Abstract
Oxidative stress is a key component in the immunosuppression of chronic kidney disease (CKD), and neutrophil function may be impaired by oxidative stress. To test the hypothesis that in uremic dogs with CKD, oxidative stress is increased and neutrophils become less viable and functional, 18 adult dogs with CKD were compared with 15 healthy adult dogs. Blood count and urinalysis were done, and the serum biochemical profile and plasma lipid peroxidation (measurement of thiobarbituric acid reactive substances) were determined with the use of commercial reagents. Plasma total antioxidant capacity (TAC) was measured with a spectrophotometer and commercial reagents, superoxide production with a hydroethidine probe, and the viability and apoptosis of neutrophils with capillary flow cytometry and the annexin V-PE system. The plasma concentrations of cholesterol (P = 0.0415), creatinine (P < 0.0001), and urea (P < 0.0001) were significantly greater in the uremic dogs than in the control dogs. The hematocrit (P = 0.0004), urine specific gravity (P = 0.015), and plasma lipid peroxidation (P < 0.0001) were significantly lower in the dogs that were in late stages of CKD than in the control group. Compared with those isolated from the control group, neutrophils isolated from the CKD group showed a higher rate of spontaneous (0.10 ± 0.05 versus 0.49 ± 0.09; P = 0.0033; median ± standard error of mean) and camptothecin-induced (18.53 ± 4.06 versus 44.67 ± 4.85; P = 0.0066) apoptosis and lower levels of superoxide production in the presence (1278.8 ± 372.8 versus 75.65 ± 86.6; P = 0.0022) and absence (135.29 ± 51.74 versus 41.29 ± 8.38; P = 0.0138) of phorbol-12-myristate-13-acetate stimulation. Thus, oxidative stress and acceleration of apoptosis occurs in dogs with CKD, the apoptosis diminishing the number of viable neutrophils and neutrophil superoxide production.
Résumé
Le stress oxydatif est un élément clé dans l’immunosuppression de maladie rénale chronique (MRC), et la fonction des neutrophiles peut être affectée par le stress oxydatif. Afin de vérifier l’hypothèse que chez les chiens urémiques avec MRC le stress oxydatif est augmenté et les neutrophiles deviennent moins viables et fonctionnels, 18 chiens adultes avec MRC ont été comparés à 15 chiens adultes en santé. Des analyses sanguines et urinaires ont été effectuées, de même que le profil biochimique sérique et la peroxydation des lipides plasmatiques (mesures des substances réactives à l’acide thiobarbiturique) ont été déterminés au moyen de réactifs commerciaux. La capacité antioxydante plasmatique totale (CAT) a été mesurée à l’aide d’un spectrophotomètre et de réactifs commerciaux, la production de superoxyde avec une sonde hydroéthidine, et la viabilité et l’apoptose des neutrophiles avec un cytomètre à flux capillaire et le système d’annexine V-PE. Les concentrations plasmatiques de cholestérol (P = 0,0415), de créatinine (P < 0,0001) et d’urée (P < 0,0001) étaient significativement plus élevées chez les chiens urémiques comparativement aux chiens témoins. L’hématocrite (P = 0,0004), la gravité spécifique de l’urine (P = 0,015), et la peroxydation des lipides plasmatiques (P < 0,0001) étaient significativement plus faibles chez les chiens dans les stades avancés de MCR que chez les chiens témoins. Comparativement aux neutrophiles provenant des chiens témoins, ceux provenant des chiens avec MRC montraient un taux plus élevé d’apoptose spontanée (0,10 ± 0,05 versus 0,49 ± 0,09; P = 0,0033; médiane ± écart-type) et d’apoptose induite par la camptothécine (18,53 ± 4,06 versus 44,67 ± 4,85; P = 0,0066) et des niveaux plus faibles de production de superoxyde en présence (1278,8 ± 372,8 versus 75,65 ± 86,6; P = 0,0022) et en absence (135,29 ± 51,74 versus 41,29 ± 8,38; P = 0,0138) de stimulation par l’acétate de phorbol-12-myristate-13. Ainsi, le stress oxydatif et l’accélération de l’apoptose surviennent chez des chiens avec MRC, l’apoptose diminuant le nombre de neutrophiles viables et la production de superoxyde par les neutrophiles.
