Abstract
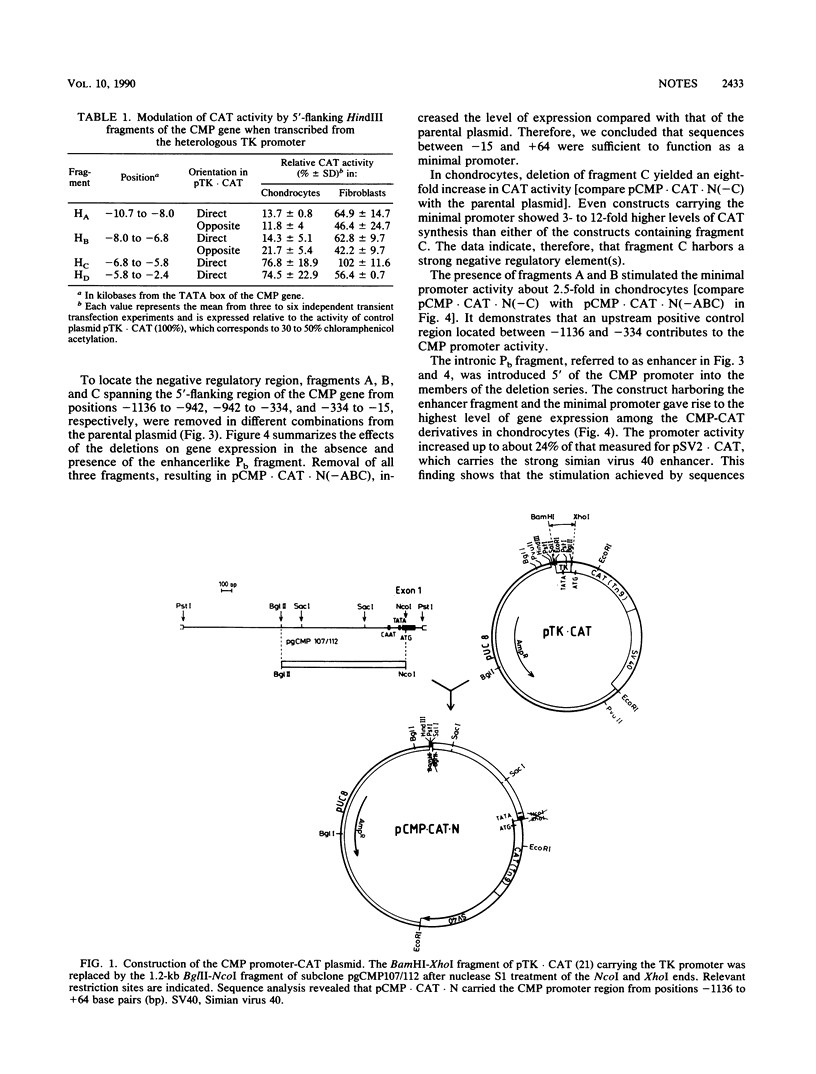
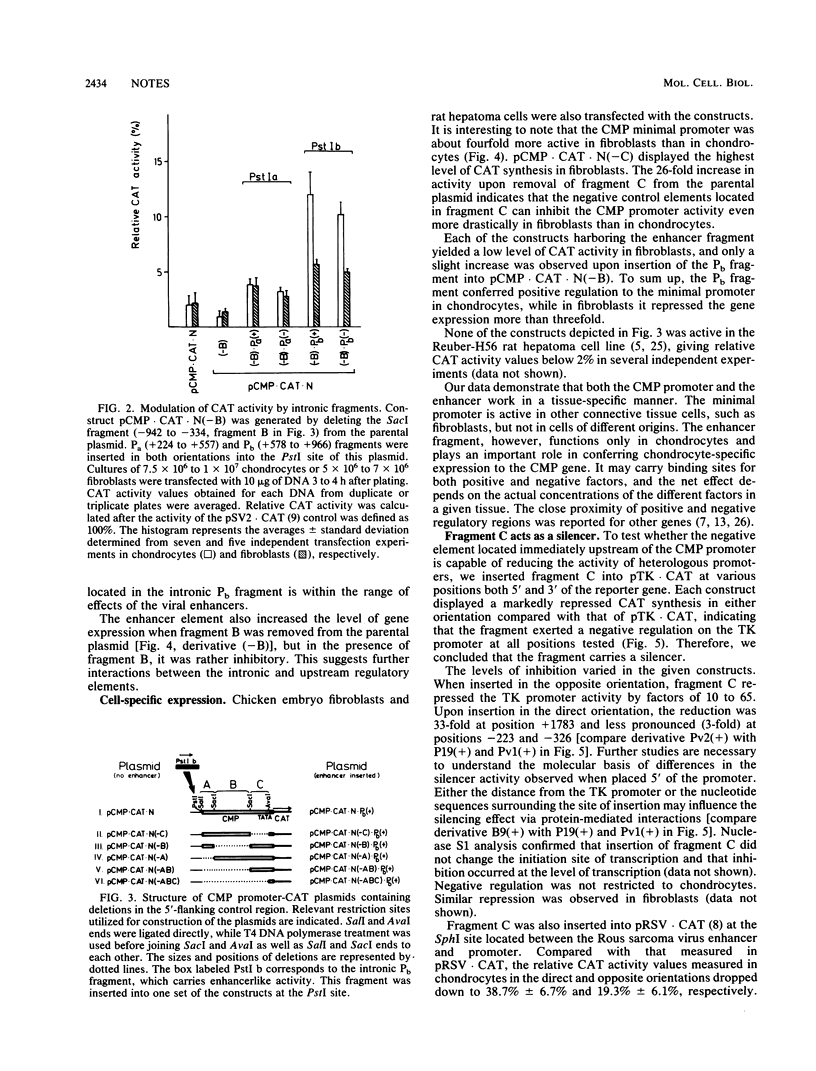
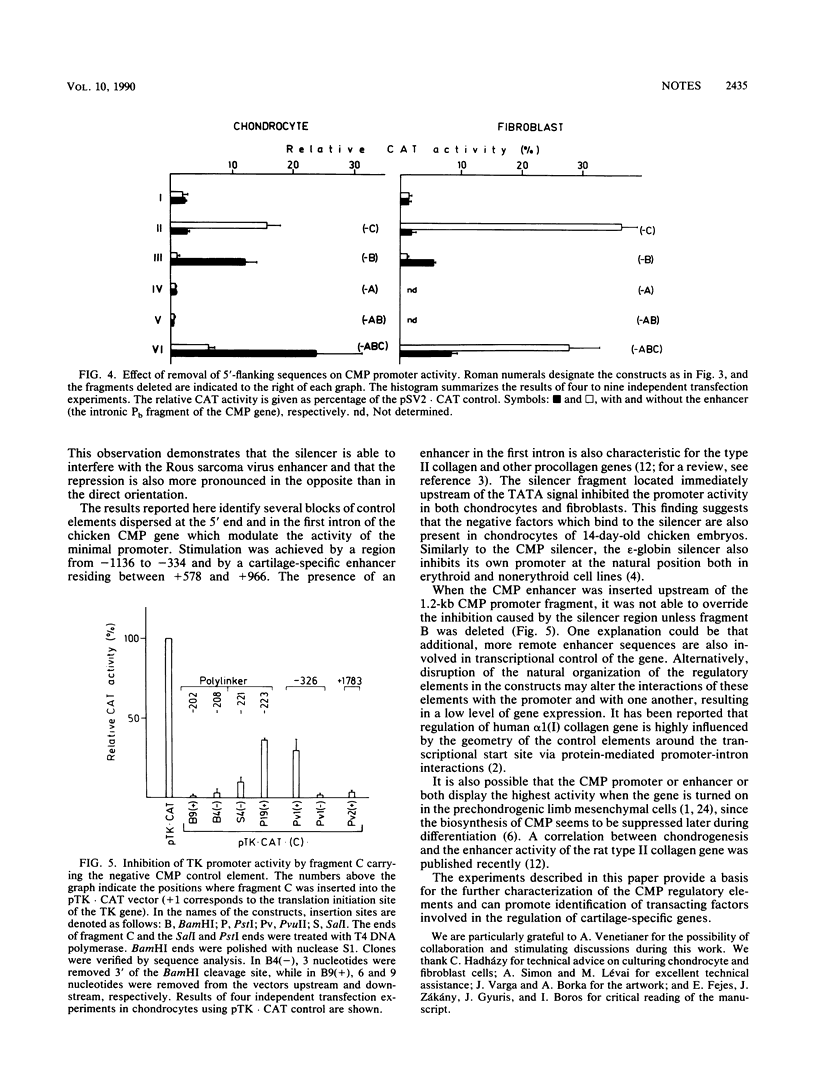
A complex pattern of regulation of the cartilage matrix protein gene was revealed by transient expression experiments. A minimal promoter from positions -15 to +64 functioned in chondrocytes and fibroblasts. An enhancer located in the first intron exerted chondrocyte-specific stimulation on the minimal promoter activity. The same fragment, however, had a negative effect in fibroblasts. Between -334 and -15, a silencer was found which inhibited the gene expression driven from its homologous as well as heterologous promoters both in chondrocytes and fibroblasts. Additional positive and negative control regions were mapped further upstream of the promoter.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argraves W. S., Deák F., Sparks K. J., Kiss I., Goetinck P. F. Structural features of cartilage matrix protein deduced from cDNA. Proc Natl Acad Sci U S A. 1987 Jan;84(2):464–468. doi: 10.1073/pnas.84.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., McKay J., Liska D. J., Apone S., Devarayalu S. Interactions between the promoter and first intron are involved in transcriptional control of alpha 1(I) collagen gene expression. Mol Cell Biol. 1988 Nov;8(11):4851–4857. doi: 10.1128/mcb.8.11.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., Sage H. Regulation of collagen gene expression. Prog Nucleic Acid Res Mol Biol. 1989;37:67–106. doi: 10.1016/s0079-6603(08)60695-9. [DOI] [PubMed] [Google Scholar]
