Abstract
Background and Aims
Interspecific hybridization and polyploidy are key processes in plant evolution and are responsible for ongoing genetic diversification in the genus Sorbus (Rosaceae). The Avon Gorge, Bristol, UK, is a world ‘hotspot’ for Sorbus diversity and home to diploid sexual species and polyploid apomictic species. This research investigated how mating system variation, hybridization and polyploidy interact to generate this biological diversity.
Methods
Mating systems of diploid, triploid and tetraploid Sorbus taxa were analysed using pollen tube growth and seed set assays from controlled pollinations, and parent–offspring genotyping of progeny from open and manual pollinations.
Key Results
Diploid Sorbus are outcrossing and self-incompatible (SI). Triploid taxa are pseudogamous apomicts and genetically invariable, but because they also display self-incompatibility, apomictic seed set requires pollen from other Sorbus taxa – a phenomenon which offers direct opportunities for hybridization. In contrast tetraploid taxa are pseudogamous but self-compatible, so do not have the same obligate requirement for intertaxon pollination.
Conclusions
The mating inter-relationships among Avon Gorge Sorbus taxa are complex and are the driving force for hybridization and ongoing genetic diversification. In particular, the presence of self-incompatibility in triploid pseudogamous apomicts imposes a requirement for interspecific cross-pollination, thereby facilitating continuing diversification and evolution through rare sexual hybridization events. This is the first report of naturally occurring pseudogamous apomictic SI plant populations, and we suggest that interspecific pollination, in combination with a relaxed endosperm balance requirement, is the most likely route to the persistence of these populations. We propose that Avon Gorge Sorbus represents a model system for studying the establishment and persistence of SI apomicts in natural populations.
Keywords: Hybridization, evolution, polyploidy, apomixis, pseudogamy, self-incompatibility, Sorbus
INTRODUCTION
Interspecific hybridization and polyploidy are important processes in plant evolution, both separately, and, more commonly, in combination through the formation of allopolyploids (Stebbins, 1950; Grant, 1981; Arnold, 1997; Rieseberg and Willis, 2007; Doyle et al., 2008; Soltis and Soltis, 2009). There are many examples of new species that have arisen through these processes (Arnold, 1997), many of which have become models for studying plant speciation at genetic, genomic and ecological levels (Hegarty and Hiscock, 2005, 2008; Rieseberg and Willis, 2007; Abbott et al., 2010). Even though homoploid hybrid speciation (i.e. hybrid speciation without a change in chromosome number) is now being recognized more extensively (Rieseberg, 1997; Soltis and Soltis, 2009; Abbott et al., 2010), most examples of hybrid speciation in plants involve allopolyploidy, which is considered to be the most important mechanism of abrupt speciation in plants (for reviews, see Grant, 1981; Hegarty and Hiscock, 2008; Leitch and Leitch, 2008). Numerous examples of allopolyploid speciation have been described in the literature, including at least six new plant species that have arisen in the past 200 years: Tragopogon mirus and T. miscellus (Soltis et al., 2004); Spartina anglica (Ainouche et al., 2003), Senecio cambrensis and S. eboracensis (Ashton and Abbott, 1992; Abbott and Lowe, 1996, 2004) and Cardamine schulzii (Urbanska et al., 1997).
The mating systems associated with plants involved in hybridization and polyploidy are critical in determining both the likelihood of hybrid and/or polyploid formation and their long-term survival (Grant, 1981; Rieseberg, 1997). In sexually reproducing plants, outbreeding often promotes hybridization and the formation of new homoploid and allopolyploid offspring, whilst inbreeding (selfing) will perpetuate new homoploids and allopolyploids, and reinforce reproductive isolation between them and their parental taxa (Grant, 1981; Rieseberg, 1997; Coyne and Orr, 2004). Asexual reproduction, through apomixis (agamospermy; the production of ‘maternal’ clonal seeds), offers a more secure means of perpetuating new hybrid and allopolyploid genotypes than selfing, and affords immediate, often total, reproductive isolation from parental taxa. Apomixis also provides a means of maintaining and propagating primary hybrids with unbalanced numbers of parental chromosomes (i.e. non-homoploids), particularly triploids. Not surprisingly therefore, apomixis is frequently associated with hybridization and polyploidy where it has been critical for the establishment of many novel taxa arising by these processes (Grant, 1981; Briggs and Walters, 1997; Hörandl, 2006; Hörandl and Paun, 2007; Whitton et al., 2008).
In Britain, the genus Sorbus (whitebeams, service trees and rowans) is a particularly good example of a genus that has undergone rapid evolution through the processes of hybridization and polyploidy – both allopolyploidy and autopolyploidy (Nelson-Jones et al., 2002; Robertson et al., 2004a, b; Chester et al., 2007; Robertson et al., 2010). ‘Hotspots’ of Sorbus evolution in Britain include: the Isle of Arran, Scotland, Cheddar Gorge, Somerset, the North Devon–Somerset coast and the Wye Valley of the Welsh Borders, but by far the richest and most important site for Sorbus diversity in Britain, and indeed probably in the world, is the Avon Gorge in Bristol, where 21 taxa have been recorded (Rich et al., 2010; Robertson et al., 2010). The Avon Gorge is a spectacular Carboniferous Limestone gorge about 2 km long and 80 m high, with extensive woodland, scrub, open rocks and quarries providing a diversity of habitats and niches that have facilitated the survival and perpetuation of newly divergent Sorbus taxa probably because of subtle pre-adaptive phenotypes (Rich et al., 2010).
We previously showed, using molecular markers, that the generation of genetic novelty within Avon Gorge Sorbus has been driven primarily by a series of interspecific hybridizations and backcrosses among closely related taxa, with each new genotype (taxon) being fixed and propagated by apomixis (Robertson et al., 2010). The result is a series of new closely related taxa, microspecies and hybrids (as defined by Rich et al., 2009), that are reproductively isolated from each other, but which occasionally participate in further hybridization events leading to a complex pattern of ongoing reticulate evolution of Sorbus within the Avon Gorge (Robertson et al., 2010). In the Avon Gorge today there are four sexual diploid species [S. aucuparia (rowan), S. aria (whitebeam), S. torminalis (wild service-tree) and S. domestica (service tree, which is not involved in hybridization and is not discussed further)], one primary diploid hybrid (S. × thuringiaca), seven polyploid taxa (S. anglica, S. bristoliensis, S. eminens, S. leighensis, S. porrigentiformis, S. whiteana and S. wilmottiana), which appear to be apomictic (Robertson et al., 2010), and four hybrids involving polyploids (S. × avonensis, S. × houstoniae, S. × proctoriana and S. × robertsonii), the mating system(s) of which have yet to be investigated. In addition to these native Sorbus taxa, there are five introduced species (S. croceocarpa, S. decipiens, S. intermedia, S. latifolia and S. glabriuscula) all of which are polyploid and believed to be apomictic. There are also populations of at least two more undescribed species and numerous puzzling individuals, which do not fit known taxa (Rich et al., 2010; Robertson et al., 2010).
The majority of diploid sexual Sorbus species investigated to date are outcrossing with a self-incompatibility mating system (Lemche, 1999; Pías and Guitián, 2006; Bailey et al., 2008; Kamm et al., 2009; Robertson et al., 2010). Self-incompatibility is a mechanism used by plants to prevent self-fertilization and consequent inbreeding depression, and is typically controlled by a single locus, S, with multiple alleles (haplotypes) (de Nettancourt, 1977; Hiscock and McInnis, 2003). Self-incompatibility has been shown to be regulated by a number of different molecular mechanisms, suggesting numerous independent origins (Allen et al., 2010). In the Rosaceae, self-incompatibility is controlled gametophytically, that is to say the self-incompatible (SI) phenotype of the pollen is determined by its own (haploid) S genotype (de Nettancourt, 1977). At a molecular level, gametophytic self-incompatibility in the Rosaceae is regulated by a pistil-expressed S-RNase and a pollen-expressed F-box protein, S locus F-box (SLF) (McClure et al., 2011; Meng et al., 2011), a system shared with species of the Solanaceae and Plantaginaceae. In this form of gametophytic self-incompatibility, incompatible pollen will germinate, but accumulation of pistil-produced S-RNases in the pollen tube indirectly inhibits further protein synthesis, causing growth to terminate within the style with a characteristic swelling of the tube tip (Luu et al., 2000).
Rosaceous genera, particularly close relatives of Sorbus, also frequently contain self-compatible (SC) and apomictic (both obligate and facultative) polyploidy species (Dickinson et al., 2007). Breakdown of self-incompatibilty (leading to self-compatibility) in polyploids is well documented among species with gametophytic self-incompatibility, particularly Rosaceae (Mable, 2004), and is associated with ‘competitive interaction’ between two different S alleles (usually there is just one) in the pollen (Chawla et al., 1997; Golz et al., 2000, 2001; Meng et al., 2011). Polyploid apomictic species of Rosaceae usually display pseudogamy (Liljefors, 1954), where pollination and fertilization of the central cell nucleus/nuclei is required for the formation of endosperm) even though no paternal genetic contribution is made to the embryo. This need for fertilization means the self-compatibility generally arising from polyploidy is highly advantageous in pseudogamous apomicts because it ensures uniparental reproduction (Noirot et al., 1997; Hörandl, 2010). Thus for Sorbus, and Rosaceae more generally, it is fair to expect diploid sexual taxa to be outcrossers with self-incompatibility, and polyploids, including all pseudogamous polyploids, to be self-compatible.