(Traduit par Docteur Serge Messier)
Introduction
In humans, kidney disease is an important cause of immunosuppression (1) that increases the risk of death from bacterial infection owing to the neutrophil dysfunction (2) associated with oxidative stress (3).
Neutrophils produce reactive oxygen species (ROS) when nicotinamide adenine dinucleotide phosphate oxidase is activated, generating the superoxide anion essential to the bactericidal function of neutrophils (4). Although the ROS derived from superoxide are required for the defence mechanism of neutrophils, free radicals produced in excess can damage a number of cellular structures, thus inducing lipid peroxidation and accelerating apoptosis (5). The increase in superoxide anion in the neutrophils of humans with chronic kidney disease (CKD) alters the function of endothelial cells, mesangial cells, and podocytes and reduces renal sodium flow and excretion (6,7).
The immunosuppressive effect associated with CKD has rarely been investigated in veterinary medicine. Recently, Kralova, Leva, and Toman (8) verified that, unlike that which occurs in humans, CKD in dogs does not alter the phagocytic ability of neutrophils. In contrast, Barbosa, Mori, and Ciarlini (9) verified in vitro that, as in humans, the oxidative metabolism and apoptosis of neutrophils is affected in uremic dogs. Oxidative stress due to a decrease in plasma antioxidant capacity and an increase in neutrophil oxidative metabolism has also been observed in cats with CKD (10). It is accepted that the viability and function of human neutrophils are affected by oxidative stress and uremic toxins (11), and there is evidence in late stages of CKD of increased apoptosis and decreased superoxide production in human neutrophils (12). However, investigators who evaluated the total and endogenous antioxidant status, such as by measuring uric acid and albumin levels, in humans with CKD reported conflicting results (13,14).
Since the progression of CKD in humans (14) and cats (10) is associated with the harmful effects of oxidative stress, it is important to investigate whether such stress also occurs in dogs and whether it alters superoxide production and neutrophil survival. Therefore, the hypothesis that oxidative stress occurs in dogs with CKD and that in this condition neutrophils become less viable and functional was tested.
Materials and methods
Animal selection
For the CKD group, 18 uremic adult dogs were selected that were being treated at the Veterinary Hospital of the Araçatuba College of Veterinary Medicine, São Paulo, Brazil, and had CKD equivalent to stage III or IV in the International Renal Interest Society (IRIS) staging system (15). The control group consisted of 15 adult dogs with no history of chronic or systemic disease and normal results of physical examination, blood count, urinalysis, and measurement of the plasma concentrations of albumin, cholesterol, creatinine, urea, and uric acid. The experimental groups were formed after all the dogs, which were of various breeds and both sexes, had been examined on at least 2 occasions; the control animals had to show no change in physical and laboratory findings. Dogs with a history of recent treatment that could affect renal function or leukocytes were not included. The study was approved by the Animal Experimentation Ethics Committee of the Faculty of Veterinary Medicine of São Paulo State University, and samples were obtained with consent of the owners.
Sample preparation
Blood was collected from the dogs into polypropylene heparinized vacuum tubes (Vacutainer Plus Plastic Sodium Heparin; Becton, Dickinson and Company, Rutherford, New Jersey, USA) that were protected from light; immediately, 4 mL was used for neutrophil isolation, and 3 mL was centrifuged to obtain plasma, which was stored at −20°C, protected from light, for a maximum of 2 wk, pending biochemical analyses, quantification of lipid peroxidation, and determination of total antioxidant capacity (TAC). Another 3 mL of whole blood was collected into plastic tubes containing potassium ethylene diamine tetraacetic acid (K2EDTA) (Vacutainer Plus Plastic K2EDTA; Becton Dickinson) for a complete blood cell count, which was done immediately.
