Abstract
This study evaluated the influence of temperature and organic load on the effectiveness of domestic bleach (DB), Surface Decontamination Foam (SDF), and Virkon in inactivating Geobacillus stearothermophilus spores, which are a surrogate for Bacillus anthracis spores. The spores were suspended in light or heavy organic preparations and the suspension was applied to stainless steel carrier disks. The dried spore inoculum was covered with the disinfectants and the disks were then incubated at various temperatures. At −20°C, the 3 disinfectants caused less than a 2.0 log10 reduction of spores in both organic preparations during a 24-h test period. At 4°C, the DB caused a 4.4 log10 reduction of spores in light organic preparations within 2 h, which was about 3 log10 higher than what was achieved with SDF or Virkon. In heavy organic preparations, after 24 h at 4°C the SDF had reduced the spore count by 4.5 log10, which was about 2 log10 higher than for DB or Virkon. In general, the disinfectants were most effective at 23°C but a 24-h contact time was required for SDF and Virkon to reduce spore counts in both organic preparations by at least 5.5 log10. Comparable disinfecting activity with DB only occurred with the light organic load. In summary, at temperatures as low as 4°C, DB was the most effective disinfectant, inactivating spores within 2 h on surfaces with a light organic load, whereas SDF produced the greatest reduction of spores within 24 h on surfaces with a heavy organic load.
Résumé
Cette étude a permis d’évaluer l’influence de la température et de la charge organique sur l’efficacité de javellisant domestique (DB), de mousse de décontamination de surface (SDF) et de Virkon pour inactiver les spores de Geobacillus stearothermophilus, un substitut pour les spores de Bacillus anthracis. Les spores ont été suspendues dans des préparations organiques légères ou denses et la suspension étaient appliquées sur des disques d’acier inoxydable. L’inoculum séché de spores était recouvert avec les désinfectants et les disques étaient ensuite incubés à différentes températures. À −20 °C les trois désinfectants ont entrainé une réduction de moins de 2 log10 du nombre de spores dans les deux préparations organiques durant une période d’essai de 24 h. À 4 °C, le DB a causé, en dedans de 2 h, une réduction de 4,4 log10 de la quantité de spores dans les préparations organiques légères, à peu près 3 log10 plus élevé que ce qui a été atteint par la SDF ou le Virkon. Dans les préparations organiques denses, après 24 h à 4 °C la SDF avait réduit le dénombrement de spores par 4,5 log10, ce qui était à peu près 2 log10 plus élevé que ce qui a été obtenu avec le DB ou le Virkon. En général, les désinfectants étaient les plus efficaces à 23 °C mais un temps de contact de 24 h était requis pour la SDF et le Virkon pour réduire le nombre de spores dans les deux préparations organiques par au moins 5,5 log10. Une activité désinfectante comparable avec le DB n’a été observée qu’avec une charge organique légère. En résumé, à des températures aussi basse que 4 °C, le DB était le désinfectant le plus efficace inactivant les spores en moins de 2 heures sur des surfaces avec des charges organiques légères, alors que la SDF a causé la plus grande réduction de spores en-dedans de 24 h sur des surfaces avec une charge organique élevée.
(Traduit par Docteur Serge Messier)
Introduction
Anthrax is a highly lethal infectious disease caused by the endospore-forming bacterium Bacillus anthracis, which is a Gram-positive soil organism commonly found in nature. Although anthrax can affect all mammals including humans, it is primarily a disease of herbivores, such as cattle, sheep, horses, pigs, goats, and camels, with hyperacute or acute symptoms and usually with a fatal outcome. Anthrax is not transmitted from sick to healthy animals, but is generally acquired by the ingestion of spores dispersed into the environment (1). Flooding, drought, and other natural or man-made disturbances can bring the spores up to the soil surface where grazing animals are at risk of exposure to the organism. The disease is characterized by outbreaks, usually involving a small number of animals, but may at times turn into an epidemic with serious consequences. According to reports by the Canadian Food Inspection Agency, anthrax outbreaks occur sporadically in the Canadian prairie provinces, often during the summer and sometimes in the colder months, and can affect hundreds of animals (2).