Here we report the first extensive analysis of mating systems in Sorbus occurring in the world diversity hotspot for this genus – the Avon Gorge, Bristol. We were particularly interested in elucidating the mating systems of the rare endemic Sorbus taxa and their proposed parents, to determine how mating system variation, hybridization and polyploidy interact to generate genetic diversity and thereby drive continuing evolution of Sorbus in the Avon Gorge. Using a combination of controlled and open pollinations followed by seed set analysis, pollen tube growth analysis and parent–offspring genotyping with molecular markers (microsatellites), we show that the two main diploid species, S. aria and S. torminalis, are SI and outcrossing, whereas triploid and tetraploid taxa are apomictic but require pollination and fertilization for endosperm formation, i.e. they are pseudogamous apomicts. Interestingly, we show that while tetraploid pseudogamous apomictic taxa are (as predicted) SC and therefore able to use their own pollen for endosperm formation, triploid taxa are SI and require pollination by other Sorbus taxa for endosperm formation and successful apomictic seed production. This apparent constraint on pseudogamous apomixis in triploid Sorbus taxa thereby imposes a requirement for intertaxon cross-pollination, which can facilitate genetic diversification in Sorbus evolution through rare sexual hybridization events. We discuss how outcrossing (self-incompatibility), self-pollination and facultative pseudogamy interact to facilitate the occasional hybridization and backcrossing that perpetuate ongoing genetic diversification and evolution among Sorbus in the Avon Gorge. This is also the first known report of an established natural population of pseudogamous SI apomictic plants. We hypothesize that intertaxon pollination and a relaxed endosperm balance requirement are primarily responsible for the establishment of SI apomictic Sorbus populations in the Avon Gorge, and we propose that this group of trees provides an ideal natural system for studying how pseudogamous SI apomicts become established and persist.
MATERIALS AND METHODS
Mating experiments
Mating systems and compatibility amongst Avon Gorge Sorbus (including S. aria, S. aucuparia, S. bristoliensis, S. eminens, S. leighensis, S. porrigentiformis, S. torminalis, S. whiteana and S. wilmottiana) were investigated by measuring seed set and pollen tube growth. Manual pollinations were carried out both in vivo (for seeds and pollen tubes) and ex vivo (for pollen tubes). Trees were selected for use in pollination tests if they were flowering and had inflorescences that could be reached from the ground. The hybrids S. × avonensis, S. × houstoniae and S. × robertsonii were not included in pollination assays because they grow in locations where pollination work is not practical. Sampling sites and identity codes of trees are given in the Supplementary Data Table S1.
In vivo pollinations
Perforated polythene bags were placed over inflorescences of the maternal plant at the ‘balloon’ stage (Fig. 1) to prevent cross-pollination. Pollination treatments included: (a) pollen from the same tree to test for self-compatibility; (b) anthers removed and pollen from conspecifics to test for cross-compatibility within the species; (c) anthers removed and pollen from different species to test for cross-compatibility between species; (d) anthers removed and no pollen to test for autonomous apomixis; and (e) intact flowers bagged and left to test for facilitated self-pollination (i.e. is an insect vector required for self-pollination to occur) (Table 1). Manual pollination was performed once for each flower by brushing dehiscing anthers against the receiving stigmas until pollen could be seen on the stigma surface. The number of flowers pollinated in each mating was between five and 15, and only one type of pollination treatment was applied to each inflorescence. Pollen exclusion bags were immediately replaced to prevent undesired pollination events. Two to three flowers from each mating combination were collected 48 h after pollination for pollen tube analysis (Supplementary Data Table S2). Styles were fixed in FPA solution (70 % ethyl alcohol:40 % formaldehyde:propionic acid in a 90:5:5 mixture) and stored at 4 °C until use. The remaining flowers were left for seed development. Fruit formation was monitored, with infructescences being bagged in late summer to prevent predation by birds and small mammals. Fruits were collected when ripe (red or orange in colour).
Fig. 1.

Flowers of S. aria at various stages of opening; flowers at the ‘balloon’ stage (indicated by white arrows) are still in bud, but they are larger and whiter in colour than flowers at ‘bud stage’ because they are nearer to opening (scale bar = 5 mm).
Table 1.
Pollination crosses, ploidy levels and pollen viability for each taxon; the total number of trees receiving treatment is shown in parentheses
| Pollen treatment received |
|||||||
|---|---|---|---|---|---|---|---|
| Taxon | Ploidy* | Average pollen viability (%)‡ | A | B | C | D | E |
| S. aria | 2x† | 84·75 | (10) | (7) | S. aucuparia (1); S. eminens (5); S. porrigentiformis (6) | ||
| S. aucuparia | 2x† | 93·28 | (1) | ||||
| S. torminalis | 2x† | 81·50 | (4) | (3) | S. aria (1); S. eminens (5); S. porrigentiformis (2) | ||
| S. × avonensis | 3x | N/A | |||||
| S. bristoliensis | 3x† | 38·20 | (6) | (4) | S. aria (6); S. porrigentiformis (1) | (3) | (3) |
| S. leighensis | 3x | 23·00§ | (7) | (5) | S. aria (7); S. porrigentiformis (6) | (7) | (1) |
| S. whiteana | 3x† | 63·00 | (5) | (4) | S. aria (5); S. porrigentiformis (5) | (5) | (1) |
| S. wilmottiana | 3x† | 46·50 | (7) | (6) | S. aria (5) | (4) | (3) |
| S. × robertsonii | 3x | N/A | |||||
| S. eminens | 4x† | 80·71 | (8) | (7) | S. aria (6); S. torminalis (1) | (3) | (2) |
| S. × houstoniae | 4x | N/A | |||||
| S. porrigentiformis | 4x† | 57·25 | (7) | (8) | S. aria (6) | (1) | |
Key to pollen treatments: (A) pollen from the same tree; (B) pollen from a conspecific individual; (C) pollen from a different taxon (pollen parents shown); (D) emasculation with no pollen; (E) no pollen/bagged and left.
*Ploidy levels of all taxa (except S. whiteana) have been measured by flow cytometry (Pellicer et al., 2012).
†Ploidy level measured by cytological analysis (Lemche, 1999; Bailey et al., 2008).
‡Based on stainability of pollen from herbaria specimens using Alexander's stain (Rich, 2009).
§From Rich et al. (2010).
Ex vivo pollinations
Mating crosses for pollen tube analysis were repeated under laboratory conditions. Inflorescences were collected at the ‘balloon’ stage (see Fig. 1) from the same trees used in the field crosses and brought to the lab where they were placed in water and protected by exclusion bags. Once open, flowers were removed from the inflorescence, emasculated and placed in water in wells of microtitre plates. For each individual, 3–5 flowers were used for each pollination treatment. Flowers were manually pollinated and fixed in FPA solution as described above.
Seed set analysis
The total numbers of fruits resulting from each cross were recorded. Seeds were removed from fruits, counted and scored for viability: large seeds that were well formed and firm were categorized as ‘viable’, while seeds that were very small, thin, empty, contained a deformed embryo or contained no embryo were categorized as ‘non-viable’ (Supplementary Data Table S3). Inviability in these latter types of Sorbus seed has previously been confirmed by dissection and germination testing (S. Ludwig, University of Bristol, UK, unpubl. res.). A cross that resulted in the production of at least one seed was considered to be compatible. Seed set results for each taxon were pooled per treatment to give an overall proportion of compatible results per number of crosses, and the majority (i.e. more than half) score was taken as the consensus compatibility status for the taxon.
Pollen tube analysis
All preserved pollinated flowers were assessed for pollen tube germination and growth using the following techniques. Samples were softened for 24 h in 8 n NaOH followed by a 2 h wash in running water, then stained in a 0·1 % solution of aniline blue in time intervals of 12–24 h (Preil, 1970; Kho and Baer, 1971). To prepare samples for microscopy, styles were separated from ovaries (which were inspected separately), and the styles were squashed. Pollen tubes in the samples were observed under ultraviolet light on an Olympus BX61 research microscope (Japan). The length of pollen tubes was determined using Multiple Image Analysis software.