Complete blood cell count, urinalysis, and biochemical analyses
The overall concentrations of leukocytes, erythrocytes, and hemoglobin were obtained by means of an electronic veterinary blood cell counter (model CC-530; CELM, São Paulo, Brazil), and the hematocrit was determined with the Strumia microcapillary method (centrifugation for 5 min at 12 700 × g). The differential leukocyte count was done on blood smears stained by quick Panotic dye (Instant-Prov; Newprov Produtos para Laboratórios, Pinhais, Brazil) in accordance with the guidelines and criteria outlined by Jain (16). All the biochemical tests were performed with the use of commercial reagents (BioSystems, Barcelona, Spain) at 37°C in an automated analyzer (BTS-370 plus; BioSystems) previously calibrated with commercial calibrator and the reactions monitored with control levels I and II. The plasma concentrations of albumin were determined by the bromocresol green assay, cholesterol by the oxidase/peroxidase enzymatic assay, creatinine by the alkaline picrate kinetic assay, urea by the urease/glutamate dehydrogenase-coupled ultraviolet enzymatic assay, and uric acid by the uricase/peroxidase enzymatic assay.
Measurement of oxidative stress
Besides determining plasma levels of the antioxidants albumin and uric acid, the plasma TAC was quantified in an automated analyzer by a method that inhibits 2.2′-azino diethylbenzothiazoline sulfonic acid cation formation (Randox Laboratories, London, England). The TAC analyses were monitored with a standard antioxidant specific for automation (Randox Laboratories).
Plasma lipid peroxidation was determined by quantifying plasma thiobarbituric acid reactive substances (TBARS) by means of a commercial reagent kit (TBARS Assay Kit; Cayman Chemical Company, Ann Arbor, Michigan, USA). Absorbance (at 530 nm) of the reactions was measured in a plate reader (Spectra Count Reader; Packard BioScience, Meriden, Connecticut, USA) in accordance with the manufacturer’s recommendations, starting with a standard commercial malondialdehyde (MDA) solution (500 mM) and using a computer program (GraphPad Prism, version 4; GraphPad Software, San Diego, California, USA). A curve for final concentrations of 0, 0.625, 1.25, 2.5, 5, 10, 25, and 50 nmol/mL of MDA was developed. Each point on the curve was obtained from the mean value for 10 repetitions.
Neutrophil isolation
To isolate neutrophils, 4 mL of whole blood containing 10 IU of heparin per milliliter of blood was transferred to sterile conical polypropylene tubes containing a double-gradient separation composed of equal volumes (3 mL each) of Histopaque-1119 and 1077 (Sigma Chemical Company, St. Louis, Missouri, USA). After centrifugation at 340 × g for 30 min, the layer of polymorphonuclear (PMN) cells was aspirated and washed twice with aqueous ammonium chloride (0.14 M) for complete lysis of residual erythrocytes. Next, the sample was centrifuged (at 100 × g) for 5 min in Hanks’ balanced salt solution (Sigma) without Ca2+ or Mg2+, and 1 mL of RPMI medium (Sigma) was added to the cell sediment. Cell concentration was determined in a hemocytometer, and cell viability was estimated by the trypan blue exclusion method (17). The sample of isolated PMN cells was diluted in RPMI to obtain a final cell concentration of 106/mL, with the purity and viability of the neutrophils 90% and 95% or greater, respectively.
