Abstract
Backgrounds and Aims
A current challenge in coevolutionary biology is to understand how suites of traits vary as coevolving lineages diverge. Floral scent is often a complex, variable trait that attracts a suite of generalized pollinators, but may be highly specific in plants specialized on attracting coevolved pollinating floral parasites. In this study, floral scent variation was investigated in four species of woodland stars (Lithophragma spp.) that share the same major pollinator (the moth Greya politella, a floral parasite). Three specific hypotheses were tested: (1) sharing the same specific major pollinator favours conservation of floral scent among close relatives; (2) selection favours ‘private channels’ of rare compounds particularly aimed at the specialist pollinator; or (3) selection from rare, less-specialized co-pollinators mitigates the conservation of floral scent and occurrence of private channels.
Methods
Dynamic headspace sampling and solid-phase microextraction were applied to greenhouse-grown plants from a common garden as well as to field samples from natural populations in a series of experiments aiming to disentangle the genetic and environmental basis of floral scent variation.
Key Results
Striking floral scent divergence was discovered among species. Only one of 69 compounds was shared among all four species. Scent variation was largely genetically based, because it was consistent across field and greenhouse treatments, and was not affected by visits from the pollinating floral parasite.
Conclusions
The strong divergence in floral scents among Lithophragma species contrasts with the pattern of conserved floral scent composition found in other plant genera involved in mutualisms with pollinating floral parasites. Unlike some of these other obligate pollination mutualisms, Lithophragma plants in some populations are occasionally visited by generalist pollinators from other insect taxa. This additional complexity may contribute to the diversification in floral scent found among the Lithophragma species pollinated by Greya moths.
Keywords: Lithophragma affine, L. cymbalaria, L. heterophyllum, L. parviflorum, Saxifragaceae, Prodoxidae, coevolution, obligate mutualism, pollinating floral parasite, private channel, phenotypic plasticity, plant–insect interactions
INTRODUCTION
As species coevolve, local populations diverge through adaptation and speciation. The descendent populations and species often differ in multiple traits important to a coevolving interaction (Godsoe et al., 2008), even as whole classes of other traits remain unchanged among the diverging lineages (e.g. Godsoe et al., 2008; Svensson et al., 2011) or are moulded by selection pressures beyond the coevolutionary interaction. One of the current challenges in coevolutionary biology is to understand how suites of traits, rather than individual traits, vary as coevolving lineages diverge and how the process of divergence is related to the coevolutionary interaction (Thompson, 2009).
Studies of the chemical interactions between plants and insects have been particularly useful in showing the breadth of traits that may be involved in the evolution of interactions. Many of the thousands of chemicals produced by plants are thought to have evolved as defences against insects, microbes, or a combination of these enemies (Pellmyr and Thien, 1986). These compounds, however, can act as defences, repellents or attractants (e.g. Irwin et al., 2004; Theis, 2006; Proffit et al., 2007; Theis and Adler, 2012) as plants evolve suites of chemicals that attract mutualistic taxa such as pollinators while also repelling others (Junker and Blüthgen, 2010). Whether as defences or attractants, the chemical compounds affecting interactions with other species usually occur as chemical cocktails derived from multiple chemical pathways rather than as isolated chemicals (Jones et al., 1991; Dudareva and Pichersky, 2000). Proliferation of these compounds has contributed to specialization and diversification on both sides of these interactions (e.g. Ehrlich and Raven, 1964; Thompson, 1989; Berenbaum and Zangerl, 1998, 2006).
The interactions between plants and pollinating floral parasites provide a particularly intriguing problem for the evolution of chemical traits in plants. These insects lay their eggs in the same flowers they pollinate, which means that their relationship to the plants has both antagonistic and mutualistic components (Bronstein et al., 2009). Mutualistic coevolving interactions between plants and insects often favour the evolution of networks of interacting species (Bascompte et al., 2003). Interactions with pollinating floral parasites, however, often favour either pairwise interactions or interactions among small groups of species (Thompson and Pellmyr, 1992; Jürgens et al., 1996; Fleming and Holland, 1998; Smith et al., 2009; Kawakita et al., 2010; Soler et al., 2011). The interplay between antagonism and mutualism sets these interactions apart from more general pollination systems, and the shared fitness advantage of increased pollination for both plants and pollinating floral parasites is likely to select for specialization on both sides of the interaction (Thompson, 1994).
Close interactions with pollinating floral parasites have been suggested to favour the evolution of a highly specific plant–insect communication system, in the form of unique compounds that represent ‘private channels’ for exclusive attraction of specific pollinators (Raguso, 2008; Soler et al., 2010). If so, then plant species pollinated by a single or a few obligate pollinators should include unique signal compounds or blends that diverge strongly from those produced by plants in more generalized pollination systems and, potentially, also from closely related plant species involved in specific interactions with other species of pollinating floral parasites. So far, however, there is limited evidence for the use of private channels in plants attracting these kinds of pollinator. For example, in the plant genus Yucca, which is involved in an obligate interaction with yucca moths, the three allopatric species analysed thus far show very similar floral scent profiles, which include antennally active, unique alcohol and lactone derivatives of the widespread plant volatile (E)-4,8-dimethyl-1,3,7-nonatriene (Svensson et al., 2005, 2006, 2011; G. Svensson, Lund University, Sweden, O. Pellmyr, University of Idaho, USA, and R. A. Raguso, Cornell University, USA, unpubl. res.). Pollinating yucca moths are attracted to yucca floral scent (Svensson et al., 2011), but the specific role of the novel floral volatiles in pollinator attraction awaits further examination.
Alternatively, growing evidence suggests that flower-pollinator specificity might be accomplished by way of specific blends of otherwise generic floral volatiles. Among the species that have been studied within Glochidion, Breynia and Ficus (for which bioassays have demonstrated olfactory attraction of pollinators), most show strong biosynthetic conservatism among related species, and emit floral scent cocktails composed of common floral compounds (Grison et al., 1999; Grison-Pigé et al., 2002; Okamoto et al., 2007; Hossaert-McKey et al., 2010; Svensson et al., 2010; but see Chen et al., 2009). In these cases, floral scent, combined with physical barriers (e.g. size-limiting ostioles in figs), limited visual display and finely tuned temporal dynamics of scent emission may constitute multi-modal ‘floral filters’ whose net result is pollinator-specificity (Raguso, 2008). In other cases, differences in the relative contributions of the different compounds between close relatives, or potential hidden chiral variation in certain compounds, are sufficient to allow the pollinators to discriminate among these species in experimental trials (Okamoto et al, 2007; Hossaert-McKey et al., 2010; Svensson et al., 2010).
Most interactions between plants and pollinating floral parasites include plant species involved in a completely obligate interaction with one or a few related insect species. In other interactions, the plants rely heavily on a pollinating floral parasite but the flowers are also visited, and sometimes pollinated, by other unrelated pollinator taxa (Thompson and Cunningham, 2002; Giménez-Benavides et al., 2007). In this study, we evaluated the diversification of floral scent within and among four different species of the genus Lithophragma (Saxifragaceae). These species are all pollinated by Greya moths in a highly specialized interaction, but plants in some populations are also pollinated by generalist pollinators such as solitary bees or bombyliid flies (Thompson and Pellmyr, 1992; Thompson and Cunningham, 2002). We focused on four populations in which previous work had shown co-pollinators to be rare but occasionally present (Thompson and Fernandez, 2006; Cuautle and Thompson, 2010; Thompson et al., 2010).
Past studies have provided evidence of coevolution in the interaction between Lithophragma plants and prodoxid moths in the genus Greya, and in geographic variation in the ecological outcome of these interactions, the morphology of the plants and the moths, and the behaviour of moths during pollination (Thompson and Cunningham, 2002; Thompson and Fernandez, 2006; Thompson, 2010; Thompson et al., 2010). The flowers, at least in some populations, are scented, suggesting that floral scent may play a role in these interactions. The other plant group pollinated by prodoxid moths, the yuccas, are also scented, and those plants have evolved a highly specialized and relatively invariant floral signal to attract their pollinators (Svensson et al., 2005, 2006, 2011). While the yucca moths are the exclusive pollinators of yuccas, Lithophragma plants in some populations are sometimes visited by pollinators other than Greya moths. Selection on floral scent could therefore be more variable in Lithophragma than in yucca, even though both groups coevolve with prodoxid moth pollinators.
