Abstract
Abundant lymphocyte infiltration is frequently found in canine malignant mammary tumors, but the pathological features and immunophenotypes associated with the infiltration remain to be elucidated. The aim of the present study was to evaluate the relationship between lymphocyte infiltration, histopathological features, and molecular phenotype in canine mammary carcinoma (MC). The study was done with archived formalin-fixed, paraffin-embedded samples (n = 47) by histologic and immunohistochemical methods. The degree of lymphocyte infiltration was evaluated by morphologic analysis, and the T- and B-cell populations as well as the T/B-cell ratio were evaluated by morphometric analysis; results were compared with the histologic features and molecular phenotypes. The degree of lymphocyte infiltration was significantly higher in MCs with lymphatic invasion than in those without lymphatic invasion (P < 0.0001) and in tumors of high histologic grade compared with those of lower histologic grade (P = 0.045). Morphometric analysis showed a larger amount of T-cells and B-cells in MCs with a higher histologic grade and lymphatic invasion, but the T/B ratio did not change. Lymphocyte infiltration was not associated with histologic type or molecular phenotype, as assessed from the immunohistochemical expression of epidermal growth factor receptor 2, estrogen receptor, cytokeratin 14, and p63. Since intense lymphocyte infiltration was associated with aggressive histologic features, lymphocytes may be important for tumor aggressiveness and greater malignant behavior in the tumor microenvironment.
Résumé
Une infiltration lymphocytaire abondante est fréquemment retrouvée dans les tumeurs mammaires malignes chez le chien, mais les caractéristiques pathologiques et les immunophénotypes associés avec l’infiltration restent à être élucidés. L’objectif de la présente étude était d’évaluer la relation entre l’infiltration lymphocytaire, les caractéristiques histopathologiques, et le phénotype moléculaire dans les carcinomes mammaires canins (CM). Cette étude a été réalisée en utilisant des méthodes histologiques et immunohistochimiques sur des échantillons archivés fixés à la formaline et enrobés de paraffine (n = 47). Le degré d’infiltration lymphocytaire a été évalué par analyse morphologique, et les populations de lymphocytes T et B ainsi que le ratio de cellules T/B ont été évalués par analyses morphométriques; les résultats ont été comparés avec les caractéristiques histologiques et les phénotypes moléculaires. Le degré d’infiltration lymphocytaire était significativement plus élevé dans les CM avec invasion lymphatique que dans ceux sans invasion lymphatique (P < 0,001) et dans les tumeurs de grade histologique élevé comparativement à ceux avec un grade histologique faible (P = 0,045). Les analyses morphométriques ont montré une quantité plus grande de cellules T et B dans les CM ayant un grade histologique élevé et invasion lymphatique, mais le ratio T/B n’a pas changé. L’infiltration lymphocytaire n’était pas associée avec le type histologique ou le phénotype moléculaire, tel qu’évalué par l’expression immunohistochimique du récepteur 2 du facteur de croissance épidermique, du récepteur d’estrogène, de cytokératine 14, et de p63. Étant donné que l’infiltration lymphocytaire marquée était associée avec des caractéristiques histologiques d’agressivité, les lymphocytes pourraient être importants pour l’agressivité des tumeurs et le comportement de malignité plus important dans le microenvironnement de la tumeur.
(Traduit par Docteur Serge Messier)
Introduction
The relationship between inflammation and cancer has been evaluated because the immune system is important in the tumor microenvironment (1). Large numbers of leukocytes infiltrate solid and metastasized tumors (2). Lymphocyte infiltration primarily stimulates the host immune response against tumors (3), and T-lymphocytes constitute most of the lymphocyte infiltration in human breast cancer (HBC) (2). Recently it has been suggested that a balance between the adaptive and innate immune responses mediated by lymphocytes may affect HBC progression and regression (4). Cytotoxic T-lymphocytes (CTLs) kill tumor cells directly, and T helper 1 (Th1) cells, which secrete antitumor cytokines, exert mainly antitumor effects (4–6). Regulatory T (Treg-cells) and T helper 2 (Th2) cells promote tumor development by suppressing the antitumor immune response (4,7,8).
Abundant lymphocyte infiltration is frequently found not only in HBC but also in canine malignant mammary carcinoma (MC) (9). Canine MC may be regarded as a potent, spontaneous animal model of HBC (10,11) because canine MC and HBC have similar biologic and pathological features (11). Studies have shown that various features, including histologic features, clinical course, hormone levels, molecular markers, proliferation markers, and genetic mutations, are similar in canine MC and HBC (11). Hence, investigating the pathogenesis and biologic features of canine mammary tumors could improve our understanding of HBC and help identify new therapeutics (11). Recently some articles reported the phenotypic features and prognostic implications of lymphocytes; the level of lymphocyte infiltration may be an important prognostic biomarker for canine MC (12–14). However, the identification of tumor-infiltrating lymphocytes and the relationship between various pathological features of canine MC remain to be investigated.
