Abstract
Background and Aims
Selective pressures exerted by agriculture on populations of arable weeds foster the evolution of adaptive traits. Germination and emergence dynamics and herbicide resistance are key adaptive traits. Herbicide resistance alleles can have pleiotropic effects on a weed's life cycle. This study investigated the pleiotropic effects of three acetyl-coenzyme A carboxylase (ACCase) alleles endowing herbicide resistance on the seed-to-plant part of the life cycle of the grass weed Alopecurus myosuroides.
Methods
In each of two series of experiments, A. myosuroides populations with homogenized genetic backgrounds and segregating for Leu1781, Asn2041 or Gly2078 ACCase mutations which arose independently were used to compare germination dynamics, survival in the soil and seedling pre-emergence growth among seeds containing wild-type, heterozygous and homozygous mutant ACCase embryos.
Key Results
Asn2041 ACCase caused no significant effects. Gly2078 ACCase major effects were a co-dominant acceleration in seed germination (1·25- and 1·10-fold decrease in the time to reach 50 % germination (T50) for homozygous and heterozygous mutant embryos, respectively). Segregation distortion against homozygous mutant embryos or a co-dominant increase in fatal germination was observed in one series of experiments. Leu1781 ACCase major effects were a co-dominant delay in seed germination (1·41- and 1·22-fold increase in T50 for homozygous and heterozygous mutant embryos, respectively) associated with a substantial co-dominant decrease in fatal germination.
Conclusions
Under current agricultural systems, plants carrying Leu1781 or Gly2078 ACCase have a fitness advantage conferred by herbicide resistance that is enhanced or counterbalanced, respectively, by direct pleiotropic effects on the plant phenology. Pleiotropic effects associated with mutations endowing herbicide resistance undoubtedly play a significant role in the evolutionary dynamics of herbicide resistance in weed populations. Mutant ACCase alleles should also prove useful to investigate the role played by seed storage lipids in the control of seed dormancy and germination.
Keywords: ACCase (acetyl-coenzyme A carboxylase), adaptation, agricultural ecosystem, Alopecurus myosuroides, fitness, grass weed, herbicide resistance, lipid biosynthesis, pleiotropic effect, seed dormancy, survival analysis
INTRODUCTION
Weeds growing in agricultural ecosystems are mostly annual, semelparous species (Baker, 1974). In addition to crop competition, weed populations are subjected to powerful anthropogenic disturbances aiming at eradicating them. These disturbances can be divided into agricultural practices that are applied before or after crop sowing. Pre-sowing practices such as soil cultivation or non-crop-selective herbicide applications eliminate early-germinated weed seedlings. In conventional agriculture, post-sowing practices essentially consist of crop-selective herbicide applications that eliminate weed seedlings germinated after the crop. Selective pressures exerted by agricultural practices foster the evolution of adaptive traits in weed populations. Germination has a prominent role in adaptation, because adequate germination timing allows seedlings to elude pre-sowing practices and to emerge under the best possible conditions for growth (Mortimer, 1997; Andersson et al., 2009). The ability to withstand herbicide effects, i.e. herbicide resistance, is another major adaptive trait (Powles and Yu, 2010). Both traits are crucial for weed success in agricultural ecosystems. Thus, the success of a weed species will depend on its capacity to evolve adequate germination dynamics, herbicide resistance or both, under the pressure of agricultural practices.
Complex factors determine weed seed germination. Germination is driven by both genetic determinants and the environment (Baskin and Baskin, 1998; Nonogaki et al., 2010). Dormancy maintenance and release depend on interactions between hormones. The transition to germination is triggered by signal molecules, among which reactive oxygen species play a key role (Oracz et al., 2007). The genetics of herbicide resistance is only partly elucidated. Herbicide resistance can evolve from mutations at the herbicide target as well as from variation in the weed metabolism pathways (Délye, 2005; Powles and Yu, 2010). It is widely recognized that genes conferring adaptation to a new environment often have pleiotropic effects, i.e. they affect a range of phenotypic traits (Otto, 2004; Pavlicev and Wagner, 2012). Due to antagonistic pleiotropy, plants expressing resistance to environmental stresses often display a fitness cost in the absence of the selective agent (Purrington, 2000). It has been shown that alleles endowing herbicide resistance can have pleiotropic effects on the weed life cycle, and that these effects depend on the allele (Vila-Aiub et al., 2009, 2011). A series of previous studies suggested a possible link between resistance to the major class of grass-specific selective herbicides inhibiting acetyl-coenzyme A carboxylase (ACCase, a key enzyme in fatty acid biosynthesis) and variation in seed germination, emergence and/or survival in the soil (Ghersa et al., 1994; Gill et al., 1996; Vila-Aiub et al., 2005b; Gundel et al., 2008; Menchari et al., 2008; Wang et al., 2010; Owen et al., 2011). A causative link between herbicide resistance and seed germination, emergence and/or survival in the soil would imply that herbicide applications will foster the simultaneous evolution of herbicide resistance and of other traits that are also crucial for weed success in agricultural ecosystems. Yet, none of the previous studies provided definite support for a direct, pleiotropic effect of herbicide resistance alleles. A single study to date suggested that differences in the requirements for seed germination and emergence could be associated with an identified herbicide resistance allele (Vila-Aiub et al., 2005b). However, a single population was used in this study. Association of the herbicide resistance allele with other causative genes via physical linkage or as a result of a genetic bottleneck, or simultaneous selection of resistance and the other traits could therefore not be ruled out.
Controlling the genetic background is essential when attempting to assess the pleiotropic effects of a particular allele (Vila-Aiub et al., 2011; Barrett and Hoekstra, 2012). Yet, using segregating genetic backgrounds cannot fully eliminate effects caused by genes tightly linked to the adaptive allele investigated (Purrington, 2000). Definite evidence for pleiotropy can only be obtained if the phenotypic effects observed are consistent among independent evolutionary origins of the adaptive allele. In the grass weed Alopecurus myosuroides Huds. (black-grass), resistance to herbicides inhibiting ACCase is endowed by a set of mutant ACCase alleles and by as yet unknown non-ACCase-based mechanisms (Délye, 2005; Powles and Yu, 2010). We previously demonstrated that each mutant, resistant ACCase allele originated from multiple independent mutational events in separate A. myosuroides populations (Délye et al., 2004). This provides the unique opportunity to assess the pleiotropic effects of mutant ACCase alleles by controlling both the genetic background and the evolutionary history of populations.
Five mutant ACCase alleles have been identified to date in A. myosuroides: Leu1781, Cys2027, Asn2041, Gly2078 and Ala2096 (Délye, 2005). Earlier studies identified Leu1781 as the most frequent allele (present in approx. 40 % of the populations where resistance evolved) and Gly2078 as the least frequent allele (present in <10 % of the populations where resistance evolved). The other three alleles had similar, intermediate frequencies in A. myosuroides populations (present in approx. 20 % of the populations where resistance evolved) (Menchari et al., 2006; Délye et al., 2010). Here, we investigated the pleiotropic effects of three ACCase alleles (Leu1781, Gly2078 and Asn2041) differing in frequency on the early life cycle stages of A. myosuroides (seed survival in the soil, seed germination and seedling emergence).
MATERIALS AND METHODS
Plant material
Assessment of pleiotropic effects must be performed using individuals sharing a common genetic background, except at the locus of interest (Vila-Aiub et al., 2009). We used batches of A. myosuroides seeds containing embryos that shared a genetic background but were homozygous wild-type (W/W), heterozygous mutant (M/W) or homozygous mutant (M/M) at the ACCase locus. The seed batches were produced in 2004 and 2005, and were the same as those used to assess fitness costs associated with Leu1781, Asn2041 and Gly2078 alleles on the plant-to-seed part of the life cycle of A. myosuroides (Menchari et al., 2008).