Floral scent has never been analysed in Lithophragma or in any other plant species in the Saxifragaceae (Knudsen et al., 2006). Therefore our first objective was to determine to what extent these plants emit floral scent signals. Thereafter, we evaluated three potential alternative patterns of floral scent variation within and among species that follow from previous studies of floral scent in other taxa. At the one extreme, floral scents may be composed of a small number of compounds as has been found in the scent profiles of yucca species (Svensson et al., 2005, 2006, 2011), and potential differences among species are due to a few unique compounds. Little floral scent variation has been detected at the population and species levels amongst three closely related, allopatric species of yuccas (Svensson et al., 2005, 2006, 2011). At the other extreme, floral scents may be composed of a great diversity of compounds commonly found in many plant species, and differences among species are simply variations on the theme of those common compounds (e.g. Grison-Pigé et al., 2002; Okamoto et al., 2007; Svensson et al., 2010). In between the two extremes, the floral scents may be composed of a moderate to large number of common and unique compounds that are highly divergent among species, thereby reflecting the complex relationships between Lithophragma plants, their locally adapted Greya moth pollinators, and more generalized co-pollinators.
Using both laboratory and field methods, we first describe striking floral scent variation within and between plant lineages, and determine whether these differences are genetically or environmentally based. We thereafter investigate the extent to which floral scent is temporally variable at the single flower level and also at the whole plant level as the plant ages, and we test whether visits by the pollinating floral parasite Greya politella alter floral scent production.
MATERIALS AND METHODS
Study system
The plant genus Lithophragma is endemic to the western US, and includes ten recognized taxa at the species or subspecies level (Taylor, 1965; Soltis et al., 1992; Kuzoff et al., 2001). The species that are involved in tight coevolutionary interactions with Greya moths are distributed over two lineages and are most common in the Pacific states. These two moth-pollinated clades include opposite ends of a continuum of ovary positions expressed in this genus (Kuzoff et al., 2001). The ovary position is almost completely inferior in the L. parviflorum and L. affine clade, whereas in the clade containing L. heterophyllum, L. cymbalaria and L. bolanderi the ovary is largely superior (Soltis et al., 1992; Kuzoff et al., 2001). These differences have the potential to affect where and how floral scent is produced within a flower. Lithophragma affine occurs in southern and western California and L. parviflorum grows in the mountains of California, Oregon, Washington, and Idaho, with some populations extending farther east (Taylor, 1965). The other clade forms a potential ring species around the Central Valley of California, with L. bolanderi in the Sierra Nevada, L. cymbalaria in the Transverse Ranges and southern Coast Ranges, and L. heterophyllum in the northern Coast Ranges.
The prodoxid moth species Greya politella co-occurs with one or several Lithophragma species throughout its range (Rich et al., 2008) and very few populations of the four species included in this study have been found absent of G. politella during decades of field work throughout the latitudinal range of these species in far western North America (J. N. Thompson, pers. obs.). Greya politella is an effective pollinator during oviposition into the floral ovaries, and is the major pollinator of these Lithophragma-clades, with the exception of local populations where the mutualism is sometimes swamped by nectar-foraging co-pollinators (Thompson and Pellmyr, 1992; Pellmyr and Thompson, 1996; Thompson and Cunningham, 2002: Thompson et al., 2010). A second Greya species, G. obscura, can contribute to pollination during nectaring (Thompson et al., 2010) in some Californian populations. Greya obscura is also specialized on Lithophragma plants, but oviposits in non-reproductive tissue. This species is distributed within the geographic range of G. politella (Thompson and Rich, 2011), and almost always occurs in sympatry with G. politella rather than alone.
We analysed floral scent in the four Lithophragma species that are the most common hosts of pollinating Greya moths. These four species include two from the L. parviflorum clade, L. parviflorum and L. affine, and two from the L. heterophyllum clade, L. heterophyllum and L. cymbalaria. We chose populations throughout the latitudinal range of the genus. The L. parviflorum population at Turnbull Wildlife Refuge in Washington (47 24·0'N, 117 34·0'W) occurs near the northern limit of the range for the genus, and long-term studies have shown that this population is engaged in a mutualistic relationship with G. politella in a pairwise interaction (Thompson and Cunningham, 2002; Thompson and Fernandez, 2006). The L. affine and L. heterophyllum populations occur sympatrically in central coastal California at the University of California, Berkeley Reserve at Hastings (36 23·037'N, 121 33·618'W), where both plants are involved in a mutualistic interaction with G. politella (Cuautle and Thompson, 2010, J. N. Thompson, unpubl. res.). The plants at this site are also visited by G. obscura, but the extent to which this species contributes significantly to pollination is not yet clear (Cuautle and Thompson, 2010). Lithophragma cymbalaria occurs in the Transverse Ranges of southern California within the University of California, Santa Barbara Reserve at Sedgwick (34 42·871'N, 120 2·999'W). At this site, the plant species is involved in mutualistic interactions with both G. politella and G. obscura (Thompson et al., 2010).
Plant growth conditions
Seeds were collected from the populations and cultivated in the greenhouse. From these individuals, bulbils (vegetative reproductive root structures) were collected and grown for two or more additional generations to decrease potential lingering maternal effects (cf. Roach and Wulff, 1987). Test plants were grown from bulbils in the greenhouse facilities of University of California, Santa Cruz. Plants of the different populations were placed haphazardly in the greenhouse to create a common garden and spread micro-environmental variation across the experiment. Once plants had developed flowering scapes, they were included in the floral scent collections. For details on plant growth conditions, see the Supplementary Data Methods.
Volatile collection methods
Floral volatiles were collected using either solid-phase microextraction [SPME field sampler 100-μm polydimethylsiloxane; Supelco (Sigma-Aldrich) Bellefonte, PA, USA], or dynamic headspace collections with subsequent hexane elution (see below). In the field, scent was collected using SPME techniques at ambient temperatures (typically between 20 °C and 25 °C depending on site and day). In the laboratory, plant individuals chosen for fragrance analysis were transferred from the greenhouse conditions to room temperature at least half an hour before volatile collection started. For every scent collection, a negative control of ambient air was collected using the same technique and equipment as for the floral sample.
The SPME samples were obtained by enclosing single or several flowers within a borosilicate glass vial of known volume (1·5 mL or 4 mL depending on treatment, see below) and capped with a nylon resin oven bag gasket (Reynolds®, Richmond, VA, USA) kept in place by a twist-tie. Samples were allowed to equilibrate for 30 min before the SPME fibre was inserted into the headspace chamber. The SPME fibre was exposed to the headspace air for 30 min, and thereafter each SPME unit was stored in an oven bag in a refrigerator (laboratory) or in a cooler with ice packs (field) until analysis.
Dynamic headspace collections were collected at room temperature. A known number of living, attached flowers from each individual plant were enclosed in a Reynolds® oven bag (8 cm × 14 cm) together with a Teflon tube scent trap filled with 10 mg of a Tenax® filter. The trap was connected through vinyl tubing via a Cole-Parmer (Vernon Hills, IL, USA) 65-mm direct-reading flow meter to a custom-built air pump (GroTech, Gothenburg, Sweden) maintaining a steady flow of 200 mL air per minute pulled through a small hole in the top of the oven bag, through the headspace surrounding the flowers (or through an empty control oven bag) and into the scent traps. The air flow was continuously monitored by the flow meters. Scent was collected from each sample for 2 h. Afterwards, samples were eluted in 300 µL of GC/MS quality hexane (Chromasolv®; Sigma-Aldrich, St Louis, MO, USA), and concentrated to 50 µL under a constant moderate flow of nitrogen gas (N2). An internal standard of 5 µL of a 0·03 % toluene (1·3 µg) solution in hexane was added to each sample (see Supplementary Data Methods). This procedure allowed us to estimate a standardized emission rate (SEM) to facilitate quantitative comparisons using the method from Svensson et al. (2005). This method is sufficient for quantitative comparisons of samples tested with the same protocol and under the same analytical conditions, but it provides only a rough estimate of the actual quantitative output of volatiles emitted due to differences among compounds in ionization efficiency and fragmentation. Samples were stored at –20 °C until analysis.
Floral scent variation within and among species
We compared field- and greenhouse-collected plants using SPME and dynamic headspace techniques. Two SPME field samples were collected from each population. Each sample included eight flowers (from eight different individuals), which were placed within a 4-mL glass vial. We chose flowers that had both older and younger flowers in bloom on the same scape. When possible, the corresponding SPME laboratory treatment included up to 16 flowers per sample (8–16 flowers depending on plant availability), from as many families as possible, in order to detect any compounds not detected in the field samples. These flowers were later dried at room temperature and weighed on a Mettler Toledo AB204 (Columbus, OH, USA) scale to determine differences in floral mass. Between 10 and 15 greenhouse-grown plant individuals per population were chosen for dynamic headspace collections using the above protocol. See Supplementary Data Table S1 for all sample sizes.