Measurement of neutrophil superoxide production
A hydroethidine (HE) (dihydroethidium bromide; Polysciences, Warrington, Pennsylvania, USA) probe was used to measure superoxide production from the neutrophils (18). Briefly, 180 μL of neutrophils (106/mL) suspended in RPMI medium was added to 20 μL of HE buffer solution (0.1 mmol/L) with and without 20 μL of phorbol 12-myristate 13-acetate (PMA; Sigma), 3.2 μmol/L. After incubation at 37°C for 15 min, the sample was maintained on ice and protected from light until the reading. Mean fluorescence was measured in a capillary flow cytometer (Guava EasyCyte Mini Flow Cytometry System; Guava Technologies, Hayward, California, USA) adjusted to the wavelength of maximum emission (593 nm) and excitation (473 nm) of ethidium bromide. To eliminate debris, the neutrophil population characterized by the largest cell size was selected. Ten thousand events were acquired and analyzed with a specific computer program (Guava Express CytoSoft Data Acquisition and Analysis Software, Personal Cell Analysis System, version 4.1, 2006; Guava Technologies). Superoxide production was quantified from the intensity of the fluorescence in spontaneous and stimulated oxidation of HE.
Measurement of the apoptotic index of the neutrophils
The percentages of apoptotic and viable neutrophils were determined with use of the Guava Nexin® Reagent (Guava Technologies, Millipore, Hayward, California, USA). The procedure for each test and proper instrument performance were verified in accordance with the manufacturer’s recommendations. Briefly, 2 samples of 100 μL of neutrophils (106/mL) suspended in RPMI medium were incubated at 37°C for 1 h with 100 μL of camptothecin (CAM) (19.8 mmol/L; Sigma) and with 100 μL of RPMI medium (CAM absent). After incubation, 100 μL of each sample was transferred to a microtube with 100 μL of annexin V-PE reagent and stored at room temperature, protected from light, for 10 min. In a capillary flow cytometer 10 000 events were acquired, and the size of the viable and apoptotic populations of neutrophils were determined with a specific computer program.
Statistical analysis