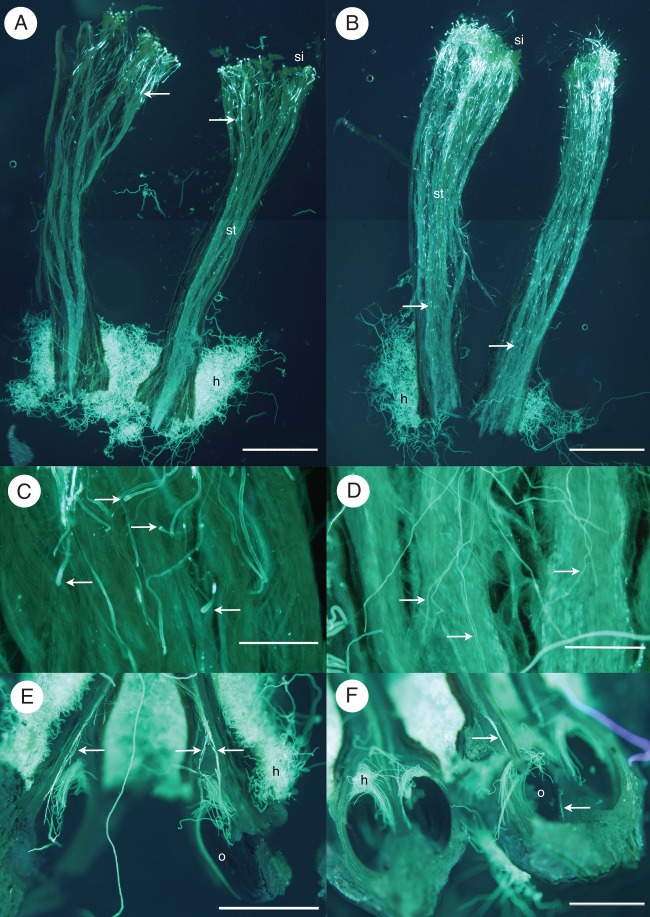
Pollen compatibility on each pistil was scored by the extent of pollen tube growth towards the ovule. Figure 2 shows example images of pollen tubes from compatible and incompatible pollinations. In the gametophytic self-incompatible (GSI) system found in Sorbus and other Rosaceae, incompatible pollen is generally inhibited in the upper half of the style before reaching the ovary (Fig. 2A); therefore, the following compatibility/incompatibility scoring system was used: growth ending in the upper or middle region of the style or showing physical signs of incompatibility (swollen tube tip) = incompatible (–) (Fig. 2A, C); growth past the lower style or entering the ovary = compatible (+) (Fig. 2B, D–F). Overall compatibility/incompatibility was determined for each individual's pollination combinations by calculating the total compatibility and incompatibility score and the majority score taken as the consensus compatibility status. Compatibility results for each taxon were then pooled per treatment to give an overall proportion of compatible results per number of crosses. Again, the majority score was taken as the consensus compatibility status for the taxon.
Fig. 2.
Example images from pollen tube analysis (si, stigma; st, style; h, hairs; o, ovule). (A) Incompatible combination from self-pollination of S. whiteana; arrows indicate that growth of pollen tubes has terminated in the upper parts of the styles (ovules have been removed from the styles). (B) Compatible combination from S. aria pollination of S. whiteana; arrows indicate pollen tube growth continues into the base of the styles. (C) Typically swollen tips of pollen tubes (arrows) that have ended incompatibly in the style of a self-pollinated S. whiteana. (D) Compatible pollen tubes (arrows) in the base of the style from S. leighensis pollinated by S. porrigentiformis. (E, F) Pollen tubes (arrows) that have penetrated ovules of S. whiteana pollinated by S. aria. Scale bars: (A, B, E, F) = 1 mm; (C, D) = 0·2 mm.
Pollen viability in the Avon Gorge Sorbus taxa was previously tested by Rich (2009) and Rich et al. (2010). Viability in the pollen of triploid taxa was estimated to range from 23·00 to 63·00 % (see Table 1). While this is lower than pollen viability for the diploid and tetraploid taxa, we consider it to be high enough not to interfere with the interpretation of data from pollination experiments (Rich, 2009).
Progeny analysis
Progeny analyses were used in order to determine whether a taxon's mating system is sexual (is it primarily selfing or outcrossing) or asexual (is it obligate or facultative). This also allowed sampling for naturally occurring hybridizations. Progeny analyses compared multilocus molecular marker phenotypes of progeny (embryos) from wild pollinations with those of their maternal parents.
Sample material
Open-pollinated seeds were used from the same trees selected for pollination testing if there was sufficient fruit set; if open-pollinated fruit set was insufficient, additional trees were sampled. These additional trees were chosen if they displayed fruit set of at least 15 ripe fruits and if fruits were within reach of long-handled pruners. For progeny DNA sampling, 15 mature berries were collected from 3–5 adult trees of each of the following Sorbus taxa located in the Avon Gorge: S. aria, S. bristoliensis, S. eminens, S. leighenis, S. porrigentiformis, S. torminalis, S. whiteana and S. wilmottiana. Two trees were sampled for S. × avonensis and one tree each for S. × houstoniae and S. × robertsonii. A single, well-formed seed was removed from each fruit and stored at 4 °C until use. For maternal DNA sampling, leaf buds were collected from each maternal tree and stored frozen at –80 °C until use. Sampling sites and identity codes of the trees are given in Supplementary Data Table S1. Additionally, progeny from two manual interspecific crosses were analysed to confirm that intertaxon pollen was being used to form endosperm: ten well-formed seeds from one S. bristoliensis × S. aria cross and ten well-formed seeds from one S. porrigentiformis × S. aria cross were randomly selected for DNA extraction and genotyping.
DNA extractions
Seeds were soaked overnight in sterile distilled water prior to DNA extraction. Embryos were then separated from the seed coat and endosperm, and DNA was extracted from individual embryos using a cetyltrimethylammonium bromide (CTAB) DNA extraction protocol (Soltis and Soltis, 2002). DNA was extracted from frozen leaf buds using the Qiagen DNAeasy plant kit following the manufacturer's instructions.
Microsatellite amplification and analysis
For all maternal trees and their progeny, three nuclear microsatellite loci were amplified by polymerase chain reaction (PCR) using primers CH01F02, MSS5 and MSS16 described by Robertson et al. (2010). Forward primers were labelled with fluorescent dye and PCRs carried out according to Robertson et al. (2010). Products were separated by capillary electrophoresis on an Applied Biosystems 3500 Genetic Analyser (Applied Biosystems, USA). Product peaks were scored using GeneMarker® genotype analysis software (SoftGenetics, USA). Although product peaks produced by fragment analysis were presumed to represent allelic products, analysis of the genetic marker variation was conducted by comparing multilocus molecular marker phenotypes of maternal parents and progeny, rather than using a strict genetic interpretation. This was largely due to the fact that allele dosage has not been investigated for the polyploid taxa so it is unknown which alleles occur in duplicate at particular loci. Ploidy levels for all taxa in this study have previously been measured by cytological analysis and/or flow cytometry analysis (Lemche, 1999; Bailey et al., 2008; Pellicer et al., 2012; see Table 1).
Analysis of molecular data was primarily qualitative. Progeny molecular data were interpreted as follows: (a) if an embryo contained non-maternal alleles it was considered to be a result of outcrossing; (b) if an embryo had a maternal molecular phenotype it was considered to be either the result of sexual reproduction with a genetically similar individual or the result of asexual reproduction; and (c) if an embryo had a reduced number of alleles compared with its mother it was considered to be the result of selfing by sexual reproduction. For each taxon, we then estimated the overall genetic diversity amongst progeny by measuring the proportion of unique progeny phenotypes (Ellstrand and Roose, 1987). This was done by calculating N(p)/N(i) [where N(p) is the number of molecular phenotypes and N(i) is the total number of progeny sampled); a proportion close to 1 would suggest a sexual mating system, whereas a proportion close to 0 would suggest an asexual mating system.
RESULTS
Pollen tube growth and seed set analyses in diploid maternal trees
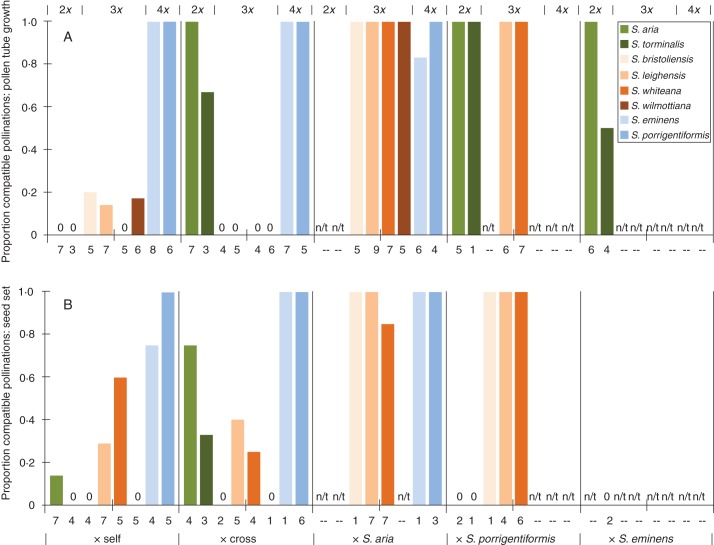
Self-pollinations of S. aria, S. aucuparia and S. torminalis resulted in incompatible pollen tubes and low or no seed set (Table 2, Fig. 3; Supplementary Data Table S2). Intraspecific pollinations amongst S. aria individuals produced compatible pollen tubes and viable seed (Table 2, Fig. 3). On the other hand, intraspecific cross-pollinations results for S. torminalis were mixed, with one combination (LEV108a × LEV100) appearing to be completely incompatible for both tests and a second (LEVG1 × LEV108a) being compatible for pollen tubes but incompatible for seed set, while the remaining mating combinations were compatible (Supplementary Data Tables S2 and S3). In interspecific matings between S. aria or S. torminalis and S. eminens or S. porrigentiformis, compatible pollen tubes were observed, but few seeds were produced and all embryos were severely deformed (Table 2).