Floral age and the importance of pollination
We evaluated effects of floral age and pollination on floral scent production by collecting scent from 13 greenhouse-grown plants (ten maternal families) of L. cymbalaria from Sedgwick and 12 greenhouse-grown plants (four families) of L. affine from Hastings using the dynamic headspace protocol on day 3, 6 and 9 after the first flower was fully developed on the scape. Before and between runs, plants were kept in a growth chamber (Conviron E-15; Pembina, ND, USA) programmed to mimic the field conditions during the plants' natural flowering period in March (L. cymbalaria, Sedgwick) and early April (L. affine, Hastings) [day 11 h light (230 µmol photons m−2 s−1), 20 °C; dusk (85 µmol m−2 s−1), 15 °C; night 11 h dark, 10 °C; dawn (85 µmol m−2 s−1 for 1 h), 15 °C].
We tested for differences in scent composition and intensity between old and young flowers of the same individual by collecting floral scent from individual flowers of different (relative) ages using SPME. Two flowers each from 12 individuals (12 maternal families) of the greenhouse population of L. cymbalaria from Sedgwick were chosen for analysis. The development of flowers on the racemose inflorescences is indeterminate, so more distal flowers have unfolded more recently than more proximal flowers. Each pair of flowers came from the same scape and were picked based on their relative position on the scape; we included the youngest (most distal, but still fully unfolded) flower, as well as the oldest (most proximal, but still in full bloom) flower in the analysis. Each flower was individually enclosed in 1·5-mL vials for SPME scent collection using the standard protocol described above.
We further explored within-population variation in floral scent and the importance of moth pollination for floral scent emission by collecting volatiles from 80 flowers (80 individuals of L. cymbalaria from the UC Sedgwick reserve). We increased the likelihood of finding pollinated flowers by picking sample flowers from scapes that included at least one fully developed flower that was younger than the focal flower. Each flower was individually enclosed in 1·5-mL glass vials, and scent was collected using SPME under field conditions. After scent collection each flower was transferred to a 1·5-mL Eppendorf tube filled with 68 % ethanol to allow preservation until dissection. Before dissection, flowers were stained with 3 µL brilliant green, which facilitated the detection of Greya moth eggs in the floral ovaries. Pollination by G. politella females while egg-laying is the most important and efficient mode of pollination in this population (Thompson et al., 2010). This means that flowers with moth eggs in the ovary can be considered to have been pollinated, whereas plants without eggs are more likely to include a higher proportion of unfertilized ovules (Thompson et al., 2010).
Floral dissections
We dissected flowers into petals and green parts (calyx + sexual organs) for flowers of each of the four focal populations, and collected scent using SPME. At each collection occasion, flowers from the participating individuals of each species/population were mixed and divided into different 4-mL vials including (a) a positive control treatment of intact flowers, (b) petals, (c) the floral green parts (stamens, carpels and calyx), and negative controls of empty vials. Depending on flower availability, we distributed between eight and 16 flowers into each treatment. Only compounds emitted from the intact flower sample were included in the analysis to avoid drawing conclusions from wound-induced compounds, and other compounds released due to the dissections. The aliphatic compounds (Z)-3-hexen-1-ol and (Z)-3-hexen-1-ol-acetate were excluded from all analyses, because these are known to be wound-induced compounds (Fall et al., 1999; D'Auria et al., 2007), and their presence could thus be a result of the handling of the sample specimens. These compounds were more common in L. parviflorum and L. affine than in L. heterophyllum and L. cymbalaria (see Supplementary Data Table S1).
Gas-chromatography/mass spectrometry (GC/MS) analysis
Both SPME samples and dynamic headspace samples were analysed using GC/MS on a Hewlett-Packard (HP) 5890 gas chromatograph that was connected to an HP 5971 mass spectrometer (electronic ionization). The GC was equipped with an EC WAX polar column (30 m long, 0·25 mm × 0·25 µm film thickness; Grace, Deerfield, IL, USA). Helium was used as carrier gas at a constant velocity of 1 mL min−1. Both SPME samples and dynamic headspace samples were analysed using the same temperature programme, starting with a 3-min hold at 60 °C. Thereafter the oven temperature was increased by 10 °C per minute for 20 min until it reached a maximum temperature of 260 °C, and the programme ended with a 7-min holding period at this temperature. The floral volatile peaks in the resulting chromatograms were manually integrated using the MS manufacturer's software (G1034 Version C.02·00; Hewlett-Packard 1989–1993). Most compounds were identified using co-chromatography with authentic standards, or by verification of MS library suggestions (NIST/Wiley) using Kovats retention index (RI) values obtained from polar wax columns equivalent to the EC-WAX column used in this study (see reference list below Supplementary Data Table S1). The remaining compounds were tentatively identified by the suggestion from the NIST/Wiley libraries or denoted as ‘unknowns’, for which the ten most abundant MS ion fragments are given in Supplementary Data Table S1.
Statistical analysis
We analysed the multivariate data on floral scent composition in PRIMER 6·1.11 by generating Bray–Curtis similarities and subsequently applying multidimensional scaling (MDS) for a graphical representation of the data. Differences between groups were explored using analysis of similarity (ANOSIM; Clarke, 1993), and we determined the average similarities and dissimilarities within and between groups using the SIMPER function (Clarke, 1993). For details on data preparations and transformations see Supplementary Data Methods.
The total hourly emission rate of floral scent per flower (the SEM) was calculated in toluene-equivalents, as described above (see also Svensson et al., 2005). The SEM, and the number of compounds emitted were analysed using the statistical software Statistica 10 (Statsoft, 2011). Data were tested in linear models when it was possible to transform data to meet the assumptions of equal variances between groups. When this was not possible, a non-parametric Kruskal–Wallis ANOVA was applied to analyse the floral scent variation.
Scent emissions from dissected floral tissues, as analysed using SPME, were analysed as qualitative results by determining whether a certain compound was (a) only emitted from the green parts, (b) only emitted from the petals, or (c) emitted from both these tissues. The frequency table of the tissue-specific emission of the four species was analysed with a Fisher exact P-test, and the same test was used in individual contrasts between each dyad of the four species with corrected alpha-values (sequential Bonferroni). Additionally, the floral dissection data were analysed graphically in a direct comparison of the scent emitted from each of the two tissues (green parts and petals). For details about how these were calculated see Supplementary Data Methods.
RESULTS
In total, 69 volatile compounds (molecular masses: 100–222 Da) were detected across all different treatments (Supplementary Data Table S1). These included volatiles from multiple biosynthetic pathways including monoterpenes (plastidic deoxy-xylulose 5-phosphate pathway; Tholl, 2006), sesquiterpenes (cytosolic mevalonate pathway; Tholl, 2006), and aromatics from pathways including both phenylalanine and tryptophan precursors (Pichersky et al., 2006; Maeda and Dudareva, 2012). The aromatics included benzenoid and phenylpropanoid esters, ethers, aldehydes and alcohols, and also nitrogenous aromatics derived from the different amino acids.
The four species differed significantly in their standardized rate of scent emission (ANOVA: species F3,67 = 62·9, P < 0·001, log-transformed), ranging from relatively low values of 14·3 ± 7·35 (ng scent flower−1) h−1 in L. heterophyllum (mean ± s.d.; n = 15) and 31·1 ± 15·9 (ng scent flower−1) h−1 in L. parviflorum (n = 23) to relatively high values of 88·2 ± 34·3 ng (ng scent flower−1) h−1 in L. affine (n = 19) and 131·5 ± 67·4 (ng scent flower−1) h−1 in L. cymbalaria (n = 14). The means of all species differed from each other, except for the comparison between L. affine and L. cymbalaria (all significant P-values <0·001, Tukey's post-hoc test) (Fig. 1A). The differences between species in emission rates could only partly be explained by differences in floral mass; L. parviflorum weighed significantly less than the other three species, which did not differ significantly from each other (ANOVA species F3,35 = 3·87; P = 0·01; see Supplementary Data Fig. S1).