Each seed batch was a segregating population obtained by crossing mother plants issued from the same A. myosuroides field sample. Field samples were collected at different localities in eastern France, in which independent mutation events were detected based on ACCase genotyping and sequencing (Menchari et al., 2006). Thirty mother plants were used to produce a segregating population. ACCase genotyping ensured that all mother plants used for producing a segregating population were M/W for the same mutant allele (e.g. Leu1781/wild-type) and did not contain any other known mutant ACCase allele (Menchari et al., 2008). Each group of 30 mother plants was planted in mid-April in a field in Dijon and isolated within a pollen-proof enclosure. All plants flowered simultaneously. Open pollination was allowed within each enclosure. Five segregating populations containing Leu1781 ACCase, three containing Asn2041 ACCase and two containing Gly2078 ACCase, i.e. a total of ten segregating populations, were produced in 2004. For a given mutant ACCase allele, each segregating population derived from a different field (Table 1). The whole procedure was repeated in 2005 so that ten new segregating populations were produced in 2005 (Table 1). The environmental conditions experienced by A. myosuroides plants during the 4 weeks preceding seed shed are critical in determining the degree of seed dormancy (Swain et al., 2006). In order to reduce environment-related heterogeneity in dormancy, a segregating population consisted of the seeds collected on the same day on all plants within an enclosure. All segregating populations produced in 2004 or in 2005 were collected on 18 August or on 26 July, respectively.
Table 1.
Alopecurus myosuroides segregating populations analysed
| Segregating populations produced in: |
No. of seeds used each year for: |
||||
|---|---|---|---|---|---|
| 2004 | 2005 | Field of origin | Segregating ACCase allele | Fresh seed experiments | Buried seed experiments |
| Leu1781-D24-04 | Leu1781-D24-05 | D24 | Leu1781 | 200 | 400 |
| Leu1781-D57-04 | Leu1781-D57-05 | D57 | Leu1781 | 200 | 400 |
| Leu1781-D98-04 | Leu1781-D98-05 | D98 | Leu1781 | 200 | 400 |
| Leu1781-D121-04 | Leu1781-D121-05 | D121 | Leu1781 | 200 | 400 |
| Leu1781-D143-04 | Leu1781-D143-05 | D143 | Leu1781 | 200 | 400 |
| Asn2041-D60-04 | Asn2041-D60-05 | D60 | Asn2041 | 333 | 666 |
| Asn2041-D83-04 | Asn2041-D83-05 | D83 | Asn2041 | 333 | 666 |
| Asn2041-D121-04 | Asn2041-D121-05 | D121 | Asn2041 | 333 | 666 |
| Gly2078-D41-04 | Gly2078-D41-05 | D41 | Gly2078 | 500 | 1000 |
| Gly2078-D83-04 | Gly2078-D83-05 | D83 | Gly2078 | 500 | 1000 |
Fresh seed and buried seed experiments
Two types of seeds were used in our experiments: freshly collected seeds and seeds buried in the soil for 1 year. For each mutant allele, a total of 3000 seeds was used each year and were equally distributed among the populations segregating for this allele (Table 1). They consisted of 1000 seeds intended for the fresh seed experiments, and 2000 seeds intended for the buried seed experiments (Table 1). Seeds intended for the fresh seed experiments were counted and used in seed germination experiments on the day they were collected.
In the field, A. myosuroides seeds can be buried by soil cultivation after crop harvest. Buried seeds will germinate when new soil cultivation is performed after the following crop unearths them, if they survived in the soil. Buried seed experiments were aimed at assessing the effect of mutant ACCase alleles on seed survival in the soil and on subsequent seed germination and emergence. To simulate natural post-maturation after seed shedding, seeds intended for buried seed experiments were allowed to mature for 40 d at room temperature before burial. Seeds from each population were then equally distributed in two sub-batches, mixed with 200 mL of soil from the INRA experimental farm in Bretenières, 13 km south-east of Dijon, and put into woven Tergal bags with a plastic identification label. Bags were subsequently heat sealed and placed at the bottom of 28 cm deep open-work baskets, with four randomly chosen bags per basket. Baskets were buried at approx. 40 cm depth at the INRA experimental farm in Bretenières, to simulate burial by ploughing. Bags prepared in 2004 were buried on 27 September and unearthed on 13 October 2005. Bags prepared in 2005 were buried on 4 September and unearthed on 19 September 2006. Seeds were manually separated from soil and immediately used for germination and pre-emergence growth experiments as described below. Soil was subsequently washed and sieved to recover the seeds not found manually. As washing induces germination (Colbach et al., 2006), the seeds thus recovered were allowed to extend a first leaf for ACCase genotyping, but were not used for germination or pre-emergence growth experiments.
Seed germination
The procedure was adapted from Colbach and Dürr (2003) and Sester et al. (2006). Seeds were laid in polystyrene boxes, directly on pleated paper (113 g m−2, double folds, 110 mm × 20 mm), with two seeds per fold and 100 seeds per box. Each seed was identified by a unique code. After addition of deionized water (40 mL), the boxes were closed hermetically to avoid water losses and placed in a growth chamber.
The expression of pleiotropic effects associated with adaptive alleles varies with the environment, and can be increased under stressful conditions (Vila-Aiub et al., 2009). In order to detect only strong effects of mutant ACCase alleles on seed germination, we used experimental conditions optimal for the germination of A. myosuroides seeds: a constant temperature of 20 °C and constant light (20 µmol m−2 s−1) to release dormancy (Froud-Williams et al., 1984; Colbach et al., 2002). During the following 3–4 months, the boxes were opened periodically. Germinated seeds with a visible rootlet were removed and their removal date registered. Germinated seeds removed from boxes were either immediately used for seedling pre-emergence growth experiments, or allowed to extend a first leaf that was collected in a 0·2 mL microcentrifuge tube labelled with the seed code and stored at –20 °C prior to ACCase genotyping.
When seed germination reached a plateau, all seeds not germinated were transferred to fresh pleated paper strips placed into new boxes. After addition of a gibberellin A3 (Sigma-Aldrich) solution (100 mg L−1, 40 mL per box), the boxes were incubated as before. Seeds germinated under gibberellin action were allowed to extend a first leaf that was collected for ACCase genotyping, but were not considered in our germination dynamics analyses. Seeds not having germinated 35 d after transfer to gibberellin were either viable but deeply dormant, or dead. To assess the proportion of seeds containing a viable embryo, 100 ungerminated seeds equally distributed among the populations segregating for a given allele were dissected each year and for each allele. Embryos with translucent tissues were considered viable, while embryos with brown, necrotic tissues were considered dead.
Seedling pre-emergence growth
This series of experiments was conducted to assess pleiotropic effects of mutant ACCase alleles on the heterotrophic growth phase of the seedlings that occurs in the soil between germination and emergence. We measured the capacity of seedlings to extend roots and shoots using the seed storage compounds as their sole carbon source. The procedure was adapted from Colbach and Dürr (2003) and Sester et al. (2006). Germinating seeds were removed from the germination boxes along with germination date recording and immediately sown 2 cm deep in 8·5 cm diameter pots filled with sand (150–200 µm; bulk density 1·47 kg dm−3). The water content of the pots (20 % w/w) was adjusted with a mineral nutrient solution before seed sowing (Colbach and Dürr, 2003). Five seeds were sown per pot, with the code and position of each seed marked on the pot. The pots were sheathed within a 20 cm high cardboard cylinder closed at the top end, covered with aluminium foil to block light and prevent photosynthesis, and transferred at 20 °C. After 15 d, sheaths were removed and the root and shoot lengths measured for each seedling. The leaf of each seedling, or the root if no leaf developed, was collected in a 0·2 mL microcentrifuge tube labelled with the seed code and stored at –20 °C prior to ACCase genotyping.