It is expected that exposure to B. anthracis could be reduced by proper disposal of infected animal carcasses and wastes and by cleaning and disinfecting contaminated surfaces. Fumigation with formaldehyde or hydrogen peroxide has been used for emergency decontamination of indoor surfaces in buildings at temperatures around 20°C (3,4). At similar temperatures, sodium hypochlorite, hydrogen peroxide, peracetic acid, and chlorine dioxide were found to be effective for routine surface decontamination in a hospital or a laboratory setting (5,6). In addition, most of these chemicals are also recommended for surface decontamination of fomites such as farm equipment, surgical instruments, and vehicles in an agricultural setting at temperatures above 10°C (7). However, as temperatures in Canada and many other countries are frequently below 10°C, more information is needed on the effectiveness of chemical disinfectants at colder temperatures.
Since the 2001 anthrax bioterrorist attacks in the United States, Geobacillus stearothermophilus spores have been used as a surrogate for B. anthracis spores to assess the effectiveness of disinfectants and decontamination processes (3,4,8). Since G. stearothermophilus spores are heat-resistant, they are also used as biological indicators of the effectiveness of heat sterilization processes (9).
The objective of this study was to evaluate the effectiveness of 3 chemical disinfectants in killing G. stearothermophilus spores at temperatures of −20°C, 4°C, 10°C, and 23°C. Domestic bleach has been recommended for inactivation of B. anthracis spores by the US Environmental Protection Agency and the US Centers for Disease Control (5). Virkon has been widely used in an effort to kill infectious agents in various environments (10–12). Surface Decontamination Foam (SDF) was recently developed for inactivating chemical and biological agents in a wide range of field environments (13,14).
Materials and methods
Carrier disks
The second tier quantitative carrier test (15) was used for evaluating the sporicidal activity of the 3 disinfectants. Disks (1 cm in diameter; 0.75 mm thick) of brushed stainless steel (AISI No. 430; Muzeen & Blythe, Winnipeg, Manitoba) were used as carriers. They were washed 3 times with distilled water and dried at 60°C for 1 h. The dried disks were sterilized at 121°C for 25 min before use.
Spore inoculum
A suspension of G. stearothermophilus American Type Culture Collection (ATCC) strain 7953 spores (Spordex) was obtained from a commercial source (Steris, Mentor, Ohio, USA). The suspension was heated at 100°C for 35 min to inactivate vegetative cells and sonicated for 5 min to break up clumps. The treated suspension was mixed with an organic preparation and the mixture containing 4.6 × 106 colony-forming units (CFUs) of spores was loaded onto a carrier disk as inoculum. There were 2 types of organic preparations: one was used to simulate a light organic challenge (equivalent to 5% to 10% serum (15) on relatively clean surfaces and the other to simulate a heavy organic challenge (light organic preparation plus 5% garden soil) on relatively dirty surfaces. The light organic preparation was a peptide and protein mixture (16) that contained 0.35% weight/volume (w/v) tryptone (Sigma, Oakville, Ontario), 0.25% w/v bovine serum albumin (Sigma), and 0.04% w/v mucin (Sigma) in 0.01 M phosphate-buffered saline (PBS, pH 7.2). The heavy organic preparation was a mixture of the light organic preparation and 5% w/v garden soil (Premium Nature Mix; Modugno-Hortibec, St. Isidore, Quebec). The garden soil was pre-sterilized at 121°C for 90 min to prevent the introduction of other live microorganisms into the tests.
Disinfectants
Standard hard water was used to prepare disinfectant solutions to avoid variations in results that may derive from differences in tap water quality. The water was prepared in accordance with AOAC 960.09 (17) to a standard hardness of 400 ppm as calcium carbonate. Solutions of the 3 disinfectants were prepared as follows. Domestic bleach (Clorox; Oakland, California, USA) containing about 5.25% sodium hypochlorite (52 500 ppm available chlorine) was diluted to obtain a chlorine concentration of 5 250 ppm for testing. Surface Decontamination Foam (SDF) (Allen Vanguard, Ottawa, Ontario) includes 3 separate reagents: GPA-2100 decontaminant (A); GPB-2100 buffer (B); and GCE-2000 surfactant (C). The SDF solution was prepared according to the manufacturer’s instructions: i) 1.8 g B and 4.5 g C were dissolved in 150 mL of water; ii) 7.8 g A was dissolved in 50 mL of water; and iii) the 2 solutions were mixed immediately before use. The 2% Virkon solution (Antec, Suffolk, United Kingdom) was prepared by dissolving 4 tablets in 1.0 L of water. For tests at −20°C, propylene glycol [1,2-propanediol, Sigma, 40% volume/volume (v/v) final concentration] was added to the disinfectant solutions as an antifreeze agent.