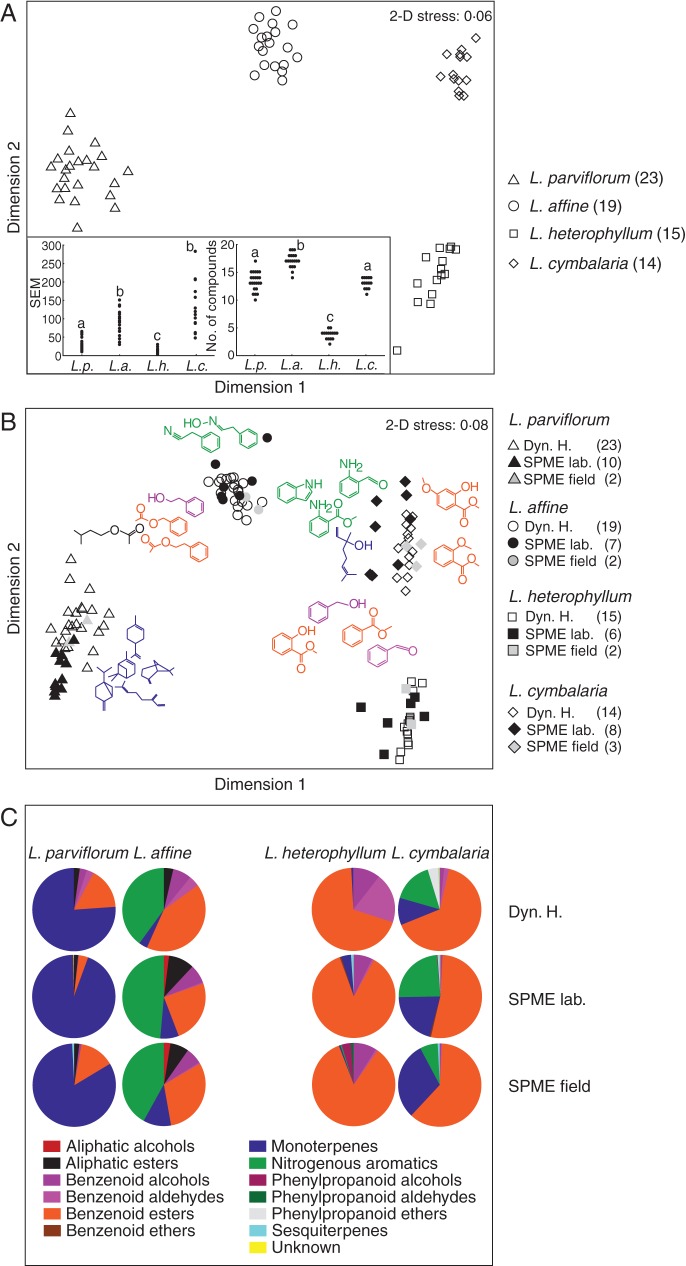
Fig. 1.
(A) Floral scent variation in the four species presented as a two-dimensional MDS plot showing within- and between-species relationships among samples generated through dynamic headspace analysis (square-root transformed data; sample sizes shown in brackets). Insert graphs show the standardized scent emission (SEM) in nanograms scent per flower per hour (estimated from the internal standard; left), and the total number of compounds emitted by the different species (right). Different letters denote significant differences between groups (P < 0·05). (B) Comparison between different techniques (SPME and dynamic headspace) and sample locations (laboratory/field) shown as a MDS plot of proportional data (arcsine-square-root transformed), including the compounds of most importance for scent variation (colour coded by compound group). (C) Pie diagrams showing the relative contribution (untransformed) of different compound groups to the floral scent bouquet of the four species.
The average number of volatile compounds present in the floral cocktail also differed among species (Kruskal–Wallis test: H(3, n = 71) = 58·5 P < 0·001), with L. heterophyllum producing a mean of 3·7 ± 0·8 detectable compounds, L. cymbalaria and L. parviflorum emitting 12 ± 1·0 and 13·3 ± 1·7 compounds, respectively, and L. affine emitting 17·1 ± 1·3 compounds (Fig. 1A). Multiple comparisons between groups revealed highly significant differences between most groups (all P < 0·0025), with the exception of the marginally significant (P = 0·053) contrast between L. cymbalaria and L. heterophyllum, and the non-significant contrast between L. cymbalaria and L. parviflorum (P = 0·84).
The combination of scents also differed significantly among species, indicating that scent variation did not result simply by adding or subtracting compounds from a base composition occurring in all species (ANOSIM: species R = 1; P < 0·01, all individual contrasts significant at the P < 0·01 level). In fact, only one scent compound (methyl salicylate) was present in all four species. The samples clustered tightly within the different species, with no overlap between species in a two-dimensional MDS plot of Bray–Curtis differences (Fig. 1A). Average similarity within groups varied from 71 % (L. cymbalaria and L. parviflorum) to 77 % (L. affine), with L. parviflorum (72 %) in between. Between groups, the most similar species were L. cymbalaria and L. heterophyllum, but the similarity between them was still as low as 32 % (average dissimilarity from the SIMPER test = 68·49 %). Lithophragma cymbalaria and L. parviflorum differed the most, with an average similarity of about 6 % (average dissimilarity = 94·04 %).
The two volatile collection methods produced very similar results, as did the results from SPME collections made in the field and those made on greenhouse-grown plants in the laboratory (Fig. 1B). The dominant compounds were the same across sampling methods and locations (Supplementary Data Table S1 and Fig. 1B, C) and consistently differed between species. However, samples analysed using SPME methods contained more compounds present in trace amounts (Supplementary Data Table S1). Lithophragma parviflorum was largely dominated by α-pinene and other monoterpenes, which together constituted over 75 % of the total volatiles emitted by this species (Fig. 1C and Supplementary Data Table S1). The PHE-derived benzenoid compounds 2-phenylethylacetate, benzyl acetate, methyl salicylate, phenylacetaldehyde and 2-phenylethanol also were common. In contrast, the closely related L. affine was dominated by nitrogenous aromatics such as phenylacetaldoxime, methyl anthranilate and indole, and, secondarily, by benzenoid esters such as 2-phenethylacetate, benzyl acetate and methyl salicylate (Fig. 1B, C and Supplementary Data Table S1).
The other clade showed similar strong divergence between the closely related species. Lithophragma heterophyllum emitted only a few compounds, and methyl salicylate was responsible for 73 % of the total scent emission (Fig. 1C and Supplementary Data Table S1). In L. cymbalaria, the benzenoid esters dimethyl salicylate and methyl salicylate together constituted more than half (54 %) of the total scent emission, and other important compounds were the nitrogenous aromatics 2-aminobenzaldehyde, methyl anthranilate and indole and the monoterpene alcohol linalool (Fig. 1C and Supplementary Data Table S1).
Floral age and the importance of pollination
Both total scent emission (paired t-test t11 = 4·12, P = 0·0017) and the number of compounds emitted (paired t-test t11 = 2·64, P = 0·023) were significantly higher in older flowers than in younger flowers (Fig. 2). These additional compounds of the older flowers were, however, emitted at low concentrations (Supplementary Data Table S1) and did not result in differences in floral scent composition between young and old L. cymbalaria–flowers in the multivariate analysis (ANOSIM (individual nested in age) R = 0·061; P = 0·13; Fig. 2).
Fig. 2.
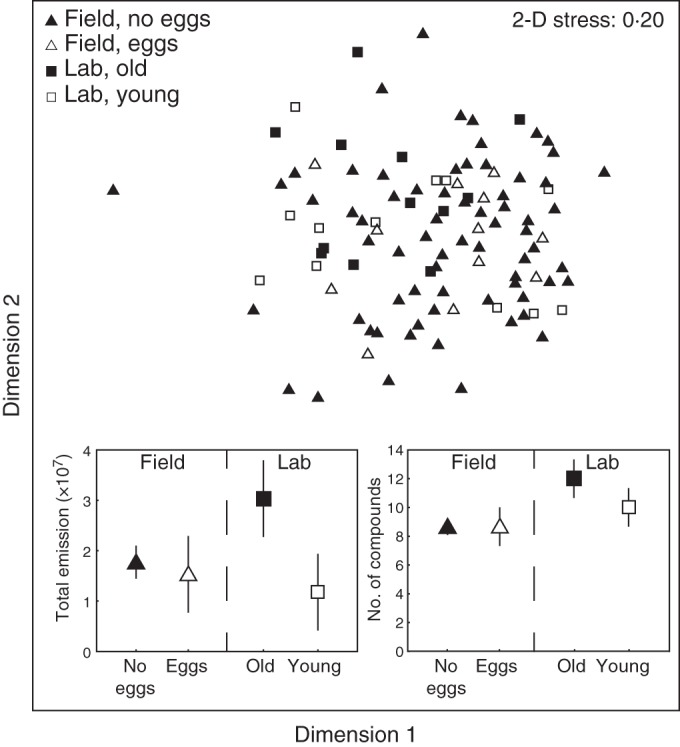
Floral scent variation in the single flower collections of L. cymbalaria including field-collected flowers with and without G. politella eggs, as well as young and old greenhouse-grown plants tested in the laboratory. The main figure shows the multivariate results as an MDS-plot. The insert on the left shows the total scent emission (the average summed peak size measured as total ion abundance in the GC/MS ± 95 % CI), and the insert on the right shows the average number of compounds present in the four groups (±95 % CI).
