Abstract
Background and Aims
The combination of clonality and a mating system promoting outcrossing is considered advantageous because outcrossing avoids the fitness costs of selfing within clones (geitonogamy) while clonality assures local persistence and increases floral display. The spatial spread of genetically identical plants (ramets) may, however, also decrease paternal diversity (the number of sires fertilizing a given dam) and fertility, particularly towards the centre of large clumped clones. This study aimed to quantify the impact of extensive clonal growth on fine-scale paternity patterns in a population of the allogamous Convallaria majalis.
Methods
A full analysis of paternity was performed by genotyping all flowering individuals and all viable seeds produced during a single season using AFLP. Mating patterns were examined and the spatial position of ramets was related to the extent of multiple paternity, fruiting success and seed production.
Key Results
The overall outcrossing rate was high (91 %) and pollen flow into the population was considerable (27 %). Despite extensive clonal growth, multiple paternity was relatively common (the fraction of siblings sharing the same father was 0·53 within ramets). The diversity of offspring collected from reproductive ramets surrounded by genetically identical inflorescences was as high as among offspring collected from ramets surrounded by distinct genets. There was no significant relationship between the similarity of the pollen load received by two ramets and the distance between them. Neither the distance of ramets with respect to distinct genets nor the distance to the genet centre significantly affected fruiting success or seed production.
Conclusions
Random mating and considerable pollen inflow most probably implied that pollen dispersal distances were sufficiently high to mitigate local mate scarcity despite extensive clonal spread. The data provide no evidence for the intrusion of clonal growth on fine-scale plant mating patterns.
Keywords: Paternity analysis, mate diversity, outcrossing, mating opportunities, AFLP, reproductive success, seed set, ramet, lily-of-the-valley, Convallaria majalis
INTRODUCTION
Many perennial flowering plants exhibit a dual reproductive mode by simultaneously producing asexual offspring by vegetative means and sexual offspring via the fusion of gametes (Fryxell, 1957; Klimeš et al., 1997). Clonal growth through the development of vegetative structures such as rhizomes, runners or bulbils typically leads to spatial clusters of genetically identical, yet morphologically independent flowering modules (ramets) derived from a single genetic individual (genet) (e.g. Chung and Epperson, 1999; Vandepitte et al., 2009). The multiplication and spread of identical ramets through clonal growth confers several advantages to a genet, including attractiveness to pollinators by increased floral density and display, local persistence when sexual recruitment fails, spread of the risks of death and disease among ramets and efficient acquisition of food resources in heterogeneous environments (Silander, 1985; Caraco and Kelly, 1991; Hutchings and Wijesinghe, 1997). However, when pollen is dispersed over short distances, clonal growth can negatively affect mating opportunities (Handel, 1985; Charpentier, 2002; Vallejo-Marín et al., 2010). Restricted pollen dispersal results from short movements of pollinating insect species, preferentially foraging among near-neighbours (Peakall and Beattie, 1991; Chittka et al., 1997), and may be common in mass-flowering species. The flowering ramets of large, spatially aggregated genets may therefore receive pollen from few sources (low mate diversity) and face increased levels of geitonogamy, i.e. self-pollination among flowers within a single genet, particularly if situated at the centre of genets (Handel, 1985).
Geitonogamous pollination and low mate diversity can be maladaptive for several reasons. In self-compatible plant species, geitonogamous pollination will increase the selfing rate and thus the likelihood of inbreeding depression (Eckert, 2000; Reusch, 2001). In plant species displaying mechanisms that encourage or enforce cross-fertilization, cross-fertilization will avoid the female fitness costs of geitonogamy while clonality alleviates the pressure on evolution towards selfing for reproductive assurance (Vallejo-Marín and O'Brien, 2007). Substantial clonal growth and associated mate scarcity can, however, reduce female sexual reproductive success when self pollen is insufficiently replaced by compatible outcross pollen (Vallejo-Marín and Uyenoyama, 2004; Honnay et al., 2006; Scobie and Wilcock, 2009). Receiving pollen from few pollen donors can also lead to low paternal diversity and may so decrease fitness by reducing the genetic quality or diversity of offspring, or by reducing the efficiency of hedging against sterility, genetic defects or selfish genetic elements present in partners (Ellstrand and Marshall, 1986; Bernasconi et al., 2004; Teixeira and Bernasconi, 2007).