Some authors have also reported on the identification and application of molecular phenotypes of canine MC because of the heterogeneity of this disease according to human classifications (15,16). The molecular phenotypes of HBC correlate with prognosis and clinical outcome and are classified as follows: luminal A [estrogen receptor (ER)+ and HER (epidermal growth factor)-2−]; luminal B (ER+/HER-2+); HER-2-overexpressing (ER−/HER-2+); basal (ER−/HER-2−/basal cell marker+); and negative/null (ER−/HER- 2−/basal cell marker−) (15–17). Luminal phenotypes are based on positive expression of ER, and basal phenotypes are determined by negative expression of ER and basal cell markers such as cytokeratin (CK) 5/6, CK14, p63, and P-cadherin (15–17). In human medicine, patients with HER-2-overexpressing cells and basal phenotypes have significantly shorter survival than those with luminal phenotypes (18,19). In canine MC, 2 previous reports correlated molecular phenotypes with survival data; however, the results were inconsistent: 1 study showed that the basal phenotype was significantly associated with lower survival rates (15), whereas the other study found that patients with the basal phenotype had a better outcome than those with the luminal phenotypes, those with the luminal B phenotype having a worse outcome than those with the luminal A phenotype (16). A high degree of lymphocyte infiltration correlated with HER-2-positive HBC (20), and HER-2 may be an appropriate target for immunotherapy because T-lymphocytes respond to HER-2 peptides (21,22). Therefore, lymphocyte infiltration is required to explore and investigate the relationship between molecular phenotypes in MC.
Because canine MC is a heterogeneous disease, it is challenging to explain the precise biologic features of MC, make a prognosis, and apply effective therapy; thus, specific tumor behavior and biologic features must be examined. In addition, the involvement of the immune response in the tumor microenvironment was recently highlighted in both human and animal cancer. Therefore, the aim of this study was to investigate whether lymphocytes infiltrate and affect a specific biologic condition or subtype of canine MC with heterogeneous biologic features by determining the total lymphocyte count, the T- and B-cell populations, and the T/B-cell ratio, correlating lymphocyte infiltration with histologic variables, and correlating the results with the molecular phenotypes.
Materials and methods
Samples
All mammary samples were originally obtained from the Veterinary Medical Teaching Hospital of Konkuk University, Seoul, Korea, or from private animal clinics. The samples had been submitted between 2006 and 2008 to the Small Animal Tumor Diagnostic Center, Department of Veterinary Pathology, Konkuk University. The 47 MC samples were obtained from purebred or mixed-breed female dogs aged 2 to 19 y [mean 10.6 ± 3.9 (standard deviation) y; median 10.6 y]. Samples of normal mammary tissue from 3 clinically healthy dogs were used as negative controls for HER-2 and as positive controls for the CK and p63 immunostaining.
Histologic examination and classification
Histopathological examination was conducted on 4-μm-thick sections of formalin-fixed, paraffin-embedded samples of canine mammary gland tissue that had been stained with hematoxylin and eosin (H&E). Canine MCs were classified according to the World Health Organization International Histological Classification of Tumors of Domestic Animals (23). Evidence of lymphatic invasion and the presence of tumor necrosis were recorded. The tumors were classified histologically as well-differentiated (grade I), moderately differentiated (grade II), or poorly differentiated (grade III) carcinomas according to the Elston and Ellis grading system (24,25).
Immunohistochemical examination and scoring
Primary antibodies included anti-CD3, anti-CD79α (DakoCytomation, Glostrup, Denmark), anti-HER-2, anti-ER (BioGenex, Fremont, California, USA), anti-CK14 (Abcam, Cambridge, England), and anti-p63 (Santa Cruz Biotechnology, Santa Cruz, California, USA). Sections were dewaxed in xylene and hydrated in graded ethanol. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 2 mM KH2PO4), pH 7.4, for 20 min at room temperature (RT), and then 3 washes in PBS were done. Antigen retrieval was accomplished by boiling CD3, ER, CK14, and p63 in tris-ethylene diamine tetraacetic acid buffer (pH 9.0) and CD79α in citric acid buffer (pH 6.0) in a microwave oven (at 750 W). Slides with ER were incubated with 5% normal goat serum for 30 min at RT. Sections were subsequently incubated with the primary antibodies, and primary antibody binding was detected with the 2-step EnVision horseradish peroxidase system (REAL EnVision Kit; DakoCytomation). The secondary polymer was applied to each slide for 40 min at RT, and the slides were washed 4 times with PBS before incubation with the appropriate substrates. The colorimetric reaction was stopped by 2 washes in distilled water, and the sections were counterstained with Harris hematoxylin. The primary antibodies, including isotype antibodies, are listed in Table I. The isotype negative controls were as follows: mouse IgG1 (eBioscience, San Diego, California, USA) for CD79α, HER, and ER; rabbit immunoglobulin fraction (DakoCytomation) for CD3; IgG3 (Abcam) for CK14; and IgG2a (BioLegend, San José, California, USA) for p63. The following positive controls were used: canine lymph node for CD3/CD79α, uterus for ER, myoepithelial cells from a normal mammary gland for CK14 and p63, and mammary carcinoma tissues for HER-2 overexpression, as in our previous study of HER-2 (26).
Table I.