At the level of the entire plant, the floral scent composition of L. affine and L. cymbalaria did not vary significantly across the lifetime of the plant, either in the full model (ANOSIM: species R = 0·902, P < 0·01; age R = –0·013, P = 0·68; Supplementary Data Fig. S2) or in separate models for each species taking the repeated measures into account [ANOSIM: L. affine age (individual nested in age) n = 12; R = 0·003, P = 0·39; L. cymbalaria; age (individual nested in age) n = 13; R = –0·013, P = 0·61]. Furthermore, there were no significant effects of plant age on overall signalling, either in terms of the standardized scent emission (repeated measure ANOVA: species F1,23 = 2·12, P = 0·16; age F2,46 = 0·68, P = 0·51; age × species F2,46 = 1·78, P = 0·18), or in the number of compounds produced (repeated measure ANOVA: species F1,23 = 2·35, P = 0·14; age F2,46 = 0·26, P = 0·77; age × species F2,46 = 0·24, P = 0·79). Three samples were outliers (see Supplementary Data Fig. S2), and exclusion of these individuals from the statistical analysis did not change the significance patterns (data not shown).
Twelve of the 80 field-collected flowers of L. cymbalaria from the Sedgwick reserve contained G. politella eggs, and could thus be determined as having been pollinated because pollination accompanying oviposition is highly efficient (Pellmyr and Thompson, 1996; Thompson et al., 2010). Plants with and without eggs emitted similar amounts of volatiles (t-test t78 = 0·60, P = 0·60), and also emitted similar average numbers of compounds (t-test t78 = 0·013, P = 99). The overall volatile composition in flowers with eggs did not differ from flowers without eggs (ANOSIM: R = –0·127; P = 0·96), and the volatile composition of these two groups did not differ from the volatile composition of the old and young flowers analysed in the laboratory (ANOSIM: R = 0·012; P = 0·38; P > 0·07 in all individual contrasts; Fig. 2).
Volatile compounds produced by different plant tissues
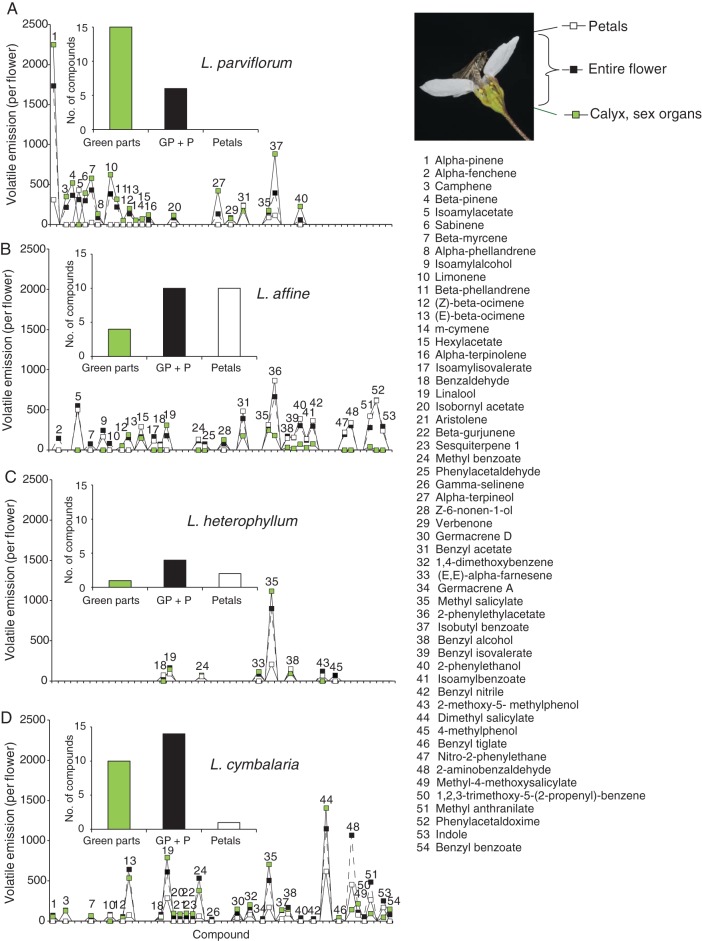
Plant tissues differed in the volatile compounds they produced (Fig. 3). Monoterpenes and sesquiterpenes were produced mostly by the green parts of the flowers, including the calyx and the sexual organs. Nitrogenous aromatics were largely emitted from the petals in L. affine and L. cymbalaria. Benzenoid compounds were emitted at higher levels from the green parts than from the petals, but this quantitative difference may be explained partly by the greater mass of the green parts. In addition, plant species differed in which compounds were produced within particular tissues. The benzenoid esters methyl salicylate and 2-phenethylacetate were more common in the green parts than in the petals in L. parviflorum, whereas the L. affine samples emitted as much or even more of these compounds from the petals (Fig. 3).
Fig. 3.
The relative contribution to the floral scent signal (square-root transformed average ion abundance in the GC/MS) from the petal tissue and the green parts of the flowers (calyx + sex organs) in comparison to the intact plant controls (see illustration) for (A) L. parviflorum (n = 4), (B) L. affine (n = 2), (C) L. heterophyllum (n = 2) and (D) L. cymbalaria (n = 2). Embedded in each figure is also a species-specific frequency diagram showing the number of compounds that are only emitted by the green parts, that are emitted by both the green parts and the petals or that are emitted exclusively from the petals.
The frequencies of compounds produced by green parts only, petals only, or both green parts and petals differed between species (Fisher exact P < 0·001; Fig. 3). The largest differences were found between L. parviflorum and L. affine [Fisher's exact P = 0·000063 (sequential Bonferroni corrected α = 0·0083); Fig. 3]. In these two closely related species, L. parviflorum emitted most compounds from green parts, whereas L. affine emitted most compounds from petals or a combination of petals and green parts. The contrast between L. affine and L. cymbalaria also was significant [Fisher's exact P = 0·0039 (α = 0·01)], and the poorly scented petals of L. parviflorum separated this species also from the two species in the other clade [L. parviflorum–L. cymbalaria, Fisher's exact P = 0·0054 (α = 0·0125); L. parviflorum–L. heterophyllum, Fisher's exact P = 0·0063 (α = 0·017)], whereas the two remaining contrasts were non-significant (L. heterophyllum–L. cymbalaria Fisher's exact P = 0·13; L. affine–L. heterophyllum Fisher's exact P = 0·85; Fig. 3).
DISCUSSION
Floral scent phenotypes in the genus Lithophragma appear to have diversified into a broad spectrum of volatiles that is dominated by common compounds found in many plant species, but also includes a few uncommon compounds (Knudsen et al., 2006). The strong floral scent divergence among closely related Lithophragma species contrasts with the lower variation found within populations (Fig. 1). This consistency of the floral scent signal among field- and greenhouse-collected individuals of the same population suggests that interspecific divergence in floral scent composition is genetically determined rather than a plastic response to the different environments at these sites. The potential importance of phenotypic plasticity is further minimized by the lack of difference in floral scent among L. affine and L. cymbalaria plants of different ages, between older and younger flowers of L. cymbalaria from the same scape, or between field-collected flowers of L. cymbalaria that had or had not been pollinated by G. politella. Egg-induced volatile emission changes have been found in several other plant species and are interpreted as responses to cues of future herbivory (e.g. Hilker and Meiners, 2006; Beyaert et al., 2012), but no such responses could be detected in L. cymbalaria in our field sample.
With so many compounds produced by each species and so little variation among individuals within each species in the emission of those compounds, the unique suite of compounds produced by each population is likely maintained by natural selection. Random genetic drift could also produce divergence in scent composition among populations, but it would be unlikely to result in consistent emission among individuals of such complex floral scents involving many compounds from several different biosynthetic pathways. A floral scent signal tightly controlled by selection is expected from studies of the synthesis of plant volatiles, because these compounds can be costly to produce (metabolic costs; Gershenzon, 1994; Wright and Schiestl, 2009), and are used as cues by both pollinator mutualists and antagonists, such as nectar thieves, seed predators and herbivores (ecological costs; Irwin et al., 2004; Theis, 2006; Proffit et al., 2007; Schiestl et al., 2011; Theis and Adler, 2012).
The variation in floral scent composition among Lithophragma species documented in this study is striking both in comparison with other genera involved in obligate pollination mutualisms (e.g. Grison et al., 1999; Grison-Pigé et al., 2002; Okamoto et al., 2007; Hossaert-McKey et al., 2010; Svensson et al., 2010), and with genera involved in other pollination systems (Levin et al., 2001; Raguso et al., 2003; Füssel et al., 2007; Hentrich et al., 2010). Strong divergence in floral scent among closely related species has been most commonly reported for species pollinated by different pollinator genera, families or orders (e.g. Stuurman et al., 2004; Shuttleworth and Johnson, 2010) rather than for species sharing an obligate pollinating floral parasite (Hossaert-McKey et al., 2010; Soler et al., 2010, Svensson et al., 2010, 2011). All the Lithophragma species reported in this study are pollinated either by the same insect species or by cryptic species embedded within the lineage (Thompson and Cunningham, 2002; Thompson and Fernandez, 2006; Rich et al., 2008; Cuautle and Thompson, 2010; Thompson et al., 2010).