ACCase genotyping of seed embryos
Genotyping seed embryos at the ACCase locus requires dissecting the seeds, collecting the embryos and extracting DNA. Genotyping can therefore not be performed prior to germination and pre-emergence growth experiments. Furthermore, attempts at genotyping embryos extracted from dissected ungerminated seeds at the ACCase locus were unsuccessful. Genotyping was therefore performed at the end of the experiments using the leaf or root fragments collected on germinated seeds, and genotypes (W/W, M/W or M/M) were retrospectively assigned to embryos. DNA extraction and genotyping were as described in Délye et al. (2010).
Analysis of germination data
All analyses were performed with R (R Development Core Team, 2012) using the package Survival 2·54. Germination data were analysed following the approach derived from survival analysis recently proposed by Onofri et al. (2010). Survival analysis enables analysis of the timing of events such as germination which occur over time, and to quantify the effects of contributing factors. Germination times were used to calculate the ‘germination probability’, i.e. the probability that a seed germinates after a specific time t, with addition of water to the germination boxes defined as t = 0. The time interval between two successive germination records varied between 1 and 8 d. Data were thus of the ‘interval’ type, as the real germination time occurred between two consecutive records. Seeds not germinated when seed germination reached a plateau were considered ‘right-censored’ observations, i.e. they were expected to germinate at an unknown time after the end of the experiment. Right-censored observations could include some dead seeds. However, assessment of embryo viability based on the dissection of seeds that had not germinated 35 d after addition of gibberellin (see ‘Seed germination’ above) indicated that the overall proportion of seeds containing dead embryos ranged from 1 to 4 %, and was thus unlikely to affect the analysis results. In the buried seed experiments, germinated seeds were found during recovery from the soil. They were considered ‘left-censored’ data, i.e. they germinated at an unknown time before the beginning of the experiment. Germination probabilities as a function of time (germination functions) were estimated using the Kaplan–Meier estimator, as implemented in the R function survfit:
where di is the number of germinations during a given time interval and ni is the number of ungerminated seeds entering the interval minus half the number of seeds having germinated during the interval (Onofri et al., 2010).
As recommended by Onofri et al. (2010), we used accelerated failure time (AFT) regression modelling as implemented in the R function survreg to compare germination functions. Experimental factors accelerating or decelerating germination shift the germination function curves to the left or right, respectively. AFT modelling assumes a linear relationship between the logarithm of time to germination and the underlying factors. As a parametric method, AFT modelling requires that an underlying distribution is defined for the germination function. Four distribution models were tested, i.e. exponential, Weibull, log-logistic and log-normal. The best distribution model was selected based on Akaike's information criterion (AIC). Model fit was checked graphically by comparing the fitted curves with those obtained using the Kaplan–Meier estimator.
Our aim was to assess the effect of the genotype at the ACCase locus on seed germination for each mutant allele investigated. AFT modelling was performed separately for the fresh and buried seed experiments, and for each mutant ACCase allele. We first considered a full model including all explanatory variables of interest, i.e. year (two levels), population (2–5 levels, Table 1) and embryo genotype (three levels, W/W, W/M or M/M), and their interactions. The experimental unit (germination box) was considered a clustering random effect and included in the model using the R frailty function. Full models were subsequently simplified by eliminating variables with non-significant effects, based on log-likelihood ratio tests. When a significant overall genotype effect was detected, the contrasting genotypes were identified by pairwise comparisons of the germination function of each genotype with those of the other two genotypes. Significant differences were assessed after sequential Bonferroni correction.
Analysis of emergence data
Seed germination does not necessarily result in successful seedling emergence. In our pre-emergence growth experiments, some germinated seeds showed no subsequent elongation of the shoot, or of both the root and the shoot. These seeds were classified as fatal germinations, i.e. seeds clearly having no chance of reaching the soil surface (Davis and Renner, 2007). The effect of genotype on germination success, a binary response variable (fatal or successful), was analysed using generalized linear modelling with a logit link function and a binomial distribution of error terms. The population and the year in which the experiment was conducted were included as random factors using the glmer function in the R package lmer4. The main effects of random factors and their interaction with genotype were assessed. When a significant overall genotype effect was detected, the contrasting genotypes were identified by pairwise comparisons of the germination success of each genotype with those of the other two genotypes. Significant differences were assessed after sequential Bonferroni correction.
The leaf and root lengths of successfully germinated seeds were square-root transformed to meet analysis of variance (ANOVA) assumptions, and the genotype effect was analysed using linear mixed-effect modelling, using the same model terms as for germination success.
RESULTS
Embryo genotypes at the ACCase locus
Attempts at genotyping embryos from ungerminated seeds were unsuccessful. Thus, only embryos from germinated seeds could be genotyped. Above 80 % of the fresh seeds used germinated and could be used for genotyping (Supplementary Data Table S1), except in populations Leu1781-D121-04 and Asn2041-D121-04, which contained high proportions of ungerminated seeds. Depending on the population, 9·5–61·5 % of the seeds buried in 2004 or in 2005 could be recovered, germinated and could be used for genotyping. A total of 9328 seedlings were thus genotyped. The number of seeds genotyped and genotyping results are given in detail in Supplementary Data Table S1.
No deviation from the expected proportions of W/W (25 %), M/W (50 %) and M/M (25 %) embryos was detected in most populations (Supplementary Data Table S1). A significant deficit in M/W embryos was observed in the buried seeds from population Leu1781-D121-04 (χ2 = 9·65; P < 0·01). A significant deficit in M/M embryos together with a significant excess of W/W embryos was observed in the buried seeds from population Leu1781-D143-05 (χ2 = 6·23; P < 0·05), in the fresh seeds from populations Gly2078-D41-04 and Gly2078-D83-04 (χ2 = 39·12; P < 0·001 and χ2 = 15·58; P < 0·001, respectively), and in the buried seeds from population Gly2078-D83-04 (χ2 = 6·01; P < 0·05).