- Cao S. X., Gutman P. D., Dave H. P., Schechter A. N. Identification of a transcriptional silencer in the 5'-flanking region of the human epsilon-globin gene. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5306–5309. doi: 10.1073/pnas.86.14.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschatrette J., Weiss M. C. Characterization of differentiated and dedifferentiated clones from a rat hepatoma. Biochimie. 1974;56(11-12):1603–1611. doi: 10.1016/s0300-9084(75)80286-0. [DOI] [PubMed] [Google Scholar]
- Franzen A., Heinegard D., Solursh M. Evidence for sequential appearance of cartilage matrix proteins in developing mouse limbs and in cultures of mouse mesenchymal cells. Differentiation. 1987;36(3):199–210. doi: 10.1111/j.1432-0436.1987.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Goodbourn S., Burstein H., Maniatis T. The human beta-interferon gene enhancer is under negative control. Cell. 1986 May 23;45(4):601–610. doi: 10.1016/0092-8674(86)90292-8. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Horton W., Miyashita T., Kohno K., Hassell J. R., Yamada Y. Identification of a phenotype-specific enhancer in the first intron of the rat collagen II gene. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8864–8868. doi: 10.1073/pnas.84.24.8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadesch T., Zervos P., Ruezinsky D. Functional analysis of the murine IgH enhancer: evidence for negative control of cell-type specificity. Nucleic Acids Res. 1986 Oct 24;14(20):8209–8221. doi: 10.1093/nar/14.20.8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Gruss P. Enhancer elements. Cell. 1983 Jun;33(2):313–314. doi: 10.1016/0092-8674(83)90410-5. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. K., Larson R. S., Corbi A. L., Dustin M. L., Staunton D. E., Springer T. A. The leukocyte integrins. Adv Immunol. 1989;46:149–182. doi: 10.1016/s0065-2776(08)60653-7. [DOI] [PubMed] [Google Scholar]