The consequences of clonality for plant mating has intrigued plant ecologists and evolutionary biologists for a long time (Vallejo-Marín and O'Brien, 2007), and has resulted in many studies relating the spatial position of ramets within genets with reproductive success (e.g. Handel, 1985; Wang et al., 2005; Araki et al., 2007). This approach does not necessarily reflect local mate diversity and associated mating opportunities alone, however, because differences in resource availability can also cause great variation in seed production within and among genets (Burd, 1994). Few studies in clonal plant populations included detailed knowledge of the paternal origin of seeds (Routley et al., 2004; Llaurens et al., 2008; Mori et al., 2009; Zipperle et al., 2011), and the potential interference of clonal growth with fine-scale mating patterns remains to be explored. To investigate the mating consequences of clonality in outcrossing plant species, we performed a full analysis of paternity in a partially self-incompatible lily-of-the-valley population (Convallaria majalis, Asparagaceae), a prime example of a herbaceous forest plant that spreads extensively through rhizomes (Chwedorzewska et al., 2008; Vandepitte et al., 2010). Our general aim was to quantify the impact of extensive clonal growth on mating patterns. Using amplified fragment length polymorphism (AFLP) (Vos et al., 1995), we genotyped all viable seeds produced during one season in the population, together with all available potential pollen donors. We then used a paternity analysis to (a) quantify paternal diversity, mating distances and the spatial extent of correlated paternity to infer the ecological mating context; and (b) investigate whether ramets face reduced paternal diversity and female fertility with increasing spatial isolation to distinct genets, as would be expected when extensive clonal spread results in local mate scarcity.
MATERIALS AND METHODS
Study species
Convallaria majalis L. occurs throughout most of Europe in ancient deciduous forests on rather acid soils. Ramets are connected by both short and far-creeping rhizomes. The two-leaf generative shoots produce white, nodding, bell-shaped hermaphroditic flowers spreading a strong, sweet fragrance. Inflorescences typically bear six to eight flowers. Flowers bloom from April to May and are visited by Hymenoptera and to a lesser extent by Diptera, which tended to alternate short and long distance flights in the studied population (K. Vandepitte, pers. obs.). Seed production after artificial self-pollination is possible but low in the study area (approx. 7 % of open-pollinated seed production under natural circumstances; Vandepitte et al., 2010), indicating leaky self-incompatibility [see also Araki et al. (2005) for pollination experiments supporting SI in the closely related Convallaria keiskei]. Within the corolla, there are six stamens with short filaments and a single style with a tripartite stigma. The ovary has three compartments (Tutin et al., 1993). Each flower can set one fruit which develops into a red berry that can contain several seeds. Seedlings emerge 2–3 years after seed release (Eriksson, 1999).
Sample collection and AFLP
The study population was situated in the northern part of Belgium near Merelbeke (Bruinbos) in a dry, recently cut forest area, where it co-occurred with Maianthenum bifolium. The population consisted of two nearby C. majalis patches (Fig. 1). The maximum distance between ramets within the population was 2·62 m. The nearest C. majalis population was located at approx. 60 m from the centre of the studied population.
Fig. 1.
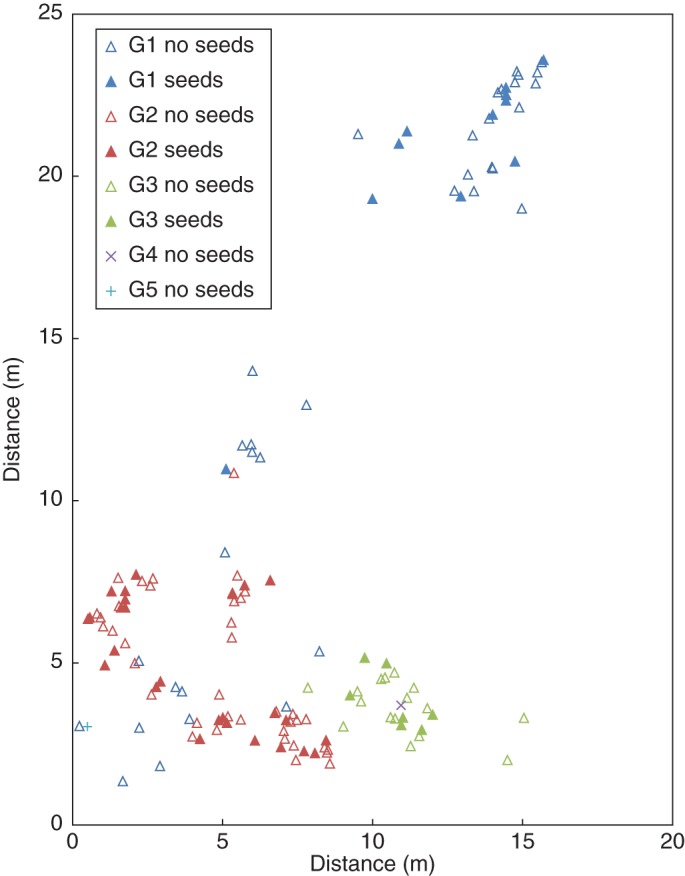
Spatial distribution of genets based on all flowering ramets present in a Convallaria majalis population in a Belgian forest. Different colours represent different genets (G), as indicated in the key.
