Abstract
Background and Aims
Several widespread tree species of temperate forests, such as species of the genus Quercus, produce recalcitrant (desiccation-sensitive) seeds. However, the ecological significance of seed desiccation sensitivity in temperate regions is largely unknown. Do seeds of such species suffer from drying during the period when they remain on the soil, between shedding in autumn and the return of conditions required for germination in spring?
Methods
To test this hypothesis, the Mediterranean holm oak (Quercus ilex) forest was used as a model system. The relationships between the climate in winter, the characteristics of microhabitats, acorn morphological traits, and the water status and viability of seeds after winter were then investigated in 42 woodlands sampled over the entire French distribution of the species.
Key Results
The percentages of germination and normal seedling development were tightly linked to the water content of seeds after the winter period, revealing that in situ desiccation is a major cause of mortality. The homogeneity of seed response to drying suggests that neither intraspecific genetic variation nor environmental conditions had a significant impact on the level of desiccation sensitivity of seeds. In contrast, the water and viability status of seeds at the time of collection were dramatically influenced by cumulative rainfall and maximum temperatures during winter. A significant effect of shade and of the type of soil cover was also evidenced.
Conclusions
The findings establish that seed desiccation sensitivity is a key functional trait which may influence the success of recruitment in temperate recalcitrant seed species. Considering that most models of climate change predict changes in rainfall and temperature in the Mediterranean basin, the present work could help foresee changes in the distribution of Q. ilex and other oak species, and hence plant community alterations.
Keywords: Quercus ilex, acorn, desiccation sensitivity, holm oak, drought, ecological filtering, germination, Mediterranean climate, recalcitrance, winter rainfall
INTRODUCTION
In most flowering plant species, seeds are in a dry quiescent state at maturity, which enables them to survive adverse environmental conditions after dispersal, such as periods of cold or drought. In these species, seed drying occurs in planta during development, after the acquisition of desiccation tolerance, i.e. the ability to withstand removal of intracellular water without irreversible damage and then to resume normal metabolism after rehydration (Leprince and Buitink, 2010). Such desiccation-tolerant seeds are called ‘orthodox’ due to their ability to survive storage for very long periods under conventional genebank conditions (Roberts, 1973).
However, about 8 % of the world's flowering plants (about 20 000 species; Tweddle et al., 2003) produce seeds that are not orthodox, i.e. they either only withstand partial drying (intermediate seeds) or are extremely sensitive to dehydration (recalcitrant seeds) (Roberts, 1973; Ellis et al., 1990). In both cases, seeds are characteristically shed from the mother plant with high moisture content and are usually not dormant. Plants in the non-orthodox categories prevail in specific habitats, such as tropical rain forests, where they represent >40 % of plant species (Tweddle et al., 2003). The most parsimonious explanation for the expansion of species displaying seed desiccation sensitivity in these particular biotopes relies on the convergent loss of seed desiccation tolerance, probably due to the lack of seasonal drought as selective pressure, from several taxonomically unrelated desiccation-tolerant ancestors (Farnsworth, 2000). Indeed, factors involved in stress tolerance, such as accumulation of heat shock proteins, may have fitness costs under conditions that are not stressful (Hoffmann, 1995; Krebs and Feder, 1998).
Although non-orthodox seed species are widespread in tropical forest systems, seed desiccation sensitivity also occurs in temperate plants. Species of the genus Quercus (Finch-Savage, 1992), Acer pseudoplatanus (Dickie et al., 1991), Aesculus hippocastanum (Tompsett and Pritchard, 1998) and Castanea sativa (Leprince et al., 1999) are temperate trees with recalcitrant seeds. The occurrence of recalcitrant seed species in temperate regions may be puzzling at first sight considering that water stress occurs seasonally in most temperate biotopes, in particular in the Mediterranean region, which is known for its periods of severe drought. Actually, many extant taxa with desiccation-sensitive seeds originated during the warm and wet global climate of the Tertiary period (65 to 2 million years BP; Xiang et al., 1998; Manos and Stanford, 2001), and thus probably lost seed desiccation tolerance for similar reasons to those mentioned above for tropical species. It has recently been suggested that such ancient species were protected from drier conditions during the Quaternary by the facilitative nurse effects of modern Quaternary species, which provided favourable microhabitats for seed germination and seedling establishment (Valiente-Banuet et al., 2006). In line with this hypothesis, one may wonder whether desiccation sensitivity is a trait which affects seed survival in temperate regions. Do seeds of recalcitrant seed species of these areas suffer from drying during the period from shedding (usually in autumn) to when the conditions required for germination return (usually in spring)? To our knowledge, no study has specifically focused on the ecological implications of seed desiccation sensitivity in temperate species, whereas various ecological aspects of this trait have been investigated in tropical biotopes (e.g. Dussert et al., 2000; Pritchard et al., 2004; Yu et al., 2008). This also contrasts with the substantial efforts made to decipher the physiological mechanisms involved in the sensitivity to drying of recalcitrant seeds (e.g. Hendry et al., 1992; Leprince et al., 1999; Roach et al., 2010).
Among the different temperate forests, the Mediterranean sclerophyllous forest provides a valuable system to investigate the impact of seed sensitivity to desiccation on the reproductive success of recalcitrant seed species. Indeed, one of the most emblematic tree species of this biome is an oak – Quercus ilex, holm oak – and is thus likely to produce recalcitrant seeds (Finch-Savage, 1992; Xia et al., 2012a). Moreover, this forest still occupies very large areas in the Mediterranean basin. In addition, the Mediterranean climate is characterized by irregular and unpredictable precipitation, thus maximizing the chance to observe contrasted autumn-to-spring cumulative rainfall across locations within a single year of study. Finally, most global and regional climate models suggest that the Mediterranean region might be especially vulnerable to global change (Gibelin and Deque, 2003; Giorgi and Lionello, 2008). These models predict significant increases in temperature and decreases in precipitation in the Mediterranean basin and, in particular, a pronounced decrease in precipitation in winter in northern Mediterranean areas. These changes may have major effects on existing Mediterranean forests, particularly on the distribution of oak species (e.g. Cheaib et al., 2012; Ruiz-Labourdette et al., 2012). In this respect, a better understanding of the significance of seed desiccation sensitivity may improve our capacity to foresee changes in plant community structure in this region.