- Kiss I., Deák F., Holloway R. G., Jr, Delius H., Mebust K. A., Frimberger E., Argraves W. S., Tsonis P. A., Winterbottom N., Goetinck P. F. Structure of the gene for cartilage matrix protein, a modular protein of the extracellular matrix. Exon/intron organization, unusual splice sites, and relation to alpha chains of beta 2 integrins, von Willebrand factor, complement factors B and C2, and epidermal growth factor. J Biol Chem. 1989 May 15;264(14):8126–8134. [PubMed] [Google Scholar]
- Kiss I., Deák F., Mestrić S., Delius H., Soos J., Dékány K., Argraves W. S., Sparks K. J., Goetinck P. F. Structure of the chicken link protein gene: exons correlate with the protein domains. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6399–6403. doi: 10.1073/pnas.84.18.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosher R. A., Gay S. W., Kamanitz J. R., Kulyk W. M., Rodgers B. J., Sai S., Tanaka T., Tanzer M. L. Cartilage proteoglycan core protein gene expression during limb cartilage differentiation. Dev Biol. 1986 Nov;118(1):112–117. doi: 10.1016/0012-1606(86)90078-3. [DOI] [PubMed] [Google Scholar]
- Lovell-Badge R. H., Bygrave A., Bradley A., Robertson E., Tilly R., Cheah K. S. Tissue-specific expression of the human type II collagen gene in mice. Proc Natl Acad Sci U S A. 1987 May;84(9):2803–2807. doi: 10.1073/pnas.84.9.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciw P. A., Bishop J. M., Varmus H. E., Capecchi M. R. Location and function of retroviral and SV40 sequences that enhance biochemical transformation after microinjection of DNA. Cell. 1983 Jul;33(3):705–716. doi: 10.1016/0092-8674(83)90013-2. [DOI] [PubMed] [Google Scholar]