Primary antibodies used for immunohistochemical analysis of canine mammary carcinomas (MCs)
| Target antigen | Antibody type | Clone | Sourcea | Antigen retrieval | Dilution | Incubation | Isotype |
|---|---|---|---|---|---|---|---|
| CD3 | Pab | IgG fraction | DakoCytomation | HIER for 10 min | 1:150 | 2.5 h, RT | Rabbit Ig fraction |
| CD79α | Mab | HM57 | DakoCytomation | HIER for 10 min | 1:150 | Overnight, 4°C | Mouse IgG1 |
| HER-2 | Mab | CB11 | BioGenex | None | 1:100 | 3 h, RT | Mouse IgG1 |
| ER | Mab | ER88 | BioGenex | HIER for 20 min | 1:60 | 3 h, RT | Mouse IgG1 |
| CK14 | Mab | LL002 | Abcam | HIER for 10 min | 1:300 | 3 h, RT | Mouse IgG3 |
| p63 | Mab | 4A4 | Santa Cruz Biotechnology | HIER for 15 min | 1:100 | Overnight, 4°C | Mouse IgG2a |
DakoCytomation, Glostrup, Denmark; BioGenex, Fremont, California, USA; Abcam, Cambridge, England; Santa Cruz Biotechnology, Santa Cruz, California, USA.
HER-2 — epidermal growth factor receptor 2; ER — estrogen receptor; CK14 — cytokeratin 14; Pab — rabbit polyclonal antibody; Mab — mouse monoclonal antibody; Ig — immunoglobulin; HIER — heat-induced epitope retrieval; RT — room temperature.
The immunohistochemical score was assigned through semi-quantitative analysis. The HER-2 immunoreactivity was scored on a 4-point scale (0 to 3+) according to the HercepTest scoring system (DakoCytomation) (27): 0 = no staining or membrane staining of less than 10% of the tumor cells; 1+ = weak, incomplete membrane staining; 2+ = weak or moderate, complete membrane staining of more than 10% of the tumor cells; and 3+ = strong, complete membrane staining of more than 10% of the tumor cells. Scores of 0 and 1+ were considered negative, whereas scores of 2+ and 3+ were consistent with HER-2 overexpression. The ER immunoreactivity was considered positive when more than 10% of the tumor cells had immunolabeled nuclei (15). Positivity for CK14 was defined as the detection of strong cytoplasmic staining in more than 1% of the invasive tumor cells (18), and positivity for p63 was defined as the detection of immunolabeling in the nuclei of more than 50% of the neoplastic cells (15). The percentage of positive cells was evaluated in 10 representative high-power fields (each with more than 1000 cells).
Morphologic and morphometric analysis of lymphocyte infiltration
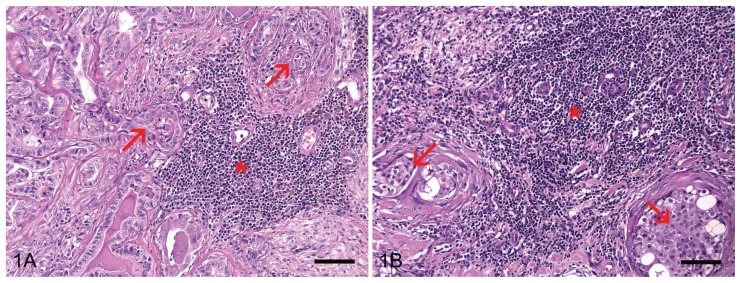
In the H&E sections the degree of lymphocytic infiltration was morphologically classified by distribution and intensity in the intratumoral areas according to previous studies (12,14). Distribution was classified as follows: focal = presence of 1 to 3 inflammatory foci; multifocal = presence of more than 3 inflammatory foci; and diffuse = even distribution of inflammatory cells in the tumor section. Density was classified as follows: 1 = discrete; 2 = moderate; and 3 = intense. The scores for distribution and intensity were multiplied. In this classification system the infiltration degree varies between 1 and 9 and is classified as follows: degree 1 = score 1 to 3; degree 2 = score 4 to 6; and degree 3 = score 7 to 9 (Figures 1A and 1B).
Figure 1.
Lymphocyte infiltration classified according to morphologic analysis. A — Inflammatory cells (asterisk) moderately infiltrate the stroma near the tumor cells (arrows). The degree of lymphocyte infiltration is 2. B — High-density lymphocyte infiltration (asterisk) around tumor nests (arrows). The degree of lymphocytic infiltration is 3. Hematoxylin and eosin. Bar = 70 μm.
Morphometric image analysis was done by a method described previously (28). Digital images were acquired with an Olympus microscope (model BX41; Olympus, Tokyo, Japan) and digital image transfer software (Leica Application Suite 2.7; Leica Microsystems, Heerbrugg, Germany). Morphometric analysis of T- and B-lymphocytes was accomplished with computerized image analysis software (Image Pro Plus 5.1; Media Cybernetics, Bethesda, Maryland, USA) according to a previous study (28). For each slide, 20 fields of immunolabeled images were acquired at a magnification of 400× (40× objective, 10× ocular). The positive areas per 1.6 mm2 were calculated by converting the positive pixels into square millimeters; the areas included the nuclear areas of the lymphocytes. The CD3-positive area divided by the CD79α-positive area represented the CD3/CD79α ratio. The total T/B-lymphocyte population was calculated by adding the CD3-positive and CD79α-positive areas.
Molecular phenotyping
The MC molecular phenotypes were defined according to a modified classification system (15,16) as follows: luminal A = ER+, HER-2−, and any CK14 or p63; luminal B = ER+, HER-2+, and any CK14 or p63; HER-2-overexpressing = ER−, HER-2+, and any CK14 or p63; basal = ER−, HER-2−, CK14+, and/or p63+); and negative null = ER−, HER-2−, CK14−, and p63−.
