Abstract
The current study tested the benefit of commercially available spray-dried bovine colostrum (The Saskatoon Colostrum Company, Saskatoon, Saskatchewan) in raising snatch-farrowed, porcine-colostrum-deprived (SF-pCD) pigs. In experiment 1, 12 SF-pCD pigs received a liquid diet composed mainly of bovine colostrum from birth to day 10; 6 remained on the same liquid diet (COL), and the other 6 were fed a diet composed mainly of milk replacer (RPL) until weaning. In experiment 2, 12 SF-pCD pigs were fed mainly bovine colostrum before weaning; after weaning, 6 were fed a starter diet containing 20% (w/w) bovine colostrum powder (STARTER-COL), and the other 6 were fed a starter diet without any bovine colostrum (STARTER-CTRL) until termination (day 42 or day 49). In experiment 1 the COL pigs had significantly fewer fever-days than did the RPL pigs. In experiment 2 diarrhea, typhlocolitis, and pancreatic degeneration developed in 4 of the STARTER-COL pigs after weaning. In both experiments all the pigs fed mainly bovine colostrum before weaning survived until termination. All pigs tested free of swine influenza virus H1N1 and H3N2, Porcine reproductive and respiratory syndrome virus, and Porcine parvovirus. In experiment 2 all the pigs tested free of Porcine circovirus type 2 (PCV2), but some in both groups tested positive for Torque teno virus genogroups 1 and 2. In conclusion, with the use of snatch-farrowing and bovine colostrum, pigs can be raised in the absence of porcine maternal antibodies with 100% survival and freedom from most porcine pathogens of biologic relevance. This model is potentially suitable for animal disease research.
Résumé
La présente étude visait à tester l’avantage du colostrum bovin déshydraté disponible commercialement pour élever des porcs captés à la misebas et privés de colostrum porcin (SF-pCD). Dans l’expérience 1, 12 porcs SF-pCD ont reçu une diète liquide composée principalement de colostrum bovin de la naissance au jour 10; 6 sont demeurés sur la même diète liquide (COL), et les 6 autres étaient nourris avec une diète composée principalement de substitut de lait (RPL) jusqu’au sevrage. Dans l’expérience 2, 12 porcs SF-pCD étaient nourris principalement avec du colostrum bovin avant le sevrage; après le sevrage, 6 étaient nourris avec une diète de début contenant 20 % (poids/poids) de poudre de colostrum bovin (STARTER-COL), et les 6 autres étaient nourris avec une diète de début mais sans le colostrum bovin (STARTER-CTRL) jusqu’à la fin de l’expérience (jour 42 ou jour 49). Dans l’expérience 1, les porcs COL avaient significativement moins de jours avec fièvre que les porcs RPL. Dans l’expérience 2, de la diarrhée, une typhlocolite et une dégénération du pancréas s’est développée chez 4 des porcs STARTER-COL après le sevrage. Dans les 2 expériences tous les porcs nourris principalement avec du colostrum bovin avant le sevrage ont survécu jusqu’à la fin de l’expérimentation. Tous les porcs se sont avérés négatifs pour les virus H1N1 et H3N2 de l’influenza porcin, le virus du syndrome reproducteur et respiratoire porcin, et le parvovirus porcin. Dans l’expérience 2, tous les porcs ont testé négatif pour le circovirus porcin de type 2 (PCV2), mais quelques-uns dans les 2 groupes ont testé positif pour le virus Torque teno des génogroupes 1 et 2. En conclusion, avec l’utilisation de la mise-bas avec captation et de colostrum bovin, les porcs peuvent être élevés en absence d’anticorps maternels porcins avec un taux de survie de 100 % et l’absence des principaux agents pathogènes porcins d’importance biologique. Ce modèle est potentiellement approprié pour la recherche sur les maladies animales.
(Traduit par Docteur Serge Messier)
Introduction
In porcine research, especially that investigating infectious diseases, obtaining pigs that are free of porcine pathogens is essential. Currently, 3 main methods are used to obtain such pigs: testing of conventional pigs for antigen and antibodies of certain pathogens, the pigs testing negative being labeled specific pathogen free (SPF); the cesarean-derived colostrum-deprived (CDCD) method; and the gnotobiotic or germ-free technique. The advantage of the SPF method is its convenience, low technical requirement, and cost efficiency. However, when the research requires freedom of infection with pathogens that are highly prevalent in pig populations, such as Porcine circovirus type 2 (PCV2), this method may be inadequate, as most pigs have antibodies against these pathogens, either maternal or acquired, or are actively infected with the pathogen of interest. As a result, researchers may have to screen a large number of farms and pigs to obtain a reliable pig source and then select pigs after the level of maternally derived antibodies has waned.
The CDCD and gnotobiotic methods use cesarean section to obtain term piglets from pregnant sows. The CDCD pigs are raised in sterile compartments for several days and then in a clean room (1). Gnotobiotic pigs are raised entirely in sterile compartments. Although the CDCD and gnotobiotic methods are reliable for obtaining pathogen-free pigs, they have several disadvantages compared with the SPF method, including the need for surgery, specialized facilities, and sterile compartments, and the greater cost. In addition, gnotobiotic experiments are limited to only a few weeks, after which the pigs outgrow the sterile compartments. There is also a risk that cesarean-derived pigs, especially if delivered before term, do not fully experience the prenatal serum cortisol surge that occurs in vaginally delivered pigs (2). This surge plays an important role in tissue maturation, immunoglobulin absorption, surfactant production, and deposition of glycogen in muscle and liver (3). This helps to explain why morbidity and mortality rates are higher in cesarean-derived piglets than in naturally born cohorts. Further, there is debate as to whether gnotobiotic pigs have the same immunologic responses as naturally born pigs because they are not exposed to microbial programming, as are piglets reared in natural environments (4).