Previous studies on other close interactions between plants and pollinating floral parasite insects have suggested a strong role of selection mediated by obligate coevolutionary interactions in the evolution of floral scents (e.g. Hossaert-McKey et al., 2010; Svensson et al., 2010, 2011). For example, the three yucca species that have been analysed for floral scent all show conserved and similar floral scents, and yucca moths behaviourally respond to the yucca volatile blend (Svensson et al., 2011). However, these species (Y. filamentosa, Y. elata and Y. glauca) are closely related, have allo- or parapatric distributions across southern North America (Pellmyr, 2003), and may not have experienced diversifying selection on floral scent composition as a prezygotic isolation mechanism. Yucca moths also show antennal sensitivity to relatively few compounds in the blend, including oxygenated C11 terpenoids that could constitute private channels for the attraction of yucca moths (Svensson et al., 2005, 2006, 2011).
The relationship between Greya moths and Lithophragma plants constitutes a useful comparison to the yucca–yucca moth interaction, because the Greya genus is closely related to the yucca-pollinating moth genera Tegeticula and Parategeticula (Pellmyr et al., 1996). Unlike in yuccas, the floral scents of Lithophragma species are largely composed of different common floral compounds (Knudsen et al., 2006), and the only compound consistently shared among all four species was methyl salicylate, which is one of the most common floral scent volatiles found among angiosperms (Knudsen et al., 2006) and has many demonstrated functions in chemical ecology (Raguso, 2008). Hence, the variation and divergence of floral scents in Lithophragma differs considerably from the lack of variation in floral scents in the yucca species analysed so far (Svensson et al., 2005, 2006, 2011). The results for Lithophragma therefore do not support the prediction of private channels in the form of unique volatile attractants (Raguso, 2008; Soler et al., 2010).
The lack of private channels, however, may reflect the historical footprint of selection from co-pollinators. In some populations the importance of the Greya moths for pollination can be swamped in some years by visits by more generalized bee and fly co-pollinators (Thompson and Cunningham, 2002; Thompson and Fernandez, 2006). In the populations included in this study, Greya moths are by far the most important pollinators, and the impact of the few visits by alternative pollinators is of much less importance for plant seed set (Thompson and Cunningham, 2002; Thompson and Fernandez, 2006; Cuautle and Thompson, 2010; Thompson et al., 2010). Even so, it is still possible that these perennial plants benefit from also attracting rare non-Greya visitors, both as a means of accomplishing long-distance gene flow (Greya moths are typically short-lived and localized), and as a buffer for years of low G. politella abundance. Under this scenario, natural selection would favour plants that attract Greya and also the local community of other insects that can serve as effective occasional co-pollinators. If so, then these results predict that floral scent should differ between Lithophragma populations that experience different suites of co-pollinators. Given that floral scent is important for host attraction also in the Greya moths, local Greya populations would be predicted to evolve preferences for the scent phenotypes present in the local plant population.
Not all compounds emitted by a flower necessarily contribute to pollinator attraction. A common pattern emerging from physiological and behavioural studies of the pollinating insects suggests that only specific subsets of the compounds in most floral scent cocktails are necessary to attract pollinators. Floral scent profiles often also include compounds that function as repellents and defences. Among the floral compounds detected in the Lithophragma, monoterpenes appear to be particularly unattractive for insect herbivores and facultative flower-visitors (Junker and Blüthgen, 2010). Thus, it is possible that selection from plant antagonists has contributed to the variation in floral scent profile, as there were large differences in the presence and contribution of monoterpenes to the scent cocktails of the four species tested here. Under this hypothesis the L. parviflorum plants should be more exposed to these antagonists than the other species, because the scent signal of L. parviflorum is dominated by monoterpenes. Nonetheless, years of field work in these populations have revealed no other insect herbivores that regularly attack Lithophragma anywhere within the geographic range of this genus (J. N. Thompson, unpubl. res.). It is difficult to determine, though, whether a substantial emission of monoterpenes explains the absence of enemies or whether the evolution of these compounds is unlinked to selection from herbivory. Behavioural and physiological studies of the Greya moth response to the various floral scent bouquets will help to determine which (if any) of the detected compounds are of importance for the mutualistic interaction.
As an alternative to direct selection, the floral scent signal could also evolve as a by-product of selection on other floral traits. The floral morphology of Lithophragma is highly variable, and the ovary position varies from completely superior in L. heterophyllum to completely inferior in L. parviflorum (Kuzoff et al., 2001). If different compounds are produced in different floral structures, and selection is acting divergently on these structures, then variation in floral scent would likely mirror that of floral morphology. The floral dissections performed in this study divided flowers into petals and green parts (calyx and sexual organs) and revealed patterns of tissue-specific volatile production, and variation between species in the tissue most important for scent production. A certain group of compounds was typically produced in the same tissue across species, which means that selection on these tissues could potentially affect floral scent variation. However, at the species and lineage scale adopted in this study, we can conclude that this is likely not the case, since the floral scent variation did not reflect the ovary position or the depth of the flowers.
Another plant with strongly compartmentalized scent production is Silene latifolia, which is pollinated by Hadena moths (Noctuidae) that, like Greya moths, are also floral parasites (Jürgens et al., 1996). In S. latifolia most compounds are emitted from the petals, whereas the lilac aldehyde and lilac alcohol compounds that are highly attractive to the moths are emitted from the anthophores, located near the nectaries at the base of floral tissues (Dötterl and Jürgens, 2005). This implies a role for floral volatiles as nectar or oviposition guides of importance for this specific interaction (Dötterl and Jürgens, 2005). Furthermore, both Silene and Lithophragma plants are visited also by other pollinators, and at large-enough co-pollinator densities the direction of the interaction with the associated pollinating floral parasites can shift from mutualism to antagonism (Thompson and Cunningham, 2002; Kephart et al., 2006; Jolivet and Bernasconi, 2006; Reynolds et al., 2012). These genera also share several similarities in terms of floral scent composition, which in both cases comprises numerous compounds from different biosynthetic pathways (Dötterl and Jürgens, 2005; Dötterl et al., 2005). However, chemical variation thus far documented between different Silene species and different geographically distant populations is much smaller than we show here for Lithophragma (Dötterl et al., 2005; Wälti et al., 2008, 2009).
In conclusion, our study has demonstrated striking patterns of floral scent variation in chemical composition, emission rate, and the volatile source tissue among four species of Lithophragma. This is the first study to report floral scent variation in the family Saxifragaceae, and the study also provides two new insights into our understanding of floral scent variation in plants involved in close mutualistic interactions with pollinating floral parasites. First, it is one of only a few studies that disentangle genetic from plastic components for scent variation (see Majetic et al., 2009) by showing a consistent signal among plants growing in different environments and among plants grown from bulbils for multiple generations. The potential for fast greenhouse cultivation of these small herbs positions Lithophragma as a model system for studies on floral scent in geographic and coevolutionary frameworks. Secondly, the larger variation in floral scent detected in Lithophragma in comparison to other plants involved in close mutualistic relationships with insect pollinators implies a role also for local variation in the generalist pollinator community to affect floral scent evolution. In comparison to other model systems of obligate mutualism, the Lithophragma plants are sometimes visited also by pollinators not involved in the tight coevolutionary interaction. This scenario suggests a role for local floral scent evolution to affect the coevolutionary interaction with the moths and to generate patterns of diversification of traits and species across the geographical range of the interaction.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We are grateful to Kate McCurdy (Sedgwick UC Reserve), Michael Rule (Turnbull National Wildlife Refuge) and Mark Stromberg (Hastings UC Reserve) for their hospitality and assistance, to Jim Velzy (UCSC Greenhouse), Galen Pelzmann, Lindsey Roark and Aliya Ingersoll for help in the laboratory, and Rob Franks at the Marine Analytical Lab, UCSC for invaluable assistance with the GC/MS equipment. This work was supported by the Swedish Research Council; the Fulbright Commission and the Crafoord Foundation to M.F., and from the National Science Foundation to J.N.T. (NSF grant number DEB-0839853) and R.A.R. (NSF grant numbers DEB-0746106 and IOS-0923765).
