Abstract
Moderate caloric restriction (CR) without malnutrition increases healthspan in virtually every species studied, including nonhuman primates. In mice, CR exerts significant microvascular protective effects resulting in increased microvascular density in the heart and the brain, which likely contribute to enhanced tolerance to ischemia and improved cardiac performance and cognitive function. Yet, the underlying mechanisms by which CR confer microvascular protection remain elusive. To test the hypothesis that circulating factors triggered by CR regulate endothelial angiogenic capacity, we treated cultured human endothelial cells with sera derived from Macaca mulatta on long-term (over 10 years) CR. Cells treated with sera derived from ad-libitum-fed control monkeys served as controls. We found that factors present in CR sera upregulate vascular endothelial growth factor (VEGF) signaling and stimulate angiogenic processes, including endothelial cell proliferation and formation of capillary-like structures. Treatment with CR sera also tended to increase cellular migration (measured by a wound-healing assay using electric cell–substrate impedance sensing [ECIS] technology) and adhesion to collagen. Collectively, we find that circulating factors induced by CR promote endothelial angiogenic processes, suggesting that increased angiogenesis may be a potential mechanism by which CR improves cardiac function and prevents vascular cognitive impairment.
Key Words: Dietary restriction, Vascular aging, Angiogenesis, Microcirculation, Cardiovascular system.
The process of angiogenesis, new capillary formation from existing blood vessels, is critical for maintenance of the microvasculature and cardiovascular homeostasis. Previous studies in laboratory rodents demonstrate that aging is associated with a progressive deterioration of microvascular homeostasis due to age-related impairment of angiogenic processes (1–5). We and others have proposed that these changes have a key role in age-related microvascular rarefaction (6), decreasing tissue blood supply, impairing adaptation to hypoxia (7–9), and suppressing wound healing. Age-related decreases in microvascular density in the heart are thought to contribute to a severe impairment in cardiac pump function during aging leading to an increased occurrence of ventricular failure (10). Age-related impairment in endothelial angiogenic capacity and decreases in cerebromicrovascular density in the hippocampus and the cingulate, retrosplenial, and motor cortex have also been causally linked to a decline of local cerebral blood flow (11) and an age-related impairment of higher brain functions, including cognitive decline (12). These results are consistent with findings in elderly patients demonstrating that age-related microvascular alterations and impairment of regional cerebral blood flow contribute to the development of mild cognitive impairment in humans (13,14).
Moderate caloric restriction (CR) without malnutrition has been shown to slow the rate of aging and increase life span and/or healthspan in most species studied, including invertebrate model organisms (15), laboratory rodents, and nonhuman primates (16–27). In laboratory rodents, moderate CR was shown to exert significant microvascular protective effects (28,29) increasing microvascular density in the heart (30) and the brain (11,12). Furthermore, explanted aorta segments isolated from rats maintained on an every other day feeding regimen, compared with control vessels, showed increased endothelial sprouting in collagen matrix (31). CR was also shown to promote revascularization in response to hindlimb ischemia in mice (32) and increase angiogenic activity in the rabbit testis (33). The aforementioned findings suggest that CR exerts beneficial effects on microvascular health (29), but the underlying mechanisms by which CR regulates angiogenic processes and confers endothelial protection remain elusive.
CR is associated with changes in the level of neuroendocrine mediators present in the circulation, which readily reach microvascular endothelial cells and elicit a variety of cytoprotective responses (29,34). CR-induced changes in circulating factors were also shown to be responsible for activation of evolutionarily conserved survival pathways in rats and mice (35,36). Important to the present discussion are our previous observations that in vitro treatment of cultured endothelial cells with sera from CR-fed rats mimics many of the vascular effects observed in vivo during CR in rodents (28). These studies support a key role of neuroendocrine factors in CR-mediated endothelial protection. However, no studies have investigated whether CR-induced changes in circulating factors mediate proangiogenic effects of CR. Furthermore, despite the rapid progress of aging research in the last few years (37–73), there are no studies to our knowledge investigating the role of CR-induced changes in circulating factors in endothelial protection in primates.
The present study was designed to elucidate the role of circulating factors induced by CR in the nonhuman primate Macaca mulatta in modulation of endothelial angiogenic capacity. Using cultured human coronary arterial endothelial cells (CAECs) as a model system, we tested the hypothesis that factors present in sera derived from CR monkeys stimulate angiogenic processes (including cell proliferation, adhesion, migration, and formation of capillary-like structures) through (a) an increase in NO bioavailability, (b) activation of Nrf2-dependent pathways, and/or (c) upregulation of vascular endothelial growth factor (VEGF) signaling.
Methods
Animals and Diet
Sera obtained from ad-libitum-fed control (C, n = 5) and calorie-restricted (CR, n = 5) rhesus macaques (M mulatta) enrolled in an ongoing longitudinal study at the Wisconsin National Primate Research Center (16,22,24,74,75) were used. The median life expectancy of rhesus monkeys in captivity is ~26 years with some of the monkeys in this colony living into their late thirties (76). The control animals included in the present study had an average age of 25.5±1.4 years and CR animals had an average age of 25.4±1.4 years, representing their very late middle age. All the animals were originally randomized to either the control or CR diet at between 8 and 14 years of age. Food allotments for CR animals (Teklad diet 93,131, enriched by 30% in vitamins and minerals) were reduced to reach a 30% CR. Control animals were provided with ~20g more than their average daily intake to assure ad-libitum access to food (purified lactalbumin-based diet containing 10% fat and 15% protein [Teklad #85387, Madison, WI]). All animal procedures were performed at the Wisconsin National Primate Research Center under approved protocols from the Institutional Animal Care and Use Committee of the Graduate School of the University of Wisconsin, Madison.
Cell Cultures
Primary human CAECs (purchased from Cell Applications, San Diego, CA, after passage 4; age of the donors is unknown) were initially cultured in MesoEndo Endothelial Cell Growth Medium (Cell Applications, Inc) followed by Endothelial Basal Medium supplemented with 10% fetal calf serum until the time of serum treatment, as described (77–80). For treatment, fetal calf serum was replaced with serum (10%) collected from control or CR-fed M mulatta, as previously described (28). Cells cultured in Endothelial Basal Medium supplemented with 10% fetal calf serum served as an additional control. To induce angiogenic processes, CAECs were treated with recombinant human VEGF (100ng/mL; R&D Systems, Minneapolis, MN). All reagents used in this study were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated.
Cell Adhesion Assays
Angiogenesis is a multistep process involving cell adhesion, proliferation, migration, and morphogenesis (81). To determine the effects of CR-induced circulating factors in regulation of the adhesion capacity of endothelial cells, we used electric cell–substrate impedance sensing (ECIS) technology (Applied Biophysics, Troy, NY) to monitor adhesion of CAECs to collagen, as reported (82). Briefly, cells were treated with sera obtained from control or CR-fed M mulatta. After 48 hours, the cells were collected and counted. VEGF (100ng/mL)-stimulated cells were seeded in collagen-coated (50 µg/mL) 96-well array culture dishes containing gold film surface electrodes (ECIS 96W1E; in each well, one active electrode and a large counter electrode). The same numbers of cells were added to each well (2.5 × 105 cells/well). The arrays were placed in an incubator and the time course for changes of capacitance (measured at 60kHz) due to the adhesion of cells to the active electrode was obtained. Time to reach 50% cell adhesion was used as an index of adhesiveness (100% change corresponds to the maximum level of cell coverage reached on the active electrode). To assay barrier function, the time course of changes in capacitance (measured at 32kHz) and resistance (measured at 1000 Hz) were monitored in parallel. Additional increases in resistance after capacitance has reached its minimum are indicative of barrier function associated with the formation of intercellular junctions (83).
Cell Proliferation Assay
Cell proliferation capacity was assessed in CAEC treated with control or CR sera using the flow cytometry–based Guava Cell Growth assay (Guava Technologies, Hayward, CA), as reported (82). Briefly, cells were collected, resuspended in phosphate-buffered saline containing 0.1% bovine serum albumin and stained with 16 µmol/L carboxyfluorescein diacetate succinimidyl ester for 15 minutes at 37°C. This dye diffuses into cells and is cleaved by intracellular esterases to form an amine-reactive product that produces a detectable fluorescence and binds covalently to intracellular lysine residues and other amine sources. Upon cell division, carboxyfluorescein diacetate succinimidyl ester divides equally into the daughter cells halving the concentration of the mother cell; therefore, there is an inverse correlation between the fluorescence intensity and the proliferation capacity of the cells. After incubation, unbound dye was quenched with serum-containing medium. Cells were washed three times, treated with control sera or CR sera for 24 hours (in the presence of 100ng/mL VEGF). Finally, cells were collected, washed, stained with propidium iodide (to gate out dead cells), and analyzed with a flow cytometer (Guava EasyCyte 8HT, Millipore, Billerica, MA). The inverse of the fluorescence intensity was used as an index of proliferation.
Assessment of Cell Migration by ECIS-Based Wound-Healing Assay
The ECIS technology was used to monitor migration of CAECs treated with control sera or CR sera in a wound-healing assay, as reported (82). Briefly, CAECs (2.5 × 105 cells/well) were seeded in 96-well array culture dishes (ECIS 96W1E), placed in an incubator (37°C), and changes in resistance and impedance were continuously monitored. When impedance reached a plateau, cells in each well were subjected to an elevated field pulse (wounding) of 5 mA applied for 20 seconds at 100kHz, which killed the cells present on the small active electrode due to severe electroporation. The detachment of the dead cells was immediately evident as a sudden drop in resistance (monitored at 4000 Hz) and a parallel increase in conductance. VEGF (100ng/mL) was immediately added to each well. CAECs surrounding the active electrode that had not been subjected to the wounding then migrated inward to replace the detached dead cells resulting in resistance recovery (continuously monitored at 4000 Hz for up to 24 hours). Time to reach 50% resistance recovery (corresponding to 50% confluence on the active electrode) was determined for control and CR sera–treated cells, and this parameter and the known physical dimensions of the electrode were used to calculate the migration rate (expressed as µm/h).