- Miksicek R., Heber A., Schmid W., Danesch U., Posseckert G., Beato M., Schütz G. Glucocorticoid responsiveness of the transcriptional enhancer of Moloney murine sarcoma virus. Cell. 1986 Jul 18;46(2):283–290. doi: 10.1016/0092-8674(86)90745-2. [DOI] [PubMed] [Google Scholar]
- Paulsson M., Heinegård D. Matrix proteins bound to associatively prepared proteoglycans from bovine cartilage. Biochem J. 1979 Dec 1;183(3):539–545. doi: 10.1042/bj1830539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R. Amino acid sequence of the murine Mac-1 alpha chain reveals homology with the integrin family and an additional domain related to von Willebrand factor. EMBO J. 1988 May;7(5):1371–1378. doi: 10.1002/j.1460-2075.1988.tb02953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirpe N. S., Goetinck P. F. Gene regulation during cartilage differentiation: temporal and spatial expression of link protein and cartilage matrix protein in the developing limb. Development. 1989 Sep;107(1):23–33. doi: 10.1242/dev.107.1.23. [DOI] [PubMed] [Google Scholar]
- Venetianer A., Pintér Z., Gál A. Examination of glucocorticoid sensitivity and receptor content of hepatoma cell lines. Cytogenet Cell Genet. 1980;28(4):280–283. doi: 10.1159/000131541. [DOI] [PubMed] [Google Scholar]
- Wasylyk C., Wasylyk B. The immunoglobulin heavy-chain B-lymphocyte enhancer efficiently stimulates transcription in non-lymphoid cells. EMBO J. 1986 Mar;5(3):553–560. doi: 10.1002/j.1460-2075.1986.tb04246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