Table 2.
Summary of pollen tube growth and seed set results from controlled pollinations on diploid maternal trees; the proportion of successful compatible crosses out of the total number of crosses performed is shown in parentheses
| Self-pollination |
Cross-pollination |
Interspecific (× S. porrigentiformis) |
Interspecific (× S. eminens) |
|||||
|---|---|---|---|---|---|---|---|---|
| Taxon | Pollen tube | Seed | Pollen tube | Seed | Pollen tube | Seed | Pollen tube | Seed |
| S. aria | – | – | + | + | + | – | + | NT |
| (0/7) | (1/7) | (7/7) | (3/4) | (5/5) | (0/2) | (6/6) | ||
| S. torminalis | – | – | + | – | + | – | +/– | – |
| (0/3) | (0/4) | (2/3) | (1/3) | (1/1) | (0/1) | (2/4) | (0/2) | |
+, compatible combination or viable seed set; –, incompatible combination or no viable seed set; +/–, inconclusive result; NT, not tested.
Fig. 3.
Proportion of compatible pollinations based on pollen tube growth (A) and seed set (B). The horizontal axis gives the type of pollination combination carried out. Combinations resulting in the production of no compatible pollen tubes or seeds are indicated by 0 to distinguish them from mating combinations that were not tested (indicated by n/t). For each pollination category, taxa occur in the same order across the chart, as shown in the chart legend (left to right). Numbers below bars indicate the total number of crosses carried out for a particular mating combination.
Pollen tube growth and seed set analyses in triploid maternal trees
Self-pollinations and intraspecific cross-pollinations in the triploid taxa (S. bristoliensis, S. leighensis, S. whiteana and S. wilmottiana) resulted in incompatible pollen tubes and low rates of viable seed production for all four species (Table 3, Fig. 3, and see Fig. 2). All triploid species allowed compatible pollen tube development and/or viable seed production when crossed with pollen from S. aria or S. porrigentiformis (Table 3, and see Fig. 2). Testing for autonomous apomixis (anthers removed, no pollen) resulted in no or low seed set (Table 3). Excluding pollinators from intact triploid flowers resulted in little or no pollen being deposited on the stigmas (Table 3; Supplementary Data Table S2); in these flowers, no compatible pollen tubes were observed, and only one treatment resulted in the production of a single viable seed (Table 3).
Table 3.
Summary of pollen tube growth and seed set results from controlled pollinations on triploid maternal trees; the proportion of successful compatible crosses out of the total number of crosses performed is shown in parentheses
| Self-pollinated |
Cross-pollinated |
Interspecific (× S. aria) |
Interspecific (× S. porrigentiformis) |
Emasculated; bagged |
Bagged and left |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taxon | Pollen tube | Seed | Pollen tube | Seed | Pollen tube | Seed | Pollen tube | Seed | Pollen tube | Seed | Pollen tube | Seed |
| S. bristoliensis | – | – | – | – | + | + | NT | + | NT | – | – | – |
| (1/5) | (0/4) | (0/4) | (0/2) | (5/5) | (1/1) | (1/1) | (0/3) | (0/3) | (0/2) | |||
| S. leighensis | – | – | – | – | + | + | + | + | NT | – | NT | + |
| (1/7) | (2/7) | (0/5) | (2/5) | (9/9) | (7/7) | (6/6) | (4/4) | (1/7) | (1/1) | |||
| S. whiteana | – | +/– | – | – | + | + | + | + | NT | – | NT | – |
| (0/5) | (3/5) | (0/4) | (1/4) | (7/7) | (6/7) | (7/7) | (6/6) | (0/5) | (0/1) | |||
| S. wilmottiana | – | – | – | – | + | NT | NT | NT | NT | – | – | – |
| (1/6) | (0/5) | (0/6) | (0/1) | (5/5) | (0/4) | (0/3) | (0/2) | |||||
Definitions of +, – and +/– as for Table 2; NT, not tested.
Pollen tube growth and seed set analyses in tetraploid maternal trees
Self-pollinations and intraspecific cross-pollinations in the tetraploid species S. eminens and S. porrigentiformis resulted in compatible pollen tubes and a high rate of viable seed set for both species (Table 4, Fig. 3). Interspecific pollination of either tetraploid species with S. aria pollen resulted in compatible pollen tubes and viable seed set for both tetraploids (Table 4, Figure 3), while interspecific pollination of S. eminens with S. torminalis pollen produced incompatible pollen tubes (Supplementary Data Table S2). Unusually, pollen tubes that formed in the S. eminens × S. torminalis cross often ended with characteristic signs of incompatibility in the base of the style rather than in the upper or middle regions of the style, suggesting possible partial compatibility. As found in the triploid taxa, the test for autonomous apomixis resulted in no seed set for either of the tetraploid species (Table 4). For tetraploid trees in which insects were excluded from intact flowers, compatible pollen tubes were seen in one sample (although these were fewer in number than the incompatible pollen tubes observed), while the other was poorly pollinated, with pollen tubes ending in the upper parts of the style (Table 4; Supplementary Data Table S2).
Table 4.
Summary of pollen tube and seed results from controlled pollinations on tetraploid maternal trees; proportion of successful compatible crosses out of the total number of crosses performed is shown in parentheses
| Self pollination |
Cross pollination |
Interspecific (× S. aria) |
Emasculated; bagged |
Bagged and left |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Taxon | Pollen tube | Seed | Pollen tube | Seed | Pollen tube | Seed | Pollen tube | Seed | Pollen tube | Seed |
| S. eminens | + | + | + | + | + | + | Not pollinated | – | – | NT |
| (8/8) | (3/4) | (7/7) | (1/1) | (5/6) | (1/1) | (0/2) | (0/2) | |||
| S. porrigentiformis | + | + | + | + | + | + | NT | – | NT | NT |
| (6/6) | (5/5) | (5/5) | (6/6) | (4/4) | (3/3) | (0/1) | ||||
Definitions of +, – and +/– as for Table 2; NT, not tested.
Microsatellite analysis of maternal parents and progeny
A high level of genetic variability was detected in progeny from S. aria and S. torminalis, with high proportions of distinguishable multilocus molecular phenotypes and high proportions of progeny with non-maternal alleles (Table 5; Supplementary Data Table S4). A small proportion of progeny differed from their mothers in having a reduced number of alleles, and the remaining small proportion of progeny displayed maternal molecular phenotypes (Table 5). All progeny for each triploid taxon (S. × avonensis, S. bristoliensis, S. leighensis, S. whiteana and S. wilmottiana) had molecular phenotypes identical to those of their maternal parents (Table 5; Supplementary Data Table S5). All seeds collected from the single S. × robertsonii tree were deformed and contained no embryos; no molecular marker analysis was therefore possible for this taxon. Like the triploid progeny, embryos sampled from the tetraploid species (S. eminens, S. × houstoniae and S. porrigentiformis) showed a high degree of similarity to their maternal parents (Table 5). However, a small number of progeny for each taxon differed from their maternal parents in the loss of alleles at one or two loci (Table 5; Supplementary Data Table S6). For progeny resulting from crosses between S. bristoliensis × S. aria and S. porrigentiformis × S. aria, all progeny molecular phenotypes were identical to their maternal parents (Table 6).
Table 5.
Summary of adult and progeny multilocus molecular marker phenotypes detected as a result of parent–offspring molecular analysis
| Taxon | Ploidy | N(i) adults | N(p) adults | No. of progeny sampled per adult | N(i) progeny | N(p) progeny | N(p)/N(i) progeny | No. of progeny with non-maternal phenotype | No. of progeny with non-maternal allele(s) | No. of progeny with reduced number of alleles |
|---|---|---|---|---|---|---|---|---|---|---|
| S. aria | 2x | 5 | 5 | 11–15 | 71 | 58 | 0·82 | 68 | 61 | 7 |
| S. torminalis | 2x | 5 | 5 | 14–15 | 72 | 50 | 0·69 | 63 | 45 | 18 |
| S. × avonensis | 3x | 1 | 1 | 10 | 10 | 1 | 0·10 | 0 | 0 | 0 |
| S. bristoliensis | 3x | 5 | 1 | 15 | 75 | 1 | 0·01 | 0 | 0 | 0 |
| S. leighensis | 3x | 5 | 1 | 9–10 | 49 | 1 | 0·02 | 0 | 0 | 0 |
| S. whiteana | 3x | 3 | 1 | 5–10 | 25 | 1 | 0·04 | 0 | 0 | 0 |
| S. wilmottiana | 3x | 4 | 1 | 10 | 40 | 1 | 0·02 | 0 | 0 | 0 |
| S. eminens | 4x | 5 | 1 | 15 | 75 | 3 | 0·04 | 2 | 0 | 2 |
| S. × houstoniae | 4x | 1 | 1 | 15 | 15 | 2 | 0·13 | 1 | 0 | 1 |
| S. porrigentiformis | 4x | 5 | 1 | 15 | 75 | 2 | 0·03 | 1 | 0 | 1 |
N(i), total number of individuals sampled; N(p), number of molecular phenotypes seen; N(p)/N(i), proportion of distinguishable phenotypes amongst progeny (as per Ellstrand and Roose, 1987).