Tube Formation Assay
To investigate the influence of control sera or CR sera on tube formation ability, CAECs were plated on Geltrex Reduced Growth Factor Basement Membrane Matrix (Invitrogen, Carlsbad, CA) in Medium 200PRF (Invitrogen). Briefly, 150 µL/well of Geltrex was distributed in ice-cold 24-well plates. The gel was allowed to solidify while incubating the plates for 30 minutes at 37°C. CAECs were then seeded at a density of 5 × 104 cells/well and placed in the incubator for 24 hours. Microscopic images were captured using a Nikon Eclipse Ti microscope equipped with a 10× phase-contrast objective (Nikon Instruments Inc., Melville, NY). The extent of tube formation was quantified by measuring total tube length in five random fields per well using NIS-Elements Microscope Imaging Software (Nikon Instruments), as recently reported (82). The mean of the total tube length per total area imaged (µm tube/mm2) was calculated for each well. Experiments were run in quadruplicates. The experimenter was blinded to the groups throughout the period of analysis.
Apoptosis Assay
To determine whether CR circulating factors exert antiapoptotic effects, CAECs were treated with control and CR sera for 24 hours. Apoptotic cell death was assessed by measuring caspase activities using the Caspase-Glo 3/7 assay kit (Promega, Madison, WI) as previously reported (84,85). In 96-well plates, 50-µL sample was mixed for 30 seconds with 50-µL Caspase-Glo 3/7 reagent and incubated for 2 hours at room temperature. Lyses buffer with the reagent served as blank. Luminescence of the samples was measured using an Infinite M200 Plate Reader (Tecan, Research Triangle Park, NC). Luminescent intensity values were normalized to the sample protein concentration.
Measurement of Endothelial NO Production
Production of NO in CAECs treated with control sera or CR sera was assessed by using 4,5-diaminofluorescein diacetate (DAF-2DA) as described (86). DAF-2DA is nonfluorescent but reacts with NO to form the highly fluorescent compound triazolofluorescein (DAF-2T). ECs were loaded with 5 µM DAF-2DA for 30 minutes at 37°C, washed three times, and DAF-2T fluorescence intensities were measured using a Tecan Infinite M200 plate reader (Tecan U.S.). Hoechst 33258 fluorescence, representing cellular DNA content, was used for normalization.
Assessment of the Effects of CR Sera Treatment on the Transcriptional Activity of Nrf2
The effect of circulating factors induced by CR on Nrf2 activity in CAECs was assessed using a reporter gene assay as described (78,79,82,87–89). We used an Antioxidant Response Element (ARE) reporter comprised of tandem repeats of the ARE transcriptional response element upstream of firefly luciferase (SA Biosciences, Frederick, MD) and a renilla luciferase plasmid under the control of the cytomegalovirus (CMV) promoter (as an internal control). Transfections in CAECs were performed using the Amaxa Nucleofector technology (Amaxa, Gaithersburg, MD), as we have previously reported (78,79,87). Firefly and renilla luciferase activities were assessed after 24 hours using the Dual Luciferase Reporter Assay Kit (Promega) and a Tecan Infinite M200 plate reader.
Quantitative Real-Time RT-PCR
A quantitative real-time reverse transcription polymerase chain reaction (RT-PCR) technique was used to analyze the effect of circulating factors induced by CR on mRNA expression of NFE2L2 (Nrf2), the Nrf2 target genes NQO1, HMOX1, and GCLC, VEGF receptors, as well as known target genes of VEGF in CAECs, as previously reported (54,90–94). In brief, total RNA was isolated using a TaqMan Cells-to-CT Kit (Applied Biosystems, Foster City, CA) and was reverse transcribed using Superscript III RT (Invitrogen) as described previously (90,95). A real-time RT-PCR technique was used to analyze mRNA expression using a Strategen MX3000, as reported (95). Amplification efficiencies were determined using a dilution series of a standard vascular sample. Quantification was performed using the efficiency-corrected ∆∆Cq method. The relative quantities of the reference genes HPRT, GAPDH, and ACTB were determined and a normalization factor was calculated based on the geometric mean for internal normalization. Oligonucleotides used for quantitative real-time RT-PCR are listed in Table 1.
Table 1.
Oligonucleotides for Real-Time RT-PCR
| mRNA Targets | Descriptions | Sense | Antisense |
| NFE2L2 | NF-E2-related factor 2 (Nrf2) | CAACTCAGCACCTTATATCTC | TTCTTAACATCTGGCTTCTTAC |
| Nqo1 | NAD(P)H:27uinine oxidoreductase 1 (NAD(P)H dehydrogenase, 27uinine 1) | AGACCTTGTGATATTCCAGTTC | GGCAGCGTAAGTGTAAGC |
| Gclc | Gamma-glutamylcysteine synthetase (glutamate-cysteine ligase, catalytic subunit) | CAGTGGTGGATGGTTGTG | ATTGATGATGGTGTCTATGC |
| Hmox1 | Heme oxygenase-1 | AAGTATCCTTGTTGACACG | TGAGCCAGGAACAGAGTG |
| VEGFR2 | Vascular endothelial growth factor receptor 2 | GAGTTCGTTGTGCTGTTTCTG | ATGTCCTTTCTTTGTGGTATTCTG |
| VEGFR1 | Vascular endothelial growth factor receptor 1 | ATTCCACTATCTCACACTAATCTG | CCCAGGCAAGTTTCAAAGC |
| RCAN1 | Regulator of calcineurin 1 | CATCGCTGTCCAAGTGTT | TTCTGTAAGGAGCATACTGTG |
| IL8 | Interleukin-8 | AATTCATTCTCTGTGGTATC | CCAGGAATCTTGTATTGC |
| NDRG1 | N-myc downstream regulated 1 | TAGCATCCTCTTAATGTG | CAGAAAGCAGAACTAAAG |
| ANGPT2 | Angiopoietin 2 | AGCACAAAGGATGGAGACAAC | CTTGAGCGAATAGCCTGAGC |
| DNAJB9 | DnaJ (Hsp40) homolog, subfamily B | AAAATAAGAGCCCGGATGCT | TGACTGCTCAAAAGAACTTCCA |
| KLF4 | Kruppel-like factor 4 | GCCTTGCTGATTGTCTAT | AAGTCAACGAAGAGAAGAA |
| IGFBP3 | Insulin-like growth factor binding protein 3 | CCAAAGTTTAGAAAGAGGTTT | GATAGGAAGCGACAAGAA |
| MEF2C | Myocyte enhancer factor 2C | AGCAATCCAAGCCACATA | ACAACAGAATCCGTCACT |
| Hprt | Hypoxanthine phosphoribosyltransferase 1 | CCGTGTGTTAGAAAAGTAAGAAGC | AACTGCTGACAAAGATTCACTGG |
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase | AACGAATTTGGCTACAGC | AGGGTACTTTATTGATGGTACAT |
| Actb | Beta-actin | CGGTGAAGGTGACAGCAG | TGTGTGGACTTGGGAGAGG |
RT-PCR = Reverse transcription polymerase chain reaction.
Measurement of Mitochondrial 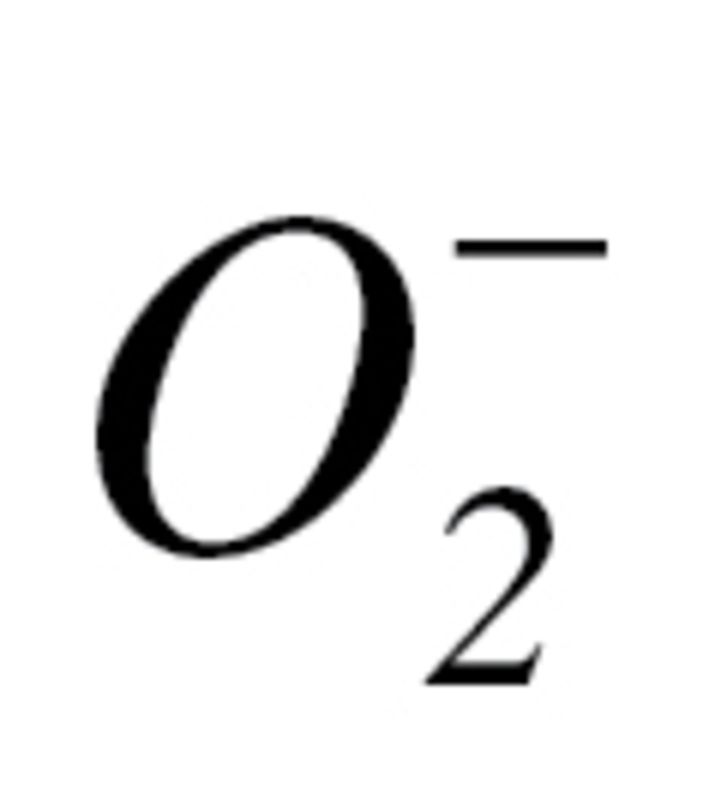 Production
Production
The effect of circulating factors induced by CR on mitochondrial 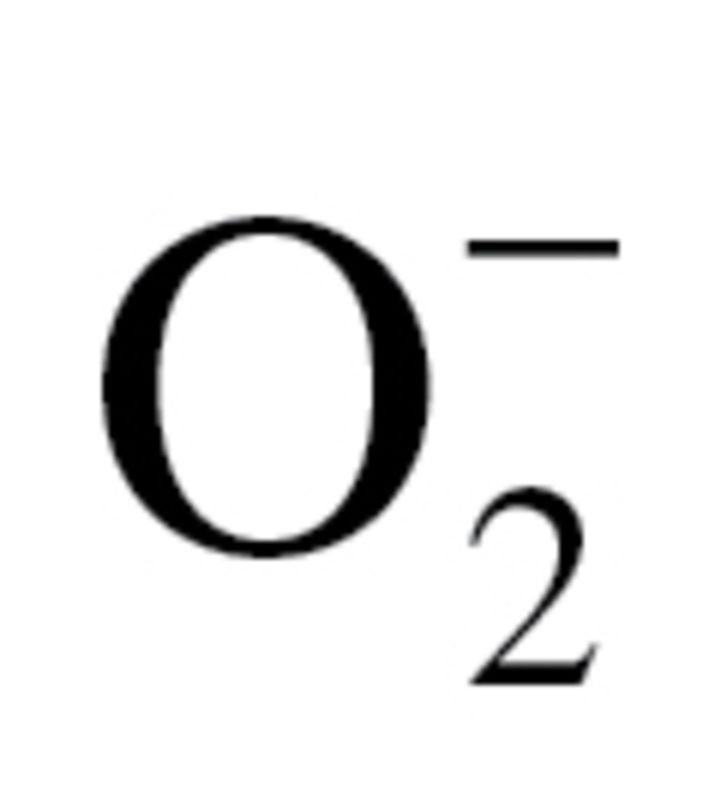 production in CAECs was measured by flow cytometry (Guava) using MitoSOX Red (Invitrogen), a mitochondrion-specific hydroethidine-derivative fluorescent dye, as previously reported (80,96,97). Cell debris (low forward and side scatter) and dead cells (Sytox Green) were gated out for analysis.
production in CAECs was measured by flow cytometry (Guava) using MitoSOX Red (Invitrogen), a mitochondrion-specific hydroethidine-derivative fluorescent dye, as previously reported (80,96,97). Cell debris (low forward and side scatter) and dead cells (Sytox Green) were gated out for analysis.