Neutralizer
A neutralizer was used to immediately stop the activity of the disinfectants at the end of a test period in order to provide an accurate contact time. The neutralizer solution contained 1.56 g of sodium thiosulfate (Na2S2O2 · 5H2O; Thermo Fisher Scientific, Ottawa, Ontario), 0.07 g of lecithin (Thermo Fisher Scientific), and 0.1 mL of Tween 80 (Thermo Fischer Scientific) in 100 mL of PBS and was sterilized at 121°C for 15 min before use.
Test procedure
Contact time course experiments were carried out to evaluate the disinfectants. Six time points were included for each experiment: 5 min, 15 min, 30 min, 1 h, 2 h, and 24 h. Duplicate sample disks and duplicate control disks were prepared for each time point. Ten microliters of the spore inoculum was applied to the surface of each disk and the discs were air dried in a biosafety cabinet for 1 h. Each disk, with the inoculum side up, was then placed in a 30-mL Nalgene polypropylene straight-side vial (Thermo Fisher Scientific). Disinfectant solution (50 μL) was added to each test disk to cover the dried inoculumn and 50 μL of PBS was added to each control disk. Vials containing the disks were incubated at −20°C, 4°C, 10°C, or 23°C for specific periods up to 24 h to assess the activity of bleach and SDF and at −20°C, 4°C, and 23°C for periods up to 24 h for Virkon. At the end of each contact time, 9.95 mL of neutralizer solution was immediately added to 2 vials with test disks and 2 vials with control disks to stop the activity of the disinfectants and the vials and contents were vortexed for 1 min. A 10-fold serial dilution was made of the suspension in each vial. Each of the serial dilutions was passed through a 0.2-μm membrane filter (Supor 200 membrane filter; Pall, Ann Arbor, Michigan, USA) in a magnetic filter holder (Pall). The filter unit was subsequently rinsed 3 times with 10 mL of PBS to maximize spore recovery. The membrane filter was removed from the unit and placed on the surface of a tryptic soy agar (TSA; Voigt Global Distribution, Lawrence, Kansas, USA) plate and incubated at 56°C in a relative humidity of approximately 60%. After incubation for 2 d and 5 d, colonies were counted.
Statistical analysis
Duplicate contact time course experiments were conducted to evaluate each disinfectant and duplicate sets of test and control disks were used for each time point. The difference in recovery of live spores from control and test disks was recorded as spore reduction (log10) at each time point to indicate the sporicidal efficiency of disinfectants for a specific contact time. The data presented were the means of spore reduction from duplicate sets of control and test disks in duplicate experiments. Student’s t-test was used to determine statistical significance (P < 0.05) in difference in spore reduction among various temperatures with 1 disinfectant solution or among various disinfectant solutions at 1 temperature. The comparison was done with either light or heavy organic preparation.
Results
The recovery of G. stearothermophilus spores from control disks ranged from 5.5 to 5.7 log10 (data not shown) and was not influenced by time, temperature, or exposure to organic matter. The neutralizer solution had no effect on the survival of the spores (data not shown), but the effectiveness of all disinfectants was influenced by the treatment variables.
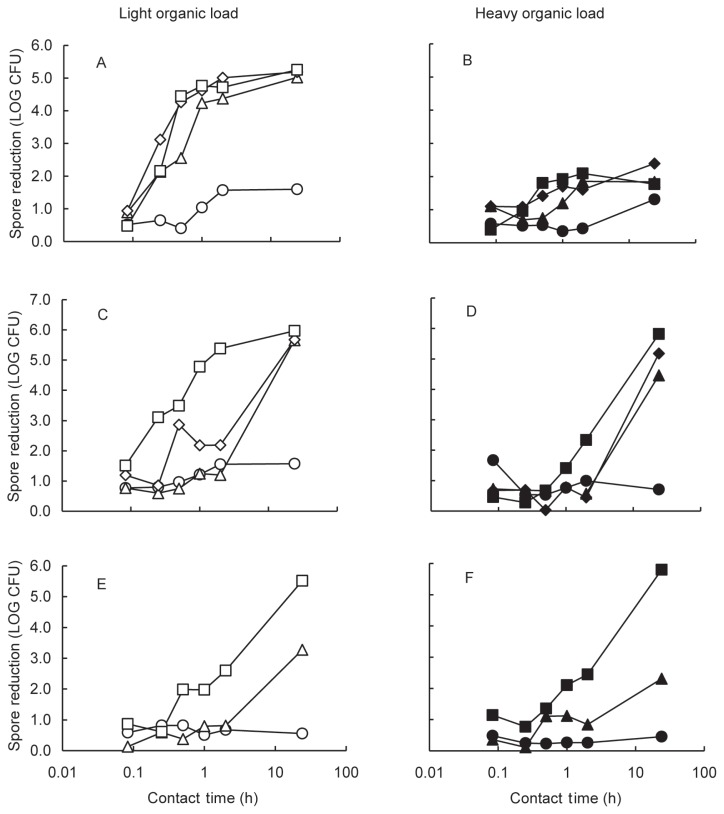
The propylene glycol that was added to the disinfectant solutions prevented freezing during the 24-h test period at −20°C, but under these conditions, the disinfectants produced less than a 2.0 log10 reduction of spores. With the light organic load, after a contact period of 2 h at 4°C or 10°C, the DB had reduced spore counts by 4.4 and 4.7 log10, respectively. These reductions were about 3 log10 higher than those produced by SDF or Virkon (4°C only) and the differences were significant (P < 0.05). After 24 h at 4°C or 10°C, both DB and SDF had reduced spore counts by more than 5.0 log10. Virkon was not tested at 10°C but spore reduction after 24 h at 4°C was about 2 log10 lower than for the other disinfectants (Figures 1, A, C, and E).