Statistical analysis
Differences in the degree of lymphocyte infiltration, histologic categorical variables, and molecular phenotypes were evaluated with Pearson’s chi-square test for categorical analysis. The normality of the distribution was assessed by the Kolmogorov–Smirnov test, and the associations between the means for the 2 groups were examined by Student’s t-test or the Mann–Whitney U-test. Analysis of variance or the Kruskal–Wallis test was used to compare more than 2 groups. Statistical significance was established at P < 0.05. Statistical analysis was done with the use of SPSS, version 17.0 (SPSS, Chicago, Illinois, USA).
Results
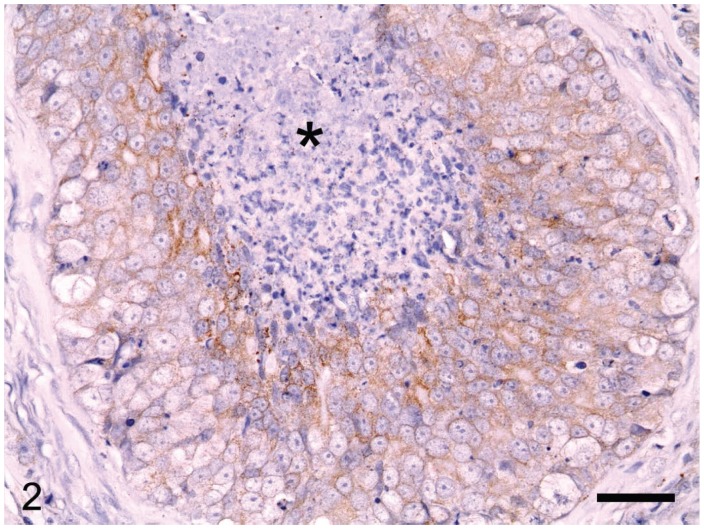
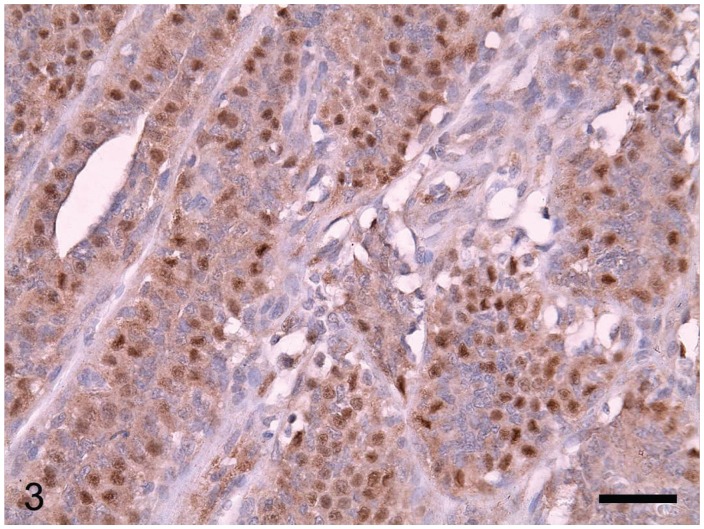
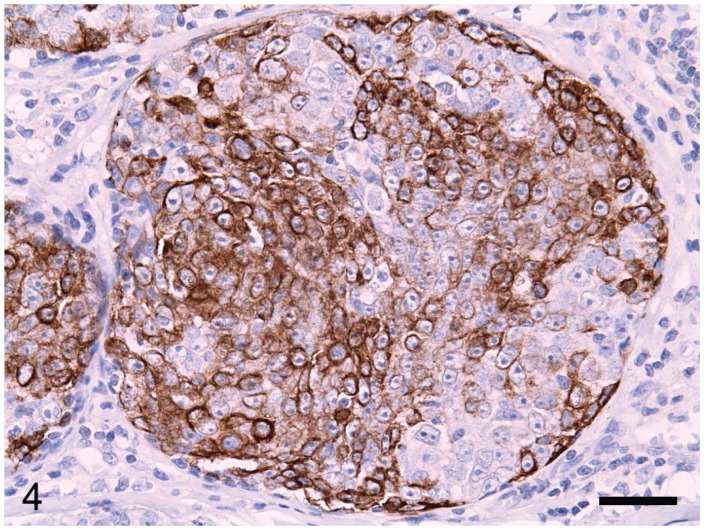
The canine MCs included simple carcinomas (n = 22), complex carcinomas (n = 3), carcinomas in benign mixed tumors (n = 7), and mammary squamous cell carcinomas (n = 15). Lymphatic invasion of tumor cells was identified in 21 cases. Of the 47 tumors, 11 were grade I, 14 grade II, and 22 grade III. The HER-2 protein was detected by immunohistochemical staining of the membrane of tumor cells (Figure 2) in 16 (34%) of the 47 MCs. Nuclear ER immunoreactivity (Figure 3) was positive in 21 (45%) of the 47 MCs, and positivity for CK14 or p63 was detected in 76%, as determined from immunolabeling in the cytoplasm and the nuclei of the tumor cells, respectively (Figures 4 and 5). From the immunohistochemical staining, the molecular phenotypes of the MCs were classified as follows: luminal A, 14; luminal B, 7; basal, 15; HER-2-overexpressing, 9; and negative/null, 2.