Analysis of VEGF-Induced Activation of Signaling Pathways
To assess whether circulating factors induced by CR alter VEGF signaling, CAECs, cultured in the presence of control or CR sera, were treated with VEGF (100ng/mL) and cells were harvested at 0, 5, 15, and 30 minutes posttreatment. The time course of VEGF-induced phosphorylation of stress-activated protein kinase/Jun-amino-terminal kinase SAPK/JNK, the serine/threonine protein kinase Akt, p38 MAP kinase (MAPK), and heat shock protein (HSP) 27 (which is phosphorylated by MAPKAP kinase 2 as a result of the activation of the p38 MAPK kinase pathway) were analyzed using a magnetic multiplex bead array (Millipore), as reported (88). The sample protein content was determined by a spectrophotometric quantitation method using bicinchoninic acid reagent (Pierce Chemical, Rockford, IL). Average fold changes in cellular content of phospho-SAPK/JNK, phospho-Akt, phospho-p38 MAPK, and phospho-HSP27 as a function of time are reported.
To determine whether CR-induced changes in circulating factors alter the transcriptional response of the cells to VEGF, we assessed VEGF (100ng/mL)-induced expression of known VEGF target genes (98), including IL8, NDRG1, MEF2C, ANGPT2, DNAJB9, KLF4, IGFBP3, and RCAN1. VEGF-induced transcription of many of these genes depends on p38 MAPK activation (99).
Data Analysis
Statistical analyses were performed using one-way analysis of variance. p < .05 was considered statistically significant. Data are expressed as means ± SEM.
Results
Effect of Treatment With cr Sera on Adhesion of CAECs to Collagen
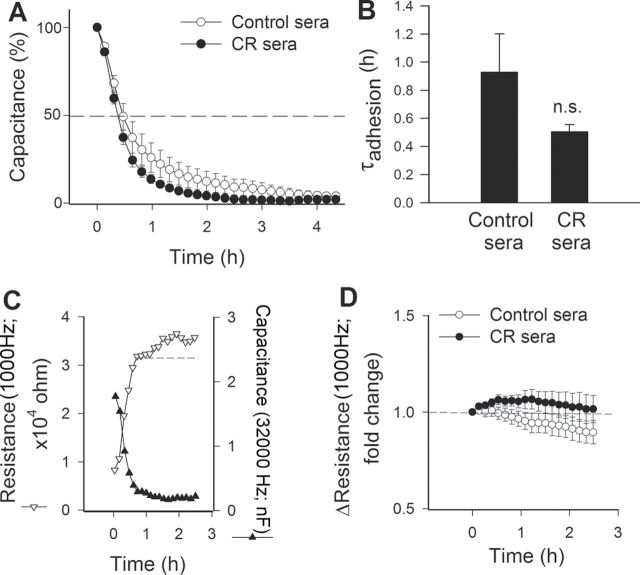
Endothelial cell adhesion events are known to have an important role in angiogenesis. We used ECIS technology to monitor changes of capacitance (at 60kHz) due to the adhesion of VEGF (100ng/mL)-stimulated cells to the collagen-coated active electrode (Figure 1A). The time constant (τ), calculated from an exponential curve fitting, was used as an index of adhesiveness. Treatment of CAECs with CR sera resulted in a decrease in τ (ie, less time was needed to reach 50% cell adhesion; Figure 1B). Although these differences reached only marginal statistical significance, the data indicate that CR sera may increase the ability of VEGF-treated CAECs to adhere to collagen.
Figure 1.
Effect of treatment with sera collected from caloric-restricted (CR) Macaca mulatta on vascular endothelial growth factor (VEGF)-induced adhesion of human coronary arterial endothelial cells (CAECs). VEGF (100ng/mL)-stimulated cell adhesion was monitored by electric cell–substrate impedance sensing (ECIS) technology (see the Methods section). (A) Time course of changes of capacitance (at 32kHz) after addition of CAECs to collagen-coated wells. Hundred percent change corresponds to the maximum level of cell coverage reached on the active electrode. Data are mean ± SEM. (n = 5 in each group). We calculated the time constant (τ) from each individual data set, which was used as an index of adhesiveness. (B) The summary data for τadhesion in CAECs treated with sera from control and CR animals are depicted here. Data are means ± SEM. (n = 5 in each group). There was a trend for a shorter τ in cells treated with CR sera, indicating that circulating factors triggered by CR tend to increase the adhesiveness of CAECs. (C) To assay barrier function, the time course of changes in capacitance (measured at 32kHz) and resistance (measured at 1,000 Hz) were monitored in parallel. (D) The time course of increases in resistance after capacitance has reached its minimum is indicative of barrier function associated with the formation of intercellular junctions.
Decline in capacitance due to adhesion of CAECs was associated with a parallel increase in resistance (Figure 1C). Comparison of the time course of additional increases in resistance (Figure 1D), after capacitance has reached its minimum (after, eg, 1.2 hours; Figure 1C), suggests that circulating factors induced by CR do not significantly alter barrier function associated with the formation of intercellular junctions.
Effect of Treatment With cr Sera on Proliferative Capacity of CAECs
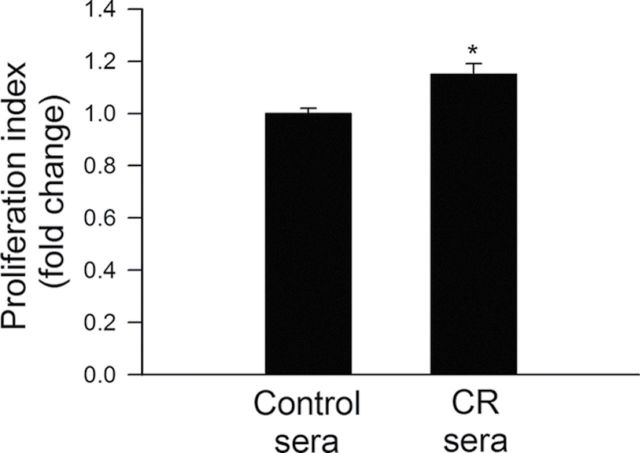
Proliferation represents a key step in angiogenesis. Proliferative capacity of CAECs treated with control or CR sera was compared. We found that treatment with CR sera significantly decreased carboxyfluorescein diacetate succinimidyl ester fluorescence (resulting in an increased proliferation index) in CAECs, indicating that proliferation capacity is significantly increased by circulating factors induced by CR (Figure 2).
Figure 2.
Treatment with sera collected from caloric-restricted (CR) Macaca mulatta significantly increases proliferation capacity of human coronary arterial endothelial cells (CAECs). Cell proliferation capacity was assessed in CAEC stimulated with vascular endothelial growth factor (VEGF; 100ng/mL) using the flow cytometry–based Guava Cell Growth assay (see Methods section). The inverse of the fluorescence intensity of the indicator dye carboxyfluorescein diacetate succinimidyl ester (CFSE) was used as an index of proliferation capacity of the cells. Data are means ± SEM (n = 5 in each group), *p < .05 versus control.
Effect of Treatment With cr Sera on the Migratory Capability of CAECs
The migratory capability of vascular endothelial cells has a pivotal role in the maintenance of microvascular integrity and angiogenesis. An ECIS-based wound-healing assay was used to assess the effect of CR serum factors on migratory capability of VEGF-treated CAECs. Treatment with CR sera tended to decrease the time for the cells to reach 50% of the maximum confluence (Figure 3A). Figure 3B indicates that the increase in the calculated migration rate in CAECs with CR sera treatment did not reach statistical significance.
Figure 3.
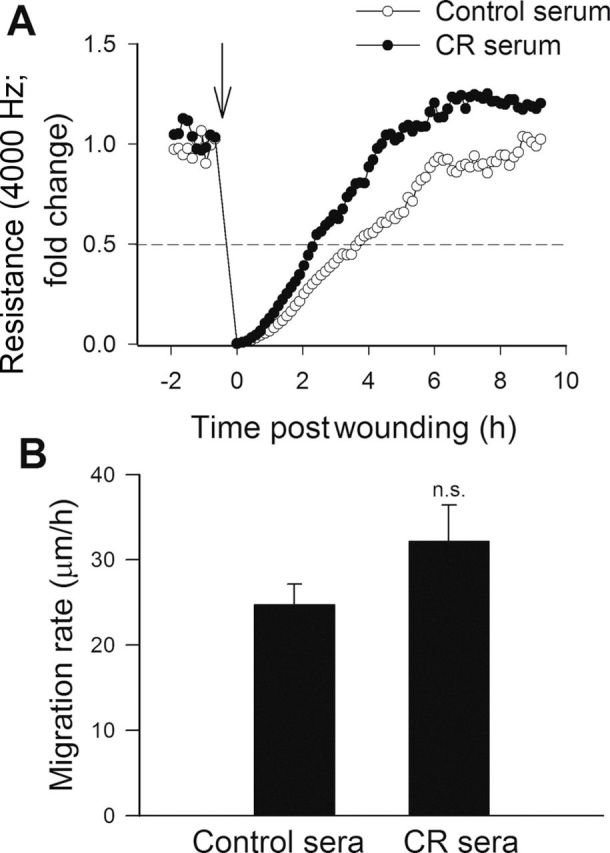
Effect of treatment with sera collected from caloric-restricted (CR) Macaca mulatta on migration capacity of human coronary arterial endothelial cells (CAECs). Vascular endothelial growth factor (VEGF; 100ng/mL)-stimulated cell migration was monitored by electric cell–substrate impedance sensing (ECIS) technology in a wound-healing assay (see Methods section). (A) Representative figure showing the time course of resistance recovery after wounding (arrow indicates the time, when an electric pulse of 5 mA for 20 seconds at 100kHz was applied; 100% represents prewounding levels of resistance measured at 4000 Hz). Resistance (at 4000 Hz) was monitored for every 160 seconds. Time to reach 50% resistance recovery (corresponding to 50% confluence on the active electrode) was determined for CAECs treated with control sera or CR sera, and this parameter and the known physical dimensions of the electrode were used to calculate the migration rate (expressed as µm/h). (B) The summary data for migration rate in CAECs treated with control sera or CR sera are depicted. Data are means ± SEM (n = 5 in each group).