Figure 1.
Effects of disinfectants on reduction of the spores of Geobacillus stearothermophilus at temperatures of −20°C (○ and ●), 4°C (△ and ▲), 10°C (◇ and ◆), and 23°C (□ and ■). The spores in the light (equivalent to 5 ~ 10% serum, open symbols) and the heavy (equivalent to 5 ~ 10% serum plus 5% garden soil, solid symbols) organic preparations were treated with domestic bleach (A and B), SDF (C and D), and Virkon (E and F) at contact times of 5 min, 15 min, 30 min, 1 h, 2 h, and 24 h. The data were the mean difference of the spore counts recovered from duplicate sets of the control and the sample disks in duplicate experiments and the standard deviations were less than 1.2 log10 colony-forming units (CFUs). The recovery of spores from untreated control disks ranged from 5.5 to 5.7 log10.
With the heavy organic load, the disinfectants reduced spore counts by only less than 2 log10 within 2 h at either 4°C or 10°C. After 24 h at these temperatures, however, the SDF had reduced spore counts by at least 4.5 log10. These reductions were significantly higher (P < 0.05) than those produced by DB or Virkon (Figures 1, B, D, and F).
With both the light and heavy organic load, the disinfectants were usually most effective at 23°C but contact for at least 24 h was required for SDF and Virkon to kill at least 5.5 log10 of spores. The DB was comparable in its effectiveness with the light organic load, but not with the heavy load. It was concluded that at temperatures as low as 4°C, DB was the most effective disinfectant for inactivating spores within 2 h on surfaces with a light organic load. In comparison, SDF produced the greatest reduction of spores within 24 h on surfaces with a heavy organic load.
Discussion
The spores of G. stearothermophilus, Bacillus atrophaeus, Bacillus cereus, and Bacillus subtilis have all been used as surrogates for spores of B. anthracis in previous studies (3,4,8,18). Geobacillus stearothermophilus was selected for use in this study because its spores have high heat resistance that aided in separating them from other microorganisms. It is also possible that the G. stearothermophilus spores are more resistant to oxidizing chemicals than are Bacillus surrogates (3,8). This may account for the fact that the disinfectants used in this study had to be in contact with the preparations of spores for up to 24 h in order to inactivate approximately 6 log10 of G. stearothermophilus.
The laboratory study described here was designed to simulate the disinfection that would be required to inactivate B. anthracis spores on the surfaces of farm equipment under field conditions. For this reason, both light and heavy organic challenges were included for evaluating the disinfectants. The light organic preparation, which is equivalent to 5% to 10% serum, has been widely used in evaluating disinfectants in medical settings and other environments (16). Such organic loads may be found on pre-cleaned farm equipment and veterinary tools. The heavy organic preparation, which is equivalent to 5% to 10% serum plus 5% sterilized garden soil, was intended to provide more stringent challenges to disinfection, such as may be encountered on inadequately cleaned tractors, trucks, and other equipment. As discussed in other studies, organic matter may reduce the effectiveness of disinfectants by interacting with the active ingredients and by forming a physical barrier that could hinder contact between disinfectants and microorganisms (19).