In May 2008, all flowering ramets were mapped to the nearest centimetre. Next, leaf material from all flowering ramets (n = 141) was collected, immediately frozen in liquid nitrogen and later transferred to a refrigerator at –80 °C. From July 2008 on, the site was visited twice a week to collect all ripe berries produced throughout the season. In October 2008, the flesh of the dried berries was peeled and 154 clean seeds were sown in Petri dishes on wet Whatman paper. Each dish contained the seeds of one berry. After 3 months at 5 °C in a refrigerator, dishes were treated with a fungicide solution (0·0003 % w/v ethylmercury thiosalicylic acid) and transferred to a growing chamber at 15 °C. During the subsequent 4 months, seedling radicles were harvested as they appeared and immediately stored in Eppendorf tubes at –80 °C. After 4 months, germination was 87·7 % (n = 135). As no new radicles appeared during the last month of the experiment, the few remaining seeds were considered non-viable and not further considered. The radicles harvested are henceforth referred to as the offspring (n = 135).
For AFLP genotyping (Vos et al., 1995), DNA was extracted from milled leaf or radicle material using the Nucleospin DNA-extraction kit (Macherey Nagel, Germany). Per individual, 400 ng of high quality DNA was used as source material. Two primer combinations were used: EcoRI-AGT/MseI-CTCG and EcoRI-AGC/MseI-CTGC. Fluorescently labelled AFLP fragments were separated on an ABI 3130 sequencer on 50 cm capillaries (Applied Biosystems). The quality of profiles was visually checked using GeneMapper 3·7 software (Applied Biosystems). Two adult and five offspring samples of low quality were removed. Next, for each primer combination, bin sets were automatically generated between 50 and 450 bp using a minimum detection threshold of 300 relative fluorescent units as to avoid artefact signals. Profiles were then automatically dominantly scored on the resultant bin set using a lower threshold of 50 relative fluorescent units s and visually inspected. Bins in which signals were not distinctly present or absent were removed.
Ten samples were repeats from independent DNA extractions. After the reproducibility of the applied procedure was confirmed (the average number of mismatches between repeats was 1·7 % after the removal of two error-prone loci from the entire dataset), replicates were randomly deleted from the final dataset of 139 adults and 130 offspring genotyped at 78 unambiguous and polymorphic loci.
Data analysis
In clonal plants, ramets derived from a single genet may differ at a small portion of AFLP loci, mostly due to PCR and scoring errors (Arnaud-Haond et al., 2007). Therefore, ramets were assigned to genets allowing a maximum of two-marker difference, a threshold slightly more conservative than the error rate (1·7 %), using the software GENOTYPE (Meirmans and Van Tienderen, 2004).
The software FaMoZ was used for reconstruction of father–offspring relationships (Gerber et al., 2003). A likelihood-based approach was applied to detect the most likely father using the logarithm of the likelihood ratio (LOD score) for dominant markers (Meagher, 1986; Gerber et al., 2000). The most frequent AFLP genotype of each genet served as parental input data. Individuals were assigned to the most likely paternal genet applying a LOD = 1·3 threshold (the assigned father was at least 20 times more likely to be the father than an uncharacterized outside paternal source) and an error rate of 1·7 %. Because preliminary analyses applying different inbreeding coefficients (FIS) among the parental genets all supported a high outcrossing rate, an FIS of 0·1 was assumed. Progeny displaying three or more alleles absent from the parental pool were automatically classified as sired by outside sources, regardless of their LOD score. The simulation procedure implemented in FaMoZ to determine a LOD and ΔLOD threshold was inconclusive, possibly due to the low number of parental genets.
Based on paternity assignments, we computed the overall outcrossing rate, the amount of pollen inflow and the number of distinct fathers assigned to seed collected from each individual flowering ramet and from each flower. To estimate the correlation of paternity among maternal sibs (full or half sibs collected from a single ramet), we also computed the proportion of full-sib pairs among pairs of maternal sibs, Pfull-sibs, either for each maternal family or as an average per fruit (Hardy et al., 2004). To determine whether nearby ramets were likely to receive the same pollen load we correlated the proportion of pairs among mothers (ramets) sharing the same siring genet and the pairwise spatial distance between mothers using a Mantel test (Hardy et al., 2004; Llaurens et al., 2008). Offspring sired by outside sources were not included in the calculation of shared paternity among mothers. Variables were computed using the statistical environment R 2.14.1 (scripts available from TDM upon request). The Mantel test was performed using the EcoDist R-package.