Recent attempts to raise snatch-farrowed, porcine-colostrum-deprived (SF-pCD) pigs with bovine colostrum have provided an alternative method for obtaining susceptible pigs for infectious disease experiments (5,6). In this method, pigs are fed bovine colostrum for 3 d and a porridge of milk replacer and dry feed from day 4 to 14 and then are weaned onto a dry diet on day 15. The advantages of this method are that the pigs experience natural vaginal delivery and bacterial colonization of their intestines and thus are more representative of conventional pigs than are CDCD or gnotobiotic pigs. However, the survival rates in these studies were at most 80%, mostly because of deaths from septicemia due to Escherichia coli or staphylococci (5,6). A further disadvantage was that the pigs were weaned much earlier than in the commercial industry and at an age when there may not be enough digestive enzymes to efficiently cope with solid feed.
In the present study a method of raising SF-pCD pigs is described that used commercially available bovine colostrum products (HeadSTART and Calf’s Choice Total HiCal; The Saskatoon Colostrum Company, Saskatoon, Saskatchewan). With this method the pigs were weaned at 21 or 24 d of age, all survived, and they were free of major porcine pathogens and maternal antibodies, such that they were fully susceptible to challenge with pathogens and receptive to vaccination at an early age. This method is thus an alternative to cesarean-derived methods of producing porcine-colostrum-deprived pigs for infectious disease research.
Materials and methods
Snatch-farrowing, animal care, and experimental design
This work was approved by the University of Saskatchewan’s Animal Research Ethics Board and adhered to the Canadian Council on Animal Care guidelines for humane animal use (7).
Pregnant sows at the Prairie Swine Center, Saskatoon, Saskatchewan, were used as the source of SF-pCD pigs. Parturition was induced by intramuscular injection of a commercial prostaglandin analogue (Planate; Merck Animal Health Canada, Kirkland, Quebec) on day 115 of pregnancy. The perivulvar area of the sows and the pens in which the sows were housed were washed with warm water twice on the day before expected parturition and sprayed with an iodine disinfectant (Prepodyne; West Penetone, Ville d’Anjou, Quebec). Before parturition a clean drape was placed behind the sow to reduce the risk of environmental contamination of the piglets. During parturition, the piglets were snatched before contacting the floor, farrowing equipment, or barn facilities. The umbilical cords were clamped and disinfected with Prepodyne. Within 1 min of birth the piglets were placed in sealed plastic containers fitted with a high-efficiency particulate air filter, which provided filtered fresh air during transportation to a Biosafety Level 2 animal care room with positive pressure ventilation at the University of Saskatchewan. The animal room was disinfected twice at 24-h intervals before pig entry: first with 7% hydrogen peroxide solution (Peroxigard; Bayer, Toronto, Ontario) and then with 1% Virkon solution (Antec International, Lavaltrie, Quebec). The piglets were raised in groups of 2 (for experiment 2) or 3 (for experiment 1) per pen on an elevated plastic floor. The concrete floor beneath the plastic flooring was washed 2 to 3 times each week in experiment 1 and daily in experiment 2. The room temperature was set at 30°C from day 1 to day 21 and was decreased by 1°C weekly thereafter. Before weaning, a heat lamp was provided for each pen and the height of the lamps adjusted according to the pigs’ comfort level (pigs sleep comfortably on their sides under the heat lamp with their abdomens exposed).
After entry to the animal care room the piglets were fed a liquid diet (Table I) hourly for the first 6 h, every 2 h from 6 to 24 h of age, and 4 times per day (8 am, 12 am, 4 pm, and 10 pm) thereafter until weaning. The pigs were initially bottled-fed, and the liquid diet was also provided in a liquid feeder (product BPW4; Miller Manufacturing Company, Eagan, Minnesota, USA) in the pen to encourage drinking from the feeder. Once a pig was observed to be drinking from the feeder, bottle-feeding was discontinued for that pig. All pigs began to drink from the feeder within 48 h of age. The liquid diets contained combinations of the following ingredients: spray-dried bovine colostrum powder containing at least 25% bovine immunoglobulin G (bIgG) (HeadSTART), spray-dried bovine colostrum powder containing at least 14% bIgG (Calf’s Choice Total HiCal), commercial pig milk replacer (WetNurse; Prairie Micro-Tech, Regina, Saskatchewan), spray-dried whole egg powder containing polyclonal antibodies to E. coli K88 (Hyper-Egg K88; J.H. Hare & Associates, Winnipeg, Manitoba), and an oral iron supplement (Enfamil Fer-In-Sol syrup; Mead Johnson & Company, Ottawa, Ontario; 30 mg of elemental iron per 5 mL). The targeted dry matter fed to each pig in the liquid diet gradually increased from 66 g/d on day 1 to 500 g/d on day 20, and the feeding volume increased from 150 to 3300 mL/d over the same period. The exact feeding amount and volume varied somewhat according to appetite. The pigs were weaned on day 24 (in experiment 1) or day 21 (in experiment 2) to a custom starter diet without porcine by-products (Table II); the treatment group in experiment 2 was weaned to a diet that contained bovine colostrum powder.