In the present study, DB was the most effective disinfectant against spores in the light organic preparations, in which it killed at least 4 logs of spores within 2 h at 4°C, 10°C, or 23°C. These findings are in agreement with a report by Best, Springthorpe, and Sattar (20). However, DB was not effective against spores in the heavy organic preparation at any of these temperatures. The effectiveness of SDF in both light and heavy organic preparations may be attributed to its surfactant ingredients. In agreement with earlier reports (10–12), the sporicidal effect of Virkon was compromised by organic loads.
Environmental temperature varies widely in many countries and in this study, the disinfectants applied to solid surfaces at −20°C reduced G. stearothermophilus spore counts by only approximately 2 log10. These results differed substantially from those of other studies (21,22), which reported that disinfectants killed at least 6 log10 of B. subtilis spores in suspension tests at temperatures from 0 to ~ −40°C. The disinfectants used in those studies were solutions made of sodium hypochlorite, peracetic acid, or ß-propiolactone. Although tests on solid surfaces or suspensions have been officially accepted by international organizations for evaluating sporicidal disinfectants (23), a much longer contact time was required to inactivate anthrax spores by alcoholic peracetic acid on a solid surface than in a suspension (24). It is therefore evident that test results could be influenced by many factors, including the type of spores used as surrogates, the type of disinfectants, temperature, and the nature of the tests.
For this study, the tests were carried out on solid surfaces in order to simulate conditions that would exist on farms. It is evident, however, that cleaning and disinfection could only reduce the levels of B. anthracis spores in the environment since farm equipment is often difficult to clean and spores washed from the equipment could survive in the soil and be picked up by boots and tires. Nevertheless, this study suggests that DB and SDF, applied at temperatures ranging from 4°C to 23°C, could substantially reduce the load of B. anthracis counts on prewashed farm equipment and thereby reduce the chances for spread of the disease.
Acknowledgments
This study is a part of the CRTI 08-0122TD project entitled “Verification of Decontamination Processes in the AgriFood Context,” which is funded by the Canadian Chemical, Biological, Radiological, and Nuclear (CBRNE) Research and Technology Initiative (CRTI). The authors thank Dr. S.A. Sattar of the University of Ottawa in Ottawa, Ontario for introducing the second tier quantitative carrier test method to assess the sporicidal activity of chemical disinfectants and Dr. J.L. Spencer, emeritus research scientist at the Canadian Food Inspection Agency in Ottawa, Ontario, for discussing and reviewing the manuscript.
References
- 1.Fasanella A, Galante D, Garofolo G, Jones MH. Anthrax undervalued zoonosis. Vet Microbiol. 2010;140:318–331. doi: 10.1016/j.vetmic.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Epp T, Argue C, Waldner C, Berke O. Spatial analysis of an anthrax outbreak in Saskatchewan, 2006. Can Vet J. 2010;51:743–748. [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers JV, Sabourin CLK, Choi YW, et al. Decontamination assessment of Bacillus anthracis, Bacillus subtilis, and Geobacillus stearothermophilus spores on indoor surfaces using a hydrogen peroxide gas generator. J Appl Microbiol. 2005;99:739–748. doi: 10.1111/j.1365-2672.2005.02686.x. [DOI] [PubMed] [Google Scholar]
- 4.Rogers JV, Choi YW, Richter WR, et al. Formaldehyde gas inactivation of Bacillus anthracis, Bacillus subtilis, and Geobacillus stearothermophilus spores on indoor surface materials. J Appl Microbiol. 2007;103:1104–1112. doi: 10.1111/j.1365-2672.2007.03332.x. [DOI] [PubMed] [Google Scholar]
- 5.Heninger SJ, Anderson CA, Beltz G, Onderdonk AB. Decontamination of Bacillus anthracis spores: Evaluation of various disinfectants. Appl Biosaf. 2009;14:7–10. doi: 10.1177/153567600901400103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatuev BA, Peterson JW. Analysis of the sporicidal activity of chlorine dioxide disinfectant against Bacillus anthracis (Sterne strain) J Hosp Infect. 2010;74:178–183. doi: 10.1016/j.jhin.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guidelines for the Surveillance and Control of Anthrax in Humans and Animals. 3rd edition. World Health Organization (WHO); 2003. [Last accessed on December 5, 2012]. [homepage on the Internet] Available from: http://www.who.int/csr/resources/publications/anthrax/whoemczdi986text.pdf. [Google Scholar]