Seed germination
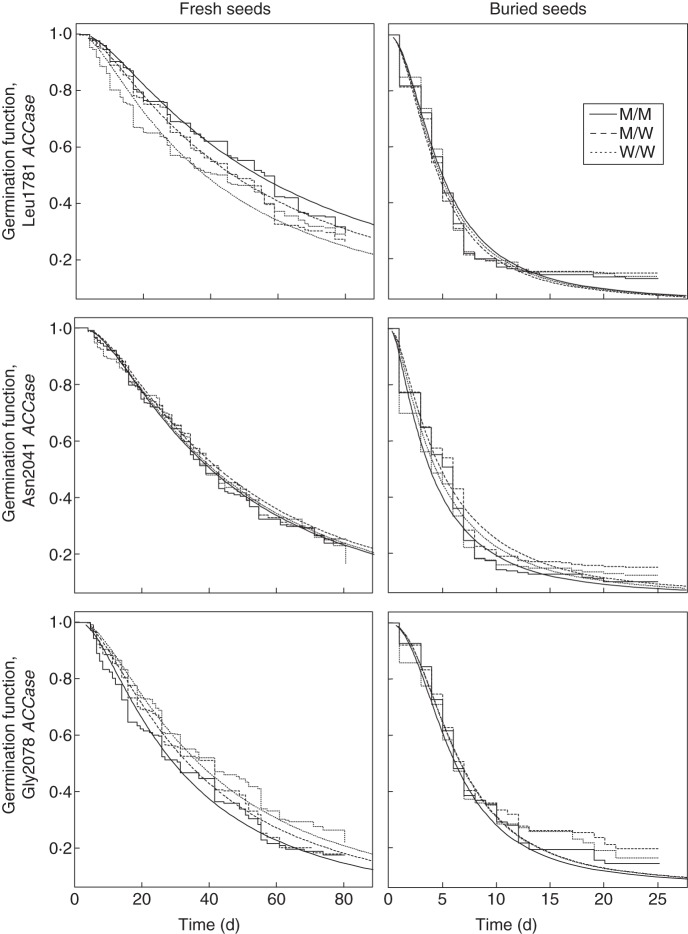
Seed dormancy was greatly reduced after burial, with substantially faster germination. The seed germination plateau was reached in 81 d for fresh seeds, and in 10–15 d for buried seeds (Fig. 1). In AFT modelling, the most appropriate distributions were always log-normal or log-logistic (Table 2). Visual comparison of the Kaplan–Meier germination functions with AFT models indicated an adequate fit (illustrated in Fig. 1).
Fig. 1.
Kaplan–Meier estimates of germination functions (step curves) together with the best fitted AFT models (continuous curves). Left column, fresh seed germination; right column, buried seed germination.
Table 2.
Estimates of T50 (time to 50 % germination) obtained for each ACCase genotype using accelerated failure time (AFT) modelling
|
T50 estimates (d)* |
|||||
|---|---|---|---|---|---|
| Experiment | ACCase allele | Best fitting AFT model | W/W | M/W | M/M |
| Fresh seeds | Leu1781 | Log-normal | 38·4 (3·0)a | 46·7 (3·3)b | 54·2 (4·5)b |
| Asn2041 | Log-normal | 43·6 (3·5)a | 46·4 (3·3)a | 44·6 (3·6)a | |
| Gly2078 | Log-normal | 40·0 (2·0)a | 36·5 (1·6)ab | 31·9 (2·0)b | |
| Buried seeds | Leu1781 | Log-logistic | 4·8 (0·4)a | 4·4 (0·4)a | 4·6 (0·4)a |
| Asn2041 | Log-normal | 3·6 (0·2)a | 4·6 (0·2)b | 4·1 (0·2)ab | |
| Gly2078 | Log-logistic | 5·8 (0·4)a | 6·3 (0·4)a | 6·3 (0·4)a | |
*The s.e. is given in parentheses. Identical letters on the same line indicate T50 values not significantly different at P < 0·05 after sequential Bonferroni correction.
AFT modelling (Supplementary Data Tables S2 and S3) identified a significant year effect on the germination function in all cases except buried seeds from populations segregating for Leu1781 ACCase. A significant population effect was not systematically observed. Overall, seeds produced in 2005 germinated faster than seeds produced in 2004. Time to reach 50 % germination (T50) values for fresh seeds were 38·7 and 46·0 d in 2005 and in 2004, respectively. T50 values were 4·9 and 6·2 d for buried seeds in 2005 and in 2004, respectively. A significant effect of the embryo genotype on the germination function was observed for fresh seeds from populations segregating for Leu1781 or Gly2078 ACCase, and for buried seeds from populations segregating for Asn2041 ACCase. The effect of the interaction of the embryo genotype with the other variables was never significant.
Pairwise comparisons of the germination functions of the different embryo genotypes (M/M, M/W and W/W) showed that, in fresh seeds from populations segregating for Leu1781 ACCase, germination of seeds containing M/M and M/W embryos was strongly delayed compared with seeds containing W/W embryos (Fig. 1, P < 0·001 and P < 0·01 for M/M and M/W embryos, respectively). In these populations, there was an average increase of 15·8 and 8·3 d in the T50 of seeds containing M/M and M/W embryos, respectively, compared with seeds containing W/W embryos (Table 2). This corresponded to a 1·41- and a 1·22-fold increase, respectively, in T50. In fresh seeds from populations segregating for Gly2078 ACCase, germination of seeds containing M/M embryos was noticeably accelerated compared with seeds containing W/W embryos (P < 0·01), while germination of seeds containing M/W embryos was intermediate (Fig. 1). In these populations, there was an average decrease of 8·1 and 3·5 d in the T50 of seeds containing M/M and M/W embryos, respectively, compared with seeds containing W/W embryos (Table 2). This corresponded to a 1·25- and a 1·10-fold decrease, respectively, in T50. Finally, in buried seeds from populations segregating for Asn2041 ACCase, germination of seed containing M/W embryos was delayed compared with seeds containing W/W embryos (P < 0·001), while germination of seeds containing M/M embryos was intermediate (Fig. 1). In these populations, there was an average increase of 1 and 0·5 d in the T50 of seeds containing M/W and M/M embryos, respectively, compared with seeds containing W/W embryos (Table 2). This corresponded to a 1·06- and a 1·02-fold increase, respectively, in T50.
Fatal germinations
Overall, a significant year or population effect was not systematically observed (Supplementary Data Tables S4 and S5). A significant effect of the embryo genotype on the proportion of fatal germinations was observed in fresh seeds from populations segregating for Leu1781 ACCase, and in buried seeds from populations segregating for Asn2041 ACCase. The effect of the interaction of the embryo genotype with the other variables was never significant, except in fresh seeds from populations segregating for Gly2078 ACCase, where a significant year × genotype interaction was observed.