The potential impact of ramet position and the impact of surrounding genetically identical inflorescences on paternal diversity, as a direct proxy of mate diversity, and on maternal sexual reproductive success were analysed using generalized linear models (GLMs) with a negative binomial distribution and a loglink function to accommodate our overdispersed zero-inflated count data. Dependent variables were (a) paternal diversity as the number of outcrossed fathers minus one (to obtain zero-inflated data), (b) fruiting success as the number of berries produced (including ramets producing no berries) and (c) seed production (the number of seeds produced per flowering ramet including ramets producing no seeds). In each of the three models, independent variables were the minimum distance of a ramet towards the nearest neighbouring genet and the distance of a ramet to its genet centre, calculated as the mean of x- and y-co-ordinates. Independent variables were nested within genet ID to account for potential differences among genets. All models included an intercept. Only genets consisting of several flowering ramets were included. For the calculation of paternal diversity per seed-producing ramet, multiple seeds sired by outside sources were assumed to result from a single external source. Because of the division of the G1 genet into two spatially distinct patches (see Fig. 1), G1 ramets with a y-co-ordinate above 1·80 m and a y-co-ordinate below 1·50 m were treated as two distinct groups to compute the distance of each ramet to the genet centre. Statistical analyses were conducted in SPSS 17·0 (SPSS Inc.).
RESULTS
Applying a two marker difference threshold, there were five flowering genets present in the studied population (Fig. 1), hereafter referred to as G1–5. The average marker difference between genets ranged from 5 to 21·01, underpinning the efficacy of our marker set in distinguishing between genets. Genets varied in size and degree of intermingling with other genets. Genets largely appeared in two spatial clusters. G1 flowering ramets (n = 48) were present in both clusters; yet all but one seed-producing G1 ramets lay spatially isolated in the smaller upper cluster. G2 (n = 66) and G3 (n = 23) were confined to the large lower cluster. G3 had a mostly aggregated distribution, while G2 was intermingled with the G1 ramets present in the lower cluster. The maximal distance was 26·3 m between G1 ramets, 9·5 m between G2 ramets and 7·3 m between G3 ramets.
Twelve out of 48 G1 ramets, 26 out of 66 G2 ramets and 7 out of 23 G3 ramets produced berries. G1 produced 34 viable seeds in total, G2 produced 72 and G3 produced 26 (Fig. 2A). On average, the fruiting G1, G2 and G3 ramets produced, respectively, 2·46 (±1·90 s.d.), 2·50 (±2·25 s.d.) and 3·64 (±3·40 s.d.) viable seeds. G4 and G5 each consisted of a single ramet and did not fruit (Fig. 1). GLM models testing the impact of ramet position (distance to the genet centre and to the nearest distinct genet) on fruiting success and seed production did not detect significant effects (all P > 0·05; Table 1), indicating that neither the spatial isolation of ramets with respect to distinct genets nor the extent to which a given ramet is surrounded by genetically identical inflorescences substantially affected female reproductive success.
Fig. 2.
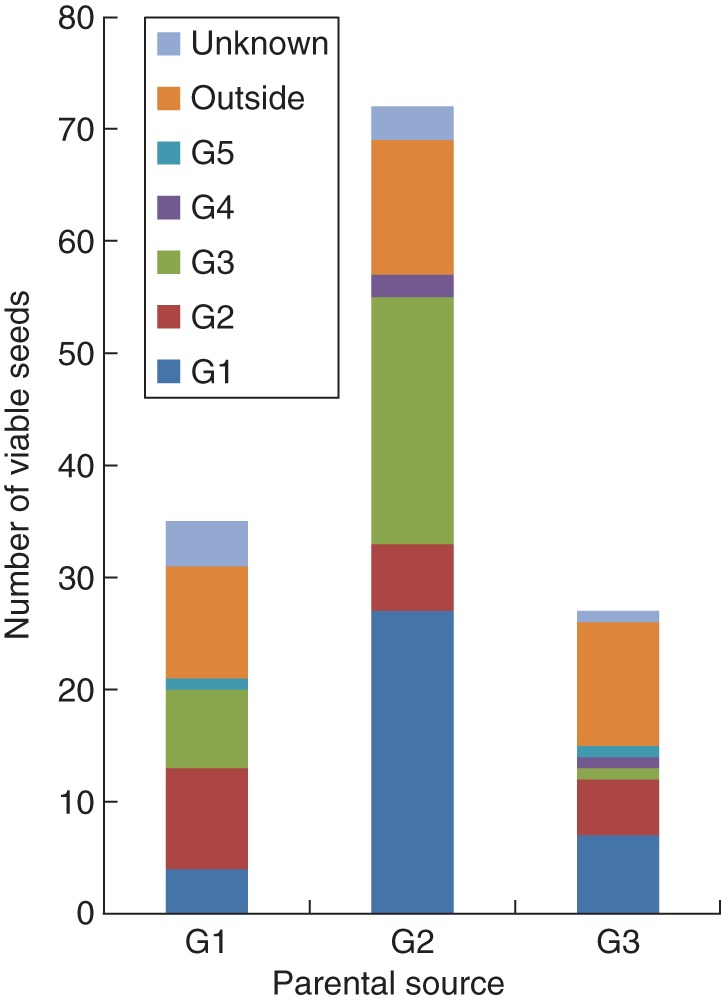
The number of viable seeds produced per Convallaria majalis genet and the respective paternal source. Offspring with ambiguous assignment results and unsuccessfully genotyped offspring were classified as unknown. Different colours represent different genets (G), as indicated in the key. The category ‘outside’ represents offspring sired by uncharacterized sources from outside the studied population.