Figure 2.
Overexpression of epidermal growth factor 2 leads to strong, complete staining of the tumor cell membrane according to the HercepTest scoring system (DakoCytomation, Glostrup, Denmark). Intratumoral necrosis is also observed (asterisk). EnVision horseradish peroxidase immunohistochemistry system (REAL EnVision Kit; DakoCytomation) with hematoxylin counterstain. Bar = 36 μm.
Figure 3.
Strong immunoreaction for estrogen receptors in the nuclei of the tumor cells. EnVision system with hematoxylin counterstain. Bar = 36 μm.
Figure 4.
Tumor cells are diffusely immunolabeled with cytokeratin 14 in the cytoplasm. EnVision system with hematoxylin counterstain. Bar = 36 μm.
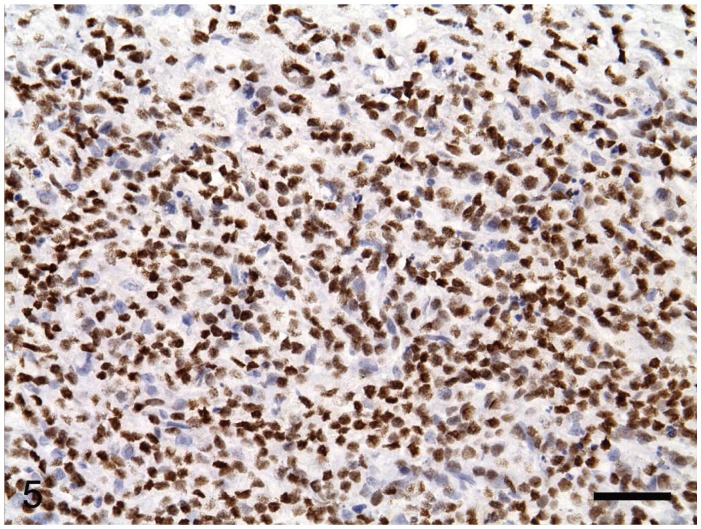
Figure 5.
Positive p63 immunostaining in the nuclei of the tumor cells. EnVision system with hematoxylin counterstain. Bar = 36 μm.
The degree of lymphocyte infiltration was not associated with histologic type of the MC (P = 0.042), presence of intratumoral necrosis (P = 0.178), or molecular phenotype (P = 0.052) according to morphologic analysis (Table II). However, a high degree of lymphocyte infiltration (3+) was most frequent in the squamous cell subtype, whereas a low degree (1+) was most frequent in the simple subtype. The degree of lymphocyte infiltration was significantly higher in MCs with lymphatic invasion than in those without lymphatic invasion (P < 0.0001) and in tumors of high histologic grade compared with those of low histologic grade (P = 0.045).
Table II.
Evaluation of the degree of lymphocyte infiltration by morphologic analysis of histologic features and molecular phenotype
| Degree of infiltration; number (and %) of MCs | ||||
|---|---|---|---|---|
|
|
||||
| Variable (n = 47) | 1 | 2 | 3 | P-valuea |
| Histologic type | NS | |||
| Simple (n = 22) | 10 (45) | 4 (18) | 8 (36) | |
| Complex (n = 3) | 1 (33) | 1 (33) | 1 (33) | |
| Mixed (n = 7) | 2 (29) | 3 (43) | 2 (29) | |
| Squamous cell (n = 15) | 1 (7) | 7 (47) | 7 (47) | |
| Lymphatic invasion | P < 0.0001 | |||
| Presence (n = 21) | 1 (5) | 6 (28) | 14 (67) | |
| Absence (n = 26) | 13 (50) | 9 (35) | 4 (15) | |
| Histologic grade | ||||
| I (n = 11) | 7 (64) | 3 (27) | 1 (9) | P = 0.045 |
| II (n = 14) | 4 (28) | 4 (28) | 6 (43) | |
| III (n = 22) | 3 (14) | 8 (36) | 11 (50) | |
| Necrosis | NS | |||
| Presence (n = 27) | 5 (18) | 10 (37) | 12 (44) | |
| Absence (n = 20) | 9 (45) | 5 (25) | 6 (30) | |
| Molecular phenotype | NS | |||
| Luminal A (n = 14) | 7 (50) | 5 (36) | 2 (14) | |
| Luminal B (n = 7) | 1 (14) | 3 (43) | 3 (43) | |
| Basal (n = 15) | 3 (20) | 4 (27) | 8 (53) | |
| HER-2-overexpressing (n = 9) | 2 (22) | 3 (33) | 4 (44) | |
With Pearson’s chi-square test.
NS — not significant. infiltration
Morphometric image analysis showed significantly greater infiltration of CD3+ T-lymphocytes in MCs of high histologic grade (P = 0.035) and MCs undergoing lymphatic invasion (P = 0.008) and of CD79α+ B-lymphocytes in high-grade MCs (P = 0.011) and tumors undergoing lymphatic invasion (P = 0.001), compared with MCs of lower histologic grade and those not invading the lymphatics (Table III). Total lymphocytes were abundant in high-grade MCs (P = 0.020) and those with lymphatic invasion (P = 0.005). Again, no statistical associations were observed between lymphocyte and histologic type, necrosis, and molecular phenotype. The histologic features and molecular phenotypes were not associated with the CD3/CD79α ratio.