The holm oak is an evergreen oak which is native to the Mediterranean region. It is found from Turkey to Spain on the European side of the Mediterranean Sea, and from Morocco to Tunisia on the African side, and it has also colonized the majority of Mediterranean islands (Barbero et al., 1992). Most Q. ilex forests are regarded as rare cases of woodlands that have undergone very little silvicultural management, and are therefore of great value for ecological observations (Lumaret et al., 2008). In France, the holm oak occupies the entire Mediterranean rim and Corsica. In the present study, to test whether environmental conditions after seed dispersal affect seed viability in temperate recalcitrant seed species, the relationships between winter climate, topography, acorn morphological traits, and seed water status and viability after the winter period were investigated in 42 populations of Q. ilex sampled over the entire French distribution of the species.
MATERIALS AND METHODS
Study area, topography and mesoclimatic data
This study covered the entire French distribution area of Quercus ilex where the climate is Mediterranean (Fig. 1). The area where Q. ilex occurs naturally was recently updated by the French national institute of geographical information, IGN (Inventaire forestier national, 2011; http://www.ifn.fr). Our sampling area covered three administrative regions (‘Languedoc-Roussillon’, ‘Rhône-Alpes’ and ‘Provence-Alpes-Côte d'Azur’) ranging from latitude 42 °27′08''N to 44 °51′08″N and from longitude 02 °07′24″E to 07 °02′26″E. A total of 41 holm oak woodlands were sampled in continental France (Fig. 1; Supplementary Data Table S1). An additional sample was collected in Corsica (latitude 42 °20'23''N; longitude 09 °32'20''E). Three locations were sampled two or three times (Supplementary Data Table S1). In these cases, two of three microsites within a location differed in their topography. The topography of each microsite was characterized by two qualitative variables (shade and soil cover) and two quantitative variables (slope and exposure). Slope was calculated using numerical topographic maps (http://www.geoportail.gouv.fr/accueil). Exposure corresponded to the azimuth (0 to +180 °) measured between north and the main gap in the canopy. Shade was assessed according to the density of trees and exposure: 0, low density woodlands and borders of groves; 1, medium density woodlands and low density woodlands with no or very little direct sunlight; and 2, understorey of high density woodlands. The soil cover was also categorized into three types: litter, moss or grass, and bare soil. The climatic conditions (rainfall, minimum and maximum temperatures) experienced by acorns from shedding to the collection date were estimated using climatic data from 1 November 2011 to 31 March 2012 recorded by the nearest meteorological station (data were purchased from the public library of Météo France, https://public.meteofrance.com/). The distance between a sampling site and the meteorological station was always <5 km. Temperature data were corrected using the international standard atmosphere adiabatic lapse rate of 0·65 °C 100 m−1 and the difference in altitude between each sampling site and its corresponding meteorological station (Supplementary Data Table S1).
Fig. 1.
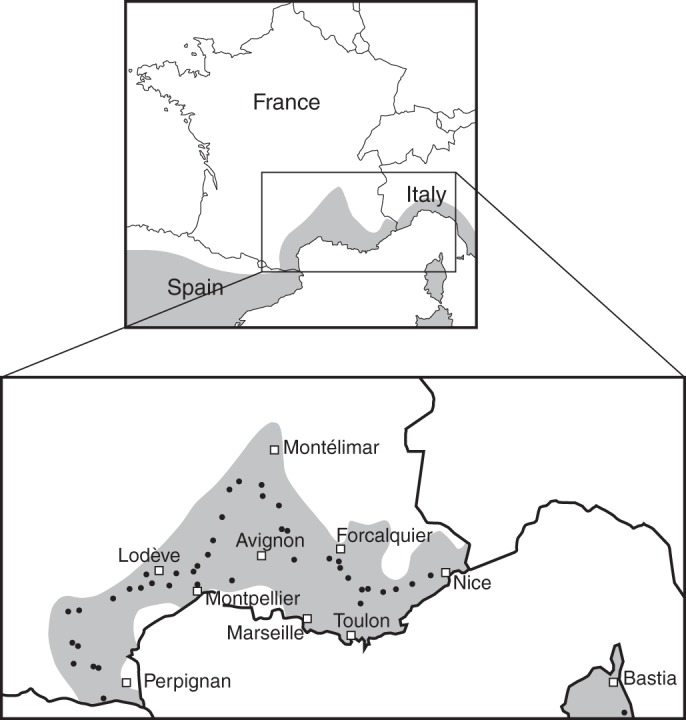
Geographical distribution of Quercus ilex (shaded) and locations of the 42 woodlands sampled (dots). The distribution area in the upper map is roughly adapted from Lumaret et al. (2002), while that in the lower map was based on data provided by the French National Institute of Geographical Information, IGN (Inventaire forestier national, 2011).
Biological material
Acorns of Q. ilex are abiotically dispersed by gravity in autumn. They may also be biotically dispersed, mainly by the European jay Garrulus glandarius and to a lesser extent by the woodmouse Apodemus sylvaticus (Gómez, 2003; Gómez et al., 2008). This study focused on gravity-dispersed acorns which remained on the surface of the ground, i.e. those that had not been removed by dispersers, damaged by insects or consumed by ungulates such as the wild boar Sus scrofa. Acorns were collected from 26 March to 2 April 2012. In each site sampled, about 200 acorns were collected from the ground underneath at least four randomly selected Q. ilex trees, immediately enclosed in a hermetically sealed plastic bag, and stored in the dark at ambient temperature for a maximum of 2 d until transport to the IRD laboratory (Montpellier, France). Acorns were then directly processed and all experiments were carried out using sound acorns, which were sorted from unsound ones by visual screening after the pericarp has been removed. The moisture content, expressed on a fresh weight basis, and the dry mass of seeds and pericarps were determined gravimetrically after oven drying for 17 h at 103 °C, using ten seeds per sample.
Viability assays
Seed viability was assessed using both percentage germination and the percentage of normal seedling development. For each location sampled, six batches of six seeds were placed on 18 g of vermiculite fully moistened with 45 mL of sterile water in closed plastic Magenta© boxes and kept at 25 °C in the dark. Germination was recorded when the radicle had grown at least 10 mm after 1 week of culture, therefore ensuring that protrusion was not due to mechanical elongation of the hypocotyl but to true growth of the radicle. Normal seedling development was recorded using the criteria of radicle geotropic growth and opening of primary leaves after 4 weeks of culture.