Effect of Treatment With cr Sera on Formation of Capillary-Like Structures by CAECs
We performed an in vitro tube formation assay to model the reorganization stage of angiogenesis. In vitro tube formation is a multistep process involving cell adhesion, migration, differentiation, and growth. We assessed the effect of treatment with control or CR sera on the ability of endothelial cells to form capillary-like structures. When seeded onto Geltrex matrices, endothelial cells cultured in the presence of control sera formed elaborated capillary networks in the presence of VEGF (Figure 4A). We found that treatment with CR sera significantly increased the formation of capillary-like structures by CAECs (Figure 4B). Summary data (expressed as tube length per area in µm/mm2) are shown in Figure 4C.
Figure 4.
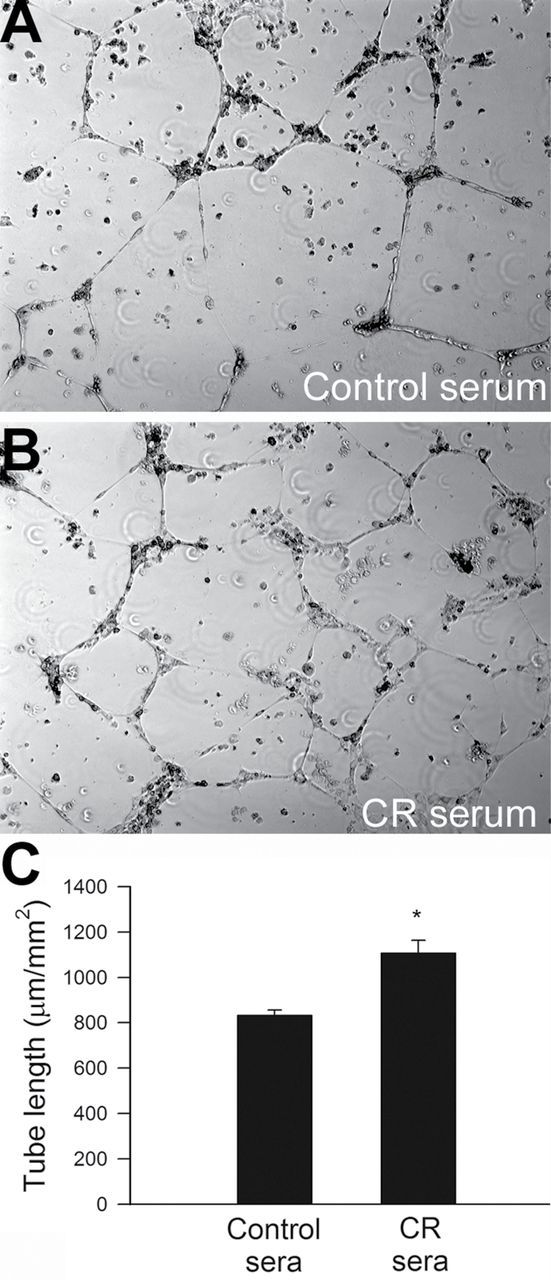
(A) Treatment with sera collected from caloric-restricted (CR) Macaca mulatta significantly increases the formation of capillary-like structures by human coronary arterial endothelial cells (CAECs). CAECs were plated on Geltrex-coated wells, and tube formation was induced by treating CAECs with vascular endothelial growth factor (VEGF; 100ng/mL, for 24 hours). Representative examples of capillary-like structures are shown in (A). Summary data, expressed as total tube length per total area scanned (µm tube/mm2), are shown in (B). Data are means ± SEM, (n = 5 in each group), *p < .05 versus control.
Treatment With cr Sera Attenuates Apoptosis in CAECs
Induction of endothelial apoptosis is an important mechanism that inhibits angiogenesis promoting microvascular rarefaction. We found that treatment with CR sera attenuated endothelial apoptosis as shown by the decreased caspase 3/7 activity (Figure 5A).
Figure 5.
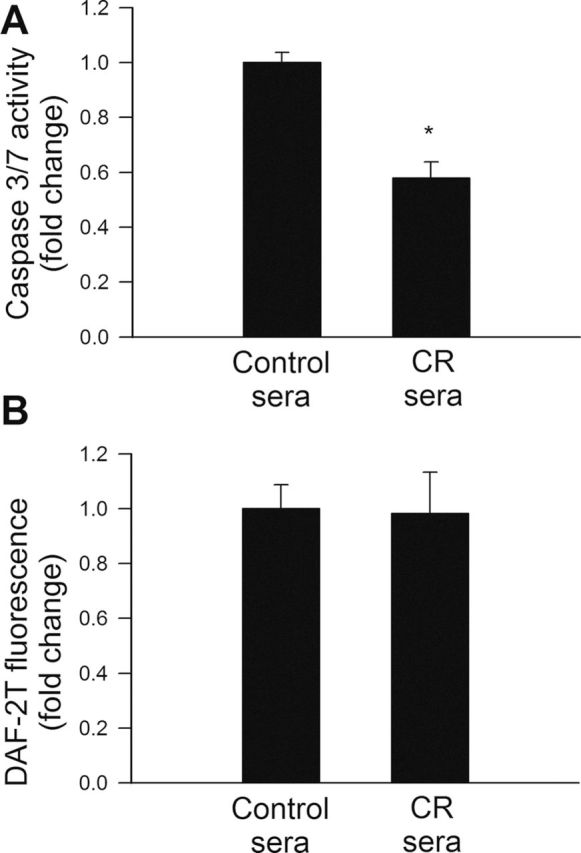
(A) Treatment with sera collected from caloric restricted (CR) Macaca mulatta significantly inhibits apoptosis in human coronary arterial endothelial cells (CAECs). Apoptotic cell death was assessed by measuring caspase 3/7 activity in cell lysates. *p < .05 versus control serum treated. Data are mean ± SEM. (n = 5 for each group). (B) In cultured CAECs treatment with CR sera did not alter cellular NO production, as compared with cells grown in the presence of sera obtained from control animals. Cellular NO production was assessed using the triazolofluorescein (DAF-2T) fluorescence method. Data are mean ± SEM. (n = 5 for each group).
Effects of Treatment of CAECs With CR Sera on NO Production
NO is an important mediator of angiogenesis that confers significant antiapoptotic effects. To test the hypothesis that treatment with CR sera confers proangiogenic and antiapoptotic effects by increasing NO bioavailability, DAF-2 was used for measurements of NO production in CAECs. We found that treatment with CR sera did not significantly affect basal cellular NO production (Figure 5B).
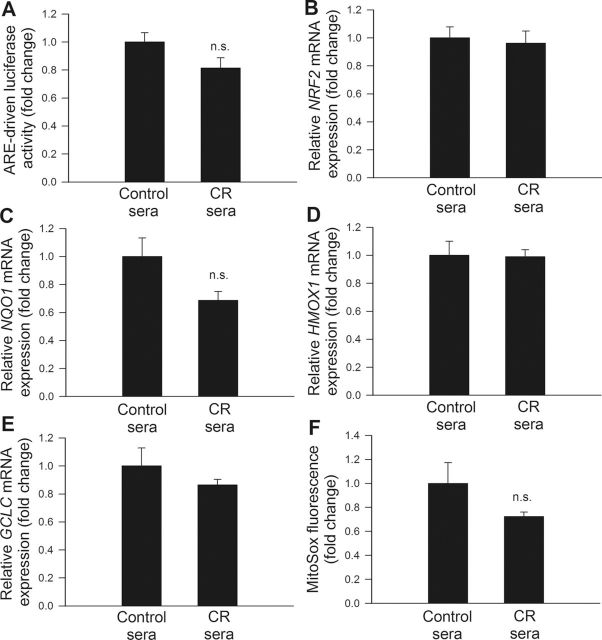
Effects of Treatment With CR Sera on Transcriptional Activity of Nrf2 in CAECs
Previous studies reported that CR in mice is associated with activation of Nrf2-driven antioxidant defense mechanisms (100) and that Nrf2 activation confers important proangiogenic and antiapoptotic effects in human endothelial cells (82). We determined the effects of serum factors triggered by CR in M mulatta on the Nrf2-driven antioxidant response. Using a reporter gene assay, no significant change in Nrf2 activation was noted in CAECs treated with CR sera (Figure 6A). In CAECs treated with CR sera, as compared with cells treated with sera from control animals, mRNA expression of Nrf2 and the Nrf2 target genes, GCLC, NQO1, and HMOX1, expression (Figure 6B, C, D, and E, respectively) were also unaltered.
Figure 6.
(A) Reporter gene assay showing the effects of treatment with sera collected from caloric-restricted (CR) Macaca mulatta on Nrf2/ARE reporter activity in cultured primary human coronary arterial endothelial cells (CAECs). Cells were transiently cotransfected with ARE-driven firefly luciferase and CMV-driven renilla luciferase constructs. At the end of the culture, period cells were then lysed and subjected to luciferase activity assay. After normalization, relative luciferase activity was obtained from four to six independent transfections. Data are mean ± SEM (n = 5 for each group). (B–E). Quantitative real-time reverse transcription polymerase chain reaction (RT-PCR) data showing the effect of treatment with CR sera on mRNA expression of NFE2L2 (Nrf2), NQO1, HMOX1, and GCLC in cultured primary CAECs. Data are mean ± SEM. (n = 5 in each group). (F) Effect of treatment with CR sera on mitochondrial 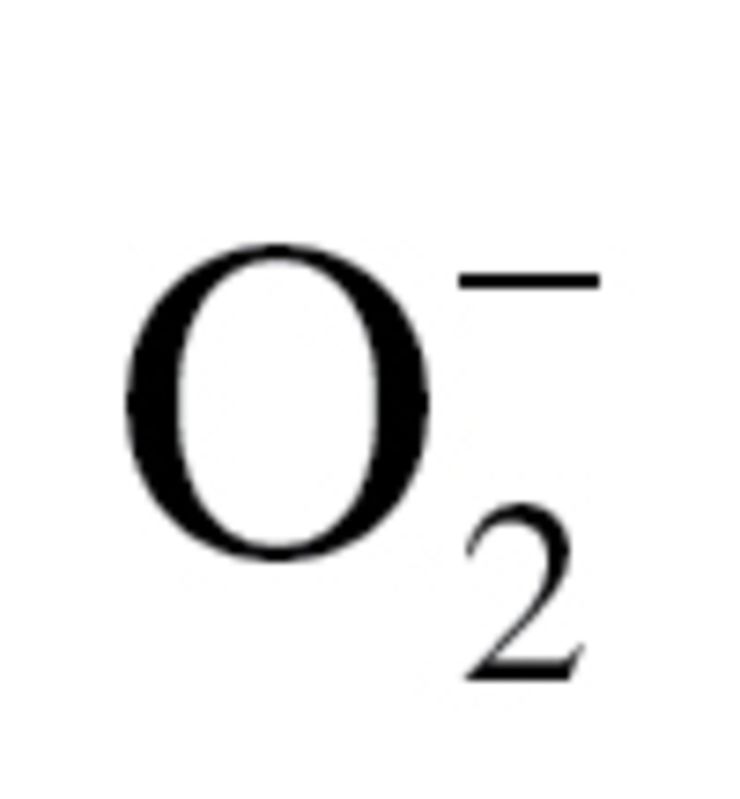 production in CAECs, assessed using the MitoSOX Red fluorescence method. Data are mean ± SEM. (n = 5 for each group).
production in CAECs, assessed using the MitoSOX Red fluorescence method. Data are mean ± SEM. (n = 5 for each group).