LITERATURE CITED
- Bascompte J, Jordano P, Melían CJ, Olesen JM. The nested assembly of plant–animal mutualistic networks. Proceedings of the National Academy of Sciences of the USA. 2003;100:9383–9387. doi: 10.1073/pnas.1633576100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum MR, Zangerl AR. Chemical phenotype matching between a plant and its insect herbivore. Proceedings of the National Academy of Sciences of the USA. 1998;95:13743–13748. doi: 10.1073/pnas.95.23.13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum MB, Zangerl AR. Parsnip webworms and host plants at home and abroad: trophic complexity in a geographic mosaic. Ecology. 2006;87:3070–3081. doi: 10.1890/0012-9658(2006)87[3070:pwahpa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Beyaert I, Köpke D, Stiller J, et al. Can insect egg deposition ‘warn’ a plant of future feeding damage by herbivorous larvae? Proceedings of the Royal Society of London B: Biological Sciences. 2012;279:101–108. doi: 10.1098/rspb.2011.0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein JL, Huxman T, Horvath B, Farabee M, Davidowitz G. Reproductive biology of Datura wrightii: the benefits of a herbivorous pollinator. Annals of Botany. 2009;103:1435–1443. doi: 10.1093/aob/mcp053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Song Q, Proffit M, Bessière J-M, Li Z, Hossaert-McKey M. Private channel: a single unusual compound assures specific pollinator attraction in Ficus semicordata. Functional Ecology. 2009;23:941–950. [Google Scholar]
- Clarke KR. Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology. 1993;18:117–143. [Google Scholar]
- Cuautle M, Thompson JN. Diversity of floral visitors to sympatric Lithophragma species differing in floral morphology. Oecologia. 2010;162:71–80. doi: 10.1007/s00442-009-1424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Auria JC, Pichersky E, Schaub A, Hansel A, Gershenzon J. Characterization of a BAHD acyltransferase responsible for producing the green leaf volatile (Z)-3-hexen-1-yl acetate in Arabidopsis thaliana. The Plant Journal. 2007;49:194–207. doi: 10.1111/j.1365-313X.2006.02946.x. [DOI] [PubMed] [Google Scholar]
- Dudareva N, Pichersky E. Biochemical and molecular genetic aspects of floral scents. Plant Physiology. 2000;122:627–634. doi: 10.1104/pp.122.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dötterl S, Jürgens A. Spatial fragrance patterns in flowers of Silene latifolia: lilac compounds as olfactory nectar guides. Plant Systematics and Evolution. 2005;255:99–109. [Google Scholar]
- Dötterl S, Wolfe LM, Jürgens A. Qualitative and quantitative analyses of flower scent in Silene latifolia. Phytochemistry. 2005;66:203–213. doi: 10.1016/j.phytochem.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Ehrlich PR, Raven PH. Butterflies and plants: a study in coevolution. Evolution. 1964;18:586–604. [Google Scholar]
- Fall R, Karl T, Hansel A, Jordan A, Lindinger W. Volatile organic compounds emitted after leaf wounding: on-line analysis by proton-transfer-reaction mass spectrometry. Journal of Geophysical Research. 1999;104:15963–15974. [Google Scholar]
- Fleming TH, Holland JN. The evolution of obligate pollination mutualisms: senita cactus and senita moths. Oecologia. 1998;114:368–375. doi: 10.1007/s004420050459. [DOI] [PubMed] [Google Scholar]
- Füssel U, Dötterl S, Jürgens A, Aas G. Inter and intraspecific variation in floral scent in the genus Salix and its implication for pollination. Journal of Chemical Ecology. 2007;33:749–765. doi: 10.1007/s10886-007-9257-6. [DOI] [PubMed] [Google Scholar]
- Gershenzon J. Metabolic costs of terpenoid accumulation in higher plants. Journal of Chemical Ecology. 1994;20:1281–1328. doi: 10.1007/BF02059810. [DOI] [PubMed] [Google Scholar]
- Giménez-Benavides L, Dötterl S, Jürgens A, Escudero A, Iriondo JM. Generalist diurnal pollination provides greater fitness in a plant with nocturnal pollination syndrome: assessing the effects of a Silene–Hadena interaction. Oikos. 2007;116:1461–1472. [Google Scholar]
- Godsoe W, Yoder JB, Smith CI, Pellmyr O. Coevolution and divergence in the Joshua tree/Yucca moth mutualism. The American Naturalist. 2008;171:816–823. doi: 10.1086/587757. [DOI] [PubMed] [Google Scholar]
- Grison L, Edwards AA, Hossaert-McKey M. Interspecies variation in floral fragrances emitted by tropical Ficus species. Phytochemistry. 1999;52:1293–1299. [Google Scholar]
- Grison-Pigé L, Hossaert-McKey M, Greeff JM, Bessière J-M. Fig volatile compounds: a first comparative study. Phytochemistry. 2002;61:61–71. doi: 10.1016/s0031-9422(02)00213-3. [DOI] [PubMed] [Google Scholar]
- Hentrich H, Kaiser R, Gottsberger G. Floral biology and reproductive isolation by floral scent in three sympatric aroid species in French Guiana. Plant Biology. 2010;12:587–596. doi: 10.1111/j.1438-8677.2009.00256.x. [DOI] [PubMed] [Google Scholar]
- Hilker M, Meiners T. Early herbivore alert: insect eggs induce plant defense. Journal of Chemical Ecology. 2006;32:1379–1397. doi: 10.1007/s10886-006-9057-4. [DOI] [PubMed] [Google Scholar]
- Hossaert-McKey M, Soler C, Schatz B, Proffit M. Floral scents: their roles in nursery pollination mutualisms. Chemoecology. 2010;20:75–88. [Google Scholar]
- Irwin RE, Adler LS, Brody AK. The dual role of floral traits: pollinator attraction and plant defense. Ecology. 2004;85:1503–1511. [Google Scholar]
- Jolivet C, Bernasconi G. Experimental analysis of constitutive and induced defence in a plant–seed–predator system. Functional Ecology. 2006;20:966–972. [Google Scholar]
- Jones CG, Firn RD, Malcolm SB. On the evolution of plant secondary chemical diversity (and discussion) Philosophical Transactions of the Royal Society of London B: Biological Sciences. 1991;333:273–280. [Google Scholar]
- Junker RR, Blüthgen N. Floral scents repel facultative flower visitors, but attract obligate ones. Annals of Botany. 2010;105:777–782. doi: 10.1093/aob/mcq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens A, Witt T, Gottsberger G. Reproduction and pollination in Central European populations of Silene and Saponaria species. Botanica Acta. 1996;109:316–324. [Google Scholar]
- Kawakita A, Okamoto T, Goto R, Kato M. Mutualism favours higher host specificity than does antagonism in plant-herbivore interaction. Proceedings of the Royal Society of London B: Biological Sciences. 2010;277:2765–2774. doi: 10.1098/rspb.2010.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kephart S, Reynolds RJ, Rutter MT, Fenster CB, Dudash MR. Pollination and seed predation by moths on Silene and allied Caryophyllaceae: evaluating a model system to study the evolution of mutualisms. New Phytologist. 2006;169:667–680. doi: 10.1111/j.1469-8137.2005.01619.x. [DOI] [PubMed] [Google Scholar]
- Knudsen JY, Eriksson R, Gershenzon J. Diversity and distribution of floral scent. The Botanical Review. 2006;72:1–120. [Google Scholar]
- Kuzoff RK, Hufford L, Soltis DE. Structural homology and developmental transformations associated with ovary diversification in Lithophragma (Saxifragaceae) American Journal of Botany. 2001;88:196–205. [PubMed] [Google Scholar]
- Levin RA, Raguso RA, McDade LA. Fragrance chemistry and pollinator affinities in Nyctaginaceae. Phytochemistry. 2001;58:429–440. doi: 10.1016/s0031-9422(01)00257-6. [DOI] [PubMed] [Google Scholar]
- Maeda H, Dudareva N. The Shikimate pathway and aromatic amino acid biosynthesis in plants. Annual Review of Plant Biology. 2012;63:73–105. doi: 10.1146/annurev-arplant-042811-105439. [DOI] [PubMed] [Google Scholar]
- Majetic CJ, Raguso RA, Ashman T-L. Sources of floral scent variation: can environment define floral scent phenotype? Plant Signaling & Behavior. 2009;4:129–131. doi: 10.4161/psb.4.2.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Kawakita A, Kato M. Interspecific variation of floral scent composition in Glochidion and its association with host-specific pollinating seed parasite (Epicephala) Journal of Chemical Ecology. 2007;33:1065–1081. doi: 10.1007/s10886-007-9287-0. [DOI] [PubMed] [Google Scholar]