After studying the distributions of the variables for normality and homoscedasticity, as recommended by Zar (19), statistical differences between groups were determined with use of the unpaired t-test, the unpaired t-test with the Welch correction, and the Mann–Whitney test. Statistical analyses were performed with statistical software [SAS System, release 9.2 (2008); SAS Institute, Cary, North Carolina, USA]. A P-value of less than 0.05 was considered significant for all tests.
Results
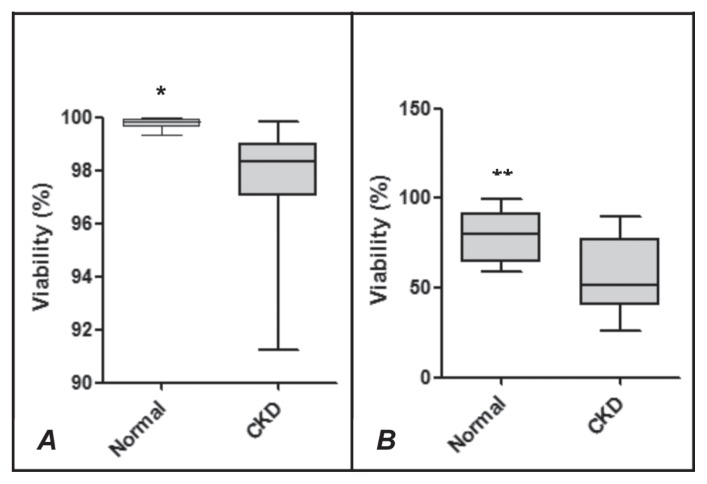
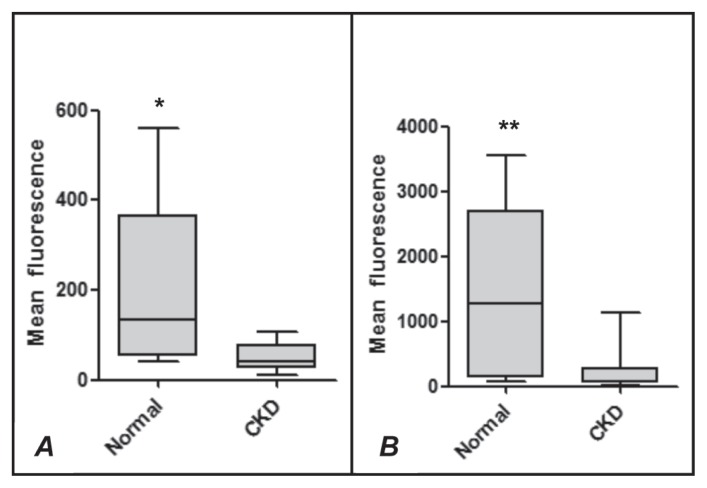
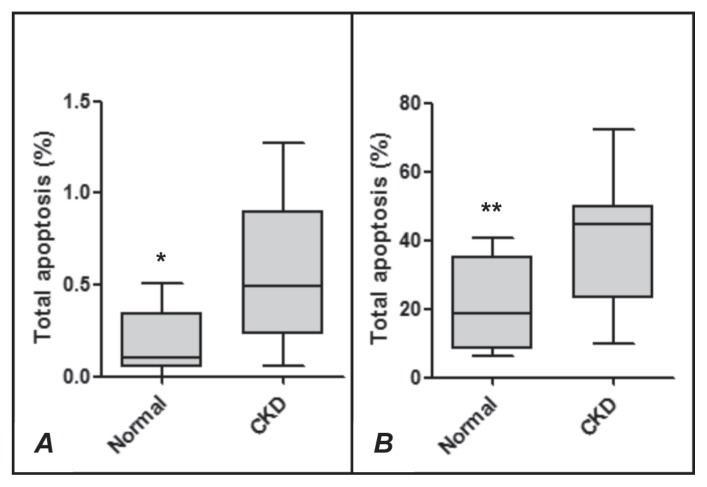
The hematologic profile and results of urinalysis and plasma biochemical analysis are summarized in Table I. The hematocrit, urine specific gravity, plasma albumin concentration, and TBARS and TAC concentrations were significantly lower in the uremic dogs than in the control dogs. The plasma concentrations of cholesterol, creatinine, and urea in the dogs with CKD were significantly above baseline, but there was no difference between the groups for uric acid. The viability (Figure 1) and superoxide production (Figure 2) of neutrophils from the dogs with CKD were lower both at rest and after stimulation with CAM (Figure 1) or PMA (Figure 2). An increase in the rate of neutrophil apoptosis was also observed in the dogs with CKD (Figure 3).
Table I.
Laboratory findings in healthy control dogs and dogs with chronic kidney disease (CKD)a
| Mean and standard deviation | |||
|---|---|---|---|
|
|
|||
| Measure | Control | CKD | P-value |
| Hematocrit (%) | 48.27 ± 2.9b | 31.26 ± 13.93c | < 0.0004** |
| Leukocyte count (× 103/μL) | 13 ± 3.37b | 21.05 ± 11.51c | < 0.0203** |
| Urine specific gravity | 1.044 ± 0.018b | 1.016 ± 0.002c | < 0.0105** |
| Plasma concentration | |||
| Albumin (g/L) | 30.36 ± 3.29b | 25.73 ± 3.7c | < 0.0033* |
| Cholesterol (mg/dL) | 208.0 ± 76.74b | 299 ± 121.78c | < 0.0415* |
| Creatinine (mg/dL) | 1.07 ± 0.13b | 4.55 ± 3.02c | < 0.0001** |
| Urea (mg/dL) | 46.45 ± 9.85b | 315.21 ± 159.95c | < 0.0001** |
| Uric acid (mg/dL) | 1.33 ± 0.59b | 1.05 ± 0.82b | < 0.3589* |
| TBARS (nmol/mL) | 43.4 ± 8.39b | 21.51 ± 9.39c | < 0.0001* |
| Plasma TAC (mmol/L) | 2.73 ± 0.24b | 2.08 ± 0.43c | < 0.0002** |
Noncoincident letters in the same row represent significant differences in the unpaired t-test (*) and the unpaired t-test with Welch’s correction (**).
TBARS — thiobarbituric acid reactive substances, representing plasma lipid peroxidation; TAC — total antioxidant capacity.
Figure 1.