Table 1.
Type III effects of generalized linear models examining the impact a ramet's position (distance to the genet center and distance to the nearest genet) has on mating opportunities and female reproductive success
| Variable | Source of variation | Wald χ2 | d.f. | P |
|---|---|---|---|---|
| Paternal diversity | Distance_to_genet_centre | 0·77 | 1 | 0·38 |
| Distance_to_distinct_genet | 1·40 | 1 | 0·24 | |
| Distance_to_genet_centre (Genet_ID) | 0·62 | 2 | 0·73 | |
| Distance_to_distinct_genet (Genet_ID) | 1·73 | 2 | 0·42 | |
| Genet_ID | 2·45 | 2 | 0·29 | |
| Fruiting success | Distance_to_genet_centre | 0·21 | 1 | 0·72 |
| Distance_to_distinct_genet | 0·01 | 1 | 0·97 | |
| Distance_to_genet_centre (Genet_ID) | 0·50 | 2 | 0·78 | |
| Distance_to_distinct_genet (Genet_ID) | 1·27 | 2 | 0·53 | |
| Genet_ID | 2·78 | 2 | 0·23 | |
| Seed production | Distance_to_genet_centre | 0·64 | 1 | 0·43 |
| Distance_to_distinct_genet | 0·01 | 1 | 0·95 | |
| Distance_to_genet_centre (Genet_ID) | 1·38 | 2 | 0·50 | |
| Distance_to_distinct_genet (Genet_ID) | 1·72 | 2 | 0·42 | |
| Genet_ID | 5·43 | 2 | 0·07 |
Of the 126 assigned offspring (out of the 135 viable seeds produced by the studied population; 94·7 %), 11 were sired by the mother genet (8·7 %), 82 were sired by a different genet from within the population (65·8 %), and 33 were sired by outside sources (26·1 %). In total, 38 seeds were assigned to G1 (on average 0·79 per ramet), 20 to G2 (0·30 per ramet), 30 to G3 (1·30 per ramet). Three seeds were assigned to G4 and two to G5, both genets consisting of a single ramet. Multiple paternity was common. Of the on average 2·64 (±2·36 s.d.) genotyped offspring per fruiting ramet, the estimated number of genetically distinct fathers was 1·55 (±0·84 s.d.). Paternal diversity was not affected by ramet position (P > 0·05; Table 1). Fruiting G1 ramets were on average sired by 1·62 (±0·87 s.d.) distinct sources, G2 by 1·50 (±0·86 s.d.) and G3 by 1·57 (±0·79 s.d.) sources. The mean number of seeds per fruit was 1·84 (±0·99 s.d.) and the mean number of distinct fathers per fruit was 1·36 (±0·52 s.d.). The estimate of the fraction of outcrossed maternal sibs that share the same father was 0·53 ± 0·40 (s.d.) per fruiting ramet, which was almost equal to the fraction within multi-seeded fruits (0·54 ± 0·37 s.d.). We observed no significant decrease in correlated paternity among mothers with spatial distance (Mantel-r = –0·04; P = 0·38).
DISCUSSION
In outcrossing species, clonal growth is predicted to decrease mate diversity or the number of compatible sires to a ramet, as ramets multiply and spread into space (Handel, 1985; Charpentier, 2002). One available genetic study examining the paternity of seed families in a population of the self-incompatible insect-pollinated clonal Arabidopsis halleri, however, could not confirm this prediction and demonstrated exceptionally high paternal diversity within ramets and fruits (Llaurens et al., 2008). The fraction of siblings sharing the same father was as low as 0·04, much lower even than the values commonly reported for non-clonal plants [e.g. 0·20 in Centaurea corymbosa (Hardy et al., 2004), 0·37–0·65 in Rutidosis leptorrhychoides (Wells and Young, 2002) and 0·31–0·54 in Grevillea iaspicula (Hoebee and Young, 2001)]. Yet, as genets of A. halleri comprise no more than a few ramets (Van Rossum et al., 2004), clonal growth was likely to be too weak to affect mating opportunities. Our genetic analyses confirm that Convallaria majalis relies strongly on clonal reproduction, a situation that was possibly intensified by previous shady conditions suppressing sexual reproduction (Vandepitte et al., 2010). The studied population of 141 flowering ramets consisted of only three main genets, each expanding across at least 7 m (Fig. 1).