Desiccation tolerance assays
Freshly shed mature acorns were collected from a single plot close to Montpellier (Pic Saint-Loup, 43 °47′17″N, 03 °48′22″E) in October 2011. After pericarp removal, seeds were desiccated by equilibration over various saturated salt solutions for 20 d at 25 °C in the dark as previously described by Dussert et al. (1999). For the measurement of seed desiccation sensitivity, batches of 36 seeds were desiccated over NH4NO3 [62 % relative humidity (RH)], NaCl (75 % RH), KCl (85 % RH), KNO3 (92 % RH) and K2SO4 (97 % RH) saturated solutions. Seed viability after desiccation was assessed using percentage of germination as described above.
Statistical analyses
All statistics were carried out using Statistica software (Statsoft, Tulsa, OK, USA). Desiccation sensitivity was quantified using the previously developed quantal response model (Dussert et al., 1999) and tested using the least square regression computed by the Quasi-Newton method. Correlations between environmental variables, seed water content and seed viability were analysed by linear regression using Pearson's correlation coefficient. The effect of two qualitative microtopography descriptors, shade and soil cover, was tested by analysis of variance (ANOVA). Post-hoc comparison of means was performed using the Newman and Keuls test. Principal component analysis (PCA) was used to analyse the respective contributions of mesoclimatic and microtopographic variables to the variation in seed water content and viability.
RESULTS
Desiccation sensitivity of fresh mature Quercus ilex seeds
To our knowledge, the level of tolerance to drying of holm oak seeds has never been investigated. The desiccation sensitivity of fresh mature holm oak seeds was first measured in vitro, using mature acorns collected in autumn from trees of a single location close to Montpellier (Pic Saint-Loup). The initial water content of these seeds was 40·7 % fresh weight (f. wt). When germination was plotted against water content, the typical S-shaped pattern of desiccation-sensitive seeds was observed (Fig. 2A, open squares). The seed water content (WC) at which 50 % of the initial viability was lost, WC50, was very high (30·6 % f. wt) and corresponded to an equilibrium RH50 of 93·4 %. This level of desiccation sensitivity is in full agreement with that previously observed in freshly shed mature seeds of Q. rubra and Q. robur (Pritchard, 1991; Finch-Savage, 1992; Finch-Savage and Blake, 1994). On the basis of these results, Q. ilex seeds can undoubtedly be categorized as recalcitrant.
Fig. 2.
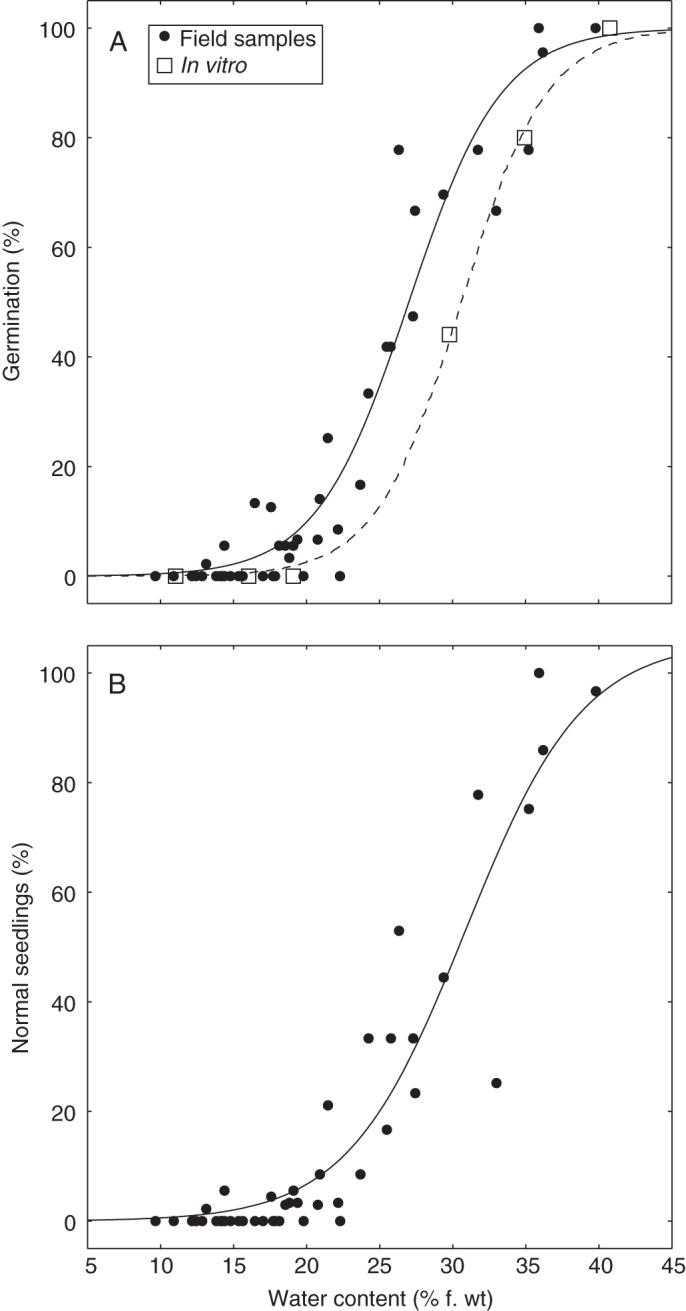
Relationships between the viability of Quercus ilex seeds collected in 42 woodlands in the South of France in spring 2012 and their water content (circles). Viability was estimated by the percentage both of germination (A) and that of normal seedling development (B). Seed desiccation sensitivity was also quantified in vitro using fresh mature acorns collected near Montpellier, France (squares). Solid and dashed lines correspond to the fitted patterns using the quantal response model described in Dussert et al. (1999).