Effect of Treatment With CR Sera on Mitochondrial ROS Production in CAECs
Increased mitochondrial oxidative stress has been shown to impair angiogenic functions (101) and promote apoptosis in endothelial cells. We tested the hypothesis that CR sera confer antiapoptotic effects by decreasing mitochondrial oxidative stress. We found that treatment of CAECs with CR sera tended to attenuate MitoSox fluorescence indicating a decreased mitochondrial reactive oxygen species production (Figure 6F), however, this effect did not reach statistical significance.
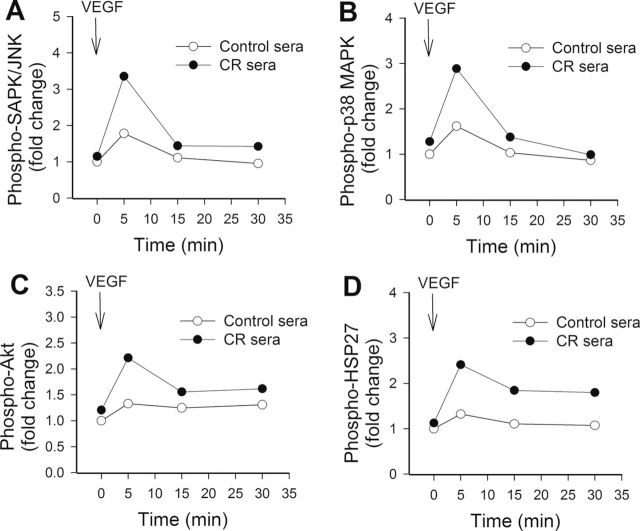
Effects of Treatment With CR Sera on VEGF Signaling in CAECs
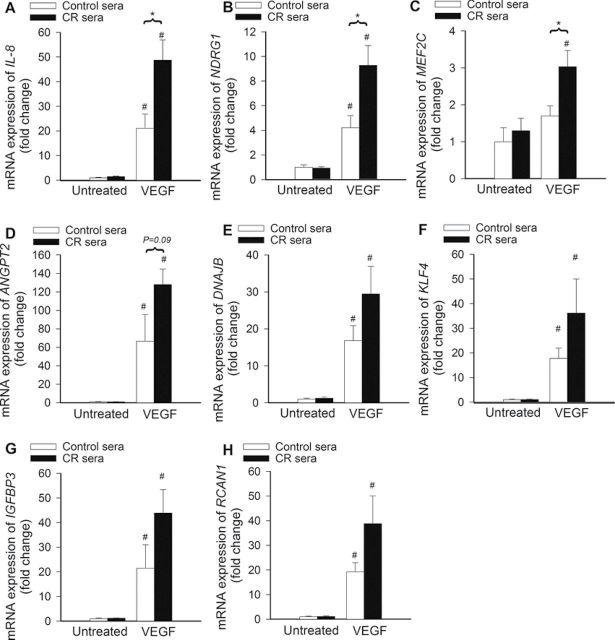
To test the hypothesis that CR sera confer proangiogenic effects by upregulating VEGF signaling, the time course for phosphorylation of SAPK/JNK, p38 MAPK, Akt, and HSP27 after VEGF stimulation was obtained (Figure 7). We found that the treatment with VEGF resulted in a more pronounced and sustained phosphorylation of SAPK/JNK (Figure 7A), p38 MAPK (Figure 7B), Akt (Figure 7C), and HSP27 (Figure 7D) in CAECs cultured in the presence of CR sera than in CAECs cultured in the presence of control sera. Treatment of CAECs with sera collected from CR-fed M mulatta did not alter expression of VEGFR2 and VEGFR1 in CAECs (Figure 8A and 8B, respectively). In contrast, treatment with CR sera upregulated VEGF (100ng/mL)-induced expression of known VEGF target genes, including IL-8 (Figure 9A), NDRG1 (Figure 9B), and MEF2C (Figure 9C). VEGF induction increases in expression of ANGPT2 (Figure 9D), DNAJB9 (Figure 9E), KLF4 (Figure 9F), IGFBP3 (Figure 9G), and RCAN1 (Figure 9H) also tended to be more pronounced in CAECs treated with CR sera than in cells treated with control sera, although the differences did not reach statistical significance.
Figure 7.
Treatment with sera collected from caloric restricted (CR) Macaca mulatta upregulates vascular endothelial growth factor (VEGF) signaling in human coronary arterial endothelial cells (CAECs). The time course for phosphorylation of SAPK/JNK (A), p38 MAPK (B), Akt (C), and HSP27 (D) in CAECs exposed to 100ng/mL VEGF for 5, 15, and 30 minutes was shown. Protein phosphorylation was analyzed using a magnetic multiplex bead array (Millipore; see the Methods section).
Figure 8.
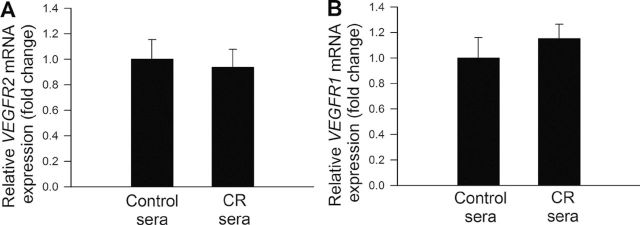
Quantitative real-time reverse transcription polymerase chain reaction (RT-PCR) data showing the effect of treatment with CR sera on mRNA expression of VEGFR2 (A) and VEGFR1 (B) in cultured primary coronary artery endothelial cells (CAECs). Data are mean ± SEM (n = 5 in each group).
Figure 9.
Treatment with sera collected from caloric-restricted (CR) Macaca mulatta upregulates vascular endothelial growth factor (VEGF; 100ng/mL)-induced expression of its target genes in human coronary artery endothelial cells (CAECs). Quantitative real-time reverse transcription polymerase chain reaction (RT-PCR) data show the effect of treatment with CR sera on VEGF-induced mRNA expression of IL-8 (A) , NDRG1 (B), MEF2C (C), ANGPT2 (D), DNAJB9 (E), KLF4 (F), IGFBP3 (G), and RCAN1 (H) in cultured primary CAECs. Data are mean ± SEM (n = 5 in each group). *p < .05 versus control sera treated and #p < .05 versus no VEGF.
Discussion
Moderate CR in laboratory rodents was shown to exert significant microvascular protective effects (11,12,28–30). However, the mechanisms that contribute to CR-induced increases in microvascular density in the heart (30) and the brain (11,12) that underlie progressive improvements in tissue blood supply and organ function are only partially understood. The principal new finding of this study is that circulating factors induced by CR in the nonhuman primate M mulatta upregulates endothelial angiogenic capacity. Major steps of the angiogenic process, including proliferation and formation of capillary-like structures, are significantly enhanced in CAECs treated with CR sera, whereas the rate of apoptosis is significantly decreased.
Here, we have devised an in vitro approach to determine the role of circulating factors that mediate the proangiogenic effects of CR. Our data show for the first time that circulating factors within the plasma of caloric-restricted nonhuman primates activate multiple angiogenic processes in endothelial cells. Although the effects of circulating factors present in the sera of CR monkeys on endothelial adhesion capacity (Figure 1) and cell migration (Figure 3) were relatively modest, sera from CR monkeys significantly stimulated endothelial cell proliferation (Figure 2) and capillary morphogenesis (Figure 4). These findings support the concept that circulating factors triggered by CR, preserve structural integrity of the microcirculation, at least in part, by stimulating angiogenesis. It should be noted that angiogenesis is required for invasive tumor growth and metastasis. Because CR confers resistance against cancer, future studies are warranted to determine whether CR exerts different effects on the tumor micro circulation (102).
It is generally accepted that increased apoptotic cell death contributes to the age-related microvascular rarefaction. Accordingly, in laboratory rodents, the percentage of apoptotic endothelial cells significantly increases with age (91,103,104). Aging is also associated with enhanced endothelial apoptosis in nonhuman primates (105). Another potentially important finding of our study is that treatment of cells in vitro with sera from CR animals mimics the known antiapoptotic effects of CR in vivo (106,107), preventing the induction of apoptosis in cultured endothelial cells (Figure 5A). In comparable fashion, sera from caloric-restricted rats also confers significant antiapoptotic effects in endothelial cells in vitro (28). We propose that antiapoptotic effects of circulating factors triggered by CR will increase the angiogenic capacity of endothelial cells in vivo and importantly contribute to the microvascular protective effects of CR. Previous studies have demonstrated that age-related oxidative stress in endothelial cells impairs bioavailability of the prosurvival factor NO. Thus, the reduction in NO is thought to be a strong inducer of endothelial apoptosis (103,108). Because CR in vivo was shown to significantly increase endothelial NO production by upregulating eNOS (28,29,109), we anticipated that this effect would be recapitulated in vitro by treatment of CAECs with sera derived from caloric-restricted M mulatta. Contrary to our prediction, we found that treatment with CR sera did not affect cellular NO production (Figure 5B). Thus, our data failed to support the hypothesis that increased endogenous NO generation mediates the antiapoptotic activity of circulating factors triggered by CR in the present cohort of M mulatta.