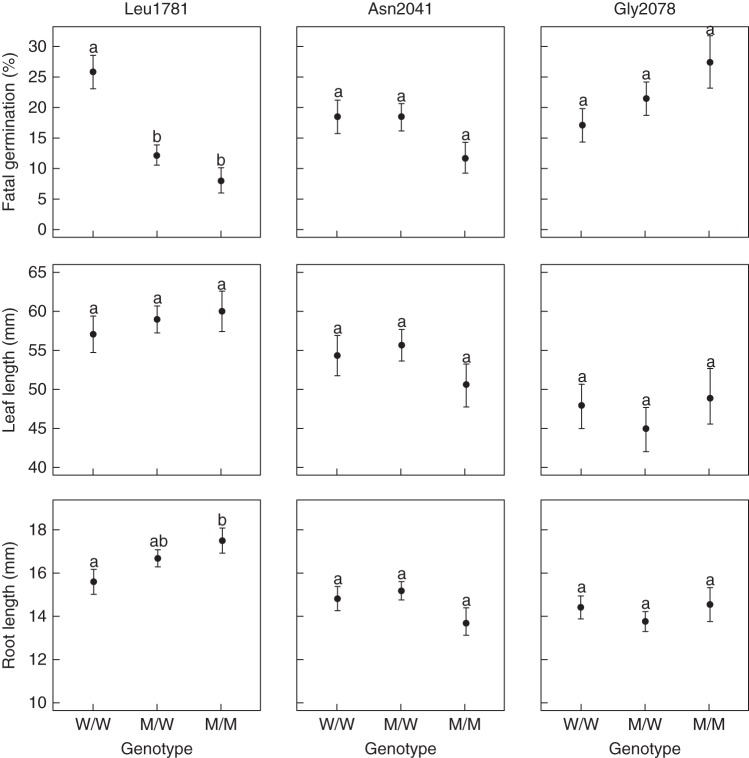
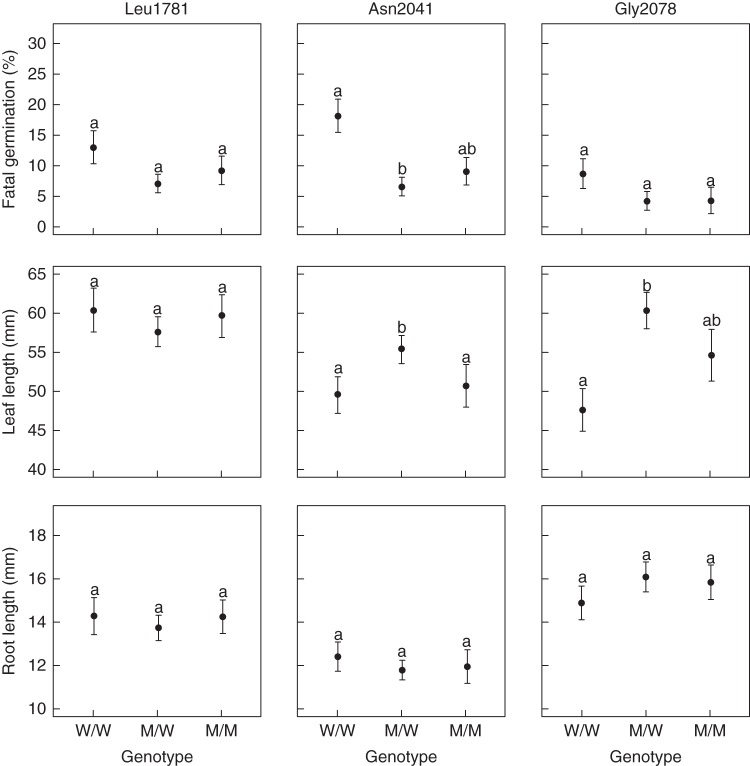
Pairwise comparisons of the different embryo genotypes in fresh seeds from populations segregating for Leu1781 ACCase showed a significantly lower frequency of fatal germinations in seeds containing M/M and M/W embryos compared with seeds containing W/W embryos (Fig. 2, top; P < 0·001). In these populations, there was a 3·24- and a 2·12-fold reduction in fatal germination in seeds containing M/M and M/W embryos compared with seeds containing W/W embryos, respectively. In buried seeds from populations segregating for Asn2041 ACCase, seeds containing M/W embryos showed a significantly lower frequency of fatal germinations than seeds containing W/W embryos (P < 0·01), while the frequency of fatal germination of seeds containing M/M embryos was intermediate (Fig. 3, top). There was a 2·71- and a 1·36-fold decrease in fatal germination in seeds containing M/W and M/M embryos compared with seeds containing W/W embryos, respectively.
Fig. 2.
Fresh seed fatal germination and emergence. Mean value ± s.e. of the proportion of fatal germination (top), leaf length (middle) and root length (bottom) computed for each category of embryo genotype and each mutant ACCase allele investigated. Identical letters within one graph indicate values not significantly different at the P < 0·05 threshold after χ2 tests (fatal germination) or F-tests (leaf or root length) with Bonferroni correction of pairwise comparisons.
Fig. 3.
Buried seed fatal germination and emergence. Mean value ± s.e. of the proportion of fatal germination (top), leaf length (middle) and root length (bottom) computed for each category of embryo genotype and each mutant ACCase allele investigated. Identical letters within one graph indicate values not significantly different at the P < 0·05 threshold after χ2 tests (fatal germination) or F-tests (leaf or root length) with Bonferroni correction of pairwise comparisons.
Although a genotype effect was not significant in fresh seeds from populations segregating for Gly2078 ACCase, a significant year × genotype interaction was observed (P < 0·001; Supplementary Data Table S4). We therefore analysed the germination success of the different embryo genotypes in these populations for each year separately. In 2004, the genotype effect was non-significant (P = 0·06). In 2005, the genotype effect was highly significant overall (P < 0·001) and for each pairwise comparison between genotypes (P < 0·001, P < 0·05 and P < 0·01 for M/M vs. W/W, M/W vs. W/W and M/M vs. M/W, respectively). There was an 8·81- and a 4·64-fold increase in fatal germination in seeds containing M/M and M/W embryos compared with seeds containing W/W embryos, respectively.
Seedling pre-emergence growth
Overall, no significant year or population effect on leaf or root length was systematically observed. The effect of the interaction of the embryo genotype with the other variables was never significant (Supplementary Data Tables S6–S9).
A significant effect of the embryo genotype on leaf length was only observed in buried seeds from populations segregating for Asn2041 or for Gly2078 ACCase (Supplementary Data Tables S6 and S7). Pairwise comparisons of the different embryo genotypes in buried seeds from populations segregating for Asn2041 ACCase showed a significantly higher leaf length for seeds containing M/W embryos compared with seeds containing M/M or W/W embryos (Fig. 3, middle, P < 0·05), with a 1·09- and a 1·12-fold increase in leaf length in seeds containing M/W embryos compared with seeds containing M/M and W/W embryos, respectively. In buried seeds from populations segregating for Gly2078 ACCase, seeds containing M/W embryos showed a higher leaf length compared with seeds containing W/W embryos (P < 0·001), while leaf length of seeds containing M/M embryos was intermediate (Fig. 3, middle). In these populations, there was a 1·28- and a 1·15-fold increase in leaf length in seeds containing M/W and M/M embryos compared with seeds containing W/W embryos, respectively.
A significant effect of the embryo genotype on root length was only observed for fresh seeds from populations segregating for Leu1781 ACCase (Supplementary Data Tables S8 and S9). Pairwise comparisons of the different embryo genotypes showed a significantly higher root length for seeds containing M/M embryos compared with seeds containing W/W (P < 0·05), while root length of seeds containing M/W embryos was intermediate (Fig. 2, bottom). There was a 1·12- and a 1·07-fold increase in root length in seeds containing M/M and M/W embryos compared with seeds containing W/W embryos, respectively.
DISCUSSION
Direct effects of resistance alleles on germination and emergence
Several previous studies investigated a possible link between resistance to ACCase inhibitors and variation in seed germination dynamics without reaching definite conclusions (Ghersa et al., 1994; Gill et al., 1996; Vila-Aiub et al., 2005b; Gundel et al., 2008; Menchari et al., 2008; Wang et al., 2010; Owen et al., 2011). This is probably because none of these studies compared plants sharing a genetic background and containing a known copy number of a single, clearly identified resistance allele using several different genetic backgrounds and evolutionary histories, as we did here. All the populations used in our study were field populations that underwent selection by ACCase-inhibiting herbicides. No reduction in the genome-wide genetic diversity of these populations was observed when compared with ‘naive’, unselected populations (Menchari et al., 2007). Thus, the pleiotropic effects observed here were not influenced by a loss of diversity at the segregating genetic background. Each mutant ACCase allele used in this study had been shown to evolve from independent mutational events in different A. myosuroides populations (Délye et al., 2004). We accordingly sampled A. myosuroides in different fields to generate the segregating populations studied here. Different evolutionary origins of the same mutant ACCase allele consistently endowed the same pleiotropic effects, whatever the population of origin, indicating that pleiotropic effects were stable across independent evolutionary histories. It is thus extremely unlikely that the effects we observed were systematically due to loci specifically linked to each mutant ACCase allele. This work is therefore the first clear and unambiguous demonstration of a direct effect of herbicide resistance alleles (i.e. mutant ACCase alleles) on seed germination dynamics and seedling emergence.