In situ drying is a major cause of Q. ilex seed mortality during winter
Very high variability in seed viability was observed among the 42 locations sampled (Fig. 1; Supplementary Data Table S1). Complete loss of viability was observed in 18 samples, and, in the remaining samples, seed germination ranged from 2 to 100 %, and only nine samples had a germination percentage >60 %. Seed viability after the winter period was very nicely explained by the water status of seeds at the moment of collection (Fig. 2A). The proportion of variance explained by the quantal response model was very high (94 %), demonstrating a very homogeneous response of Q. ilex seeds to drying, independent of their origin. Germination was highly significantly (P < 10−6) correlated with seed water content within the 20–40 % f. wt hydration window. A similar trend was obtained when normal seedling development was chosen as the criterion for seed viability (Fig. 2B). The relationship between viability and water content obtained with seeds which remained on the soil during the whole winter period was almost identical to that observed in vitro with fresh mature seeds dehydrated in controlled conditions (Fig. 2A). The level of desiccation tolerance of seeds collected in the spring, as quantified by WC50 (27·0 % f. wt), was also very close to that of fresh seeds. The large number of samples displaying complete loss of viability (two-fifths of the sites sampled) clearly shows that acorns that remain on the soil until spring are subject to high environmental risks. Climatic parameters and/or microsite topography would thus be expected to be important factors in determining seed survival.
Rainfall and maximum temperatures during the winter period have a direct impact on seed water content and survival
The 42 locations sampled throughout the French distribution area of Q. ilex displayed very high variability in cumulative rainfall during the 2011–2012 winter, ranging from 13 to 293 mm (Supplementary Data Table 1). The average minimum and maximum temperatures also varied significantly from –3 to +8 °C and from +9 to +16 °C, respectively. Relationships between seed water content and survival and environmental factors were analysed by linear regression (Table 1, Fig. 3). Neither the average minimum temperature (Table 1, Fig. 3B) nor the absolute minimum temperature (which ranged from –18·5 to –0·8 °C, Table 1) was significantly correlated with seed water content and seed viability. The proportion of variance explained by these variables was <2 % (with P > 0·05), suggesting that holm oak acorns are highly tolerant to frost.
Table 1.
Relationships between water content and viability of Quercus ilex seeds collected during spring in 42 locations throughout the French distribution area of the species, and their mass, winter climatic conditions and microtopography of the microsites sampled
| Seed water content (% f. wt) |
Germination (%) |
|||
|---|---|---|---|---|
| R | P | R | P | |
| Seed dry mass (g) | –0·082 | 0·592 | –0·096 | 0·531 |
| Rainfall (mm) | 0·563 | 0·000 | 0·513 | 0·000 |
| Tmin (°C) | 0·124 | 0·418 | 0·030 | 0·845 |
| Tmin aver (°C) | 0·140 | 0·360 | 0·046 | 0·762 |
| Tmax aver (°C) | –0·455 | 0·002 | –0·489 | 0·001 |
| Taver (°C) | –0·179 | 0·240 | –0·275 | 0·067 |
| T°C range (°C) | –0·386 | 0·009 | –0·336 | 0·024 |
| Days rain <2 mm | –0·167 | 0·272 | –0·161 | 0·289 |
| Days rain <5 mm | –0·373 | 0·012 | –0·314 | 0·036 |
| Slope (%) | 0·253 | 0·093 | 0·194 | 0·202 |
| Exposure (° to North) | –0·540 | 0·000 | –0·504 | 0·000 |
Tmin, absolute minimum temperature; Tmin aver, average minimum temperatures; Tmax aver, average maximum temperatures; Taver, average daily temperatures; T°C range, average daily temperature range; Days rain <2 mm, number of consecutive days with <2 mm daily rainfall; Days rain <5 mm, number of consecutive days with <5 mm daily rainfall.
Significant correlations are in bold.
Fig. 3.
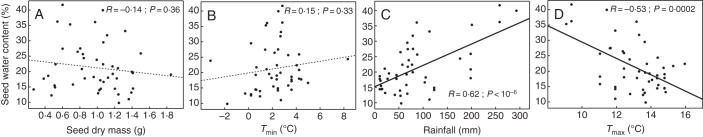
Correlations between the water content of Quercus ilex seeds collected in 42 woodlands in the South of France in spring 2012 and their dry mass (A), and three climatic parameters used to describe the environmental conditions in winter: average minimum winter temperature (B), winter rainfall (C) and average maximum winter temperatures (D). R and P are Pearson's linear correlation coefficient and probability of significance, respectively.
In contrast, a highly significant (P < 10−6) positive correlation was observed between seed water content and winter cumulative rainfall from December to March (Table 1, Fig. 3C). This climatic variable explained a significant proportion (38 %) of the variance for seed water content. In the 12 locations which experienced a severe winter drought (<50 mm cumulative rainfall), seed water content was never higher than 21 % f. wt, and percentage germination was always <15 % (Fig. 3C). In contrast, the seed water content of acorns collected in the few locations which received >250 mm rainfall during the same period was very high (35–40 % f. wt). The local average maximum temperature also displayed highly significant (P = 2 × 10−4) negative correlation with seed water content and explained >28 % of variance (Table 1, Fig. 3D). The seed water content of acorns sampled in 13 locations with high winter maximum temperatures (above +14 °C) was always lower than 23 %, while acorns with a high water content (35–40 % f. wt) at the end of winter were all sampled in the few woodlands with low maximal temperatures (less than +10 °C).
Microsite topography also contributes to seed survival
The effect of two qualitative microsite topography parameters – shade and ground cover – was assessed using ANOVA and a post-hoc Newman and Keuls test. There was a highly significant effect of both parameters on seed water content and survival, as assessed by percentage germination and the percentage of normal seedling development (Table 2). Seeds collected in sites with little or no shade had a very low water content at the end of winter and were in fact almost all dead. Among the three types of soil cover at our sample sites, leaf litter best prevented seed drying and subsequent loss of viability (Table 2). However, grass and moss were considerably less detrimental than bare soils. The influence of two other quantitative topographic parameters – exposure and slope – was tested by linear regression. While slope had no apparent effect on the status of seeds in spring, exposure had a significant impact on seed water content, and northern exposure was clearly less injurious (Table 1).
Table 2.
