):2135–2143. doi: 10.1249/01.mss.0000147628.78551.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molenberghs G, Verbeke G. Models for Discrete Longitudinal Data. New York (NY): Springer; 2005. pp. 151–186. [Google Scholar]
- 23.Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr. 2008;152(2):201–206. doi: 10.1016/j.jpeds.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Ness AR, Leary SD, Mattocks C, et al. Objectively measured physical activity and fat mass in a large cohort of children. PLoS Med. 2007;4(3):e97. doi: 10.1371/journal.pmed.0040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pate RR, Wang CY, Dowda M, Farrell SW, O’Neill JR. Cardiorespiratory fitness levels among US youth 12 to 19 years of age: findings from the 1999–2002 National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 2006;160(10):1005–1012. doi: 10.1001/archpedi.160.10.1005. [DOI] [PubMed] [Google Scholar]
- 26.Peralta LR, Jones RA, Okely AD. Promoting healthy lifestyles among adolescent boys: the Fitness Improvement and Lifestyle Awareness Program RCT. Prev Med. 2009;48(6):537–542. doi: 10.1016/j.ypmed.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 28.Sallis JF, Buono MJ, Roby JJ, Micale FG, Nelson JA. Seven-day recall and other physical activity self-reports in children and adolescents. Med Sci Sports Exerc. 1993;25(1):99–108. doi: 10.1249/00005768-199301000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Shaw-Perry M, Horner C, Trevino RP, Sosa ET, Hernandez I, Bhardwaj A. NEEMA: a school-based diabetes risk prevention program designed for African-American children. J Natl Med Assoc. 2007;99(4):368–375. [PMC free article] [PubMed] [Google Scholar]
- 30.Studies to Treat or Prevent Pediatric Type 2 Diabetes Prevention Study Group. Prevalence of the metabolic syndrome among a racially/ ethnically diverse group of U.S. eighth-grade adolescents and associations with fasting insulin and homeostasis model assessment of insulin resistance levels. Diabetes Care. 2008;31(10):2020–2025. doi: 10.2337/dc08-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanasescu M, Leitzmann MF, Rimm EB, Hu FB. Physical activity in relation to cardiovascular disease and total mortality among men with type 2 diabetes. Circulation. 2003;107:2435–2439. doi: 10.1161/01.CIR.0000066906.11109.1F. [DOI] [PubMed] [Google Scholar]
- 32.The Healthy Study Group. A school-based intervention for diabetes risk reduction. N Engl J Med. 2010;363(5):445–453. doi: 10.1056/NEJMoa1001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 34.Tukey JW. The future of data analysis. Ann Math Stat. 1962;33:18. [Google Scholar]
- 35.van Sluijs EM, McMinn AM, Griffin SJ. Effectiveness of interventions to promote physical activity in children and adolescents: systematic review of controlled trials. BMJ. 2007;335(7622):703. doi: 10.1136/bmj.39320.843947.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Welk GJ, Meredith MD. FITNESSGRAM/ACTIVITYGRAM Reference Guide. Dallas (TX): The Cooper Institute; 2008. pp. 96–121. [Google Scholar]
- 37.Zimmet P, Alberti G, Kaufman F, et al. The metabolic syndrome in children and adolescents. Lancet. 2007;369(9579):2059–2061. doi: 10.1016/S0140-6736(07)60958-1. [DOI] [PubMed] [Google Scholar]