Pleiotropic effects depend on the ACCase allele
In buried seeds, Asn2041 ACCase showed a substantial overdominant reduction in fatal germination. The biological significance of these differences is not obvious, because they were only observed in seeds having sojourned 1 year in the soil. When seeds were not protected in Tergal bags as in our experiments, A. myosuroides annual seed decline rate values were estimated to be 73–83 % (Moss, 1985). Thus, the differences observed may not be of perceptible biological and ecological significance in the field. No other phenotypic differences due to Asn2041 ACCase have been identified here and in a previous work (Menchari et al., 2008). Phenotypic changes associated with this type of mutant ACCase allele can thus be considered extremely limited, and not likely to confer substantial fitness variation during the A. myosuroides life cycle.
As previously observed (Menchari et al., 2008) in populations segregating for Gly2078 ACCase, a segregation distortion against M/M embryos was observed in the seeds produced in 2004 only. A co-dominant substantial increase in fatal germination was observed in 2005 only. In 2004, during the 4 week period before seed shed that is crucial for A. myosuroides seed viability (Swain et al., 2006), temperatures were higher (+3·2 °C on average) and rainfall lower (21·6 mm vs. 57·8 mm) than during the same period in 2005. Peaks of high temperatures (>30 °C during 1–4 h) were observed on several occasions in 2005 during the crucial 4 week period before seed shed. Stressful conditions at critical periods may be responsible for the observed variation of pleiotropic effects. More importantly, in fresh seeds, Gly2078 ACCase caused a co-dominant acceleration in germination. Gly2078 ACCase had previously been shown to confer a substantial, recessive reduction in plant biomass and seed output under stressful conditions (Menchari et al., 2008). Here, we showed that Gly2078 ACCase also had pleiotropic effects on seed germination and emergence, and that these effects seemed to vary with the environmental conditions prevailing during seed development.
Leu1781 ACCase caused a substantial co-dominant delay in germination, and a substantial co-dominant decrease in fatal germination of fresh seeds. Delayed germination due to Leu1781 ACCase had previously been proposed in the grass weed Lolium rigidum. It could not definitely be proved because the increase in T50 observed was slight and a single population had been studied (Vila-Aiub et al., 2005b). Previous studies controlling genetic background showed a non-significant trend for plants carrying Leu1781 ACCase to produce more seeds and biomass than W/W plants (Vila-Aiub et al., 2005a; Menchari et al., 2008). A recent study using a controlled genetic background in the grass Setaria sp. reported an increase in vigour, tolerance to stress and seed output, together with earlier flowering in plants containing Leu1781 ACCase (Wang et al., 2010). The authors used a single evolutionary origin of Leu1781 ACCase and could not reach a conclusion on a direct effect of Leu1781 ACCase. However, the consistent trends observed across grass species in the literature (Vila-Aiub et al., 2005a; Menchari et al., 2008; Wang et al., 2010) would suggest so. Furthermore, an increase in vigour caused by Leu1781 ACCase would be consistent with the reduction in fatal germination observed here for seeds with embryos carrying Leu1781 ACCase. In this hypothesis, Leu1781 ACCase consistently causes delayed seed germination, a reduction in fatal germination and most probably an increase in vigour and in seed and biomass production.
Our results therefore show that pleiotropic effects directly associated with mutant ACCase alleles vary substantially with the allele, and can also vary with the environment. Such results are not unique to plants. The deleterious effects on winter survival of four mutant alleles conferring the same adaptation (insecticide resistance) in the mosquito Culex pipiens had been shown to vary with the allele. For one allele, the pleiotropic effects observed also varied with the environment (Chevillon et al., 1997). In the yeast Saccharomyces cerevisiae, the pleiotropic effects of several mutant alleles endowing fungicide resistance were studied individually for each of several genes. The pleiotropic effects associated with resistance alleles could be positive or deleterious in a given environment, depending on the allele. When several environmental conditions were assessed, the pleiotropic effects associated with each allele could also be positive or deleterious, depending on the environment (Gerstein et al., 2012). Variation of associated pleiotropic effects with the resistance allele and with the environment may be thus be a general rule.
Fitness effect of mutant ACCase alleles in agricultural ecosystems
Our experimental conditions were designed to be optimal for seed germination. They were not representative of environmental conditions in the field, where resistance evolves. Yet, strong phenotypic effects were still observed under these experimental conditions, especially on seed germination. It can thus reasonably be expected that the effects we observed would also occur in the field. This is further supported by the consistency of the mutant ACCase pleiotropic effects observed across different grass species and in different experimental systems here and in previous works (Vila-Aiub et al. 2005a, b; Menchari et al., 2008; Wang et al., 2010). This suggests the results obtained on A. myosuroides are of general value.
Success of a weed species in agricultural ecosystems depends on its capacity successfully to withstand or escape pre-sowing practices and post-sowing herbicide applications. Weed seed germination in a given weed population is not synchronous (Froud-Williams et al., 1984; Mortimer, 1997). Pre-sowing practices destroy seedlings issued from early-germinating seeds. Post-sowing herbicide applications destroy herbicide-sensitive seedlings. The mutant ACCase alleles studied here all confer the capacity to survive application of the ACCase inhibitors most widely used in Europe (Délye et al., 2008). They also directly cause various phenotypic and phenological changes, especially in seed germination dynamics. Seedlings carrying Leu1781 ACCase show a delayed germination: the fitness advantage conferred by herbicide resistance is expected to be enhanced by a higher probability of escaping pre-sowing cultural practices. This advantage is further enhanced by the decrease in fatal germination conferred by this allele. In contrast, seedlings carrying Gly2078 ACCase show earlier germination: the fitness advantage conferred by herbicide resistance is expected to be counterbalanced by a higher probability to be destroyed by pre-sowing cultural practices. These differences are expected to be further enhanced during a grass weed life cycle by an increased vigour and tolerance to environmental stresses associated with Leu1781 ACCase, and a higher sensitivity to stresses associated with Gly2078 ACCase (Menchari et al., 2008).
The pleiotropic effects of the three mutant ACCase alleles studied are consistent with their observed frequencies in the field (Menchari et al., 2006; Délye et al., 2010). Gly2078 ACCase that causes potentially deleterious effects is the least frequent allele, while Leu1781 ACCase that causes potentially favourable effects is by far the most frequent allele. The idea that resistance endows fitness costs is very widespread (Purrington, 2000). Here, we demonstrated that different resistance mutations at the same gene had different, contrasting pleiotropic effects, with one of them most probably causing a fitness advantage rather than a fitness cost under current, conventional agricultural systems. Previous studies showed that, in A. myosuroides populations where resistance due to mutant ACCase has evolved, a single type of mutant ACCase allele is generally present (Menchari et al., 2006; Délye et al., 2010). Our results therefore suggest that the particular mutation acquired by a population during adaptation to herbicide application will alter its future evolutionary pathway. Pleiotropic effects must therefore be taken into account when trying to predict the outcome of herbicide selective pressure, for instance by incorporation into agronomic models (e.g. Colbach et al., 2006) to estimate the effects of practices for managing as well as minimizing the risk for selection of herbicide resistance. This will enable diversification of weed control programmes, which is the only way towards lasting weed control efficacy (Menchari et al., 2008; Birch et al., 2011).