Effect of shade and soil cover on the water content and percentage germination of Quercus ilex seeds collected during spring in 42 locations throughout the French distribution area of the species
| Factor | Category | n | Water content (% f. wt) | Germination (%) |
|---|---|---|---|---|
| Shade | Shade | 20 | 24·5a | 38·3a |
| Mid-shade | 15 | 20·8a | 16·7ab | |
| Light | 11 | 14·3b | 2·2b | |
| F | 9·0 | 5·8 | ||
| P | 0·0006*** | 0·0059** | ||
| Soil cover | Leaf litter | 9 | 26·6a | 47·0a |
| Grass and moss | 17 | 23·1a | 30·0a | |
| Bare soil | 20 | 16·5b | 6·2b | |
| F | 9·1 | 7·1 | ||
| P | 0·0005*** | 0·0022** |
Results of one-way ANOVA: F and P.
Means followed by the same letter did not differ significantly according to the Newman and Keuls test.
n, number of samples per category.
In order to examine the respective contribution of all climatic and topographic variables to the status of seeds after winter, the two topographic qualitative variables – soil cover and shade – were transformed into scores ranked according to their impact on seed viability as indicated by ANOVA. All variables were then analysed by PCA, soil cover and shade being supplementary variables. The four main principal components (PC1–PC4) explained 82 % of the variance of the whole data set (Table 3). Variance of seed viability and water content was mainly explained by PC1 and PC3 (Table 3). These two components, which accounted for about 50 % of the overall variance, were therefore chosen for the analysis of the respective contributions of climate and topography (Fig. 4). As expected from the above results, seed viability and water content vectors were collinear. Moreover, the correlation circle clearly demonstrates that the mesoclimatic variables shown to influence seed survival individually (as assessed by linear regression above) mainly contributed to PC1, while microtopographic parameters were associated with PC3. PC1 was positively correlated with seed water content (R = 0·70), germination (R = 0·66) and rainfall (R = 0·89), but negatively correlated with the average maximum temperature (R = –0·74), diurnal temperature range (R = –0·82) and the number of consecutive days without significant rainfall (R = –0·70) (Table 3). PC3 was significantly correlated with germination (R = 0·640), shade (R = –0·57) and exposure (R = –0·78). This key finding demonstrates that climate explained most between-site variation in seed survival, but microtopography explained most of the residual part of it.
Table 3.
Correlation of the four first principal components resulting from principal component analysis (PCA) of the whole data set (46 Quercus ilex samples collected in 42 locations) with individual seed traits, climatic and microtopographic variables
| Factor | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| Seed dry mass | –0·279 | –0·041 | 0·146 | –0·723 (52·3) |
| Seed water content | 0·703 (49·4) | –0·251 | 0·606 (36·7) | –0·059 |
| Germination | 0·661 (43·7) | –0·338 | 0·583 (34·0) | 0·021 |
| Rainfall | 0·888 (78·8) | –0·120 | –0·159 | –0·200 |
| Tmin | 0·409 | 0·837 (70·1) | 0·087 | –0·148 |
| Tmin aver | 0·485 | 0·861 (74·2) | 0·027 | 0·051 |
| Tmax aver | –0·738 (54·5) | 0·407 | 0·178 | –0·337 |
| Taver | –0·079 | 0·948 (89·8) | 0·131 | –0·172 |
| T°C range | –0·823 (67·8) | –0·412 | 0·087 | –0·246 |
| Days rain <2 mm | –0·675 (45·6) | 0·075 | 0·561 (31·5) | 0·198 |
| Days rain <5 mm | –0·701 (49·1) | 0·260 | 0·349 | 0·464 |
| Slope | –0·014 | –0·179 | 0·359 | –0·450 |
| Exposure | –0·209 | –0·014 | –0·782 (61·2) | –0·131 |
| Shade* | 0·106 | –0·063 | 0·566 (32·0) | –0·017 |
| Soil cover* | –0·359 | 0·171 | –0·317 | 0·008 |
| Eigen value | 4·419 (33·99) | 2·975 (22·88) | 1·996 (15·35) | 1·269 (9·77) |
All variables were active, with the exception of two qualitative variables, shade and soil cover, which were supplementary variables and are indicated by an asterisk.
Percentage contributions of variables to the principal components are indicated in parentheses for significant correlations. The Eigen value of each PC and the corresponding percentage of total variance explained (in parentheses) are also indicated in the bottom row.
Tmin, absolute minimum temperature; Tmin aver, average minimum temperatures; Tmax aver, average maximum temperatures; Taver, average daily temperatures; T°C range, average daily temperature range; Days rain <2 mm, number of consecutive days with <2 mm daily rainfall; Days rain <5 mm, number of consecutive days with <5 mm daily rainfall.
Fig. 4.
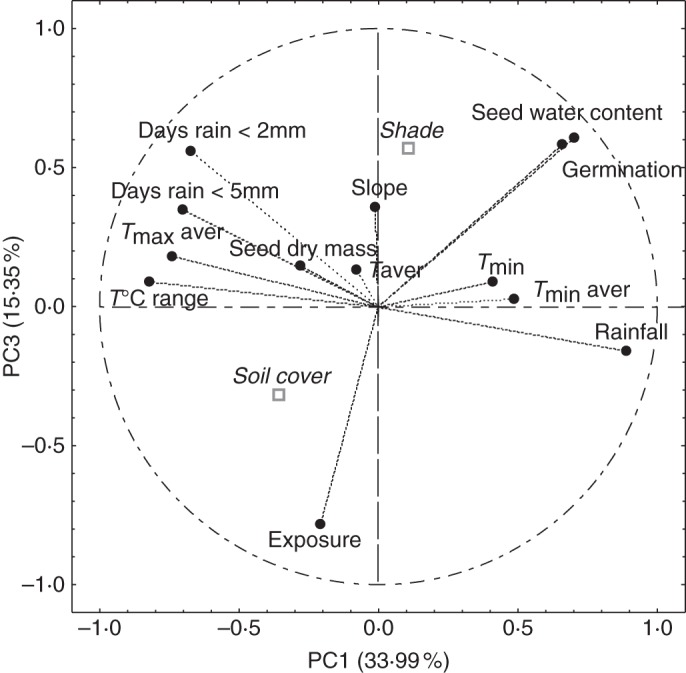
Correlation circle of all seed, climatic and topography variables using the first and the third components (PC1 × PC3) resulting from the principal component analysis (PCA) of data collected in 42 locations over the French distribution area of Quercus ilex. All variables were active (circles), with the exception of two qualitative variables, shade and soil cover, which were converted into scores for PCA but analysed as supplementary variables (squares). Taver, average daily temperatures; Tmax aver, average maximum temperatures; Tmin aver, average minimum temperatures; Tmin, absolute minimum temperature (recorded in February); T°C range, average daily temperature range; Days rain <2 mm, number of consecutive days without significant rainfall (daily rainfall not more than 2 mm).