Table I.
Ingredients in the liquid diets fed until weaning to snatch-farrowed, porcine-colostrum-deprived pigs in experiments 1 and 2
| Experiment 1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Days 1 to 3 | Days 4 to 10 | Days 11 to 15 | Days 16 to 23 | Experiment 2 | ||||||
|
|
|
|
|
|
||||||
| Ingredienta | RPL | COL | RPL | COL | RPL | COL | RPL | COL | Days 1 to 3 | Days 4 to 21 |
| Colostrum A (%, w/v) | 20 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 0 |
| Colostrum (%, w/v) B | 0 | 0 | 15 | 15 | Reduce to 5 | 15 | 5 | 15 | 0 | 15 |
| Milk replacer (%, w/v) | 0 | 0 | 0 | 0 | Increase to 13 | 0 | 13 | 0 | 0 | 0 |
| Iron (mg/kg milk solids) | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 |
| IgY K88 (g/pig/d) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Warm water (L/pig/d) | 0.3–0.53 | 0.67–1.67 | 1.84–2.5 | 2.67–3.33 | 0.3–0.53 | 0.67–3.33 | ||||
Colostrum A — HeadSTART, The Saskatoon Colostrum Company, Saskatoon, Saskatchewan; Colostrum B — Calf’s Choice Total HiCal, The Saskatoon Colostrum Company; milk replacer — WetNurse, Prairie Micro-Tech, Regina, Saskatchewan; iron — Enfamil Fer-In-Sol syrup (30 mg of elemental iron per 5 mL), Mead Johnson & Company, Ottawa, Ontario; IgY K88 — Hyper-Egg K88; J.H. Hare & Associates, Winnipeg, Manitoba. RPL — Replacement: dietary colostrum was gradually replaced with milk replacer from day 11 until 5% colostrum remained in the diet; COL — Colostrum: the diet consisted mainly of bovine colostrum until day 20.
Table II.
Ingredients and nutrient levels in the dry starter diet fed to the pigs after weaning
| Experiment 2 | |||
|---|---|---|---|
|
|
|||
| Ingredients | Experiment 1 (%) | STARTER-COL (%) | STARTER-CTRL (%) |
| Wheat | 30.90 | 44.80 | 30.90 |
| Colostrum B | 0 | 20.00 | 0 |
| White fish meal | 8.61 | 0 | 8.61 |
| Oat groat | 15.00 | 15.00 | 15.00 |
| Whey permeate | 20.00 | 11.40 | 20.00 |
| Soybean meal | 15.00 | 3.60 | 15.00 |
| NuProa | 5.00 | 0 | 5.00 |
| Canola oil | 3.00 | 2.00 | 3.00 |
| Limestone | 0.43 | 0.90 | 0.43 |
| Salt | 0.44 | 0.63 | 0.44 |
| Monocalcium phosphate | 0 | 0.63 | 0 |
| Zinc oxide | 0.40 | 0.40 | 0.40 |
| Lysine | 0.47 | 0.36 | 0.47 |
| Starter microbial phytase | 0.20 | 0.20 | 0.20 |
| Choline chloride | 0.08 | 0.08 | 0.08 |
| Methionine | 0.25 | 0.03 | 0.25 |
| Threonine | 0.20 | 0 | 0.20 |
| L-tryptophan | 0.03 | 0 | 0.03 |
| Nutrientsb | |||
| Crude protein | 21.7 | 21.5 | 21.7 |
| Crude fat | 1.5 | 8.4 | 1.5 |
| Crude fibre | 5.6 | 1.6 | 5.6 |
| Digestible energy (Mcal/kg) | 3.69 | 3.76 | 3.69 |
| Net energy (Mcal/kg) | 2.62 | 2.76 | 2.62 |
| Calcium | 0.9 | 0.9 | 0.9 |
| Available phosphorus | 0.8 | 0.7 | 0.8 |
| Sodium | 0.47 | 0.45 | 0.47 |
| Total lysine | 1.58 | 1.64 | 1.58 |
| Total threonine:lysine | 0.64 | 0.77 | 0.64 |
| Total methionine + cystine:lysine | 0.58 | 0.61 | 0.58 |
| Total tryptophan:lysine | 0.17 | 0.20 | 0.17 |
Alltech Canada, Guelph, Ontario.
As-fed basis; estimated 90% dry matter.
STARTER-COL — Diet devoid of all animal by-products except 20% (w/v) colostrum B;
STARTER-CTRL — Same starter diet as used in experiment 1.
No prophylactic parenteral antibiotic treatment was used before the pigs entered the animal care room or before weaning, and no antibiotics were added to the starter feeds. In experiment 1, when a pig’s temperature was greater than 40°C, oxytetracycline (Bio-Mycin 200; Boehringer Ingelheim, Burlington, Ontario) was administered intramuscularly (20 mg/mL) to relieve the symptoms of septicemia.