Implications of this study for the relationship between lipid biosynthesis and seed germination
As with most herbicides, ACCase inhibitors target a crucial step in plant metabolism (Délye, 2005; Powles and Yu, 2010). ACCase is a key regulatory enzyme in fatty acid biosynthesis (Ohlrogge and Jaworski, 1997). In grass seeds, lipids are major storage components of the embryo (reviewed in Barthole et al., 2012). Lipids can also represent a substantial part of the storage compounds in the albumen, especially for grasses in the family Avenaceae (Banaś et al., 2007), which includes A. myosuroides. The quantity of storage lipids has an influence on the energy available for seedling emergence, and the nature of storage lipids affects the dynamics of lipid peroxidation that is involved in dormancy release (Oracz et al., 2007). Acetyl-CoA, the substrate of ACCase, cannot cross the cytoplasmic membrane. The biosynthesis of storage lipids thus takes place in the developing seed, where it is essentially controlled by the embryo (Neuberger et al., 2008; Alonso et al., 2010). Thus, changes in seed storage lipid contents due to pleiotropic effects of herbicide-resistant ACCase alleles contained in the embryo genome could explain the variation in seed germination and emergence we observed.
Previous studies compared the enzyme activity of isoforms encoded by mutant ACCase alleles with that of wild-type ACCase. They reported no differences for Leu1781 ACCase, no difference or a slight decrease in activity for Asn2041 ACCase, and a strong reduction in activity for Gly2078 ACCase (Marles et al., 1993; Shukla et al., 1997; Délye et al., 2003, 2005; Yu et al., 2007). Impairment of ACCase activity due to ACCase inhibitors has been shown to generate active oxygen species (Cummins et al., 1999). It is possible that altered activity of Gly2078 ACCase could also cause active oxygen species release, which could enhance or accelerate lipid peroxidation and accelerate dormancy release. However, effects of mutant ACCase alleles on the quantitative and qualitative composition of seed storage lipids and on its peroxidation over time have not been investigated to date, and would require the production of appropriate weed lines producing seeds with controlled embryo genotypes.
In conclusion, we demonstrated direct effects of the selection for herbicide resistance on other traits crucial for weed seedling establishment. Depending on the ACCase mutation and the selective pressure exerted by cultural practices, these effects may enhance or counterbalance the selective advantage conferred by herbicide resistance. Different mutant ACCase alleles have contrasting effects on seed germination and seedling emergence. Our results therefore contribute to a growing body of evidence showing a key role for seed storage lipids in controlling seed dormancy and seedling emergence (Oracz et al., 2007). One final consequence of this study is the availability of mutant ACCase alleles selected in natura that should prove useful for biochemical or physiological studies aiming at a better understanding of the role played by seed storage lipids in the control of seed dormancy and germination.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
This work was supported by funding from INRA, Département Santé des Plantes et Environnement and the Conseil Régional de Bourgogne.
LITERATURE CITED
- Alonso AP, Dale VL, Sachar-Hill Y. Understanding fatty acid synthesis in developing maize embryos using metabolic flux analysis. Metabolic Engineering. 2010;12:488–497. doi: 10.1016/j.ymben.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Andersson L, Åkerblom Espeby L. Variation in seed dormancy and light sensitivity in Alopecurus myosuroides and Apera spica-venti. Weed Research. 2009;49:261–270. [Google Scholar]
- Baker HG. The evolution of weeds. Annual Review of Ecology and Systematics. 1974;5:1–24. [Google Scholar]
- Banaś A, Dexbski H, Banaś W, et al. Lipids in grain tissues of oat (Avena sativa): differences in content, time of deposition, and fatty acid composition. Journal of Experimental Botany. 2007;58:2463–2470. doi: 10.1093/jxb/erm125. [DOI] [PubMed] [Google Scholar]
- Barrett RDH, Hoekstra HE. Molecular spandrels: tests of adaptation at the genetic level. Nature Reviews Genetics. 2011;12:767–780. doi: 10.1038/nrg3015. [DOI] [PubMed] [Google Scholar]
- Barthole G, Lepiniec G, Rogowsky PM, Baud S. Controlling lipid accumulation in cereal grains. Plant Science. 2012;185–186:33–39. doi: 10.1016/j.plantsci.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Baskin CC, Baskin JM. Seeds: ecology, biogeography, and evolution of dormancy and germination. San Diego: Academic Press; 1998. [Google Scholar]
- Birch ANE, Begg GS, Squire GR. How agro-ecological research helps to address food security issues under new IPM and pesticide reduction policies for global crop production systems. Journal of Experimental Botany. 2011;62:3251–3261. doi: 10.1093/jxb/err064. [DOI] [PubMed] [Google Scholar]
- Chevillon C, Bourguet D, Rousset F, Pasteur N, Raymond M. Pleiotropy of adaptive changes in populations: comparisons among insecticide resistance genes in Culex pipiens. Genetical Research. 1997;70:195–204. doi: 10.1017/s0016672397003029. [DOI] [PubMed] [Google Scholar]
- Colbach N, Dürr C. Effects of seed production and storage conditions on blackgrass (Alopecurus myosuroides) germination and shoot elongation. Weed Science. 2003;51:708–717. [Google Scholar]
- Colbach N, Chauvel B, Dürr C, Richard G. Effect of environmental conditions on Alopecurus myosuroides germination. I. Effect of temperature and light. Weed Research. 2002;42:210–221. [Google Scholar]
- Colbach N, Dürr C, Roger-Estrade J, Chauvel B, Caneill J. AlomySys: modelling black-grass (Alopecurus myosuroides Huds.) germination and emergence, in interaction with seed characteristics, tillage and soil climate. I. Construction. European Journal of Agronomy. 2006;24:95–112. [Google Scholar]
- Cummins I, Cole DJ, Edwards R. A role for glutathione transferases functioning as glutathione peroxidases in resistance to multiple herbicides in black-grass. The Plant Journal. 1999;18:285–292. doi: 10.1046/j.1365-313x.1999.00452.x. [DOI] [PubMed] [Google Scholar]
- Davis AS, Renner KA. Influence of seed depth and pathogens on fatal germination of velvet leaf (Abutilon theophrasti) and giant foxtail (Setaria faberi) Weed Science. 2007;55:30–35. [Google Scholar]
- Délye C. Weed resistance to acetyl coenzyme A carboxylase inhibitors: an update. Weed Science. 2005;53:728–746. [Google Scholar]
- Délye C, Zhang X-Q, Chalopin C, Michel S, Powles SB. An isoleucine residue within the carboxyl-transferase domain of multidomain acetyl-CoA carboxylase is a major determinant of sensitivity to aryloxyphenoxypropionate but not to cyclohexanedione inhibitors. Plant Physiology. 2003;132:1716–1723. doi: 10.1104/pp.103.021139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Délye C, Straub C, Michel S, Le Corre V. Nucleotide variability at the acetyl coenzyme A carboxylase gene and the signature of herbicide selection in the grass weed Alopecurus myosuroides (Huds.) Molecular Biology and Evolution. 2004;21:884–892. doi: 10.1093/molbev/msh095. [DOI] [PubMed] [Google Scholar]