- Pellmyr O. Yuccas, yucca moths and coevolution: a review. Annals of the Missouri Botanical Garden. 2003;90:35–55. [Google Scholar]
- Pellmyr O, Thien LB. Insect reproduction and floral fragrances: keys to the evolution of the angiosperms? Taxon. 1986;35:76–85. [Google Scholar]
- Pellmyr O, Thompson JN. Sources of variation in pollinator contribution within a guild: the effects of plant and pollinator factors. Oecologia. 1996;107:595–604. doi: 10.1007/BF00333953. [DOI] [PubMed] [Google Scholar]
- Pellmyr O, Thompson JN, Brown JM, Harrison RG. Evolution of pollination and mutualism in the yucca moth lineage. The American Naturalist. 1996;148:827–847. [Google Scholar]
- Pichersky E, Noel JP, Dudareva N. Biosynthesis of plant volatiles: nature's diversity and ingenuity. Science. 2006;311:808–811. doi: 10.1126/science.1118510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proffit M, Schatz B, Borges RM, Hossaert-McKey M. Chemical mediation and niche partitioning in nonpollinating fig–wasp communities. Journal of Animal Ecology. 2007;76:296–303. doi: 10.1111/j.1365-2656.2007.01213.x. [DOI] [PubMed] [Google Scholar]
- Raguso RA. Wake up and smell the roses: the ecology and evolution of floral scent. Annual Review of Ecology, Evolution and Systematics. 2008;39:549–569. [Google Scholar]
- Raguso RA, Levin RA, Foose SE, Holmberg ME, McDade LA. Fragrance chemistry, nocturnal rhythms, and pollination ‘syndromes’ in Nicotiana. Phytochemistry. 2003;63:265–284. doi: 10.1016/s0031-9422(03)00113-4. [DOI] [PubMed] [Google Scholar]
- Reynolds RJ, Kula AAR, Fenster CB, Dudash MR. Variable nursery pollinator importance and its effect on plant reproductive success. Oecologia. 2012;168:439–448. doi: 10.1007/s00442-011-2095-9. [DOI] [PubMed] [Google Scholar]
- Rich KA, Thompson JN, Fernandez CC. Diverse historical processes shape deep phylogeographical divergence in the pollinating seed parasite Greya politella. Molecular Ecology. 2008;17:2430–2448. doi: 10.1111/j.1365-294X.2008.03754.x. [DOI] [PubMed] [Google Scholar]
- Roach DA, Wulff RD. Maternal effects in plants. Annual Review of Ecology and Systematics. 1987;18:209–235. [Google Scholar]
- Schiestl FP, Huber FK, Gomez JM. Phenotypic selection on floral scent: trade-off between attraction and deterrence? Evolutionary Ecology. 2011;25:237–248. [Google Scholar]
- Shuttleworth A, Johnson SD. The missing stink: sulphur compounds can mediate a shift between fly and wasp pollination systems. Proceedings of the Royal Society of London B: Biological Sciences. 2010;277:2811–2819. doi: 10.1098/rspb.2010.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CI, Drummond CS, Godsoe W, Yoder JB, Pellmyr O. Host specificity and reproductive success of yucca moths (Tegeticula spp. Lepidoptera: Prodoxidae) mirror patterns of gene flow between host plant varieties of the Joshua tree (Yucca brevifolia: Agavaceae) Molecular Ecology. 2009;18:5218–5229. doi: 10.1111/j.1365-294X.2009.04428.x. [DOI] [PubMed] [Google Scholar]
- Soler C, Proffit M, Chen C, Hossaert-McKey M. Private channels in plant–pollinator mutualisms. Plant Signaling and Behavior. 2010;5:893–895. doi: 10.4161/psb.5.7.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler C, Hossaert-McKey M, Buatois B, Bessière J-M, Schatz B, Proffit M. Geographic variation in floral scent in a highly specialized pollination mutualism. Phytochemistry. 2011;72:74–81. doi: 10.1016/j.phytochem.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Soltis DE, Soltis PS, Thompson JN, Pellmyr O. Chloroplast DNA variation in Lithophragma (Saxifragaceae) Systematic Botany. 1992;17:607–619. [Google Scholar]
- StatSoft. 2011 STATISTICA (data analysis software system), version 10. www.statsoft.com . [Google Scholar]
- Stuurman J, Hoballah ME, Broger L, Moore J, Basten C, Kuhlemeier C. Dissection of floral pollination syndromes in Petunia. Genetics. 2004;168:1585–1599. doi: 10.1534/genetics.104.031138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson GP, Hickman MO, Jr, Bartram S, Boland W, Pellmyr O, Raguso RA. Chemistry and geographic variation of floral scent in Yucca filamentosa (Agavaceae) American Journal of Botany. 2005;92:1624–1631. doi: 10.3732/ajb.92.10.1624. [DOI] [PubMed] [Google Scholar]
- Svensson GP, Pellmyr O, Raguso RA. Strong conservation of floral scent composition in two allopatric Yuccas. Journal of Chemical Ecology. 2006;32:2657–2665. doi: 10.1007/s10886-006-9189-6. [DOI] [PubMed] [Google Scholar]
- Svensson GP, Okamoto T, Kawakita A, Goto R, Kato M. Chemical ecology of obligate pollination mutualisms: testing the ‘private channel’ hypothesis in the Breynia–Epicephala association. New Phytologist. 2010;186:995–1004. doi: 10.1111/j.1469-8137.2010.03227.x. [DOI] [PubMed] [Google Scholar]
- Svensson GP, Pellmyr O, Raguso RA. Pollinator attraction to volatiles from virgin and pollinated host flowers in a yucca/moth obligate mutualism. Oikos. 2011;120:1577–1583. [Google Scholar]
- Taylor RL. Berkeley, CA: University of California Press; 1965. The genus Lithophragma (Saxifragaceae) [Google Scholar]
- Theis N. Fragrance of Canada thistle (Cirsium arvense) attracts both floral herbivores and pollinators. Journal of Chemical Ecology. 2006;32:917–927. doi: 10.1007/s10886-006-9051-x. [DOI] [PubMed] [Google Scholar]
- Theis N, Adler LS. Advertising to the enemy: enhanced floral fragrance increases beetle attraction and reduces plant reproduction. Ecology. 2012;93:430–455. doi: 10.1890/11-0825.1. [DOI] [PubMed] [Google Scholar]
- Tholl D. Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Current Opinion in Plant Biology. 2006;9:297–304. doi: 10.1016/j.pbi.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Thompson JN. Concepts of coevolution. Trends in Ecology and Evolution. 1989;4:179–183. doi: 10.1016/0169-5347(89)90125-0. [DOI] [PubMed] [Google Scholar]
- Thompson JN. The coevolutionary process. Chicago, IL: University of Chicago Press; 1994. [Google Scholar]
- Thompson JN. The coevolving web of life. The American Naturalist. 2009;173:125–150. doi: 10.1086/595752. [DOI] [PubMed] [Google Scholar]
- Thompson JN. The adaptive radiation of coevolving prodoxid moths and their host plants: Greya moths and Yucca moths In. In: Grant PR, Grant RB, editors. In search of the causes of evolution: from field observation to mechanisms. Princeton, NJ: Princeton University Press; 2010. pp. 228–246. [Google Scholar]
- Thompson JN, Cunningham BM. Geographic structure and dynamics of coevolutionary selection. Nature. 2002;417:735–738. doi: 10.1038/nature00810. [DOI] [PubMed] [Google Scholar]
- Thompson JN, Fernandez CC. Temporal dynamics of antagonism and mutualism in a geographically variable plant–insect interaction. Ecology. 2006;87:103–112. doi: 10.1890/05-0123. [DOI] [PubMed] [Google Scholar]
- Thompson JN, Pellmyr O. Mutualism with pollinating seed parasites amid co-pollinators: constraints on specialization. Ecology. 1992;73:1780–1791. [Google Scholar]
- Thompson JN, Rich KA. Range edges and the molecular divergence of Greya moth populations. Journal of Biogeography. 2011;38:551–553. [Google Scholar]
- Thompson JN, Laine A-L, Thompson JF. Retention of mutualism in a geographic diverging interaction. Ecology Letters. 2010;13:1368–1377. doi: 10.1111/j.1461-0248.2010.01529.x. [DOI] [PubMed] [Google Scholar]
- Wälti MO, Mühlemann JK, Widmer A, Schiestl FP. Floral odour and reproductive isolation in two species of Silene. Journal of Evolutionary Biology. 2008;21:111–121. doi: 10.1111/j.1420-9101.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- Wälti MO, Page PA, Widmer A, Schiestl FP. How to be an attractive male: floral dimorphism and attractiveness to pollinators in a dioecious plant. BMC Evolutionary Biology. 2009;9:190. doi: 10.1186/1471-2148-9-190. http://dx.doi.org/10.1186/1471-2148-9-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright GA, Schiestl FP. The evolution of floral scent: the influence of olfactory learning by insect pollinators on the honest signaling of floral rewards. Functional Ecology. 2009;23:841–851. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.