Experiment 1 aimed to compare the effects of 2 different liquid diet formulations on the health of SF-pCD pigs. The 12 SF-pCD pigs used in this experiment were conveniently placed, in the order they arrived at the facility, into 4 × 6 ft (1.23 × 1.85 m) pens (3 pigs per pen) equipped with a liquid feeder. Unlimited access to water was provided by a nipple drinker. All the pigs were housed in the same room throughout the experiment. The 6 pigs in 2 systematically selected pens (pens 1 and 2) were fed a liquid diet composed mainly of bovine colostrum for the first 10 d, and then the colostrum was gradually replaced with milk replacer (RPL) until 5% (w/v) colostrum was left in the diet; they were designated the RPL group. The 6 pigs in the remaining 2 pens (pens 3 and 4) were fed a liquid diet composed mainly of bovine colostrum (COL) throughout days 1 to 20 and were designated the COL group. The starter diet was introduced to all the pigs on day 20, and the pigs were fully weaned on day 24. Blood samples were drawn from the cranial vena cava of half of the pigs in each group into tubes containing ethylene diamine tetraacetic acid (EDTA) 24 h after the initial colostrum feeding and weekly thereafter. The rectal temperature was measured daily from entry to the animal care room until day 33. The experiment was terminated on day 35, when all the surviving animals were euthanized with intravenous barbiturate and underwent necropsy.
Experiment 2 aimed to evaluate the potential benefits of adding bovine colostrum powder to the dry starter diet fed after weaning. The 12 SF-pCD pigs used in this experiment were housed 2 per pen, conveniently selected. All were kept in the same room throughout the experiment. Before weaning on day 21, all the pigs were fed a liquid diet composed mainly of bovine colostrum. At weaning, the pigs were systematically allocated into 2 groups. The pigs in 3 pens (pens 2, 4, and 6) were fed a dry starter diet devoid of animal by-products except for 20% (w/w) colostrum (Calf’s Choice Total HiCal) and designated the STARTER-COL group. The pigs in the remaining 3 pens (pens 1, 3, and 5) served as the control group and were fed the same starter diet as was used in experiment 1 without additional colostrum; they were designated the STARTER-CTRL group. Blood samples were drawn from the cranial vena cava into EDTA tubes 24 h after the initial colostrum feeding and weekly thereafter. The rectal temperature was measured daily until day 30 and twice weekly thereafter. Half of the pigs in each group were euthanized by intravenous barbiturate on day 42 and the remainder on day 49. Necropsy was done immediately after euthanasia.
Packed cell volume (PCV) and plasma total protein concentration
In both experiments the PCV was determined in the whole blood samples by the capillary tube method, and the plasma total protein concentration was determined by refractometry.
Histopathological examination of tissues
Internal organs were collected at necropsy, preserved in 10% formalin, and processed for histopathological examination. Tissues examined included brain, nasal turbinate, salivary gland, tonsil, thymus, lung, heart, bronchial lymph node, stomach (fundus and pylorus), duodenum, jejunum, ileum, spiral colon, cecum, mesenteric lymph node, adrenal gland, kidney, and superficial inguinal lymph node.
Adjunct tests for porcine pathogens
In both experiments the plasma from blood collected immediately before necropsy was examined for the presence of pathogen-specific antibodies by enzyme-linked immunosorbent assay with commercial kits used according to the manufacturer’s instructions by Prairie Diagnostic Services (PDS), Saskatoon, Saskatchewan. The samples were tested for antibodies to swine influenza virus (SIV) with Swine Influenza Virus Antibody Test Kit H1N1 (part 99-06731) and Swine Influenza Virus Antibody Test Kit H3N2 (part 99-09332) from IDEXX Laboratories, Westbrook, Maine, USA, and for Porcine reproductive and respiratory syndrome virus (PRRSV) with PPRS X3 HerdChek, Porcine Reproductive and Respiratory Virus Antibody Test Kit (part 99-18070), from IDEXX Laboratories. An in-house hemagglutination (HA) inhibition assay adapted from a previously described protocol (8) was used by PDS to test for Porcine parvovirus (PPV) antibody. Briefly, PPV propagated in the laboratory from a diagnostic isolate was used as the HA antigen and was incubated at a concentration of 6 HA units with 2-fold dilutions of serum starting at 1:20. Hemagglutinating activity was assessed with the use of chicken erythrocytes. An antibody test for PCV2 was done with a previously described in-house immunoperoxidase monolayer assay (9). In both experiments polymerase chain reaction (PCR) was used to test for the presence of PCV2 DNA in plasma collected at all time points and in spleen, superficial inguinal lymph node, and tonsil collected at necropsy (10). Additionally, in experiment 2 PCR was used to test for the presence of DNA of Torque teno virus (TTV) genogroups 1 and 2 in bone marrow (11). For pigs that died or were euthanized for humane reasons before the end of the experiment, lung, spleen, and joint or abdominal fluid was cultured for bacteria under both aerobic and anaerobic conditions.
Plasma IgG concentrations
The concentration of bIgG in plasma was determined by radial immunodiffusion (RID) as previously described (12). The plasma half-life of bIgG was calculated with the following formula:
where: T1/2 is the half-life, T the total time period, ConB the beginning bIgG concentration, and ConE the ending bIgG concentration. All plasma collected in experiment 2 was also tested for porcine (p) IgG by RID as previous described (13). The plasma pIgG concentrations were not compared by statistical methods because half of the pigs in each group were euthanized on day 42 and the remainder on day 49, which substantially reduced the statistical power (n = 3).