Viability of neutrophils from healthy dogs (Normal) and dogs with chronic kidney disease (CKD) not activated (A) or activated (B) by camptothecin (CAM). The values of upper quartile, median, and lower quartile are indicated in each box, and the bars outside the box indicate semiquartile ranges. Differences between the 2 groups of dogs significant at *P = 0.0026 and **P = 0.0022.
Figure 2.
Superoxide production measured by the mean fluorescence of ethidium bromide in neutrophils from healthy dogs (Normal) and dogs with chronic kidney disease (CKD) not activated (A) or activated (B) by phorbol 12-myristate 13-acetate. The values of upper quartile, median, and lower quartile are indicated in each box, and the bars outside the box indicate semiquartile ranges. Differences between the 2 groups of dogs significant at *P = 0.0138 and **P = 0.0022.
Figure 3.
Total apoptosis of neutrophils from healthy dogs (Normal) and dogs with chronic kidney disease (CKD) not activated (A) or activated (B) by camptothecin (CAM). The values of upper quartile, median, and lower quartile are indicated in each box, and the bars outside the box indicate semiquartile ranges. Differences between the 2 groups of dogs significant at *P = 0.0033 and **P = 0.0066.
Discussion
The hematologic and biochemical profiles of the healthy dogs selected for the control group remained within the reference ranges (20), demonstrating their health status. The clinical signs, increased plasma concentrations of cholesterol, creatinine, and urea, decreased plasma albumin concentration, and nonregenerative normochromic normocytic anemia observed during selection of the CKD group persisted for at least 2 wk, demonstrating IRIS stage III or IV of kidney disease at the time of assessment (15).
Although the plasma concentration of the antioxidant uric acid was lower in the dogs with CKD than in the control group, the difference was not statistically significant. The results for plasma uric acid concentration in human patients with kidney disease are conflicting, probably owing to the use of different methodologies (13,14).
The lower level of plasma albumin observed in the dogs with CKD could have contributed to their lower TAC. According to Terawaki et al (21), albumin exerts an important antioxidant role in CKD-related oxidative stress.
The TAC was significantly lower in the dogs with CKD than in the healthy control group, confirming that the oxidative stress previously described in humans (13,14) and cats (10) also occurs in dogs. The observed difference supports the hypothesis that an antioxidant other than uric acid is decreased in dogs with CKD.
The lower antioxidant capacity did not promote the expected increase in TBARS. On the contrary, lipid peroxidation in the plasma was lower in the dogs with CKD than in the control group. The lower production of superoxide in the dogs with CKD may have contributed to reduced formation of TBARS. Although TBARS measurement is a commonly reported method of detecting MDA, it is insufficiently sensitive and is affected by interference from related species or overestimation due to stressing conditions during sample manipulation (22).
The lower superoxide production in the dogs with CKD fully supports the hypothesis that uremic toxins in dogs with IRIS stage III or IV kidney disease contribute to the diminished oxidative metabolism of the neutrophils, a potentially important mechanism that affects the innate immune response of dogs with renal failure, as previously reported for humans (23) and cats (10), as well as from in vitro experiments involving dog cells (9). However, this result is in disagreement with the observation by McLeish et al (24) and Rysz et al (3) of an increase in ROS production in human patients with uremia and the lack of observation of alterations in neutrophil oxidative metabolism in other studies of such patients (2,25,26). The results of investigations into the oxidative metabolism of human neutrophils in CKD remain highly contradictory; however, some of these differences are known to be due to the methods of neutrophil isolation and quantification of ROS (23).