Remarkably, although the low local genet diversity probably limited the number of potential fathers, multiple paternity was still relatively common (the fraction of siblings sharing the same father was 0·53 within ramets), and the diversity of offspring collected from reproductive ramets surrounded by genetically identical inflorescences was as high as among offspring collected from ramets surrounded by distinct genets. Neither the spatial isolation of ramets with respect to distinct genets nor differences among genets affected fruiting success or seed production (Table 1). Overall, these findings indicate that the fine-scale mating and associated reproductive consequences of extensive clonal growth are limited in C. majalis, as long as some genet diversity is locally maintained.
There are several lines of evidence that indicate that pollen dispersal distances were sufficiently high to counterbalance local mate scarcity. First, 27 % of the offspring were sired by outside pollen sources, while the nearest population was located at approx. 60 m. Second, there was no significant correlation between the spatial distance between ramets and the degree of shared paternity, contrary to expectations when pollen transfer distances are predominantly small (Hardy et al., 2004). Third, our observation of multiple paternity within single fruits (the fraction of siblings sharing the same father was 0·54 within multi-seeded fruits) suggests substantial pollen carryover or multiple pollinator visits per flower. The latter is in accordance with the species' entomophilous flowers producing a strong sweet scent, which must attract a variety of insects. Finally, cross-fertilization (the outcrossing rate was 91 %) may have additionally promoted paternal diversity (Llaurens et al., 2008), since selfing would reduce the number of ovules available to outcrossing and thus decrease the likelihood of multiple outcrossed sires.
As a result of intergenet pollen dispersal, the female fitness costs of extensive clonal spread are likely to be moderate in the studied population and the low female reproductive success can probably partially be attributed to resource limitation (Burd, 1994). In the closely related self-incompatible Japanese lily-of-the-valley (Convallaria keiskei), fruiting success remained well below its maximal potential when outcrossed pollen was overly supplemented (Araki et al., 2005). Partially resource-limited female reproductive success also concurs with the nutrient-poor soil conditions experienced by the studied C. majalis population. Under these environmental conditions, the production of fleshy berries, containing relatively large seeds (Eriksson, 1999), must be costly. We can, however, not rule out the possibility that pollen limitation or self-pollination contributed to the observed low reproductive success, e.g. through stigma clogging or early seed or fruit abortion (Richards, 1986; Honnay et al., 2006), without conducting appropriate experimental manipulations. Although fruiting success and seed production did not significantly differ between genets (Table 1), the number of seeds produced per ramet was highest in the smallest G3 genet, which is likely to experience the lowest degree of geitonogamous mating (Charpentier, 2002).
In conclusion, our data provide no evidence for the influence of clonal spread on fine-scale mating patterns and associated variation in female reproductive success among ramets in outcrossing plant species (Handel, 1985; Wang et al., 2005; Araki et al., 2007). Random mating and considerable pollen inflow most probably implied that pollen dispersal distances were sufficiently high to mitigate reduced mate diversity towards ramets isolated from distinct genets in the studied population. We can, however, not exclude the influence of clonality on either female or male reproductive success at the population or genet level (Routley et al., 2004; Mori et al., 2009). Paternity analyses combined with field observations of pollinator behaviour in different populations and species seem needed to assess the generality of this pattern.
ACKNOWLEDGEMENTS
We are grateful to S. Van Glabeke for assistance in the field. K.V. holds a postdoctoral fellowship of the Research Foundation – Flanders (FWO).