Statistical analysis
Differences between the groups in frequencies of lesions were compared by Fisher’s exact test. The group difference in plasma bIgG concentration on day 1 was compared by the Mann–Whitney U test. The numbers of fever-days, defined as the total number of days that individual pigs had a body temperature of 39.5°C or greater, were compared between groups by generalized linear models with the use of a Poisson regression and SPSS Predictive Analytics SoftWare Statistics 18 (SPSS, Chicago, Illinois, USA). Probabilities of less than 0.05 were considered statistically significant.
Results
Experiment 1
All the pigs learned to drink from the liquid feeder within 48 h of entry to the animal care room. Four of the six RPL pigs and all the COL pigs survived until the termination of the experiment (on day 35). One RPL pig was observed to have atresia ani on day 2, defecating by way of a rectovaginal fistula. Lethargy, anorexia, and temperature elevation (to 40.4°C) developed on day 16, and the animal was euthanized by intravenous administration of barbiturate. Acute fibrinous polyserositis and renal petechiae were noted grossly. Histopathological changes were consistent with acute septicemia, and E. coli was cultured from lung, spleen, and joint fluid. Lethargy, anorexia, and temperature elevation (to 41.4°C) developed in another RPL pig, on day 22. Gross and histological lesions indicated acute septicemia, and E. coli was cultured from lung, spleen, and abdominal fluid. Both E. coli isolates were not further characterized.
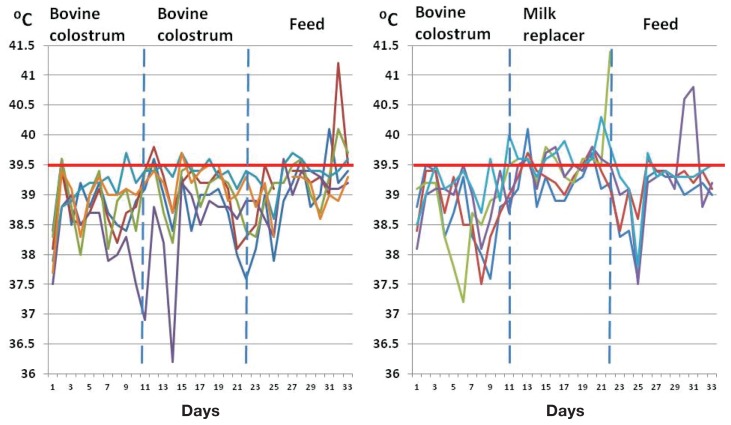
The RPL pigs had significantly more fever-days than the COL pigs from day 11 to day 20 (16 fever-days in 50 total pig-days for the RPL pigs versus 5 fever-days in 54 pig-days for the COL pigs; P = 0.009) but not from day 1 to day 10 or from day 21 to day 33 (P > 0.05) (Figure 1). Two of the 4 RPL pigs and 4 of the 6 COL pigs had mild neutrophilic infiltration in the mesenteric lymph nodes, but the difference was not statistically significant (P > 0.05). No other pathological changes were observed in the pigs that survived until day 35.
Figure 1.
Body temperatures of snatch-farrowed, porcine-colostrum-deprived (SF-pCD) pigs in experiment 1. Between days 11 and 20 of life the pigs fed bovine colostrum as the main component of their diet (left panel) had significantly fewer days of fever (P = 0.009) than the pigs for which the colostrum was gradually replaced with milk replacer from day 11 (right panel) before weaning to a dry feed.
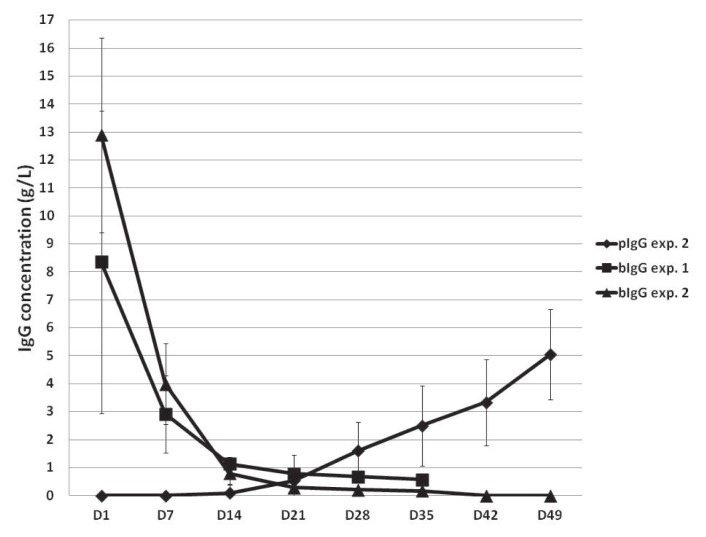
The RPL pigs had marginally but significantly higher plasma concentrations of bIgG at days 1 and 7 (P = 0.0495) than the COL pigs even though the groups had the same diet during this time. The levels were highest 24 h after initial colostrum intake and then decreased rapidly in the first 2 wk of life to negligible levels (Figure 2). The half-life of bIgG in experiment 1 was 9.2 d. The PCV and plasma total protein concentration remained within normal limits throughout the experiment (data not shown).
Figure 2.