The actual circulating factor(s) that mediate the proangiogenic effects of CR are presently unknown. Because serum adiponectin levels increase in CR rats (106,110) and previous studies demonstrate that adiponectin promotes angiogenesis via an Akt-dependent pathway (111), future studies will examine the role of adiponectin in the microvascular protective effects of CR in nonhuman primates. Our recent studies in laboratory rats suggest that neuroendocrine factors also mediate, at least in part, the antiinflammatory and antioxidative endothelial protective effects of CR (28). Further studies are needed to determine whether the humoral factors present in the circulation of caloric-restricted M mulatta also confer anti-inflammatory endothelial protective effects.
Previous studies have attributed the antioxidative effects of CR to Nrf2 activation (29). Seminal studies by de Cabo and coworkers have shown that CR activates Nrf2 signaling and causes marked increases in plasma membrane NQO1 activity in rat and murine tissues (36,112,113). The upregulation of Nrf2-driven plasma membrane redox systems that occurs during CR contributes to the decreases of the levels of cellular oxidative stress in the aged liver (36,112–114). Recent data also demonstrate that activation of the Nrf2-driven antioxidant system contributes to the protective effect of CR against cancer (100,115). In that regard, a functional Nrf2 pathway is essential for a healthy endothelial angiogenic response since all the major steps of the angiogenic process, including adhesion, proliferation, migration, and formation of capillary-like structures are compromised by disruption of Nrf2 signaling in cultured endothelial cells (82). Nrf2 blockade also suppresses angiogenesis in mouse models in vivo (116). Further, the Nrf2 targets, heme oxygenase-1 (117,118) and thioredoxin (119), were shown to confer proangiogenic effects in animal disease models. Moreover, Nrf2 confers significant antiapoptotic effects in endothelial cells (78,79,82,87,120). Nevertheless, treatment with CR sera failed to increase transcriptional activity of Nrf2 in the present study, indicating that other mechanisms may be important in the response of endothelial cells to CR in primates (Figure 6). Multiple lines of evidence suggest that homologs of the NAD+-dependent protein deacetylase SIRT1 contribute to life-span extension by CR in lower organisms (121). In mammals, SIRT1 is also inducible by circulating factors triggered by CR (35) and there is evidence that antioxidant endothelial effects induced by circulating factors during CR are, in part, mediated by pathways that involve SIRT1 (28). Importantly, previous studies demonstrate that SIRT1 regulates the angiogenic activity of endothelial cells and that disruption of SIRT1 gene expression in zebrafish and mice results in defective blood vessel formation and blunts ischemia-induced neovascularization (122). Thus, future studies should test the hypothesis that SIRT1 activation induced by circulating factors present in the blood of caloric-restricted M mulatta contributes to the proangiogenic effects of CR.
Regulation of endothelial angiogenic processes by VEGF involves activation of SAPK/JNK, Akt and p38 MAPK, and phosphorylation of HSP27 (123). Time course analysis of VEGF-induced activation of the aforementioned pathways suggest that circulating factors induced by CR in M mulatta upregulate VEGF signaling in endothelial cells (Figure 7). The available evidence suggests that this is independent of changes in the expression of VEGF receptors (Figure 8). The concept that humoral factors potentiate VEGF signaling is further supported by the observation that treatment with CR sera augments the transcriptional response of CAECs to VEGF (Figure 9). There is increasing evidence that VEGFR activity can be regulated at multiple levels, including dimerization of receptors, interaction with the trimeric G proteins Gαq/Gα11, dephosporylation of the receptors by tyrosine-specific phosphatases, and degradation of the receptors through the proteasome pathway (reviewed in Reference 123). It can be hypothesized that circulating factors triggered by CR may affect these pathway(s) modulating VEGFR2 signaling.
In conclusion, circulating factors triggered by CR activate endogenous proangiogenic and antiapoptotic mechanisms in endothelial cells. These changes in endothelial cell function and responsiveness may contribute to the cardiovascular protective effects of CR in nonhuman primates. Because serum obtained from caloric-restricted humans also confers cytoprotection in human cells in vitro (124), we predict that CR may also promote microvascular health in humans. Potentially, the mechanisms of CR could be harnessed for development of new pharmacological approaches for the prevention and treatment of diseases associated with microvascular rarefaction in elderly patients (such as vascular cognitive impairment and heart failure).
Funding
This work was supported by grants from the American Federation for Aging Research (to A.C.), the Oklahoma Center for the Advancement of Science and Technology (to A.C., Z.U., and W.E.S.), the University of Oklahoma College of Medicine Alumni Association (to A.C.), the American Heart Association (to A.C., P.T., and Z.U.), the National Institutes of Health (NIH) (AG031085 to A.C., AT006526 to Z.U., P01 AG011915 to R.W., P51 RR000167 to W.N.P.R.C., and AG038747, NS056218, and P01 AG11370 to W.E.S.), the Ellison Medical Foundation (to W.E.S.), and the Arkansas Claude Pepper Older Americans Independence Center at University of Arkansas Medical Center (to A.C.). This research was conducted in part at a facility constructed with support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01 from NCRR.
Acknowledgments
The authors would like to express their gratitude for the support of the Donald W. Reynolds Foundation, which funds aging research at the University of Oklahoma Health Sciences Center under its Aging and Quality of Life Program.
References
- 1. Tarnawski AS, Pai R, Tanigawa T, Matysiak-Budnik T, Ahluwalia A. PTEN silencing reverses aging-related impairment of angiogenesis in microvascular endothelial cells. Biochem Biophys Res Commun. 2010;394:291–296 [DOI] [PubMed] [Google Scholar]
- 2. Bach MH, Sadoun E, Reed MJ. Defects in activation of nitric oxide synthases occur during delayed angiogenesis in aging. Mech Ageing Dev. 2005;126:467–473 [DOI] [PubMed] [Google Scholar]
- 3. Sadoun E, Reed MJ. Impaired angiogenesis in aging is associated with alterations in vessel density, matrix composition, inflammatory response, and growth factor expression. J Histochem Cytochem. 2003;51:1119–1130 [DOI] [PubMed] [Google Scholar]
- 4. Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci. 2010;65:1028–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahluwalia A, Tarnawski AS. Activation of the metabolic sensor-AMP activated protein kinase reverses impairment of angiogenesis in aging myocardial microvascular endothelial cells. Implications for the aging heart. J Physiol Pharmacol. 2011;62:583–587 [PubMed] [Google Scholar]
- 6. Ungvari Z, Csiszar A. The emerging role of igf-1 deficiency in cardiovascular aging: recent advances. J Gerontol A Biol Sci Med Sci. 2012;67:599–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murugesan N, Demarest TG, Madri JA, Pachter JS. Brain regional angiogenic potential at the neurovascular unit during normal aging. Neurobiol Aging. 2012;33:1004.e1–1004.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Benderro GF, Lamanna JC. Hypoxia-induced angiogenesis is delayed in aging mouse brain. Brain Res. 2011;1389:50–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ingraham JP, Forbes ME, Riddle DR, Sonntag WE. Aging reduces hypoxia-induced microvascular growth in the rodent hippocampus. J Gerontol A Biol Sci Med Sci. 2008;63:12–20 [DOI] [PubMed] [Google Scholar]
- 10. Anversa P, Li P, Sonnenblick EH, Olivetti G. Effects of aging on quantitative structural properties of coronary vasculature and microvasculature in rats. Am J Physiol. 1994;267(3 Pt 2):H1062–H1073 [DOI] [PubMed] [Google Scholar]
- 11. Lynch CD, Cooney PT, Bennett SA, et al. Effects of moderate caloric restriction on cortical microvascular density and local cerebral blood flow in aged rats. Neurobiol Aging. 1999;20:191–200 [DOI] [PubMed] [Google Scholar]
- 12. Adams MM, Shi L, Linville MC, et al. Caloric restriction and age affect synaptic proteins in hippocampal CA3 and spatial learning ability. Exp Neurol. 2008;211:141–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. 2011;37:56–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Austad SN. Life extension by dietary restriction in the bowl and doily spider, Frontinella pyramitela. Exp Gerontol. 1989;24:83–92 [DOI] [PubMed] [Google Scholar]
- 16. Colman RJ, Anderson RM, Johnson SC, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edwards IJ, Rudel LL, Terry JG, Kemnitz JW, Weindruch R, Cefalu WT. Caloric restriction in rhesus monkeys reduces low density lipoprotein interaction with arterial proteoglycans. J Gerontol A Biol Sci Med Sci. 1998;53:B443–B448 [DOI] [PubMed] [Google Scholar]
- 18. Edwards IJ, Rudel LL, Terry JG, et al. Caloric restriction lowers plasma lipoprotein (a) in male but not female rhesus monkeys. Exp Gerontol. 2001;36:1413–1418 [DOI] [PubMed] [Google Scholar]
- 19. Ingram DK, Cutler RG, Weindruch R, et al. Dietary restriction and aging: the initiation of a primate study. J Gerontol. 1990;45:B148–B163 [DOI] [PubMed] [Google Scholar]
- 20. Kayo T, Allison DB, Weindruch R, Prolla TA. Influences of aging and caloric restriction on the transcriptional profile of skeletal muscle from rhesus monkeys. Proc Natl Acad Sci USA. 2001;98:5093–5098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kemnitz JW, Roecker EB, Weindruch R, Elson DF, Baum ST, Bergman RN. Dietary restriction increases insulin sensitivity and lowers blood glucose in rhesus monkeys. Am J Physiol. 1994;266(4 Pt 1):E540–E547 [DOI] [PubMed] [Google Scholar]
- 22. Kemnitz JW, Weindruch R, Roecker EB, Crawford K, Kaufman PL, Ershler WB. Dietary restriction of adult male rhesus monkeys: design, methodology, and preliminary findings from the first year of study. J Gerontol. 1993;48:B17–B26 [DOI] [PubMed] [Google Scholar]
- 23. Kim MJ, Roecker EB, Weindruch R. Influences of aging and dietary restriction on red blood cell density profiles and antioxidant enzyme activities in rhesus monkeys. Exp Gerontol. 1993;28:515–527 [DOI] [PubMed] [Google Scholar]