- Délye C, Zhang X-Q, Michel S, Matéjicek A, Powles SB. Molecular bases for sensitivity to acetyl-coenzyme A carboxylase inhibitors in black-grass. Plant Physiology. 2005;137:794–806. doi: 10.1104/pp.104.046144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Délye C, Matéjicek A, Michel S. Cross-resistance patterns to ACCase-inhibiting herbicides conferred by mutant ACCase isoforms in Alopecurus myosuroides Huds. (black-grass), re-examined at the recommended herbicide field rate. Pest Management Science. 2008;64:1179–1186. doi: 10.1002/ps.1614. [DOI] [PubMed] [Google Scholar]
- Délye C, Michel S, Bérard A, et al. Geographical variation in resistance to acetyl-coenzyme A carboxylase-inhibiting herbicides across the range of the arable weed Alopecurus myosuroides (black-grass) New Phytologist. 2010;186:1005–1017. doi: 10.1111/j.1469-8137.2010.03233.x. [DOI] [PubMed] [Google Scholar]
- Froud-Williams RJ, Drennan DSH, Chancellor RJ. The influence of burial and dry storage upon cyclic changes in dormancy, germination and response to light in seeds of various arable weeds. New Phytologist. 1984;96:473–481. [Google Scholar]
- Gerstein AC, Lo DS, Otto SP. Parallel genetic changes and nonparallel gene–environment interactions characterise the evolution of drug resistance in yeast. Genetics. 2012;192:241–252. doi: 10.1534/genetics.112.142620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghersa CM, Martínez-Ghersa MA, Brewer TG, Roush ML. Selection pressures for diclofop-methyl resistance and germination time of Italian ryegrass. Agronomy Journal. 1994;86:823–828. [Google Scholar]
- Gill GS, Cousens RG, Allan MR. Germination, growth and development of herbicide-resistant and susceptible populations of rigid ryegrass (Lolium rigidum) Weed Science. 1996;44:252–256. [Google Scholar]
- Gundel PE, Martínez-Ghersa MA, Ghersa CM. Dormancy, germination and ageing of Lolium multiflorum seeds following contrasting herbicide selection regimes. European Journal of Agronomy. 2008;28:606–613. [Google Scholar]
- Marles MAS, Devine MD, Hall JC. Herbicide resistance in Setaria viridis conferred by a less sensitive form of acetyl coenzyme A carboxylase. Pesticide Biochemistry and Physiology. 1993;46:7–14. [Google Scholar]
- Menchari Y, Camilleri C, Michel S, et al. Weed response to herbicides: regional-scale distribution of herbicide resistance alleles in the grass weed Alopecurus myosuroides. New Phytologist. 2006;171:861–874. doi: 10.1111/j.1469-8137.2006.01788.x. [DOI] [PubMed] [Google Scholar]
- Menchari Y, Délye C, Le Corre V. Genetic variation and population structure in black-grass (Alopecurus myosuroides Huds.), a successful, herbicide-resistant, annual grass weed of winter cereal fields. Molecular Ecology. 2007;16:3161–3172. doi: 10.1111/j.1365-294X.2007.03390.x. [DOI] [PubMed] [Google Scholar]
- Menchari Y, Chauvel B, Darmency H, Délye C. Fitness costs associated with three mutant acetylcoenzyme A carboxylase alleles endowing herbicide resistance in black-grass Alopecurus myosuroides. Journal of Applied Ecology. 2008;45:939–947. [Google Scholar]
- Mortimer AM. Phenological adaptation in weeds: an evolutionary response to the use of herbicides? Pesticide Science. 1997;51:299–304. [Google Scholar]
- Moss SR. The survival of Alopecurus myosuroides Huds. seeds in soil. Weed Research. 1985;25:201–211. [Google Scholar]
- Neuberger T, Sreenivasulu N, Rokitta M, et al. Quantitative imaging of oil storage in developing crop seeds. Plant Biotechnology Journal. 2008;6:31–45. doi: 10.1111/j.1467-7652.2007.00294.x. [DOI] [PubMed] [Google Scholar]
- Nonogaki H, Bassel GW, Bewley JD. Germination – still a mystery. Plant Science. 2010;179:574–581. [Google Scholar]
- Ohlrogge JG, Jaworski JB. Regulation of fatty acid biosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology. 1997;48:109–136. doi: 10.1146/annurev.arplant.48.1.109. [DOI] [PubMed] [Google Scholar]
- Onofri A, Gresta F, Tei F. A new method for the analysis of germination and emergence data of weed species. Weed Research. 2010;50:187–198. [Google Scholar]
- Oracz K, El-Maarouf Bouteau H, Farrant JM, et al. ROS production and protein oxidation as a novel mechanism for seed dormancy alleviation. The Plant Journal. 2007;50:462–465. doi: 10.1111/j.1365-313X.2007.03063.x. [DOI] [PubMed] [Google Scholar]
- Otto SP. Two steps forward, one step back: the pleiotropic effects of favoured alleles. Proceedings of the Royal Society B: Biological Sciences. 2004;271:705–714. doi: 10.1098/rspb.2003.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen MJ, Michael PJ, Renton M, Steadman KJ, Powles SB. Towards large-scale prediction of Lolium rigidum emergence. II. Correlation between dormancy and herbicide resistance levels suggests an impact of cropping systems. Weed Research. 2011;51:133–141. [Google Scholar]
- Pavlicev M, Wagner GP. A model of developmental evolution: selection, pleiotropy and compensation. Trends in Ecology and Evolution. 2012;27:316–322. doi: 10.1016/j.tree.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Powles SB, Yu Q. Evolution in action: plants resistant to herbicides. Annual Review of Plant Biology. 2010;61:317–347. doi: 10.1146/annurev-arplant-042809-112119. [DOI] [PubMed] [Google Scholar]
- Purrington CB. Costs of resistance. Current Opinion in Plant Biology. 2000;3:305–308. doi: 10.1016/s1369-5266(00)00085-6. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. The R project for statistical computing. 2012 http://www.r-project.org/ [Google Scholar]
- Sester M, Dürr C, Darmency H, Colbach N. Evolution of weed beet (Beta vulgaris L.) seed bank: quantification of seed survival, dormancy, germination and pre-emergence growth. European Journal of Agronomy. 2006;24:19–25. [Google Scholar]
- Shukla A, Leach GE, Devine MD. High-level resistance to sethoxydim conferred by an alteration in the target enzyme, acetyl-CoA carboxylase, in Setaria faberi and Setaria viridis. Plant Physiology and Biochemistry. 1997;35:803–807. [Google Scholar]
- Swain AJ, Hughes ZS, Cook SK, Moss SR. Quantifying the dormancy of Alopecurus myosuroides seeds produced by plants exposed to different soil moisture and temperature regimes. Weed Research. 2006;46:470–479. [Google Scholar]
- Vila-Aiub MM, Neve P, Powles SB. Resistance cost of a cytochrome P450 herbicide metabolism mechanism but not an ACCase target site mutation in a multiple resistant Lolium rigidum population. New Phytologist. 2005a;167:787–796. doi: 10.1111/j.1469-8137.2005.01465.x. [DOI] [PubMed] [Google Scholar]
- Vila-Aiub MM, Neve P, Steadman KJ, Powles SB. Ecological fitness of a multiple herbicide-resistant Lolium rigidum population: dynamics of seed germination and seedling emergence of resistant and susceptible phenotypes. Journal of Applied Ecology. 2005b;42:288–298. [Google Scholar]
- Vila-Aiub MM, Neve P, Powles SB. Fitness costs associated with evolved herbicide resistance alleles in plants. New Phytologist. 2009;184:751–767. doi: 10.1111/j.1469-8137.2009.03055.x. [DOI] [PubMed] [Google Scholar]
- Vila-Aiub MM, Neve P, Roux F. A unified approach to the estimation and interpretation of resistance costs in plants. Heredity. 2011;107:386–394. doi: 10.1038/hdy.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Picard JC, Tian X, Darmency H. A herbicide-resistant ACCase 1781 Setaria mutant shows higher fitness than wild type. Heredity. 2010;105:394–400. doi: 10.1038/hdy.2009.183. [DOI] [PubMed] [Google Scholar]
- Yu Q, Collavo A, Zheng M-Q, Owen M, Sattin M, Powles SB. Diversity of acetyl-coenzyme A carboxylase mutations in resistant Lolium populations: evaluation using clethodim. Plant Physiology. 2007;145:547–558. doi: 10.1104/pp.107.105262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.