Plasma concentrations of bovine and porcine immunoglobulin G (bIgG and pIgG) in the SF-pCD pigs in experiments 1 and 2. Squares indicate mean values for experiment 1. Triangles indicate mean values for bIgG and diamonds mean values for pIgG in experiment 2. The vertical lines represent standard deviations.
At termination, all the pigs were negative for antibodies to SIV (H1N1 and H3N2), PRRSV, and PPV. However, 1 COL pig was positive for PCV2 antibody, and PCV2 DNA was detected by PCR in the plasma from day 21 to day 35, as well as in spleen and superficial inguinal lymph node collected at necropsy. Another COL pig, reared in the same pen, remained negative for PCV2 antibody but had PCV2 DNA detected by PCR in spleen and superficial inguinal lymph node collected at necropsy.
Experiment 2
All the pigs learned to drink from the liquid feeder by 48 h. All pigs in both groups survived until the end of the experiment. The feces of all the STARTER-CTRL pigs were of normal consistency throughout the experiment. Four of the six STARTER-COL pigs had semiliquid diarrhea from day 27 (6 d after weaning) until the end of the experiment, but they remained alert and otherwise healthy. Necropsy of these pigs revealed a dilated colon and cecum that contained unformed feces. The large intestinal mucosa was reddened, and mesocolonic blood vessels were congested. Histologic examination showed typhlocolitis with combinations of the following changes: bacterial attachment on the mucosa, degeneration and necrosis of the epithelial cells, and congestion, edema, and mixed inflammatory cell infiltration of the lamina propria. No adjunct tests were carried out to determine the cause of the typhlocolitis. In addition, the pancreas was firm and had a nodular appearance on gross examination in all the STARTER-COL pigs; microscopic examination showed pancreatic glandular epithelial degeneration and necrosis with regeneration and fibrosis. In the STARTER-CTRL pigs the pancreas was normal macroscopically and microscopically except in 1 pig, which had mild microscopic pancreatic degeneration. Both groups had only 2 fever-days in total.
As in experiment 1, the plasma concentrations of bIgG were highest 1 d after initial colostrum intake, then decreased rapidly in the first 2 wk to negligible levels (Figure 2). The half-life of bIgG in experiment 2 was 5.5 d. Porcine IgG was not detectable in the plasma until day 21 and then gradually increased to 5 g/L by day 49 (Figure 2). The PCV and plasma total protein concentration remained within normal limits through the experiment (data not shown).
All the pigs were negative at the end of experiment 2 for antibodies to SIV (H1N1 and H3N2), PRRSV, PPV, and PCV2. The absence of PCV2 infection was confirmed by PCR in plasma and tonsil; however, PCR detected TTV1 DNA in 1 STARTER-COL pig and 4 STARTER-CTRL pigs, as well as TTV2 DNA in 1 of the latter 4 pigs.
Discussion
In the current study, neonatal SF-pCD pigs were successfully raised on a bovine-colostrum-based liquid diet, all remaining serologically negative for SIV, PRRSV, and PPV. Moreover, all but 1 pig remained serologically negative for PCV2, and only 2 pigs (in experiment 1) had detectable PCV2 DNA in serum or tissues. In both experiments, all the pigs fed mainly bovine colostrum before weaning survived until termination of the experiment.
The bovine colostrum was likely the main factor contributing to the high survival rate. It is well-known that the epitheliochorial placentation in pigs and other farm mammals prevents macromolecule transportation from dam to fetus. Thus, pigs are born hypogam-maglobulinemic, with only trace amounts of immunoglobulin in the blood. As a result, neonatal pigs have a low survival rate when neither sow colostrum nor immunologic supplements derived from other sources are fed, in spite of intensive treatment with antibiotics (14). In other studies using bovine colostrum in neonatal pigs, colostrum was fed for only a few days (5,6,14), and the survival rate was at most 80% (6,14). In the present experiments, the use of commercially available spray-dried bovine colostrum powder allowed more protracted use of the colostrum and enables the SF-pCD technique to be easily adopted by others. It is clear from our results that providing bovine colostrum to pigs after gut closure provides health benefits up to weaning at 3 wk of age. Although immunoglobulins cannot be absorbed in a substantial quantity after gut closure, and the plasma half-life of bIgG in these experiments was shorter than that of pIgG (at least 12 to 14 d) (15), they and the other antimicrobial substances present in colostrum may provide local immunity in the gastrointestinal tract.
It is noteworthy, however, that bovine colostrum fed after weaning to the SF-pCD pigs, in experiment 2, was associated with persistent diarrhea, typhlocolitis, and pancreatic degeneration. The mechanism for this phenomenon was not determined. The level of fat in the diet supplemented with colostrum was 8.4% compared with 1.5% in the diet without the supplementation (Table II). The additional colostrum powder in the diet after weaning, without consideration of the fat levels in the diet, may exceed the capacity of the pancreatic enzymes and cause maldigestion and diarrhea (likely osmotic). Irrespective of the reason, the finding of no benefit to supplementation with bovine colostrum after weaning substantially reduces the cost of raising these pigs.