Table III.
Comparison of lymphocyte subpopulations by morphometric analysis of histologic features and molecular phenotype
| Lymphocyte subpopulation, positive area/1.6 mm2 (mean ± standard deviation)a | ||||
|---|---|---|---|---|
|
|
||||
| Variable | CD3+ T-cells | CD79α+ B-cells | Total T/B-cells | CD3+/CD79α+ ratio |
| Histologic type | NS* | NS* | NS* | NS† |
| Simple | 0.078 ± 0.102 | 0.027 ± 0.052 | 0.106 ± 0.143 | 0.277 ± 0.79 |
| Complex | 0.028 ± 0.032 | 0.008 ± 0.012 | 0.036 ± 0.033 | 14.38 ± 0.54 |
| Mixed | 0.043 ± 0.055 | 0.011 ± 0.012 | 0.055 ± 0.066 | 2.73 ± 0.58 |
| Squamous cell | 0.119 ± 0.120 | 0.028 ± 0.035 | 0.147 ± 0.144 | 3.36 ± 0.95 |
| Lymphatic invasion | P = 0.008‡ | P = 0.001‡ | P = 0.005‡ | NS§ |
| Presence | 0.118 ± 0.112 | 0.038 ± 0.049 | 0.157 ± 0.148 | 2.70 ± 0.72 |
| Absence | 0.055 ± 0.085 | 0.013 ± 0.031 | 0.067 ± 0.105 | 3.92 ± 1.02 |
| Histologic grade | P = 0.035* | P = 0.011* | P = 0.020* | NS† |
| I | 0.025 ± 0.047 | 0.003 ± 0.004 | 0.029 ± 0.047 | 3.05 ± 1.04 |
| II | 0.072 ± 0.087 | 0.023 ± 0.040 | 0.096 ± 0.119 | 4.31 ± 0.99 |
| III | 0.118 ± 0.119 | 0.035 ± 0.049 | 0.153 ± 0.152 | 2.72 ± 0.73 |
| Necrosis | NS‡ | NS‡ | NS‡ | NS§ |
| Presence | 0.103 ± 0.113 | 0.027 ± 0.045 | 0.131 ± 0.143 | 3.26 ± 0.85 |
| Absence | 0.055 ± 0.080 | 0.020 ± 0.037 | 0.075 ± 0.113 | 3.01 ± 0.84 |
| Molecular phenotype | NS* | NS* | NS* | NS† |
| Luminal A | 0.028 ± 0.043 | 0.005 ± 0.007 | 0.033 ± 0.045 | 5.84 ± 0.59 |
| Luminal B | 0.133 ± 0.141 | 0.049 ± 0.052 | 0.182 ± 0.181 | 2.53 ± 0.76 |
| Basal | 0.111 ± 0.111 | 0.038 ± 0.059 | 0.148 ± 0.157 | 2.91 ± 0.83 |
| HER-2-overexpressing | 0.076 ± 0.095 | 0.016 ± 0.015 | 0.092 ± 0.097 | 2.09 ± 0.76 |
Statistical significance determined by
the Kruskal–Wallis test,
analysis of variance,
the Mann–Whitney U-test, and
Student’s t-test.
Discussion
Mammary gland tumors are the most common neoplasms in female dogs and are a heterogeneous group with different prognoses (29). Although the classification of histologic features and biologic and molecular identification for making a prognosis have been explored, the immune response and the relationship between tumor behavior and lymphocyte infiltration are still unclear.
In this study, lymphocyte infiltration of canine MC tissues was assessed by morphologic and morphometric image analyses, and the results from these 2 methods consistently showed that lymphocyte infiltration was associated with an aggressive histologic grade and lymphatic invasion. Morphologic evaluation revealed that a high degree of lymphocyte infiltration was significantly correlated with lymphatic invasion and a high histologic grade. A previous morphologic analysis of inflammatory cells found that a high intensity of lymphocyte infiltration was associated with lymph node metastasis, advanced clinical stage, and low survival rate (12); our results are consistent with these results. Although we have no clinical or survival data, we suggest that an abundance of tumor-infiltrating lymphocytes in canine MC tissue is associated with aggressive tumor behavior and a higher degree of malignancy.
By morphometric image analysis, greater numbers of CD3+ T-cells, CD79α+ B-cells, and total lymphocytes were observed in MCs of high histologic grade with lymphatic invasion. Recent studies reported that an abundance of intratumoral CD3+ T-cells correlated with tumor invasiveness and shorter overall survival in canines with malignant mammary tumors (13,14). One of those studies showed that the number of T-cells increased more in the malignant mammary tumors than in benign tumors (14), whereas the other study showed that the number of intratumoral T-cells was higher in benign tumors than in malignant tumors (13). The T-cell count is likely different in benign and malignant tumors; however, for malignant tumors, a high degree of T-cell infiltration was correlated with a poor prognosis in both studies. In addition, the latter study showed that a greater abundance of intratumoral T-cells was associated with tumor invasiveness (13); our T-cell infiltration results are consistent with this observation. For B-cells, the infiltration likely increases in canine MCs compared with benign tumors, although this difference is not statistically significant (14). Another study showed that the percentage of B-cells in MCs is higher in dogs with lymph node metastasis (12). In HBC, B-cell infiltration is associated with progression of malignant tumors (30). Because our results revealed that more B-cells are observed in high-grade MCs with lymphatic invasion, we suggest that greater infiltration of B-cells is associated with aggressive tumor behavior in canine MC.