LITERATURE CITED
- Araki K, Yamada E, Ohara M. Breeding system and floral visitors of Convallaria keiskei. Plant Species Biology. 2005;20:149–153. [Google Scholar]
- Araki K, Shimatani K, Ohara M. Floral distribution, clonal structure, and their effects on pollination success in a self-incompatible Convallaria keiskei population in northern Japan. Plant Ecology. 2007;189:175–186. [Google Scholar]
- Arnaud-Haond S, Duarte CM, Alberto F, Serrao EA. Standardizing methods to address clonality in population studies. Molecular Ecology. 2007;16:5115–5139. doi: 10.1111/j.1365-294X.2007.03535.x. [DOI] [PubMed] [Google Scholar]
- Bernasconi G, Ashman TL, Birkhead TR, et al. Evolutionary ecology of the pre-zygotic stage. Science. 2004;303:971–975. doi: 10.1126/science.1092180. [DOI] [PubMed] [Google Scholar]
- Burd M. Bateman principle and plant reproduction: the role of pollen limitation in fruit and seed set. Botanical Review. 1994;60:83–139. [Google Scholar]
- Caraco T, Kelly CK. On the adaptive value of physiological integration in clonal plants. Ecology. 1991;72:81–93. [Google Scholar]
- Charpentier A. Consequences of clonal growth for plant mating. Evolutionary Ecology. 2002;15:521–530. [Google Scholar]
- Chittka L, Gumbert A, Kunze J. Foraging dynamics of bumble bees: correlates of movements within and between plant species. Behavioral Ecology. 1997;8:239–249. [Google Scholar]
- Chung MG, Epperson BK. Spatial genetic structure of clonal and asexual reproduction in populations of Adenophora grandiflora (Campanulaceae) Evolution. 1999;53:1068–1078. doi: 10.1111/j.1558-5646.1999.tb04522.x. [DOI] [PubMed] [Google Scholar]
- Chwedorzewska KJ, Galera H, Kosinski I. Plantations of Convallaria majalis L. as a threat to the natural stands of the species: genetic variability of the cultivated plants and natural populations. Biological Conservation. 2008;141:2619–2624. [Google Scholar]
- Eckert CG. Contributions of autogamy and geitonogamy to self-fertilization in a mass-flowering, clonal plant. Ecology. 2000;81:532–542. [Google Scholar]
- Ellstrand NC, Marshall DL. Patterns of multiple paternity in populations of Raphanus sativus. Evolution. 1986;40:837–842. doi: 10.1111/j.1558-5646.1986.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Eriksson O. Seed size variation and its effect on germination and seedling performance in the clonal herb Convallaria majalis. Acta Oecologia. 1999;20:61–66. [Google Scholar]
- Fryxell PA. Mode of reproduction of higher plants. Botanical Review. 1957;23:135–233. [Google Scholar]
- Gerber S, Streiff R, Bodénès C, Mariette S, Kremer A. Comparison of microsatellites and AFLP markers for parentage analysis. Molecular Ecology. 2000;9:1037–1048. doi: 10.1046/j.1365-294x.2000.00961.x. [DOI] [PubMed] [Google Scholar]
- Gerber S, Chabrier P, Kremer A. FaMoz: a software for parentage analysis using dominant, codominant and uniparentally inherited markers. Molecular Ecology Notes. 2003;3:479–481. [Google Scholar]
- Handel SN. The intrusion of clonal growth patterns on plant breeding systems. American Naturalist. 1985;125:367–383. [Google Scholar]
- Hardy OJ, Santiago C, González-Martínez BC, Freville H, Mignot A, Olivieri I. Fine-scale genetic structure and gene dispersal in Centaurea corymbosa (Asteraceae). II. Correlated paternity within and among sibships. Genetics. 2004;168:1601–1614. doi: 10.1534/genetics.104.027714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebee SE, Young AG. Low neighbourhood size and high interpopulation differentiation in the endangered shrub Grevillea iaspicula McGill (Proteaceae) Heredity. 2001;86:489–496. doi: 10.1046/j.1365-2540.2001.00857.x. [DOI] [PubMed] [Google Scholar]
- Honnay O, Jacquemyn H, Roldán-Ruiz I, Hermy M. Consequences of prolonged clonal growth on local and regional genetic structure and fruiting success of the forest perennial Maianthemum bifolium. Oikos. 2006;112:21–30. [Google Scholar]
- Hutchings MJ, Wijesinghe DK. Patchy habitat, division of labour, and growth dividends in clonal plants. Trends in Ecology and Evolution. 1997;12:390–394. doi: 10.1016/s0169-5347(97)87382-x. [DOI] [PubMed] [Google Scholar]
- Klimeš L, Klimešova J, Hendriks R, van Groenendael J. Clonal plant architecture: a comparative analysis of form and function. In: de Kroon H, van Groenendael J, editors. The ecology and evolution of clonal plants. Leiden: Backhuy; 1997. pp. 1–29. [Google Scholar]
- Llaurens V, Castric V, Austerlitz F, Vekemans X. High paternal diversity in the self-incompatible herb Arabidopsis halleri despite clonal reproduction and spatially-restricted pollen dispersal. Molecular Ecology. 2008;17:1577–1588. doi: 10.1111/j.1365-294X.2007.03683.x. [DOI] [PubMed] [Google Scholar]