The normal PCV of the SF-pCD pigs indicates that oral iron supplementation was sufficient to prevent anemia. The industry-standard practice of administering iron dextran parenterally was not used in this study since there was a concern that any injection would increase the risk of septicemia. For the same reason, only half of the pigs were bled in experiment 1. However, in experiment 2, when all the pigs were bled weekly, no septicemia occurred in any pig. Thus, injections and blood collection can be done in SF-pCD pigs without health compromise, provided the procedures are conducted hygienically. Indeed, in a subsequent SF-pCD experiment in the same laboratory, iron dextran was administered intramuscularly in the neck without ill effects (data not shown).
The roles of polyclonal antibodies to E. coli K88, which reportedly prevented diarrhea due to K88 (F4) E. coli(16), were not characterized in this study. This component was included in the diet to help avoid potential illness due to K88 E. coli because F4 colibacillosis is a common disease of young pigs in the commercial swine industry, and vaccination of dams before farrowing is routine.
Pyrexia was rare in experiment 2, in contrast to experiment 1. This may be due to the higher level of sanitation during experiment 2, as the concrete under the plastic floor was cleaned more frequently (daily) than in experiment 1 (2 to 3 times weekly), discouraging the accumulation of fecal microorganisms in the environment. The benefit of high sanitation is further emphasized by the fact that when SF-pCD pigs were raised on a concrete floor bedded with straw, diarrhea and septicemia due to E. coli developed in 4 of 9 pigs (5). Thus, good hygiene practices are strongly recommended for the health of SF-pCD pigs.
The results of this experiment demonstrate that it is possible to raise SF-pCD pigs that remain free of porcine pathogens, including SIV, PRRSV, PPV, and PCV2. The source farm was serologically free of PRRSV but positive for SIV, PPV, and PCV2. Snatch-farrowing, preventing contact with barn facilities, minimizing exposure to barn air, and disinfecting the sows and piglets are likely all critical in preventing horizontal transmission of these pathogens to SF-pCD pigs. Although the source of PCV2 infection in 2 pigs in experiment 1 was not definitively identified, retrospective evaluation of the biosecurity protocols guiding entry into the animal care room and the timing of the infections implicated a bottle of antibiotic previously used on a PCV2-positive farm. In experiment 1 all the pigs remained PCV2-negative before day 21, which indicates that vertical transmission of PCV2 was unlikely. After the biosecurity measures were improved, all the pigs in experiment 2 remained PCV2-negative. Thus, it is possible, although not guaranteed, that PCV2-negative pigs can be obtained from a PCV2-positive farm using the SF-pCD method, provided the piglets are not infected prenatally. A recent study showed that 39.9% of the piglets on 5 North American farms were born PCV2-viremic (17). Although in that study most of the piglets were born of gilts, which may be more likely to vertically infect their progeny in utero, the putatively high rate of viremia at birth on some farms highlights that PCV2-free status is not guaranteed. We recommend excluding PCV2-viremic sows to increase the probability of obtaining PCV2-free piglets.
Similarly, the presence of TTV1 and TTV2 in some of the SF-pCD pigs in the present study was not unexpected since vertical transmission of TTV had been reported (18,19). Even gnotobiotic derivation cannot guarantee freedom from infection with TTV or other vertically transmitted pathogens.
The SF-pCD method has advantages over the conventional SPF method in obtaining pathogen-free pigs and is less technically demanding than CDCD and gnotobiotic models. Since conventional SPF pigs have suckled their dams and remain on the farm before their inclusion in an experiment, there is a high risk of exposure and possibly infection with organisms circulating on the farm. Moreover, the presence of maternally derived antibodies against endemic pathogens means that SPF piglets cannot be used for experiments until passive immunity has decayed. Unlike the CDCD and gnotobiotic methods, SF-pCD derivation requires neither surgery nor sterile compartments. Thus, the technical requirements are low and easily adapted to any laboratory. The natural birth of SF-pCD pigs allows them to be exposed to the vaginal flora and the potential for infection by the vaginal microbiota and any pathogens present. The technique also avoids the need to sacrifice the donor sow after surgery.
In conclusion, this study established a method to raise SF-pCD pigs with high health and survival that balances convenience, freedom from major pathogens, animal welfare, cost, and pathogen susceptibility. The SF-pCD model is a good alternative to current methods of producing pigs for infectious disease research. The main disadvantages are the intensive labor associated with bottle-feeding for the first 48 h, the risk of contamination by vaginal microbiota, and the risk of septicemia if the environment is not hygienic.
Acknowledgments
This research was made possible with a contribution from Saskatchewan Agriculture and Food through the Agriculture Development Fund. The bovine colostrum powder and the bIgG assay kits were generously donated by The Saskatoon Colostrum Company, Saskatoon, Saskatchewan. The Hyper-Egg K88 was donated by J.H. Hare & Associates, Winnipeg, Manitoba. The authors appreciated the help of diagnosticians at Prairie Diagnostic Services, Saskatoon, and Dr. Malachy Young, of Gowan’s Feed Consulting, Wainwright, Alberta, for formulating the starter diets. Daniel Petri’s expertise in raising colostrum-deprived pigs and the assistance of Crissie Auckland, Shrijana Dhakal, Rayna Gundvalsen, Blake Balog, Atul Desai, and the staff at the Animal Care Unit of Western College of Veterinary Medicine, University of Saskatchewan, Saskatoon, was critical to the success of this research.