- 8.Sabbah S, Springthorpe S, Sattar SA. Use of a mixture of surrogates for infectious bioagents in a standard approach to assessing disinfection of environmental surfaces. Appl Environ Microbiol. 2010;76:6020–6022. doi: 10.1128/AEM.00246-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe T, Turukawa S, Hirata J, Koyama T, Ogihara H, Yamasaki M. Inactivation of Geobacillus stearothermophilus spores by high-pressure carbon dioxide treatment. Appl Environ Microbiol. 2003;69:7124–7129. doi: 10.1128/AEM.69.12.7124-7129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herńndez A, Martró E, Matas L, Martin M, Ausina V. Assessment of in-vitro efficacy of 1% Virkon against bacteria, fungi, viruses and spores by means of AFNOR guidelines. J Hosp Infect. 2000;46:203–209. doi: 10.1053/jhin.2000.0818. [DOI] [PubMed] [Google Scholar]
- 11.Angelillo IF, Bianco A, Nobile CGA, Pavia M. Evaluation of the efficacy of glutaraldehyde and peroxygen for disinfection of dental instruments. Lett Appl Microbiol. 1998;27:292–296. [PubMed] [Google Scholar]
- 12.Coates D. Sporicidal activity of sodium dichloroisocyanurate, peroxygen and glutaraldehyde disinfectants against Bacillus subtilis. J Hosp Infect. 1996;32:283–294. doi: 10.1016/s0195-6701(96)90039-0. [DOI] [PubMed] [Google Scholar]
- 13.Love AH, Bailey CG, Hanna ML, et al. Efficacy of liquid and foam decontamination technologies for chemical warfare agents on indoor surfaces. J Hazard Mater. 2011;196:115–122. doi: 10.1016/j.jhazmat.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Technology Information Summary on Surface Decontamination Foam. US EPA; 2009. [Last accessed on December 5, 2012]. [homepage on the Internet] EPA. Available from: http://www.epa.gov/nhsrc/pubs/TISSurfaceDecontaminationFoam.pdf. [Google Scholar]
- 15.Sattar SA, Springthorpe VS, Adegbunrin O, Zafer AA, Busa M. A disc-based quantitative carrier test method to assess the virucidal activity of chemical germicides. J Virol Methods. 2003;112:3–12. doi: 10.1016/s0166-0934(03)00192-7. [DOI] [PubMed] [Google Scholar]
- 16.ASTM International. Standard Quantitative Disk Carrier Test Method for Determining the Bactericidal, Virucidal, Fungicidal, Mycobactericidal and Sporicidal Activities of Liquid Chemical Germicides, E2197-02. West Conshohocken: ASTM; 2002. [Google Scholar]
- 17.Official Methods of Analysis. AOAC (Association of Official Analytical Chemists) International; Washington DC, AOAC: [Last accessed December 5, 2012]. p. 960.09. Available from: http://www.eoma.aoac.org. [Google Scholar]
- 18.Tomasino SF, Pines RM, Cottrill MP. Determining the efficacy of liquid sporicides against spores of Bacillus subtilis on a hard nonporous surface using the quantitative three step method: Collaborative study. J AOAC Int. 2008;91:833–852. [PubMed] [Google Scholar]
- 19.Springthorpe VS, Sattar SA. Carrier tests to assess microbicidal activities of chemical disinfectants for use on medical devices and environmental surfaces. J AOAC Int. 2005;88:182–201. [PubMed] [Google Scholar]
- 20.Best MV, Springthorpe S, Sattar SA. Feasibility of a combined carrier test for disinfectants: Studies with a mixture of five types of microorganisms. Am J Infect Control. 1994;22:152–162. doi: 10.1016/0196-6553(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 21.Jones LA, Jr, Hoffman RK, Phillips CR. Sporicidal activity of peracetic acid and ß–propiolactone at subzero temperatures. Appl Microbiol. 1967;15:357–362. doi: 10.1128/am.15.2.357-362.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones LA, Jr, Hoffman RK, Phillips CR. Sporicidal activity of sodium hypochlorite at subzero temperatures. Appl Microbiol. 1968;16:787–791. doi: 10.1128/am.16.5.787-791.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Humphreys PN. Testing standards for sporicides. J Hosp Infect. 2011;77:193–198. doi: 10.1016/j.jhin.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Nattermann H, Becker S, Jacob D, Klee SR, Schwebke I, Appel B. Efficient killing of anthrax spores using aqueous and alcoholic peracetic acid solutions. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2005;48:939–950. doi: 10.1007/s00103-005-1108-4. [DOI] [PubMed] [Google Scholar]