- Meagher TR. Analysis of paternity within a natural population of Chamaelirium luteum. 1. Identification of most-likely male parents. American Naturalist. 1986;128:199–215. [Google Scholar]
- Meirmans PG, Van Tienderen PH. GENOTYPE and GENODIVE: two programs for the analysis of genetic diversity of asexual organisms. Molecular Ecology Notes. 2004;4:792–794. [Google Scholar]
- Mori Y, Nagamitsu T, Kubo T. Clonal growth and its effects on male and female reproductive success in Prunus ssiori (Rosaceae) Population Ecology. 2009;51:175–186. [Google Scholar]
- Peakall R, Beattie AJ. The genetic consequences of worker ant pollination in a self-compatible, clonal orchid. Evolution. 1991;45:1837–1848. doi: 10.1111/j.1558-5646.1991.tb02691.x. [DOI] [PubMed] [Google Scholar]
- Reusch TBH. Fitness-consequences of geitonogamous selfing in a clonal marine angiosperm (Zostera marina) Journal of Evolutionary Biology. 2001;14:129–138. doi: 10.1046/j.1420-9101.2001.00257.x. [DOI] [PubMed] [Google Scholar]
- Richards AJ. Plant breeding systems. London: George Allen & Unwin; 1986. [Google Scholar]
- Routley MB, Kron P, Husband BC. The consequences of clone size for paternal and maternal success in domestic apple (Malus × domestica) American Journal of Botany. 2004;91:1326–1332. doi: 10.3732/ajb.91.9.1326. [DOI] [PubMed] [Google Scholar]
- Scobie AR, Wilcock CC. Limited mate availability decreases reproductive success of fragmented populations of Linnaea borealis, a rare, clonal self-incompatible plant. Annals of Botany. 2009;103:835–846. doi: 10.1093/aob/mcp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silander JA. In: Microevolution in clonal plants. Jackson JBC, Buss LW, Cook RE, editors. New Haven, CT: Yale University Press; 1985. pp. 107–152. Population biology and evolution of clonal plants. [Google Scholar]
- Teixeira S, Bernasconi G. High prevalence of multiple paternity within fruits in natural populations of Silene latifolia, as revealed by microsatellite DNA analysis. Molecular Ecology. 2007;16:4370–4379. doi: 10.1111/j.1365-294X.2007.03493.x. [DOI] [PubMed] [Google Scholar]
- Tutin TG, Heywood VH, Burges VA, et al. Flora Europaea. 2nd edn. Vol. I. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- Vallejo-Marín M, O'Brien HE. Correlated evolution of self-incompatibility and clonal reproduction in Solanum (Solanaceae) New Phytologist. 2007;173:415–421. doi: 10.1111/j.1469-8137.2006.01924.x. [DOI] [PubMed] [Google Scholar]
- Vallejo-Marín M, Uyenoyama MK. On the evolutionary costs of self-incompatibility: incomplete reproductive compensation due to pollen limitation. Evolution. 2004;58:1924–1935. doi: 10.1111/j.0014-3820.2004.tb00480.x. [DOI] [PubMed] [Google Scholar]
- Vallejo-Marín M, Dorken ME, Barrett SCH. The ecological and evolutionary consequences of clonality for plant mating. Annual Review of Ecology Evolution and Systematics. 2010;41:193–213. [Google Scholar]
- Vandepitte K, Jacquemyn H, Roldán-Ruiz I, Honnay O. Extremely low genotypic diversity and sexual reproduction in isolated populations of the self incompatible lily-of-the-valley (Convallaria majalis) and the role of the local forest environment. Annals of Botany. 2010;105:769–776. doi: 10.1093/aob/mcq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandepitte K, Roldán-Ruiz I, Leus L, Jacquemyn H, Honnay O. Canopy closure shapes clonal diversity and fine-scale genetic structure in the dioecious understorey perennial Mercurialis perennis. Journal of Ecology. 2009;97:404–414. [Google Scholar]
- Van Rossum F, Bonnin I, Fenart S, Pauwels M, Petit D, Saumitou-Laprade P. Spatial genetic structure within a metallicolous population of Arabidopsis halleri, a clonal, self-incompatible and heavy-metal-tolerant species. Molecular Ecology. 2004;13:2959–2967. doi: 10.1111/j.1365-294X.2004.02314.x. [DOI] [PubMed] [Google Scholar]
- Vos P, Hogers R, Bleeker M, et al. AFLP: a new technique for DNA-fingerprinting. Nucleic Acids Research. 1995;23:4407–4414. doi: 10.1093/nar/23.21.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang QF, Guo YH, Barrett SCH. Reproductive consequences of interactions between clonal growth and sexual reproduction in Nymphoides peltata: a distylous aquatic plant. New Phytologist. 2005;165:329–335. doi: 10.1111/j.1469-8137.2004.01234.x. [DOI] [PubMed] [Google Scholar]
- Wells GP, Young AG. Effects of seed dispersal on spatial genetic structure in populations of Rutidosis leptorrhychoides with different levels of correlated paternity. Genetical Research. 2002;79:219–226. doi: 10.1017/s0016672302005591. [DOI] [PubMed] [Google Scholar]
- Zipperle AM, Coyer JA, Reise K, Stam WT, Olsen JL. An evaluation of small-scale genetic diversity and the mating system in Zostera noltii on an intertidal sandflat in the Wadden Sea. Annals of Botany. 2011;107:127–133. doi: 10.1093/aob/mcq214. [DOI] [PMC free article] [PubMed] [Google Scholar]