References
- 1.Struve R. A source of CDCD swine for research. Proc Am Assoc Swine Vet Conf. 1999;117 [Google Scholar]
- 2.Sangild PT, Holtug K, Diernaes L, Schmidt M, Skadhauge E. Birth and prematurity influence intestinal function in the new-born pig. Comp Biochem Physiol A Physiol. 1997;118:359–361. doi: 10.1016/s0300-9629(96)00319-2. [DOI] [PubMed] [Google Scholar]
- 3.Fowden AL, Li J, Forhead AJ. Glucocorticoids and the preparation for life after birth: Are there long-term consequences of the life insurance? Proc Nutr Soc. 1998;57:113–122. doi: 10.1079/pns19980017. [DOI] [PubMed] [Google Scholar]
- 4.Butler JE, Weber P, Sinkora M, et al. Antibody repertoire development in fetal and neonatal piglets. VIII. Colonization is required for newborn piglets to make serum antibodies to T-dependent and type 2 T-independent antigens. J Immunol. 2002;169:6822–6830. doi: 10.4049/jimmunol.169.12.6822. [DOI] [PubMed] [Google Scholar]
- 5.Oliveira S, Galina L, Blanco I, Canals A, Pijoan C. Naturally-farrowed, artificially-reared pigs as an alternative model for experimental infection by Haemophilus parasuis. Can J Vet Res. 2003;67:146–150. [PMC free article] [PubMed] [Google Scholar]
- 6.Blanco I, Galina-Pantoja L, Oliveira S, Pijoan C, Sanchez C, Canals A. Comparison between Haemophilus parasuis infection in colostrum-deprived and sow-reared piglets. Vet Microbiol. 2004;103:21–27. doi: 10.1016/j.vetmic.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Olfert ED, Cross BM, McWilliam AA, editors. Guide to the Care and Use of Experimental Animals. 2nd ed. Vol. 1. Ottawa, Ontario: Canadian Council on Animal Care; 1993. Available at www.ccac.ca/Documents/Standards/Guidelines/Experimental_Animals_Vol1.pdf. [Google Scholar]
- 8.Carmichael LE, Joubert JC, Pollock RV. Hemagglutination by canine parvovirus: Serologic studies and diagnostic applications. Am J Vet Res. 1980;41:784–791. [PubMed] [Google Scholar]
- 9.McNair I, Marshall M, McNeilly F, et al. Interlaboratory testing of porcine sera for antibodies to porcine circovirus type 2. J Vet Diagn Invest. 2004;16:164–166. doi: 10.1177/104063870401600214. [DOI] [PubMed] [Google Scholar]
- 10.McIntosh KA, Tumber A, Harding JCS, Krakowka S, Ellis JA, Hill JE. Development and validation of a SYBR green real-time PCR for the quantification of porcine circovirus type 2 in serum, buffy coat, feces, and multiple tissues. Vet Microbiol. 2009;133:23–33. doi: 10.1016/j.vetmic.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Kekarainen T, Sibila M, Segalés J. Prevalence of swine Torque teno virus in post-weaning multisystemic wasting syndrome (PMWS)-affected and non-PMWS-affected pigs in Spain. J Gen Virol. 2006;87:833–837. doi: 10.1099/vir.0.81586-0. [DOI] [PubMed] [Google Scholar]
- 12.Chelack BJ, Morley PS, Haines DM. Evaluation of methods for dehydration of bovine colostrum for total replacement of normal colostrum in calves. Can Vet J. 1993;34:407–412. [PMC free article] [PubMed] [Google Scholar]
- 13.Olson GL, Robine L, Rosengren LB, et al. Parturition induction 2 days prior to term decreases birth weight, lactational growth but not piglet maturity, health or post-weaning growth. Can J Anim Sci. 2009;89:219–228. [Google Scholar]
- 14.Gomez GG, Phillips O, Goforth RA. Effect of immunoglobulin source on survival, growth, and hematological and immunological variables in pigs. J Anim Sci. 1998;76:1–7. doi: 10.2527/1998.7611. [DOI] [PubMed] [Google Scholar]
- 15.Rooke JA, Carranca C, Bland IM, et al. Relationships between passive absorption of immunoglobulin G by the piglet and plasma concentrations of immunoglobulin G at weaning. Livest Prod Sci. 2003;81:223–234. [Google Scholar]
- 16.Marquardt RR, Jin LZ, Kim JW, Fang L, Frohlich AA, Baidoo SK. Passive protective effect of egg yolk antibodies against enterotoxigenic Escherichia coli K88+ infection in neonatal and early weaned piglets. FEMS Immunol Med Microbiol. 1999;23:283–288. doi: 10.1111/j.1574-695X.1999.tb01249.x. [DOI] [PubMed] [Google Scholar]
- 17.Shen H, Wang C, Madson DM, Opriessnig T. High prevalence of porcine circovirus viremia in newborn piglets in five clinically normal swine breeding herds in North America. Prev Vet Med. 2010;97:228–236. doi: 10.1016/j.prevetmed.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Guino L, Kekarainen T, Segalés J. Evidence of Torque teno virus (TTV) vertical transmission in swine. Theriogenology. 2009;71:1390–1395. doi: 10.1016/j.theriogenology.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Pozzuto T, Mueller B, Meehan B, et al. In utero transmission of porcine torque teno viruses. Vet Microbiol. 2009;137:375–379. doi: 10.1016/j.vetmic.2009.02.001. [DOI] [PubMed] [Google Scholar]