Table 6.
Multilocus molecular marker phenotypes for maternal trees and their progeny for controlled interspecific pollinations (the maternal parent is listed before the pollen parent); numbers represent allele size detected by capillary electrophoresis
| Mating combination | Locus F02 | Locus MSS5 | Locus MSS16 |
|---|---|---|---|
| S. bristoliensis LEV87 | 192, 202 | 120, 134, 144 | 166, 178, 198 |
| S. aria LEV86 | 200, 202 | 134 | 166 |
| All progeny (n = 10) | 192, 202 | 120, 134, 144 | 166, 178, 198 |
| S. porrigentiformis LEV85 | 196, 202, 206, 208 | 120, 132, 136, 142 | 166, 170, 178 |
| S. aria LEV86 | 200, 202 | 134 | 166 |
| All progeny (n = 10) | 196, 202, 206, 208 | 120, 132, 136, 142 | 166, 170, 178 |
DISCUSSION
Mating systems in diploid Sorbus taxa
Pollination assays confirmed self-incompatibility in the diploid sexual species S. aria. Outcrossing was further supported by success in intraspecific crossing results and as shown by progeny analysis. A high level of genotypic diversity was detected in S. aria, with the majority (96 %) of wild-pollinated progeny having non-maternal multilocus molecular phenotypes and 86 % of these progeny displaying non-maternal alleles. In light of the pollination results presented here, the small proportions of maternal molecular phenotypes (4·2 %) and phenotypes with fewer alleles than the maternal mother (9·8 %) are more likely to have resulted from matings between genetically similar parents than from selfing or apomixis.
The small sample of S. aucuparia was found to be SI (Supplementary Data Table S2), which is in agreement with previous reports (Raspé et al., 2000; Pías and Guitián, 2006). However, this species was not included in further analyses because it is a minor component of the Avon Gorge Sorbus evolutionary processes compared with S. aria and S. torminalis (Robertson et al., 2010).
While the results from S. torminalis self-pollination suggest an SI, outcrossing mating system, the results of the controlled intraspecific crosses among S. torminalis individuals gave inconclusive results, and it is difficult to draw a firm conclusion from the small sample size. Incompatible results from intraspecific crosses are most probably due to relatedness amongst the trees crossed – S. torminalis is known to reproduce vegetatively via root suckers (Rich et al., 2010), leading to local clonal patches.
Similarly to S. aria, the high genotypic diversity found in progeny of S. torminalis indicates outcrossing: most wild-pollinated S. torminalis progeny (87 %) had non-maternal molecular phenotypes, with 62 % of the progeny displaying non-maternal alleles. The 25 % of S. torminalis progeny displaying a loss of alleles from the maternal molecular phenotype, along with the 12·5 % with maternal molecular phenotypes, probably resulted from matings between genetically similar parents. This is more likely to happen for S. torminalis because of the species' relatively small population size in the Avon Gorge (n = 50) (Robertson et al., 2010). Pollination studies by Rasmussen and Kollmann (2004) in Denmark also showed S. torminalis to be an obligate outcrosser. Interestingly, however, a study by Hoebee et al. (2007) reported seed set via selfing in an isolated stand of S. torminalis in Switzerland, suggesting the potential for a mixed (outcrossing/selfing) mating system, so it is possible that some of the maternal or maternal-like progeny from wild pollinations in the Avon Gorge may have resulted from self-pollination. However, obligate outcrossing through self-incompatibility has also been detected in other diploid Sorbus species, notably S. aucuparia and S. domestica (Raspé et al., 2000; Pías and Guitián, 2006; Kamm et al., 2009), suggesting that self-incompatibility is the usual mating system in sexual diploid Sorbus.
Mating systems in polyploid Sorbus taxa: pseudogamous apomixis
Progeny analyses for five of the triploid species (S. × avonensis, S. bristoliensis, S. leighensis, S. whiteana and S. wilmottiana) revealed extremely low genotypic diversity and that all offspring had multilocus molecular phenotypes identical to their maternal parent, a pattern consistent with obligate apomixis. However, Nogler (1984) suggests that all apomixis is facultative and that sometimes sexual reproduction occurs so rarely that it is nearly undetectable in particular apomictic plants. Indeed there is evidence for a ‘leaky’ (facultative) apomictic mating system in other triploid Sorbus: even though Robertson et al. (2004b) concluded from progeny analysis that triploid S. arranensis is an obligate apomict, they also showed that it is responsible for multiple origins of S. pseudofennica on Arran, Scotland as a result of hybridizations with S. aucuparia (where S. arranensis is the female parent) (Robertson et al., 2004a). Another example is triploid S. leyana which may also be facultatively apomictic (Proctor and Groenhof, 1992) and is believed to have given rise in the wild to hybrid Sorbus × motleyi and in cultivation has been observed to hybridize frequently with other members of subgenus Sorbus (Rich et al., 2010). However, there is as yet no conclusive evidence of hybridizations involving triploid maternal trees occurring commonly in the Avon Gorge (Robertson et al., 2010). The mating system of S. × robertsonii, on the other hand, remains undefined for now. While Rich et al. (2010) reported observing well-formed seeds from this tree and a pollen stainability of 74 %, all seeds collected in 2010 were deformed and empty, suggesting sterility, or a lack of suitable pseudogamous mating events.
A similar situation to the fertile triploids was found in the tetraploids, where 97·3 % of S. eminens offspring, 98·7 % of S. porrigentiformis offspring and 93·3 % of S. × houstoniae offspring had molecular phenotypes identical to their mother trees and very low genotypic diversity (Table 5) – again a pattern consistent with nearly obligate apomixis. However, as suggested previously, perhaps the infrequency of sexual reproduction is making it more challenging to detect (Nogler, 1984). The small numbers of non-maternal tetraploid progeny are consistent with this hypothesis since their molecular phenotypes show a reduction in the number of alleles displayed, which is suggestive of selfing and therefore sexual seed production. An alternative explanation would be mutation(s) in the embryos, but we consider rare sexual reproduction to be a more parsimonious explanation.
Insect exclusion/emasculation experiments showed that the apomictic Sorbus require pollen for seed set, thus confirming that they are all pseudogamous. Similar pollination experiments by Liljefors (1954), who observed pollen tubes in the micropyles of apomicts, also suggest pseudogamy in apomictic Sorbus, as does Jankun and Kovanda's (1987) observation of single-sperm fertilization of the fused central nuclei in triploid S. bohemica. Our ongoing analyses of apomictic Sorbus seeds using flow cytometry and endosperm genotyping further suggests that pseudogamy is the mode of apomixis in polyploid Sorbus (S. Ludwig, University of Bristol, UK, unpubl. res.).
Mating systems in polyploid Sorbus taxa: self-(in)compatibility
Results from self-pollination and intraspecific crosses in the triploid Sorbus species indicate that these triploids are unable to use either self- or conspecific pollen for pseudogamous agamospermy. This observation is intriguing because it implies that the triploids are either SI or male sterile. The latter is unlikely because previous pollen viability tests showed that pollen from triploid Sorbus taxa in the Avon Gorge is viable (see Table 1). Additionally, pollen tube analyses for the present study show that triploid pollen is able to germinate on the stigma, with pollen tubes growing into the style before termination, indicating viability. Thus it appears that the triploid taxa are expressing gametophytic self-incompatibility, and because all individuals of each triploid taxon are genetically identical clones (apomictic single-origin hybrids) they share the same S alleles, making them all interincompatible. It is well known that autopolyploidy and allopolyploidy are usually associated with loss of gametopytic self-incompatibility (resulting in self-compatibility) (Golz et al., 2000; Mable, 2004), so the presence of gametopytic self-incompatibility in triploid Sorbus was unexpected and demands further investigation. Nevertheless, while breakdown of gametopytic self-incompatibility in polyploids has been widely described in the Solanaceae, examples of GSI polyploids, notably Prunus cerasus, have been recorded in the Rosaceae (Hauck et al., 2002), suggesting that the mechanisms of RNase-mediated gametopytic self-incompatibility in the Rosaceae and Solanaceae have fundamental differences (Hauck et al., 2002, 2006).