As in humans (27), CKD in dogs causes a significantly higher rate of spontaneous and induced neutrophil apoptosis than in healthy controls. Our results contradict the report by Kralova, Leva, and Toman (8) that CKD does not alter the production of reactive oxygen radicals in neutrophils from dogs. Those investigators used chemiluminescence, a method that is not specific for superoxide detection, as well as different procedures for isolation and incubation of the neutrophils. The results of the current study are consistent with the observations reported by Barbosa, Mori, and Ciarlini (9), who obtained in vitro evidence that uremic toxins in dogs promote the initial activation of the oxidative metabolism of neutrophils that subsequently induces the acceleration of apoptosis and diminished superoxide production. These findings support the hypothesis of Cendoroglo et al (12) that in the early stages of CKD an increase in the oxidative metabolism of neutrophils occurs, such that oxidants accumulate and eventually cause cell damage capable of accelerating apoptosis and affecting superoxide production at a later stage.
How the mechanisms of oxidative stress in late stages of CKD accelerate apoptosis and diminish the oxidative metabolism of neutrophils in dogs remains to be determined.
In conclusion, oxidative stress occurs in dogs with CKD, as demonstrated by the lower plasma TAC in those dogs as compared with healthy controls. This change occurs concomitantly with inhibition of oxidative metabolism and acceleration of neutrophil apoptosis.
Acknowledgment
This work was graciously funded by the São Paulo Research Foundation.
References
- 1.Vanholder R, Van Laecke S, Glorieux G. What is new in uremic toxicity? Pediatr Nephrol. 2008;23:1211–1221. doi: 10.1007/s00467-008-0762-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anding K, Gross P, Rost JM, et al. The influence of uraemia and haemodialysis on neutrophil phagocytosis and antimicrobial killing. Nephrol Dial Transplant. 2003;18:2067–2073. doi: 10.1093/ndt/gfg330. [DOI] [PubMed] [Google Scholar]
- 3.Rysz J, Kasielski M, Apanasiewicz J, et al. Increased hydrogen peroxide in the exhaled breath of uraemic patients unaffected by haemodialysis. Nephrol Dial Transplant. 2004;19:158–163. doi: 10.1093/ndt/gfg499. [DOI] [PubMed] [Google Scholar]
- 4.Huimin J, Qinjun X, Peijun Z, et al. Impaired GP-91PHOX gene expression and dysfunction of peripheral blood neutrophils in patients maintaining hemodialysis. Chin Med J. 2000;113:120–123. [PubMed] [Google Scholar]
- 5.Kato S, Chmielewski M, Honda H, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3:1526–1533. doi: 10.2215/CJN.00950208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaziri ND, Dicus M, Ho ND, et al. Oxidative stress and dysregulation of superoxide dismutase and NADPH oxidase in renal insufficiency. Kidney Int. 2003;63:179–185. doi: 10.1046/j.1523-1755.2003.00702.x. [DOI] [PubMed] [Google Scholar]
- 7.Nistala R, Whaley-Connell A, Sowers JR. Redox control of renal function and hypertension. Antioxid Redox Signal. 2008;10:2047–2089. doi: 10.1089/ars.2008.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kralova S, Leva L, Toman M. Polymorphonuclear function in naturally occurring renal failure in dogs. Vet Med (Praha) 2009;54:236–243. [Google Scholar]
- 9.Barbosa TS, Mori CK, Ciarlini PC. Efeito inibidor do soro urêmico sobre o metabolismo oxidativo dos neutrófilos de cães. Arq Bras Med Vet Zootec. 2010;62:1352–1358. [Google Scholar]
- 10.Keegan RF, Webb CB. Oxidative stress and neutrophil function in cats with chronic renal failure. J Vet Intern Med. 2010;24:514–519. doi: 10.1111/j.1939-1676.2010.0498.x. [DOI] [PubMed] [Google Scholar]
- 11.Chonchol M. Neutrophil dysfunction and infection risk in end-stage renal disease. Semin Dial. 2006;19:291–296. doi: 10.1111/j.1525-139X.2006.00175.x. [DOI] [PubMed] [Google Scholar]
- 12.Cendoroglo M, Bertrand LJ, Balakrishnan VS, Perianayagam M, King AJ, Pereira BJG. Neutrophil apoptosis and dysfunction in uremia. J Am Soc Nephrol. 1999;10:93–100. doi: 10.1681/ASN.V10193. [DOI] [PubMed] [Google Scholar]
- 13.Clermont G, Lecour S, Lahet JJ, et al. Alteration in plasma antioxidant capacities in chronic renal failure and hemodialysis patients: A possible explanation for the increased cardiovascular risk in these patients. Cardiovasc Res. 2000;47:618–623. doi: 10.1016/s0008-6363(00)00117-6. [DOI] [PubMed] [Google Scholar]
- 14.Erdogan C, Ünlücerci Y, Türkmen A, et al. The evaluation of oxidative stress in patients with chronic renal failure. Clin Chim Acta. 2002;322:157–161. doi: 10.1016/s0009-8981(02)00173-0. [DOI] [PubMed] [Google Scholar]
- 15.International Renal Interest Society. IRIS Staging of CKD. 2006. [(last accessed 2013 Jan 11)]. Available at http://www.iris-kidney.com/guidelines/en/staging_ckd.shtml.