The size and pericarp thickness of acorns did not influence their water status after winter
To test whether the variability of acorn morphology among the 42 locations sampled, possibly caused by intraspecific genetic variability or phenotypic plasticity, also contributed to the hydration and viability status of seeds after the winter period, the relationships between seed water content and germination, seed mass and pericarp thickness were tested by linear regression. The size of seeds, estimated by their dry mass, did not significantly influence their water status in spring (Fig. 3A). Similarly, the thickness of the acorn pericarp, estimated by the ratio of the pericarp to seed dry mass, was not significantly correlated with seed water content in a sub-set of 18 samples (R = 0·26; P = 0·37, data not shown).
DISCUSSION
It is now well established that environmental conditions can affect various traits of developing seeds (Donohue, 2009), including size (Daws and Jensen, 2011), desiccation tolerance (Daws et al., 2004) and longevity (Kochanek et al., 2010). For instance, latitudinal gradient was shown to cause climatic variations (sum of temperatures) that were large enough to trigger phenotypic variation in the level of desiccation sensitivity of seeds in Acer pseudoplatanus, probably due to variation in seed maturity at the end of the summer period (Daws et al., 2006a). Immature seeds are indeed usually shorter lived and more sensitive to desiccation than those that have reached peak maturity (e.g. Probert et al., 2007). In contrast, our results demonstrate a very homogeneous response of Q. ilex seeds to drying which is independent of their origin. The relationship between germination and water content was highly consistent over the entire French distribution of the species, and very similar to that obtained in vitro with fresh mature acorns, suggesting that full maturity was reached in all the locations sampled (Supplementary Data Table S1). This important finding strengthens the value of the Mediterranean holm oak forest as a model to investigate the ecological filtering effect of winter environmental conditions: results are not biased by the occurrence of a geographical variation in seed tolerance to drying before winter.
Similarly, one may hypothesize that the genetic variability detected among Q. ilex populations in the South of France (Lumaret et al., 2002) influences the pattern of seed desiccation tolerance in this region. The homogeneity of seed response to drying observed throughout the French distribution of Q. ilex rather suggests that this intraspecific genetic variation has no or very little impact on this trait. Neither did we find any evidence of a relationship between the size of holm oak seeds, the thickness of the pericarp and their survival. This finding may appear inconsistent with recent studies on the role played by seed size and seed coat thickness and anatomy in surviving pre-germination environmental conditions (Daws et al., 2006b; Hill et al., 2012; Xia et al., 2012b). However, it is worth noting that up to now, all significant relationships identified between these traits resulted from studies carried out at the interspecific level and not within a single species. Besides, a recent study demonstrated that it is necessary to use descriptors more sophisticated than those used in the present work to investigate the role played by the anatomy of the pericarp in Quercus sp. (Xia et al., 2012b).
Until now, little attention has been paid to the significance of seed desiccation sensitivity in temperate ecosystems. To our knowledge, this is the first study to investigate whether this trait can influence recruitment in recalcitrant seed species. Using Q. ilex as a model system, we established that temperate desiccation-sensitive seeds may suffer from drying during the period between shedding and the return of favourable conditions for germination. Within its French distribution area, the length of the period when Q. ilex seeds remain on the ground is very long since seed dispersal takes place in autumn and seedlings emerge at the beginning of spring. Our results demonstrate that winter mortality of desiccation-sensitive seeds can be particularly high in Mediterranean environments where the onset of the wet spring season is preceded by winter rainfall events that may be erratic in timing and amount. In addition to the major impact of winter rainfall, we showed that in Mediterranean forest ecosystems, the water content of seeds on the soil, and subsequently their viability, is dramatically influenced by winter maximum temperatures and microtopography. There is a significant amount of literature on the regeneration of oak species which focused either on mortality resulting from biotic factors (pathogenic fungi as well as insect and mammal predation) or on the death of newly emerged seedlings caused by frost or summer drought (Gomez, 2004; Garcia and Houle, 2005; Pons and Posas, 2007; Smit et al., 2008; Monnier et al., 2012; Perez-Ramos et al., 2012). Our study establishes that seed drying during the winter period is another major cause of mortality in the transient soil seed bank. Henceforth, this issue should be taken into account in studies dedicated to recruitment in temperate recalcitrant seed species.
Our study also revealed that the characteristics of a given microsite may account for the part of the between-location variation in seed water status and viability which is not explained by mesoclimatic variables. This finding corroborates the increasing number of studies which established the crucial importance of the microhabitat for seedling survival and growth in oak species (Monnier et al., 2012; Perez-Ramos et al., 2012). We observed that drying and subsequent mortality were significantly lower when acorns were dispersed in leaf litter than in grass, moss or on bare soil. Leaf litter, and even burial by dispersers, has already been suggested to protect holm oak acorns against desiccation (Kollmann and Schill, 1996; Garcia et al., 2002; Gomez, 2004). Our study also suggests that shade plays a significant role in Q. ilex seed survival in winter. Similarly, shade provided by shrubs has been shown to facilitate seedling recruitment in many Quercus species, including Q. ilex (Callawey, 1992; Smit et al., 2008). The topography parameters analysed in the present study were simply recorded as qualitative descriptors with a limited number of categories. Considering the significance of the results obtained with these two parameters, further characterization of microsites using complementary variables – such as light availability based on canopy openness, light spectral quality, litter composition and depth, soil moisture and temperature – should improve our understanding of the effects of microhabitat characteristics on the water status of seeds that remain on the soil throughout the winter period.