Despite the general trend of self-incompatibility among Sorbus triploids (Fig. 3), some (most notably S. whiteana) did occasionally produce a few seeds when self-pollinated. While it is possible that this could be the result of pollen contamination, it seems unlikely due to the precautions taken. Instead, seed set resulting from self-pollination could be due to slow but persistent pollen tube growth in ‘incompatible’ pollen tubes, as has been observed in Nicotiana (Lush and Clarke, 1997). Unlike pollen tube analysis, for which flowers are harvested 48 h after pollination, seed set analysis occurs months after pollination, allowing much longer for pollen tube growth and the possibility of self-fertilization of the polar nuclei.
In contrast to the diploid and triploid Sorbus taxa, all tetraploid Sorbus taxa sampled were SC being able to form pollen tubes and set viable seed when pollinated with either self- or conspecific pollen (Fig. 3). The presence of self-compatibility in the tetraploids was entirely predictable because gametopytic self-incompatibility breakdown in polyploids, especially tetraploids, is a well-known phenomenon (Golz et al., 2000; Mable, 2004). The cause for this breakdown of self-incompatibility in derived polyploid species and individuals has not yet been fully resolved (Mable, 2004) but in species with gametopytic self-incompatibility it is widely believed to be the result of ‘competitive interaction’ between different S alleles in polyploid (usually diploid) pollen (Chawla et al., 1997; Golz et al., 2000). This can be explained by the ‘inhibitor model’ for RNase-mediated gametopytic self-incompatibility (Golz et al., 2001; Meng et al., 2011), which proposes that the pollen S gene product (SLF) is, or functions indirectly as, an inhibitor of stylar S-RNases (the female S determinant) but SLF proteins are somehow unable to inhibit the S-RNase with which they are ‘allelic’, i.e. SLF1 cannot inhibit S-RNase1 but can inhibit S-RNases 2, 3, 4 etc. (Meng et al., 2011). Despite the exact mechanism of S-RNase inhibition in compatible pollen–pistil interactions being unclear (McClure et al., 2011), it follows that, in the diploid pollen of a tetraploid with S genotype S1S1S2S2, the presence of both SLF1 and SLF2 will result in self-compatibility by virtue of each SLF protein ‘inhibiting’ its non-allelic S-RNase. Even so, some polyploid plants in the Rosaceae do maintain gametopytic self-incompatibility as tetraploids (Hauck et al., 2002), and gametopytic self-incompatibility appears to be active in the triploid Sorbus taxa sampled, indicating that the maintenance and regulation of gametopytic self-incompatibility in polyploids is far from straightforward.
The significance of interspecific pollen use in Sorbus evolution and the establishment of triploid populations
Crossing experiments showed that the apomictic polyploids are able to utilize pollen from S. aria in pseudogamous seed production. Triploids also appear to be compatible with pollen from S. porrigentiformis. On the other hand, when S. eminens was pollinated by S. torminalis, the combinations were incompatible overall, although the possibility of partial compatibility is suggested by the extent of pollen tube growth in some samples. The finding of only maternal offspring from S. bristoliensis × S. aria and S. porrigentiformis × S. aria crosses provides further evidence that interspecific pollen was used only to fertilize the endosperm and initiate apomictic embryo production in the polyploids.
The use of pollen from a related species or taxon for endosperm formation, though rare, has been reported, for instance in a hexaploid cytotype of Pennisetum setaceum (Simpson and Bashaw, 1969) and in a pollen-sterile facultative triploid of Crataegus (Dickinson and Phipps, 1986; Talent and Dickinson, 2007). In other instances, pseudogamous apomictic plants have the ability rather than requirement to use intertaxon pollen, including Potentilla (Smith, 1963a, b; Asker, 1978; Nyléhn et al., 2003) and Rubus (Haskell, 1960), which could be beneficial when conspecific pseudogamous mates are infrequent or for providing hybrid vigour via heterotic endosperm (Haskell, 1960).
This type of system – one requiring or allowing interspecific pollination for pseudogamous apomixis – increases the potential for interspecific hybridizations. However, molecular analyses of the open-pollinated offspring did not detect any evidence for interspecific hybridization among the Sorbus taxa sampled. This most probaby reflects the rarity of any such hybridizations, or, as the results of the interspecific crosses (diploid × tetraploid) suggest, may reflect intrinsic post-zygotic reproductive barriers, because while compatible pollen tubes were observed, no viable seeds were formed. Abnormalities in seed development from interploidy crosses due to endosperm imbalance have been frequently cited and could be a major contributing factor to seed abortion in these types of Sorbus crosses (Lin, 1984; Haig and Westoby, 1991; Scott et al., 1998; Bushell et al., 2003). Additionally, obligate apomixis in the Avon Gorge triploid taxa provides a strong block to tetraploid formation via a triploid bridge, thus favouring the formation of triploids via a tetraploid bridge.
Established natural populations of SI pseudogamous apomictic plants have not been previously reported, and data on this type of system are extremely rare (Noirot et al., 1997; Stewart-Cox et al., 2005; Hörandl, 2010). It is predicted that the ultimate reason SI pseudogams are unable to become established is a lack of suitable mates, thereby forcing the SI genotype to succumb to extinction via a minority disadvantage (Levin, 1975; Noirot et al., 1997; Hörandl, 2010). Generally, it is assumed that fecundity is reduced in these plants either because they are pollinated with incompatible pollen, or because of seed abortion as a consequence of endosperm imbalance resulting from interploidy crosses (Hörandl, 2010). It is therefore predicted that pseudogamous apomicts must be SC in order for them to overcome mating disadvantages (Noirot et al., 1997). Hörandl (2010) additionally suggests that SI populations could persist if they are facultatively apomictic with a high S-allele diversity, if the endosperm balance requirement is relaxed or if the mating system was to shift to an autonomous system in which pollen is not required for endosperm formation.
We argue that Sorbus present an exception to these assumptions and suggest that the triploid populations were able to become established in spite of their SI pseudogamous mating system primarily because of their compatibility with sympatric S. aria and S. porrigentiformis pollen, facilitated by a relaxed endosperm balance requirement. Normally balanced endosperm in sexually reproducing angiosperms has a maternal:paternal ratio of 2m:1p whereas in triploid Sorbus, the endosperm ratio is usually 3m:1p (6m:2p, both sperm cells used; S. Ludwig, University of Bristol, UK, unpubl. res.) which, though close to 2m:1p, is still considered ‘abnormal’. Clearly, there must be a relaxation of this requirement in triploid Sorbus, as has been found in Crataegus (Talent and Dickinson, 2007).
The triploids also avoid reproductive exclusion by having flowering periods that coincide with S. aria and S. porrigentiformis and by sharing the same pollinators (S. Ludwig, University of Bristol, UK, unpubl. res.). Their relative adult fitness levels are probably high enough to allow them to coexist with other Sorbus species, and via apomixis they produce equally fit progeny to form subsequent generations. Reproductive fitness in the triploids, therefore, could potentially be reduced by a lack of sympatric compatible pollen donors (e.g. if S. aria neighbours do not bloom some years or are cut down/die), lack of pollinator activity or competition with S. aria for pollen if triploids become locally dominant in the population. A negative consequence of having an intertaxon mating requirement is that these triploids will only be able to colonize a new area if a compatible pollen source is already present.
Conclusions
The mating inter-relationships among Avon Gorge Sorbus taxa are complex and their interactions with hybridization and polyploidy appear to be the driving force behind the generation of genetic diversity and continuing evolution of Sorbus in the Gorge. In this first extensive analysis of the mating systems of taxa in this evolving Sorbus ‘syngameon’, we have shown that the two primary diploid species, S. aria and S. torminalis, are SI (GSI) and outcrossing, the polyploid taxa are all pseudogamous apomicts, but while the tetraploid taxa can use their own pollen to sire the endosperm, i.e. they are SC, the triploid taxa cannot, because, like the diploids they are SI, so require pollination by other Sorbus taxa for endosperm formation and successful apomictic seed production.
This apparent dependency of triploid Sorbus on intertaxon pollen for apomictic seed set increases the likelihood of hybridization through fertilization of rare sexual embryo sacs associated with facultative apomixis. This in turn will fuel the continuing evolution of new Sorbus taxa through such rare hybridization and backcrossing events, as proposed by Robertson et al. (2010). This is the most likely explanation for the recent origin of tetraploid S. × houstoniae, from the combination of an unreduced triploid S. bristoliensis egg with a reduced S. aria pollen grain.
Such hybridization events depend heavily on local flowering phenology and pollen dispersal vectors. Flowering of all Sorbus taxa in the Avon Gorge overlaps with the long flowering period of S. aria (S. Ludwig, University of Bristol, UK, unpubl. res.), thereby providing ample opportunity for hybridization events. Observations of Sorbus pollinators (S. Ludwig, University of Bristol, UK, unpubl. res.) indicate that bees and bumble-bees are the most frequent visitors to all Sorbus taxa in the Avon Gorge, as shown for other Sorbus elsewhere (Raspé et al., 2000; Oddou-Muratorio et al., 2006). Further detailed investigation of insect visitation is important to quantify pollen movement within and between Sorbus trees in the Avon Gorge because the type of pollen transfer required differs for each type of mating system: the sexual outcrossing species require pollen movement between individuals; the SC apomictic tetraploids require pollen movement within flowers at the very least; and the SI apomictic triploids require pollen movement between different taxa.