- 16.Jain NC. Schalm’s Veterinary Hematology. 4th ed. Philadelphia: Lea & Febiger; 1986. p. 5p. [Google Scholar]
- 17.Metcalf JA, Gallin JI, Nauseef WM, Root RK. Laboratory Manual of Neutrophil Function. New York: Raven Press; 1986. p. 10p. [Google Scholar]
- 18.Walrand S, Valeix S, Rodriguez C, et al. Flow cytometry study of polymorphonuclear neutrophil oxidative burst: A comparison of 3 fluorescent probes. Clin Chim Acta. 2003;331:103–110. doi: 10.1016/s0009-8981(03)00086-x. [DOI] [PubMed] [Google Scholar]
- 19.Zar JH. Biostatistical Analysis. 2nd ed. Englewood Cliffs, New Jersey: Prentice Hall; 1984. pp. 93–95. [Google Scholar]
- 20.Stockham SL, Scott MA. Fundamentals of Veterinary Clinical Pathology. Ames, Iowa: Iowa State University Press; 2002. p. 384. [Google Scholar]
- 21.Terawaki H, Yoshimura K, Hasegawa T, et al. Oxidative stress is enhanced in correlation with renal dysfunction: Examination with the redox state of albumin. Kidney Int. 2004;66:1988–1993. doi: 10.1111/j.1523-1755.2004.00969.x. [DOI] [PubMed] [Google Scholar]
- 22.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Sardenberg C, Suassuna P, Andreoli MCC, et al. Effects of uraemia and dialysis modality on polymorphonuclear cell apoptosis and function. Nephrol Dial Transplant. 2006;21:160–165. doi: 10.1093/ndt/gfi095. [DOI] [PubMed] [Google Scholar]
- 24.McLeish KR, Klein JB, Lenderer EL, et al. Azotemia, TNF alpha, and LPS prime the human neutrophil oxidative burst by distinct mechanisms. Kidney Int. 1996;50:407–416. doi: 10.1038/ki.1996.330. [DOI] [PubMed] [Google Scholar]
- 25.Paul JL, Roch-Arveiller M, Man NK, Luong N, Moatti N, Raichvarg D. Influence of uremia on polymophonuclear leukocytes oxidative metabolism in end-stage renal disease and dialyzed patients. Nephron. 1991;57:428–432. doi: 10.1159/000186308. [DOI] [PubMed] [Google Scholar]
- 26.Gastadello K, Husson C, Wens R, et al. Role of complement and platelet-activating factor in the stimulation of phagocytosis and reactive oxygen species production during haemodialysis. Nephrol Dial Transplant. 2000;15:1638–1646. doi: 10.1093/ndt/15.10.1638. [DOI] [PubMed] [Google Scholar]
- 27.Majewska E, Baj Z, Sulowska Z, et al. Effects of uraemia and haemodialysis on neutrophil apoptosis and expression of apoptosis-related proteins. Nephrol Dial Transplant. 2003;18:2582–2588. doi: 10.1093/ndt/gfg441. [DOI] [PubMed] [Google Scholar]