For long-lived species such as holm oak, sporadic winter drought events are not sufficient to compromise natural recruitment. However, an increase in the frequency of prolonged winter drought periods and a rise in winter temperatures could hamper the regeneration of non-orthodox seed species. In the context of climate change, one may thus assume that seed desiccation sensitivity is a key functional trait that could dramatically affect vegetation composition. In particular, climate projections consistently predict significant increases in temperature and decreases in precipitation in the Mediterranean basin (Gibelin and Deque, 2003; Giorgi and Lionello, 2008), suggesting possible future changes in the distribution of holm oak. For example, changes in precipitation were shown to have dramatic effects on Quercus emoryi recruitment in woodlands in southeastern Arizona (Weltzin and McPherson, 2000). Our results therefore illustrate the importance of considering the behaviour of seeds, particularly their sensitivity to drying, which is often overlooked when predicting the response of woody plants to impending climate change in Mediterranean regions.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
The authors wish to thank François Luro (INRA, San Guliano) for sending acorns from Corsica.
LITERATURE CITED
- Barbero M, Loisel R, Quezel P. Biogeography, ecology and history of Mediterranean Quercus ilex ecosystems. Plant Ecology. 1992;99–100:19–34. [Google Scholar]
- Callaway RM. Effect of shrubs on recruitment of Quercus douglasii and Quercus lobata in California. Ecology. 1992;73:2118–2128. [Google Scholar]
- Cheaib A, Badeau V, Boe J, et al. Climate change impacts on tree ranges: model intercomparison facilitates understanding and quantification of uncertainty. Ecology Letters. 2012;15:533–544. doi: 10.1111/j.1461-0248.2012.01764.x. [DOI] [PubMed] [Google Scholar]
- Daws MI, Jensen M. Effects of developmental heat sum on fruit traits of clonal lines of Quercus petraea grown under controlled conditions. Plant Growth Regulation. 2011;64:203–206. [Google Scholar]
- Daws MI, Lydall E, Chmierlarz P, Leprince O, Matthews S, Thanos CA, Pritchard HW. Developmental heat sum influences recalcitrant seed traits in Aesculus hippocastanum across Europe. New Phytologist. 2004;162:157–166. [Google Scholar]
- Daws MI, Cleland H, Chmielarz P, et al. Variable desiccation tolerance in Acer pseudoplatanus seeds in relation to developmental conditions: a case of phenotypic recalcitrance? Functional Plant Biology. 2006a;33:59–66. doi: 10.1071/FP04206. [DOI] [PubMed] [Google Scholar]
- Daws MI, Garwood NC, Pritchard HW. Prediction of desiccation sensitivity in seeds of woody Species: a probabilistic model based on two seed traits and 104 species. Annals of Botany. 2006b;97:667–674. doi: 10.1093/aob/mcl022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie JB, Mary K, Morris SVA, Titley SE. The effects of desiccation on seed survival in Acer platanoides L. and Acer pseudoplatanus L. Seed Science Research. 1991;1:149–162. [Google Scholar]
- Donohue K. Completing the cycle: maternal effects as the missing link in plant life histories. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:1059–1074. doi: 10.1098/rstb.2008.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussert S, Chabrillange N, Engelmann F, Hamon S. Quantitative estimation of seed desiccation sensitivity using a quantal response model: application to nine species of the genus Coffea L. Seed Science Research. 1999;9:135–144. [Google Scholar]
- Dussert S, Chabrillange N, Engelmann F, Anthony F, Louarn J, Hamon S. Relationship between seed desiccation sensitivity, seed water content at maturity and climatic characteristics of native environments of nine Coffea L. species. Seed Science Research. 2000;10:293–300. [Google Scholar]
- Ellis RH, Hong TD, Roberts EH. An intermediate category of seed storage behaviour? I. Coffee. Journal of Experimental Botany. 1990;41:1167–1174. [Google Scholar]
- Farnsworth E. The ecology and physiology of viviparous and recalcitrant seeds. Annual Review of Ecology and Systematics. 2000;31:107–138. [Google Scholar]
- Finch-Savage WE. Embryo water status and survival in the recalcitrant species Quercus robur L.: evidence for a critical moisture content. Journal of Experimental Botany. 1992;43:663–669. [Google Scholar]
- Finch-Savage WE, Blake PS. Indeterminate development in desiccation-sensitive seeds of Quercus robur L. Seed Science Research. 1994;4:127–133. [Google Scholar]
- Garcia D, Houle G. Fine-scale spatial patterns of recruitment in red oak (Quercus rubra): what matters most, abiotic or biotic factors ? Ecoscience. 2005;12:223–235. [Google Scholar]
- Garcia D, Banuelos MJ, Houle G. Differential effects of acorn burial and litter cover on Quercus rubra recruitment at the limit of its range in eastern North America. Canadian Journal of Botany. 2002;80:1115–1120. [Google Scholar]
- Gibelin AL, Deque M. Anthropogenic climate change over the Mediterranean region simulated by a global variable resolution model. Climate Dynamics. 2003;20:327–339. [Google Scholar]
- Giorgi F, Lionello P. Climate change projections for the Mediterranean region. Global and Planetary Change. 2008;63:90–104. [Google Scholar]
- Gómez JM. Spatial patterns in long-distance dispersal of Quercus ilex acorns by jays in an heterogeneous landscape. Ecography. 2003;26:573–584. [Google Scholar]
- Gómez JM. Importance of microhabitat and acorn burial on Quercus ilex early recruitment: non-additive effects on multiple demographic processes. Plant Ecology. 2004;172:287–297. [Google Scholar]
- Gómez JM, Puerta-Pineiro C, Schupp EW. Effectiveness of rodents as local seed dispersers of Holmoaks. Oecologia. 2008;155:529–537. doi: 10.1007/s00442-007-0928-3. [DOI] [PubMed] [Google Scholar]