It would seem that triploid Sorbus have survived and multiplied in the Avon Gorge using a mating system ideal for overcoming the challenges of polyploid establishment, and they have therefore escaped extinction by minority exclusion through the ability to use pollen from the most locally abundant diploid and tetraploid species. This group of Sorbus provides opportunities to explore further the relationship between the coexistence of long-lived diploid, triploid and tetraploid plants and the relative fitness of each species. This information could then be used to model fluctuations in species abundance, overall population dynamics and the likelihood for new triploid taxa to become established in the Avon Gorge in relation to their mating systems and fitness levels. In combination with our mating system work, this natural Sorbus system could provide valuable insights into the establishment and persistence of SI polyploid plants.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Bristol City Council, Natural England, The National Trust, and the Avon Gorge and Downs Wildlife Project for collaboration and support, and Jane Memmott for advice on pollinator identification. This work was supported by a research grant from the Leverhulme Trust; a studentship from the National Environment Research Council to S.L.; a small grant from the Botanical Society of the British Isles; and a small grant from the School of Biological Sciences Research Committee, University of Bristol.
LITERATURE CITED
- Abbott RJ, Lowe AJ. A review of hybridization and evolution in British Senecio. In: Hind DJN, Beentje HJ, editors. Compositae, systematics. Kew, UK: Royal Botanic Gardens; 1996. pp. 679–689. [Google Scholar]
- Abbott RJ, Lowe AJ. Origins, establishment and evolution of new polyploid species: Senecio cambrensis and S. eboracensis in the British Isles. Biological Journal of the Linnean Society. 2004;82:467–474. [Google Scholar]
- Abbott RJ, Hegarty MJ, Hiscock SJ, Brennan AC. Homoploid hybrid speciation in action. Taxon. 2010;59:1375–1386. [Google Scholar]
- Ainouche ML, Baumel A, Salmon A, Yannic G. Hybridization, polyploidy and speciation in Spartina (Poaceae) New Phytologist. 2003;161:165–172. [Google Scholar]
- Allen AM, Lexer C, Hiscock SJ. Comparative analysis of pistil transcriptomes reveals conserved and novel genes expressed in dry, wet, and semi-dry stigmas. Plant Physiology. 2010;154:1347–1360. doi: 10.1104/pp.110.162172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold ML. Natural hybridization and evolution. New York: Oxford University Press; 1997. [Google Scholar]
- Ashton PA, Abbott RJ. Multiple origins and genetic diversity in the newly arisen allopolyploidy species, Senecio cambrensis Rosser (Compositae) Heredity. 1992;68:25–32. [Google Scholar]
- Asker S. Pseudogamy, hybridization and evolution in Potentilla. Hereditas. 1978;87:179–184. [Google Scholar]
- Bailey JP, Kay QON, McAllister H, Rich TCG. Chromosome numbers in Sorbus L. (Rosaceae) in the British Isles. Watsonia. 2008;27:69–72. [Google Scholar]
- Briggs D, Walters SM. Plant variation and evolution, 3rd edn. Cambridge, UK: Cambridge University Press; 1997. [Google Scholar]
- Bushell C, Spielman M, Scott RJ. The basis of natural and artificial postzygotic hybridization barriers in Arabidopsis species. The Plant Cell. 2003;15:1430–1442. doi: 10.1105/tpc.010496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla B, Bernatzky R, Liang W, Marcotrigiano M. Breakdown of self-incompatibility in tetraploid Lycopersicon peruvianum: inheritance and expression of S-related proteins. Theoretical and Applied Genetics. 1997;95:992–996. [Google Scholar]
- Chester M, Cowan RS, Fay MF, Rich TCG. Parentage of endemic Sorbus L. (Rosaceae) species in the British Isles – evidence from plastid DNA. Botanical Journal of the Linnean Society. 2007;154:291–304. [Google Scholar]
- Coyne JA, Orr HA. Speciation. Sunderland, MA: Sinauer Associates; 2004. [Google Scholar]
- Dickinson TA, Lo E, Talent N. Polyploidy, reproductive biology, and Rosaceae: understanding evolution and making classifications. Plant Systematics and Evolution 26. 2007;6:59–78. [Google Scholar]
- Dickinson TA, Phipps JB. Studies in Crataegus (Rosaceae: Maloideae) XIV. The breeding system of Crataegus crus-galli sensu lato in Ontario. American Journal of Botany. 1986;73:116–130. doi: 10.1002/j.1537-2197.1986.tb09687.x. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Flagel LE, Paterson AH, et al. Evolutionary genetics of genome merger and doubling in plants. Annual Review of Genetics. 2008;42:443–461. doi: 10.1146/annurev.genet.42.110807.091524. [DOI] [PubMed] [Google Scholar]
- Ellstrand NC, Roose ML. Patterns of genotypic diversity in clonal plant species. American Journal of Botany. 1987;74:123–131. [Google Scholar]
- Golz JF, Clarke AE, Newbigin E. Mutational approaches to the study of self-incompatibility: revisiting the pollen-part mutants. Annals of Botany. 2000;85:95–103. [Google Scholar]
- Golz JF, Oh H-Y, Su V, Kusaba M, Newbigin E. Genetic analysis of Nicotiana pollen-part mutants is consistent with the presence of an S-ribonuclease inhibitor at the S-locus. Proceedings of the National Academy of Sciences, USA. 2001;98:15372–15376. doi: 10.1073/pnas.261571598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant V. Plant speciation. 2nd edn. New York: Columbia University Press; 1981. [Google Scholar]
- Haig D, Westoby M. Genomic imprinting in endosperm: its effect on seed development in crosses between species, and between different ploidies of the same species, and its implications for the evolution of apomixis. Philosophical Transactions of the Royal Society B: Biological Sciences. 1991;333:1–13. [Google Scholar]
- Haskell G. Role of the male parent in crosses involving apomictic Rubus species. Heredity. 1960;14:101–113. [Google Scholar]
- Hauck NR, Yamane H, Tao R, Iezzoni AF. Self-compatibility and incompatibility in tetraploid sour cherry (Prunus cerasus L.) Sexual Plant Reproduction. 2002;15:39–46. [Google Scholar]
- Hauck NR, Yamane H, Tao R, Iezzone AF. Accumulation of nonfunctional S-haplotypes results in the breakdown of gametophytic self-incompatibility in tetraploid Prunus. Genetics. 2006;172:1191–1198. doi: 10.1534/genetics.105.049395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty MJ, Hiscock SJ. Hybrid speciation in plants: new insights from molecular studies. New Phytologist. 2005;165:411–423. doi: 10.1111/j.1469-8137.2004.01253.x. [DOI] [PubMed] [Google Scholar]
- Hegarty MJ, Hiscock SJ. Genomic clues to the evolutionary success of polyploidy plants. Current Biology. 2008;18:R435–R444. doi: 10.1016/j.cub.2008.03.043. [DOI] [PubMed] [Google Scholar]
- Hiscock SJ, McInnis SM. The diversity of self-incompatibility systems in flowering plants. Plant Biology. 2003;5:23–32. [Google Scholar]
- Hoebee SE, Arnold U, Düggelin C, et al. Mating patterns and contemporary gene flow by pollen in a large continuous and a small isolated population of the scattered forest tree Sorbus torminalis. Heredity. 2007;99:47–55. doi: 10.1038/sj.hdy.6800962. [DOI] [PubMed] [Google Scholar]
- Hörandl E. The complex causality of geographical parthenogenesis. New Phytologist. 2006;171:525–538. doi: 10.1111/j.1469-8137.2006.01769.x. [DOI] [PubMed] [Google Scholar]
- Hörandl E. The evolution of self-fertility in apomictic plants. Sexual Plant Reproduction. 2010;23:73–86. doi: 10.1007/s00497-009-0122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörandl E, Paun O. Patterns and sources of genetic diversity in apomictic plants: implications for evolutionary potentials and ecology. In: Hörandl E, Grossniklaus U, Van Dijk PJ, Sharbel T, editors. Apomixis: evolution, mechanisms and perspectives. Ruggell, Liechtenstein: ARG-Gantner; 2007. pp. 169–154. [Google Scholar]
- Jankun A, Kovanda M. Apomixis and origin of Sorbus bohemica (Embryological studies in Sorbus no. 2) Preslia. 1987;59:97–116. [Google Scholar]
- Kamm U, Rotach P, Gugerli F, Siroky M, Edwards P, Holderegger R. Frequent long-distance gene flow in a rare temperate forest tree (Sorbus domestica) at the landscape scale. Heredity. 2009;103:476–482. doi: 10.1038/hdy.2009.70. [DOI] [PubMed] [Google Scholar]