- 24. Ramsey JJ, Colman RJ, Binkley NC, et al. Dietary restriction and aging in rhesus monkeys: the University of Wisconsin study. Exp Gerontol. 2000;35:1131–1149 [DOI] [PubMed] [Google Scholar]
- 25. Zainal TA, Oberley TD, Allison DB, Szweda LI, Weindruch R. Caloric restriction of rhesus monkeys lowers oxidative damage in skeletal muscle. FASEB J. 2000;14:1825–1836 [DOI] [PubMed] [Google Scholar]
- 26. Maswood N, Young J, Tilmont E, et al. Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson’s disease. Proc Natl Acad Sci USA. 2004;101:18171–18176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matochik JA, Chefer SI, Lane MA, et al. Age-related decline in striatal volume in rhesus monkeys: assessment of long-term calorie restriction. Neurobiol Aging. 2004;25:193–200 [DOI] [PubMed] [Google Scholar]
- 28. Csiszar A, Labinskyy N, Jimenez R, et al. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130:518–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res. 2008;102:519–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Katare RG, Kakinuma Y, Arikawa M, Yamasaki F, Sato T. Chronic intermittent fasting improves the survival following large myocardial ischemia by activation of BDNF/VEGF/PI3K signaling pathway. J Mol Cell Cardiol. 2009;46:405–412 [DOI] [PubMed] [Google Scholar]
- 31. Facchetti F, Monzani E, Cavallini G, Bergamini E, La Porta CA. Effect of a caloric restriction regimen on the angiogenic capacity of aorta and on the expression of endothelin-1 during ageing. Exp Gerontol. 2007;42:662–667 [DOI] [PubMed] [Google Scholar]
- 32. Kondo M, Shibata R, Miura R, et al. Caloric restriction stimulates revascularization in response to ischemia via adiponectin-mediated activation of endothelial nitric-oxide synthase. J Biol Chem. 2009;284:1718–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carvalho M, Mateus L, Afonso F, et al. Testicular angiogenic activity in response to food restriction in rabbits. Reproduction. 2009;137:509–515 [DOI] [PubMed] [Google Scholar]
- 34. de Cabo R, Fürer-Galbán S, Anson RM, Gilman C, Gorospe M, Lane MA. An in vitro model of caloric restriction. Exp Gerontol. 2003;38:631–639 [DOI] [PubMed] [Google Scholar]
- 35. Cohen HY, Miller C, Bitterman KJ, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392 [DOI] [PubMed] [Google Scholar]
- 36. Hyun DH, Emerson SS, Jo DG, Mattson MP, de Cabo R. Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proc Natl Acad Sci USA. 2006;103:19908–19912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Behrens MI, Silva M, Schmied A, et al. Age-dependent increases in apoptosis/necrosis ratios in human lymphocytes exposed to oxidative stress. J Gerontol A Biol Sci Med Sci. 2011;66:732–740 [DOI] [PubMed] [Google Scholar]
- 38. Carnes BA, Riesch R, Schlupp I. The delayed impact of parental age on offspring mortality in mice. J Gerontol A Biol Sci Med Sci. 2012;67:351–357 [DOI] [PubMed] [Google Scholar]
- 39. Chandrashekara KT, Shakarad MN. Aloe vera or resveratrol supplementation in larval diet delays adult aging in the fruit fly, Drosophila melanogaster. J Gerontol A Biol Sci Med Sci. 2011;66:965–971 [DOI] [PubMed] [Google Scholar]
- 40. Chung E, Diffee GM. Effect of aging on power output properties in rat skinned cardiac myocytes. J Gerontol A Biol Sci Med Sci. 2011;66:1267–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dekker P, van Baalen LM, Dirks RW, et al. Chronic inhibition of the respiratory chain in human fibroblast cultures: differential responses related to subject chronological and biological age. J Gerontol A Biol Sci Med Sci. 2012;67:456–464 [DOI] [PubMed] [Google Scholar]
- 42. Ding J, Berryman DE, Jara A, Kopchick JJ. Age- and sex-associated plasma proteomic changes in growth hormone receptor gene-disrupted mice. J Gerontol A Biol Sci Med Sci. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Eghbalieh SD, Chowdhary P, Muto A, et al. Age-related neointimal hyperplasia is associated with monocyte infiltration after balloon angioplasty. J Gerontol A Biol Sci Med Sci. 2012;67:109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Florez H, Ma Y, Crandall JP, et al. Parental longevity and diabetes risk in the Diabetes Prevention Program. J Gerontol A Biol Sci Med Sci. 2011;66:1211–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Forman K, Vara E, García C, et al. Effect of a combined treatment with growth hormone and melatonin in the cardiological aging on male SAMP8 mice. J Gerontol A Biol Sci Med Sci. 2011;66:823–834 [DOI] [PubMed] [Google Scholar]
- 46. Gesing A, Masternak MM, Wang F, et al. Expression of key regulators of mitochondrial biogenesis in growth hormone receptor knockout (GHRKO) mice is enhanced but is not further improved by other potential life-extending interventions. J Gerontol A Biol Sci Med Sci. 2011;66:1062–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gunin AG, Kornilova NK, Vasilieva OV, Petrov VV. Age-related changes in proliferation, the numbers of mast cells, eosinophils, and cd45-positive cells in human dermis. J Gerontol A Biol Sci Med Sci. 2011;66:385–392 [DOI] [PubMed] [Google Scholar]
- 48. Hozawa A, Sugawara Y, Tomata Y, et al. Relationship between serum adiponectin levels and disability-free survival among community-dwelling elderly individuals: The Tsurugaya Project. J Gerontol A Biol Sci Med Sci. 2012;67:530–536 [DOI] [PubMed] [Google Scholar]
- 49. Inzitari M, Arnold AM, Patel KV, et al. Subclinical vascular disease burden and risk for death and cardiovascular events in older community dwellers. J Gerontol A Biol Sci Med Sci. 2011;66:986–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Katsumata Y, Todoriki H, Higashiuesato Y, et al. Metabolic syndrome and cognitive decline among the oldest old in Okinawa: in search of a mechanism. The KOCOA Project. J Gerontol A Biol Sci Med Sci. 2012;67:126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Labbé A, Garand C, Cogger VC, et al. Resveratrol improves insulin resistance hyperglycemia and hepatosteatosis but not hypertriglyceridemia, inflammation, and life span in a mouse model for Werner syndrome. J Gerontol A Biol Sci Med Sci. 2011;66:264–278 [DOI] [PubMed] [Google Scholar]
- 52. Lesniewski LA, Durrant JR, Connell ML, Folian BJ, Donato AJ, Seals DR. Salicylate treatment improves age-associated vascular endothelial dysfunction: potential role of nuclear factor kappaB and forkhead Box O phosphorylation. J Gerontol A Biol Sci Med Sci. 2011;66:409–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lushchak OV, Gospodaryov DV, Rovenko BM, et al. Balance between macronutrients affects life span and functional senescence in fruit fly Drosophila melanogaster. J Gerontol A Biol Sci Med Sci. 2012;67:118–125 [DOI] [PubMed] [Google Scholar]
- 54. McFarlane D, Wolf RF, McDaniel KA, White GL. Age-associated alteration in innate immune response in captive baboons. J Gerontol A Biol Sci Med Sci. 2011;66:1309–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mitterberger MC, Mattesich M, Klaver E, Piza-Katzer H, Zwerschke W. Reduced insulin-like growth factor-I serum levels in formerly obese women subjected to laparoscopic-adjustable gastric banding or diet-induced long-term caloric restriction. J Gerontol A Biol Sci Med Sci. 2011;66:1169–1177 [DOI] [PubMed] [Google Scholar]
- 56. Mojtahedi MC, Thorpe MP, Karampinos DC, et al. The effects of a higher protein intake during energy restriction on changes in body composition and physical function in older women. J Gerontol A Biol Sci Med Sci. 2011;66:1218–1225 [DOI] [PubMed] [Google Scholar]
- 57. Murias JM, Kowalchuk JM, Ritchie D, Hepple RT, Doherty TJ, Paterson DH. Adaptations in capillarization and citrate synthase activity in response to endurance training in older and young men. J Gerontol A Biol Sci Med Sci. 2011;66:957–964 [DOI] [PubMed] [Google Scholar]
- 58. Pérez VI, Cortez LA, Lew CM, et al. Thioredoxin 1 overexpression extends mainly the earlier part of life span in mice. J Gerontol A Biol Sci Med Sci. 2011;66:1286–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rochon J, Bales CW, Ravussin E, et al. Design and conduct of the CALERIE study: comprehensive assessment of the long-term effects of reducing intake of energy. J Gerontol A Biol Sci Med Sci. 2011;66:97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tazearslan C, Cho M, Suh Y. Discovery of functional gene variants associated with human longevity: opportunities and challenges. J Gerontol A Biol Sci Med Sci. 2012;67:376–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yubero-Serrano EM, Gonzalez-Guardia L, Rangel-Zuñiga O, et al. Mediterranean diet supplemented with coenzyme Q10 modifies the expression of proinflammatory and endoplasmic reticulum stress-related genes in elderly men and women. J Gerontol A Biol Sci Med Sci. 2012;67:3–10 [DOI] [PubMed] [Google Scholar]
- 62. Deak F, Sonntag WE. Aging, synaptic dysfunction, and insulin-like growth factor (IGF)-1. J Gerontol A Biol Sci Med Sci. 2012;67:611–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fok WC, Zhang Y, Salmon AB, et al. Short-term treatment with rapamycin and dietary restriction have overlapping and distinctive effects in young mice. J Gerontol A Biol Sci Med Sci. 2012. doi:10.1093/gerona/gls127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cesari M, Kritchevsky SB, Nicklas B, et al. Oxidative damage, platelet activation, and inflammation to predict mobility disability and mortality in older persons: results from the health aging and body composition study. J Gerontol A Biol Sci Med Sci. 2012;67A:671–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Craft S, Foster TC, Landfield PW, Maier SF, Resnick SM, Yaffe K. Session III: mechanisms of age-related cognitive change and targets for intervention: inflammatory, oxidative, and metabolic processes. J Gerontol A Biol Sci Med Sci. 2012;67:754–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Horan MP, Pichaud N, Ballard JW. Quantifying mitochondrial dysfunction in complex diseases of aging. J Gerontol A Biol Sci Med Sci. 2012. doi:10.1093/gerona/glr263. [DOI] [PubMed] [Google Scholar]
- 67. Kosik KS, Rapp PR, Raz N, Small SA, Sweatt JD, Tsai LH. Mechanisms of age-related cognitive change and targets for intervention: epigenetics. J Gerontol A Biol Sci Med Sci. 2012;67:741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Miller RA. Genes against aging. J Gerontol A Biol Sci Med Sci. 2012;67:495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pilling LC, Harries LW, Powell J, Llewellyn DJ, Ferrucci L, Melzer D. Genomics and successful aging: grounds for renewed optimism?. J Gerontol A Biol Sci Med Sci. 2012;67:511–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Poon LW, Woodard JL, Stephen Miller L, et al. Understanding dementia prevalence among centenarians. J Gerontol A Biol Sci Med Sci. 2012;67:358–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Solesio-Jofre E, Lorenzo-López L, Gutiérrez R, López-Frutos JM, Ruiz-Vargas JM, Maestú F. Age-related effects in working memory recognition modulated by retroactive interference. J Gerontol A Biol Sci Med Sci. 2012;67:565–572 [DOI] [PubMed] [Google Scholar]