- Hendry GAF, Finch-Savage WE, Thorpe PC, et al. Free radical processes and loss of seed viability during desiccation in recalcitrant species Quercus robur L. New Phytologist. 1992;122:273–279. doi: 10.1111/j.1469-8137.1992.tb04231.x. [DOI] [PubMed] [Google Scholar]
- Hill JP, Edwards W, Franks PJ. Size is not everything for desiccation-sensitive seeds. Journal of Ecology. 2012;100:1131–1140. [Google Scholar]
- Hoffmann AA. Acclimation: increasing survival at a cost. Trends in Ecology and Evolution. 1995;10:1–2. [Google Scholar]
- Kollman J, Schill HP. Spatial patterns of dispersal, seed predation and germination during colonization of abandoned grassland by Quercus petraea and Corylus avellana. Vegetatio. 1996;125:193–205. [Google Scholar]
- Kochanek J, Buckley YM, Probert RJ, Adkins S, Steadman KJ. Pre-zygotic parental environment modulates seed longevity. Austral Ecology. 2010;35:837–848. [Google Scholar]
- Krebs RA, Feder ME. Hsp70 and larval thermotolerance in Drosophila melanogaster: how much is enough and when is too much? Journal of Insect Physiology. 1998;44:1091–1101. doi: 10.1016/s0022-1910(98)00059-6. [DOI] [PubMed] [Google Scholar]
- Leprince O, Buitink J. Desiccation tolerance: from genomics to the field. Plant Science. 2010;179:554–564. [Google Scholar]
- Leprince O, Buitink J, Hoekstra FA. Axes and cotyledons of recalcitrant seeds of Castanea sativa Mill. exhibit contrasting responses of respiration to drying in relation to desiccation sensitivity. Journal of Experimental Botany. 1999;50:1515–1524. [Google Scholar]
- Lumaret R, Mir C, Michaud H, Raynal V. Phylogeographical variation of chloroplast DNA in holm oak (Quercus ilex L.) Molecular Ecology. 2002;11:2327–2336. doi: 10.1046/j.1365-294x.2002.01611.x. [DOI] [PubMed] [Google Scholar]
- Manos PS, Stanford AM. The historical biogeography of Fagaceae: tracking the tertiary history of temperate and subtropical forests of the northern hemisphere. International Journal of Plant Science. 2001;162:S77–S93. [Google Scholar]
- Monnier Y, Prevosto B, Ripert C, Corbani AC, Fernandez C. Forest microhabitats differentially influence seedling phenology of two co-existing Mediterranean oak species. Journal of Vegetation Science. 2012;23:260–270. [Google Scholar]
- Perez-Ramos IM, Urbieta IR, Zavala MA, Maranon T. Ontogenetic conflicts and rank reversals in two Mediterranean oak species: implications for coexistence. Journal of Ecology. 2012;100:467–477. [Google Scholar]
- Pons J, Pausas JG. Rodent acorn selection in a Mediterranean oak landscape. Ecology Research. 2007;22:535–541. [Google Scholar]
- Pritchard HW. Water potential and embryonic axes viability in recalcitrant seeds of Quercus rubra. Annals of Botany. 1991;67:43–49. [Google Scholar]
- Pritchard HW, Daws MI, Fletcher BJ, Gaméné CS, Msanga HP, Omondi W. Ecological correlates of seed desiccation tolerance in tropical African dryland trees. American Journal of Botany. 2004;9:863–870. doi: 10.3732/ajb.91.6.863. [DOI] [PubMed] [Google Scholar]
- Probert RJ, Adams J, Coneybeer J, Crawford A, Hay FR. Seed quality for conservation is critically affected by pre-storage factors. Australian Journal of Botany. 2007;55:326–335. [Google Scholar]
- Roberts EH. Predicting the storage life of seeds. Seed Science and Technology. 1973;1:499–514. [Google Scholar]
- Roach T, Beckett RP, Minibayeva FV, et al. Extracellular superoxide production, viability and redox poise in response to desiccation in recalcitrant Castanea sativa seeds. Plant, Cell and Environment. 2010;33:59–75. doi: 10.1111/j.1365-3040.2009.02053.x. [DOI] [PubMed] [Google Scholar]
- Ruiz-Labourdette D, Nogués-Bravo D, Saínz Ollero H, Schmitz MF, Pineda FD. Forest composition in Mediterranean mountains is projected to shift along the entire elevational gradient under climate change. Journal of Biogeography. 2012;39:162–176. [Google Scholar]
- Smit C, den Ouden J, Diaz M. Facilitation of Quercus ilex recruitment by shrubs in Mediterranean open woodlands. Journal of Vegetation Science. 2008;19:193–200. [Google Scholar]
- Tompsett PB, Pritchard HW. The effect of chilling and moisture status on the germination, desiccation tolerance and longevity of Aesculus hippocastanum L. seeds. Annals of Botany. 1998;82:249–261. [Google Scholar]
- Tweddle JC, Dickie JB, Baskin CC, Baskin JM. Ecological aspects of seed desiccation sensitivity. Journal of Ecology. 2003;91:294–304. [Google Scholar]
- Valiente-Banuet A, Rumebe AV, Verdú M, Callaway RM. Modern Quaternary plant lineages promote diversity through facilitation of ancient Tertiary lineages. Proceedings of the National Academy of Sciences, USA. 2006;103:16812–16817. doi: 10.1073/pnas.0604933103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltzin JF, McPherson GR. Implications of precipitation redistribution for shifts in temperate savanna ecotones. Ecology. 2000;81:1902–1913. [Google Scholar]
- Xia K, Daws MI, Hay FR, Chen WY, Zhou ZK, Pritchard HW. A comparative study of desiccation responses of seeds of Asian Evergreen Oaks, Quercus subgenus Cyclobalanopsis and Quercus subgenus Quercus. South African Journal of Botany. 2012a;78:47–54. [Google Scholar]
- Xia K, Daws MI, Stuppy W, Zhou ZK, Pritchard HW. Rates of water loss and uptake in recalcitrant fruits of quercus species are determined by pericarp anatomy. PLoS ONE. 2012b;7 doi: 10.1371/journal.pone.0047368. e47368. http://dx.doi.org/10.1371/journal.pone.0047368 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang QY, Crawford DJ, Wolfe AD, Tang YC, DePamphilis CW. Origin and biogeography of Aesculus L. (Hippocastanaceae): a molecular phylogenetic perspective. Evolution. 1998;52:988–997. doi: 10.1111/j.1558-5646.1998.tb01828.x. [DOI] [PubMed] [Google Scholar]
- Yu Y, Baskin JM, Baskin CC, Tang Y, Cao M. Ecology of seed germination of eight non-pioneer tree species from a tropical seasonal rain forest in southwest China. Plant Ecology. 2008;197:1–16. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