- Kho YO, Baër J. Fluorescence microscopy in botanical research. Zeiss Information. 1971;76:54–57. [Google Scholar]
- Leitch AR, Leitch IJ. Genomic plasticity and the diversity of polyploid plants. Science. 2008;320:481–483. doi: 10.1126/science.1153585. [DOI] [PubMed] [Google Scholar]
- Lemche EB. Darwin College, Cambridge: UK; 1999. The origins and interactions of British Sorbus species. PhD thesis. [Google Scholar]
- Levin DA. Minority cytotype exclusion in local plant populations. Taxon. 1975;24:35–43. [Google Scholar]
- Liljefors A. Studies on propagation, embryology and pollination in Sorbus. Acta Horti Bergiani. 1954;16:277–329. [Google Scholar]
- Lin BY. Ploidy barrier to endosperm development in maize. Genetics. 1984;107:103–115. doi: 10.1093/genetics/107.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lush WM, Clarke AE. Observations of pollen tube growth in Nicotiana alata and their implications for the mechanism of self-incompatibility. Sexual Plant Reproduction. 1997;10:27–35. [Google Scholar]
- Luu DT, Qin X, Morse D, Cappadocia M. S-RNase uptake by compatible pollen tubes in gametophytic self-incompatibility. Nature. 2000;407:649–651. doi: 10.1038/35036623. [DOI] [PubMed] [Google Scholar]
- Mable BK. Polyploidy and self-compatibility: is there an association? New Phytologist. 2004;162:803–811. doi: 10.1111/j.1469-8137.2004.01055.x. [DOI] [PubMed] [Google Scholar]
- McClure BA, Cruz-Garcia F, Romero C. Compatibility and incompatibility in S-RNase-based systems. Annals of Botany. 2011;108:647–658. doi: 10.1093/aob/mcr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Sun P, Kao T-h. S-RNase-based self-incompatibility in Petunia inflata. Annals of Botany. 2011;108:637–646. doi: 10.1093/aob/mcq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson-Jones EB, Briggs D, Smith AG. The origin of intermediate species of the genus Sorbus. Theoretical and Applied Genetics. 2002;105:953–963. doi: 10.1007/s00122-002-0957-6. [DOI] [PubMed] [Google Scholar]
- de Nettancourt D. Incompatibility in angiosperms. Berlin: Springer-Verlag; 1977. [Google Scholar]
- Nogler GA. Gametophytic apomixis. In: Johri BM, editor. Embryology of angiosperms. Berlin: Springer-Verlag; 1984. pp. 475–518. [Google Scholar]
- Noirot M, Couvet D, Hamon S. Main role of self-pollination rate on reproductive allocations in pseudogamous apomicts. Theoretical and Applied Genetics. 1997;95:479–483. [Google Scholar]
- Nyléhn J, Hamre E, Nordal I. Facultative apomixis and hybridization in arctic Potentilla section Niveae (Rosaceae) from Svalbard. Botanical Journal of the Linnean Society. 2003;142:373–381. [Google Scholar]
- Oddou-Muratorio S, Klein EK, Demesure-Musch B, Austerlitz F. Real-time patterns of pollen flow in the wild-service tree, Sorbus torminalis (Rosaceae). III. Mating patterns and the ecological maternal neighborhood. American Journal of Botany. 2006;93:1650–1659. doi: 10.3732/ajb.93.11.1650. [DOI] [PubMed] [Google Scholar]
- Pellicer J, Clermont S, Houston L, Rich TCG, Fay MF. Cytotype diversity in the Sorbus complex (Rosaceae) in Britain: sorting out the puzzle. Annals of Botany. 2012;110:1185–1193. doi: 10.1093/aob/mcs185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pías B, Guitián P. Breeding systems and pollen limitation in the masting tree Sorbus aucuparia L. (Rosaceae) in the NW Iberian Peninsula. Acta Oecologica. 2006;29:97–103. [Google Scholar]
- Preil W. Observing the growth of pollen tubes in pistil and ovarian tissue by means of fluorescence microscopy. Zeiss Information. 1970;75:24–25. [Google Scholar]
- Proctor MCF, Groenhof AC. Peroxidase isozyme and morphological variation in Sorbus L. in South Wales and adjacent areas, with particular reference to S. porrigentiformis E.F. Warb. Watsonia. 1992;19:21–37. [Google Scholar]
- Rasmussen KK, Kollmann J. Poor sexual reproduction on the distribution limit of the rare tree Sorbus torminalis. Acta Oecologica. 2004;25:211–218. [Google Scholar]
- Raspé O, Findlay C, Jacqueman AL. Sorbus aucuparia L. Journal of Ecology. 2000;88:910–930. [Google Scholar]
- Rich TCG. Pollen stainability in British Sorbus L. (Rosaceae) Plant Ecology & Diversity. 2009;2:85–88. [Google Scholar]
- Rich TCG, Harris SA, Hiscock SJ. Five new Sorbus (Rosaceae) taxa from the Avon Gorge, England. Watsonia. 2009;27:217–228. [Google Scholar]
- Rich TCG, Houston L, Robertson A, Proctor MCF. Whitebeams, rowans and service trees of Britain and Ireland. A monograph of British and Irish Sorbus L. London: Botanical Society of the British Isles; 2010. B.S.B.I. Handbook No. 14. [Google Scholar]
- Rieseberg LH. Hybrid origins of plant species. Annual Review of Ecology and Systematics. 1997;28:359–389. [Google Scholar]
- Rieseberg LH, Willis JH. Plant speciation. Science. 2007;317:910–914. doi: 10.1126/science.1137729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson A, Newton AC, Ennos RA. Multiple hybrid origins, genetic diversity and population genetic structure of two endemic Sorbus taxa on the Isle of Arran, Scotland. Molecular Ecology. 2004a;13:123–143. doi: 10.1046/j.1365-294x.2003.02025.x. [DOI] [PubMed] [Google Scholar]
- Robertson A, Newton AC, Ennos RA. Breeding systems and continuing evolution in the endemic Sorbus taxa on Arran. Heredity. 2004b;93:487–495. doi: 10.1038/sj.hdy.6800528. [DOI] [PubMed] [Google Scholar]
- Robertson A, Rich TCG, Allen AM, et al. Hybridization and polyploidy as drivers of continuing evolution and speciation in Sorbus. Molecular Ecology. 2010;19:1675–1690. doi: 10.1111/j.1365-294X.2010.04585.x. [DOI] [PubMed] [Google Scholar]
- Scott RJ, Spielman M, Bailey J, Dickinson HG. Parent-of-origin effects on seed development in Arabidopsis thaliana. Development. 1998;125:3329–3341. doi: 10.1242/dev.125.17.3329. [DOI] [PubMed] [Google Scholar]
- Simpson CE, Bashaw EC. Cytology and reproductive characteristics in Pennisetum setaceum. American Journal of Botany. 1969;56:31–36. [Google Scholar]
- Smith GL. Studies in Potentilla L. I. Embryological investigations into the mechanism of agamospermy in British P. tabernaemontani Aschers. New Phytologist. 1963a;62:264–282. [Google Scholar]
- Smith GL. Studies in Potentilla L. II. Cytological aspects of apomixis in P. crantzii (Cr.) Beck ex Fritsch. New Phytologist. 1963b;62:283–300. [Google Scholar]
- Soltis DE, Soltis PS. Soltis Lab CTAB DNA extraction protocol. 2002 http://www.flmnh.ufl.edu/soltislab/Soltis_site/Protocols.html. [accessed 26 April 2012] [Google Scholar]
- Soltis DE, Soltis PS, Pires JC, Kovarik A, Tate JA, Mavrodiev E. Recent and recurrent polyploidy in Tragopogon (Asteraceae): cytogenetic, genomic and genetic comparisons. Biological Journal of the Linnean Society. 2004;82:485–501. [Google Scholar]
- Soltis PS, Soltis DE. The role of hybridization in plant speciation. Annual Review of Plant Biology. 2009;60:561–588. doi: 10.1146/annurev.arplant.043008.092039. [DOI] [PubMed] [Google Scholar]
- Stebbins GL. Variation and evolution in plants. New York: Columbia University Press; 1950. [Google Scholar]
- Stewart-Cox JA, Britton NF, Mogie M. Space mediates coexistence of females and hermaphrodites. Bulletin of Mathematical Biology. 2005;67:1273–1302. doi: 10.1016/j.bulm.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Talent N, Dickinson TA. Endosperm formation in aposporous Crataegus (Rosaceae, Spiraeoideae, tribe Pyreae): parallels to Ranunculaceae and Poaceae. New Phytologist. 2007;173:231–249. doi: 10.1111/j.1469-8137.2006.01918.x. [DOI] [PubMed] [Google Scholar]
- Urbanska KM, Hurka H, Landolt E, Neuffer B, Mummenhoff K. Hybridization and evolution in Cardamine (Brassicaceae) at Urnerboden, central Switzerland: Biosystematic and molecular evidence. Plant Systematics and Evolution. 1997;204:233–256. [Google Scholar]
- Whitton J, Sears CJ, Baack EJ, Otto SP. The dynamic nature of apomixis in the angiosperms. International Journal of Plant Sciences. 2008;169:169–182. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.