- 72. Taylor CL, Albanese E, Stewart R. The association of dementia with upper arm and waist circumference in seven low- and middle-income countries: the 10/66 cross-sectional surveys. J Gerontol A Biol Sci Med Sci. 2012;67:897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zheng JJ, Lord SR, Close JC, et al. Brain White Matter Hyperintensities, Executive Dysfunction, Instability, and Falls in Older People: A Prospective Cohort Study. J Gerontol A Biol Sci Med Sci. 2012. doi:10.1093/gerona/gls063. [DOI] [PubMed] [Google Scholar]
- 74. Colman RJ, Ramsey JJ, Roecker EB, Havighurst T, Hudson JC, Kemnitz JW. Body fat distribution with long-term dietary restriction in adult male rhesus macaques. J Gerontol A Biol Sci Med Sci. 1999;54:B283–B290 [DOI] [PubMed] [Google Scholar]
- 75. Colman RJ, Roecker EB, Ramsey JJ, Kemnitz JW. The effect of dietary restriction on body composition in adult male and female rhesus macaques. Aging (Milano). 1998;10:83–92 [DOI] [PubMed] [Google Scholar]
- 76. Gresl TA, Colman RJ, Roecker EB, et al. Dietary restriction and glucose regulation in aging rhesus monkeys: a follow-up report at 8.5 yr. Am J Physiol Endocrinol Metab. 2001;281:E757–E765 [DOI] [PubMed] [Google Scholar]
- 77. Ungvari Z, Labinskyy N, Mukhopadhyay P, et al. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H1876–H1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ungvari Z, Bagi Z, Feher A, et al. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–H24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ungvari Z, Bailey-Downs L, Gautam T, et al. Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol. 2011;300:H1133–H1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Csiszar A, Labinskyy N, Perez V, et al. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol. 2008;295:H1882–H1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Clapp C, Thebault S, Jeziorski MC, Martínez De La Escalera G. Peptide hormone regulation of angiogenesis. Physiol Rev. 2009;89:1177–1215 [DOI] [PubMed] [Google Scholar]
- 82. Valcarcel-Ares MN, Gautam T, Warrington JP, et al. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J Gerontol A Biol Sci Med Sci. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Giaever I, Keese CR. Micromotion of mammalian cells measured electrically. Proc Natl Acad Sci USA. 1991;88:7896–7900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ungvari Z, Ridgway I, Philipp EE, et al. Extreme longevity is associated with increased resistance to oxidative stress in arctica islandica, the longest-living non-colonial animal. J Gerontol A Biol Sci Med Sci. 2011;66(7):741–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bailey-Downs LC, Sosnowska D, Toth P, et al. Growth hormone and igf-1 deficiency exacerbate high-fat diet-induced endothelial impairment in obese lewis dwarf rats: implications for vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:553–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Csiszar A, Labinskyy N, Zhao X, et al. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell. 2007;6:783–797 [DOI] [PubMed] [Google Scholar]
- 87. Ungvari Z, Bailey-Downs L, Gautam T, et al. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-{kappa}B activation in the nonhuman primate Macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011;66:866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci. 2012;67:811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bailey-Downs LC, Mitschelen M, Sosnowska D, et al. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:313–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Csiszar A, Ungvari Z, Edwards JG, et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166 [DOI] [PubMed] [Google Scholar]
- 91. Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-tumor necrosis factor-alpha treatment in aging. Am J Pathol. 2007;170:388–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ungvari Z, Labinskyy N, Gupte S, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol. 2008;294:H2121–H2128 [DOI] [PubMed] [Google Scholar]
- 93. Ungvari Z, Orosz Z, Labinskyy N, et al. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–H47 [DOI] [PubMed] [Google Scholar]
- 94. Ungvari Z, Gautam T, Koncz P, et al. Vasoprotective effects of life span-extending peripubertal GH replacement in Lewis dwarf rats. J Gerontol A Biol Sci Med Sci. 2010;65:1145–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-alpha-induced activation of coronary arterial endothelial cells: role of NF-kappaB inhibition. Am J Physiol Heart Circ Physiol. 2006;291:H1694–H1699 [DOI] [PubMed] [Google Scholar]
- 96. Csiszar A, Podlutsky A, Podlutskaya N, et al. Testing the oxidative stress hypothesis of aging in primate fibroblasts: is there a correlation between species longevity and cellular ros production?. J Gerontol A Biol Sci Med Sci. 2012;67:841–852.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ungvari Z, Sosnowska D, Podlutsky A, Koncz P, Sonntag WE, Csiszar A. Free radical production, antioxidant capacity, and oxidative stress response signatures in fibroblasts from lewis dwarf rats: effects of life span-extending peripubertal gh treatment. J Gerontol A Biol Sci Med Sci. 2011;66:501–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Schweighofer B, Testori J, Sturtzel C, et al. The VEGF-induced transcriptional response comprises gene clusters at the crossroad of angiogenesis and inflammation. Thromb Haemost. 2009;102:544–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Maiti D, Xu Z, Duh EJ. Vascular endothelial growth factor induces MEF2C and MEF2-dependent activity in endothelial cells. Invest Ophthalmol Vis Sci. 2008;49:3640–3648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Pearson KJ, Lewis KN, Price NL, et al. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Natl Acad Sci USA. 2008;105:2325–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Uraoka M, Ikeda K, Kurimoto-Nakano R, et al. Loss of bcl-2 during the senescence exacerbates the impaired angiogenic functions in endothelial cells by deteriorating the mitochondrial redox state. Hypertension. 2011;58:254–263 [DOI] [PubMed] [Google Scholar]
- 102. Urits I, Mukherjee P, Meidenbauer J, Seyfried TN. Dietary restriction promotes vessel maturation in a mouse astrocytoma. J Oncol. 2012;2012:264039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics. 2004;17:21–30 [DOI] [PubMed] [Google Scholar]
- 104. Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Asai K, Kudej RK, Shen YT, et al. Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arterioscler Thromb Vasc Biol. 2000;20:1493–1499 [DOI] [PubMed] [Google Scholar]
- 106. Shinmura K, Tamaki K, Bolli R. Impact of 6-mo caloric restriction on myocardial ischemic tolerance: possible involvement of nitric oxide-dependent increase in nuclear Sirt1. Am J Physiol Heart Circ Physiol. 2008;295:H2348–H2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Edwards MG, Anderson RM, Yuan M, Kendziorski CM, Weindruch R, Prolla TA. Gene expression profiling of aging reveals activation of a p53-mediated transcriptional program. BMC Genomics. 2007;8:80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hoffmann J, Haendeler J, Aicher A, et al. Aging enhances the sensitivity of endothelial cells toward apoptotic stimuli: important role of nitric oxide. Circ Res. 2001;89:709–715 [DOI] [PubMed] [Google Scholar]
- 109. Nisoli E, Tonello C, Cardile A, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317 [DOI] [PubMed] [Google Scholar]
- 110. Shinmura K, Tamaki K, Saito K, Nakano Y, Tobe T, Bolli R. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation. 2007;116:2809–2817 [DOI] [PubMed] [Google Scholar]
- 111. Ouchi N, Kobayashi H, Kihara S, et al. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hyun DH, Hernandez JO, Mattson MP, de Cabo R. The plasma membrane redox system in aging. Ageing Res Rev. 2006;5:209–220 [DOI] [PubMed] [Google Scholar]
- 113. Hyun DH, Hunt ND, Emerson SS, Hernandez JO, Mattson MP, de Cabo R. Up-regulation of plasma membrane-associated redox activities in neuronal cells lacking functional mitochondria. J Neurochem. 2007;100:1364–1374 [DOI] [PubMed] [Google Scholar]
- 114. de Cabo R, Cabello R, Rios M, et al. Calorie restriction attenuates age-related alterations in the plasma membrane antioxidant system in rat liver. Exp Gerontol. 2004;39:297–304 [DOI] [PubMed] [Google Scholar]
- 115. Martín-Montalvo A, Villalba JM, Navas P, de Cabo R. NRF2, cancer and calorie restriction. Oncogene. 2011;30:505–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kim TH, Hur EG, Kang SJ, et al. NRF2 blockade suppresses colon tumor angiogenesis by inhibiting hypoxia-induced activation of HIF-1a. Cancer Res. 2011;71:2260–2275 [DOI] [PubMed] [Google Scholar]
- 117. Deramaudt BM, Braunstein S, Remy P, Abraham NG. Gene transfer of human heme oxygenase into coronary endothelial cells potentially promotes angiogenesis. J Cell Biochem. 1998;68:121–127 [DOI] [PubMed] [Google Scholar]
- 118. Grochot-Przeczek A, Dulak J, Jozkowicz A. Heme oxygenase-1 in neovascularisation: a diabetic perspective. Thromb Haemost. 2010;104:424–431 [DOI] [PubMed] [Google Scholar]
- 119. Samuel SM, Thirunavukkarasu M, Penumathsa SV, et al. Thioredoxin-1 gene therapy enhances angiogenic signaling and reduces ventricular remodeling in infarcted myocardium of diabetic rats. Circulation. 2010;121:1244–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ungvari Z, Bailey-Downs L, Sosnowska D, et al. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am J Physiol Heart Circ Physiol. 2011;301:H363–H372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Wood JG, Rogina B, Lavu S, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689 [DOI] [PubMed] [Google Scholar]
- 122. Potente M, Ghaeni L, Baldessari D, et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371 [DOI] [PubMed] [Google Scholar]
- 124. Allard JS, Heilbronn LK, Smith C, et al. In vitro cellular adaptations of indicators of longevity in response to treatment with serum collected from humans on calorie restricted diets. PLoS ONE. 2008;3:e3211 [DOI] [PMC free article] [PubMed] [Google Scholar]