In addition, the data for total infiltration of T- and B-cells obtained by morphometric analysis were similar to those obtained by morphologic analysis: greater lymphocyte infiltration was associated with higher histologic grade and increased lymphatic invasion. This suggests that the infiltrating lymphocytes are crucial for tumor malignancy in canine MC and supports the HBC reports that abundant lymphocyte infiltration in high-grade and invasive carcinomas correlates with metastasis and poor prognosis (4,31,32). We investigated whether the relative amount of T- and B-cells in the infiltration is related to the immune response; however, no tumor features correlated with the relative amount of T- and B-cells. Additional insights will likely require the investigation of T-cell subpopulations, including CTLs and Treg- and Th-cells, rather than estimation of the ratio of T-and B-cells.
Furthermore, the lymphocyte infiltration data were statistically evaluated to investigate a possible association with molecular phenotype because some reports have shown a correlation between molecular phenotype and tumor behavior or prognosis in both HBC and canine MC (15,16,33). Basal and HER-2-overexpressing phenotypes are aggressive and correlate with a poor prognosis for HBC patients (18,19,33). In canine MCs, although controversial results have been reported, the basal phenotype correlating with a high histologic grade (15) or a low grade (16), the molecular phenotype correlation might provide significant information on the biologic and pathological features of MCs. However, morphologic and morphometric analyses failed to show an association between lymphocyte infiltration and molecular phenotype in the present study. Further studies are thus required to clarify whether molecular phenotype correlates with prognosis and whether lymphocytes are involved in determining the molecular phenotype of canine MCs.
In conclusion, a large number of lymphocytes, including T- and B-cells, consistently infiltrate canine MCs and are associated with lymphatic invasion and a high histologic grade. The results of this study suggest that tumor-infiltrating lymphocytes are important for tumor progression and metastatic potential and not tumor killing or regression. This is the first report of the histopathological features of lymphocyte infiltration in different molecular phenotypes. Although the distinct functions of T- and B-cells remain to be determined — including by studies of the involvement of subpopulations of T-cells and the clinical implications of lymphocyte infiltration — this study has provided important data that support the relationship between lymphocytes and cancer in dogs.
Acknowledgments
This study was supported by Konkuk University in 2012. The authors thank Mrs. Rae-Hwa Jang for excellent technical assistance. They also acknowledge the private veterinary clinics for providing the canine mammary samples.
References
- 1.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Sullivan C, Lewis CE. Tumour-associated leucocytes: Friends or foes in breast carcinoma. J Pathol. 1994;172:229–235. doi: 10.1002/path.1711720302. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Baruch A. Host microenvironment in breast cancer development: Inflammatory cells, cytokines and chemokines in breast cancer progression: Reciprocal tumor–microenvironment interactions. Breast Cancer Res. 2003;5:31–36. doi: 10.1186/bcr554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: Crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moss RB, Moll T, El-Kalay M, et al. Th1/Th2 cells in inflammatory disease states: Therapeutic implications. Expert Opin Biol Ther. 2004;4:1887–1896. doi: 10.1517/14712598.4.12.1887. [DOI] [PubMed] [Google Scholar]
- 6.Munk ME, Emoto M. Functions of T-cell subsets and cytokines in mycobacterial infections. Eur Respir J Suppl. 1995;20:668s–675s. [PubMed] [Google Scholar]
- 7.Tan TT, Coussens LM. Humoral immunity, inflammation and cancer. Curr Opin Immunol. 2007;19:209–216. doi: 10.1016/j.coi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Johansson M, Tan T, de Visser KE, Coussens LM. Immune cells as anti-cancer therapeutic targets and tools. J Cell Biochem. 2007;101:918–926. doi: 10.1002/jcb.21230. [DOI] [PubMed] [Google Scholar]
- 9.Rutten VP, Misdorp W, Gauthier A, et al. Immunological aspects of mammary tumors in dogs and cats: A survey including own studies and pertinent literature. Vet Immunol Immunopathol. 1990;26:211–225. doi: 10.1016/0165-2427(90)90092-7. [DOI] [PubMed] [Google Scholar]
- 10.Rivera P, von Euler H. Molecular biological aspects on canine and human mammary tumors. Vet Pathol. 2011;48:132–146. doi: 10.1177/0300985810387939. Epub 2010 Dec 7. [DOI] [PubMed] [Google Scholar]
- 11.Queiroga FL, Raposo T, Carvalho MI, Prada J, Pires I. Canine mammary tumours as a model to study human breast cancer: Most recent findings. In Vivo. 2011;25:455–465. [PubMed] [Google Scholar]
- 12.Estrela-Lima A, Araujo MS, Costa-Neto JM, et al. Immunophenotypic features of tumor infiltrating lymphocytes from mammary carcinomas in female dogs associated with prognostic factors and survival rates. BMC Cancer. 2010;10:256. doi: 10.1186/1471-2407-10-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carvalho MI, Pires I, Prada J, Queiroga FL. T-lymphocytic infiltrate in canine mammary tumours: Clinic and prognostic implications. In Vivo. 2011;25:963–969. [PubMed] [Google Scholar]
- 14.Saeki K, Endo Y, Uchida K, Nishimura R, Sasaki N, Nakagawa T. Significance of tumor-infiltrating immune cells in spontaneous canine mammary gland tumor: 140 cases. J Vet Med Sci. 2012;74:227–230. doi: 10.1292/jvms.11-0118. Epub 2011 Sep 22. [DOI] [PubMed] [Google Scholar]
- 15.Gama A, Alves A, Schmitt F. Identification of molecular phenotypes in canine mammary carcinomas with clinical implications: Application of the human classification. Virchows Arch. 2008;453:123–132. doi: 10.1007/s00428-008-0644-3. [DOI] [PubMed] [Google Scholar]
- 16.Sassi F, Benazzi C, Castellani G, Sarli G. Molecular-based tumour subtypes of canine mammary carcinomas assessed by immunohistochemistry. BMC Vet Res. 2010;6:5. doi: 10.1186/1746-6148-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matos I, Dufloth R, Alvarenga M, Zeferino LC, Schmitt F. p63, cytokeratin 5, and P-cadherin: Three molecular markers to distinguish basal phenotype in breast carcinomas. Virchows Arch. 2005;447:688–694. doi: 10.1007/s00428-005-0010-7. Epub 2005 Oct 19. [DOI] [PubMed] [Google Scholar]
- 18.Kim MJ, Ro JY, Ahn SH, Kim HH, Kim SB, Gong G. Clinicopathologic significance of the basal-like subtype of breast cancer: A comparison with hormone receptor and Her2/neuoverexpressing phenotypes. Hum Pathol. 2006;37:1217–1226. doi: 10.1016/j.humpath.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:0869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexe G, Dalgin GS, Scanfeld D, et al. High expression of lymphocyte-associated genes in node-negative HER2+ breast cancers correlates with lower recurrence rates. Cancer Res. 2007;67:10669–10676. doi: 10.1158/0008-5472.CAN-07-0539. [DOI] [PubMed] [Google Scholar]
- 21.Baxevanis CN, Sotiriadou NN, Gritzapis AD, et al. Immunogenic HER-2/neu peptides as tumor vaccines. Cancer Immunol Immunother. 2006;55:85–95. doi: 10.1007/s00262-005-0692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milano F, Guarriera M, Rygiel AM, Krishnadath KK. Trastuzumab mediated T-cell response against HER-2/neu overexpressing esophageal adenocarcinoma depends on intact antigen processing machinery. PLoS One. 2010;5:e12424. doi: 10.1371/journal.pone.0012424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Misdorp W, Else RW, Hellmen E, Lipscomb TP. International Histological Classification of Tumors of Domestic Animals. Washington DC: Armed Forces Institute of Pathology; 1999. Histological classification of mammary tumors of the dog and the cat; pp. 18–25. [Google Scholar]
- 24.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology. 2002;41:154–161. [PubMed] [Google Scholar]
- 25.Karayannopoulou M, Kaldrymidou E, Constantinidis TC, Dessiris A. Histological grading and prognosis in dogs with mammary carcinomas: Application of a human grading method. J Comp Pathol. 2005;133:246–252. doi: 10.1016/j.jcpa.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Kim JH, Im KS, Kim NH, Yhee JY, Nho WG, Sur JH. Expression of HER-2 and nuclear localization of HER-3 protein in canine mammary tumors: Histopathological and immunohistochemical study. Vet J. 2011;189:318–322. doi: 10.1016/j.tvjl.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Reis-Filho JS, Pinheiro C, Lambros MB, et al. EGFR amplification and lack of activating mutations in metaplastic breast carcinomas. J Pathol. 2006;209:445–453. doi: 10.1002/path.2004. [DOI] [PubMed] [Google Scholar]
- 28.Kim JH, Yu CH, Yhee JY, Im KS, Kim NH, Sur JH. Canine classical seminoma: A specific malignant type with human classifications is highly correlated with tumor angiogenesis. BMC Cancer. 2010;10:243. doi: 10.1186/1471-2407-10-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Misdorp W. Tumors of the mammary gland. In: Meuten DJ, editor. Tumors in Domestic Animals. 4th ed. Ames, Iowa: Iowa State University Press; 2002. pp. 575–606. [Google Scholar]
- 30.Coronella-Wood JA, Hersh EM. Naturally occurring B-cell responses to breast cancer. Cancer Immunol Immunother. 2003;52:715–738. doi: 10.1007/s00262-003-0409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong PY, Staren ED, Tereshkova N, Braun DP. Functional analysis of tumor-infiltrating leukocytes in breast cancer patients. J Surg Res. 1998;76:95–103. doi: 10.1006/jsre.1998.5301. [DOI] [PubMed] [Google Scholar]
- 32.Chin Y, Janseens J, Vandepitte J, Vandenbrande J, Opdebeek L, Raus J. Phenotypic analysis of tumor-infiltrating lymphocytes from human breast cancer. Anticancer Res. 1992;12:1463–1466. [PubMed] [Google Scholar]
- 33.van’t Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]