Abstract
Background and Aims
Preservation of cultivar purity creates a particular challenge for plants that are self-incompatible, require insects for cross-pollination, and have easily germinating seeds and vigorously spreading rhizomes. As the fields must be planted with mixed populations, and a balance must be maintained between the cultivars to achieve effective pollination, methods for field monitoring of the relative density of different cultivars must be practical. Furthermore, a DNA-based method is needed for cultivar verification in the collections and outside of the growing season. The aim of this study was to develop both types of methods for Rubus arcticus (arctic bramble).
Methods
Morphological parameters were measured from six cultivars grown on three farms. Observations from the flowers and fruits included: petal and sepal number, flower diameter, arrangement of petals, size of calyx in relation to corolla, fruit weight, yield and soluble sugars. Observations from the leaves included: width and height of middle leaflet, shape of the base of terminal leaflet, shape of terminal leaflet, leaf margin serration and fingertip touch. The applicability of simple sequence repeat (SSR) or microsatellite DNA markers developed for red raspberry was tested on eight arctic bramble cultivars.
Key Results and Conclusions
Morphological and molecular identification methods were developed for R. arcticus. The best morphological characteristics were the length-to-width ratio of the middle leaflet and leaf margin serration. A particular characteristic, fingertip touch, was shown by electron microscopy to be related to the density and quality of the leaf hairs. Red raspberry SSR marker no. 126 proved to be applicable for differentiation of the eight arctic bramble cultivars tested. These identification methods are critical to secure the maintenance and management of R. arcticus. However, the challenges faced and approaches taken are equally applicable to other species with similar biology.
Keywords: Arctic bramble, authenticity, cultivar purity, identification, morphological markers, Rubus arcticus ssp. arcticus, self-incompatibility, SSR markers, UPOV, preservation, cultivar maintenance, genetic resources conservation
INTRODUCTION
Arctic bramble (Rubus arcticus ssp. arcticus) grows wild throughout subarctic Eurasia, mainly between 60 ° and 70 °N, but also in Asia in a broader zone, from 50 ° to 70 °N. The distribution extends into the northern parts of North America (Hulten, 1967). In spite of its aromatic and delicious fruits, genuine arctic bramble is cultivated only in Finland at a small scale, but has been used as a breeding parent with Alaskan arctic bramble (R. arcticus ssp. stellatus) and raspberry (R. idaeus). The six arctic bramble cultivars (‘Mespi’, ‘Mesma’, ‘Pima’, ‘Elpee’, ‘Marika’, ‘Muuruska’) commercially available are selections of natural accessions and their crosses (Ryynänen, 1972; Ryynänen and Dalman, 1983; Pirinen et al., 1998). The field and in vitro collections of arctic bramble and its cultivars are very important because there are clear signs of its diminishing in the wild in Finland and especially in Estonia (Ryynänen, 1973; Vool et al., 2011). As arctic bramble is self-incompatible (Larsson, 1969; Tammisola, 1988) and needs cross-pollination with insects, the cultivars can be preserved only as vegetative cultures. Rubus arcticus ssp. arcticus crosses with R. arcticus ssp. stellatus and R. arcticus ssp. × stellarcticus (Larsson, 1969), which threatens the genuineness of arctic bramble whenever these plants are grown in close proximity. Arctic bramble has superior aroma qualities compared with the other two subspecies (Kallio et al., 1980; Häkkinen et al., 1995), which is highly valued by the traditional Finnish liqueur industry.
Currently, the arctic bramble cultivars are preserved in The Finnish National Programme for Plant Genetic Resources in MTT Agrifood Research Finland Piikkiö as field collections and in Laukaa as elite plants (Aaltonen et al., 2006). There are also field and in vitro collections of arctic bramble in the University of Eastern Finland. Seed collections of arctic bramble are kept in the United States National Germplasm repository, the plants being collected from the wild in Finland, Alaska and Russia (USDA-ARS, 2011). There are ex situ collections of wild arctic bramble in the University of Agriculture in Estonia (IPGRI, 2002). In the Svalbard Global seed Vault, three accessions of arctic bramble can be found in seed collections (FAO, 2010; SGSV, 2010). Of the seven Finnish arctic bramble cultivars mentioned above only four are present in the national collections in MTT and of those four cultivars only two are maintained as elite plants (Aaltonen et al., 2006). Aaltonen et al. (2006) published guidelines for the long-term preservation of arctic bramble, according to which plots of different cultivars should not be planted less than 2 m apart in order to prevent growth of the rhizomes to the neighbouring plots. Mechanical barriers can also be used to prevent mixing of the plant material (Ryynänen and Dahlman, 1983). This is important as the rhizome of arctic bramble can spread 0·5 m yr−1 under favourable conditions (Saastamoinen, 1930; Ervi et al., 1955; Ryynänen, 1973). However, in the guidelines for gene banks, Reed et al. (2004) point out that the easily outcrossing grasses have to be isolated from potential pollinators in field collections. Similarly, Chebotar et al. (2003) conclude that in open-pollinating plants the distance between regeneration plots should be maximized.
To assess the genuineness of a cultivar, it must be clearly described. The closest relatives of arctic bramble, for which The International Union for the Protection of New Varieties of Plants (UPOV) guidelines for the conduct of tests for distinctness, uniformity and stability are available, are raspberry (Rubus idaeus) and strawberry (Fragaria) (UPOV, 2003, 2012), and these guidelines obviously need to be adjusted for any related rare species. In general, differentiation of arctic bramble cultivars is difficult in the field, and only an experienced observer is able to identify them based on morphological characteristics. Although descriptions are available on each of the arctic bramble cultivars separately, these are not of practical use for the distinction of the plants in the field. Pirinen et al. (1998) have suggested guidelines for arctic bramble cultivar identification. Also, Ryynänen (1972) and Ryynänen and Dalman (1983) specified morphological characteristics distinctive to the cultivars ‘Pima’, ‘Mespi’ and ‘Mesma’. However, there is a need for a simple tool for cultivar identification in the field. It might also be necessary sometimes to identify cultivars at an early growth stage when the leaves are not opened and the morphological features are not fully developed. DNA-based methods, such as RAPD (random amplification of polymorphic DNA), AFLP (amplified fragment length polymorphism) and SSR (simple sequence repeat), provide improved accuracy in all conditions for cultivar identification.
In an earlier study, different arctic bramble cultivars were distinguished from each other with RAPD analysis (Pirinen et al., 1998). However, as the method is prone to errors and the reproducibility is poor between laboratories and DNA extraction methods, RAPD is not recommended for routine use in cultivar identification. In a study of the genetic diversity of arctic bramble, AFLP was used to distinguish between different cultivars (Lindqvist-Kreuze et al., 2003). However, the method is laborious and requires high-quality DNA for good reproducibility. PCR-based SSR markers are by far the best option for cultivar identification, as they are reliable and the quality of DNA is not as critical as with AFLP. It is therefore increasingly the preferred method for cultivar identification, markers being developed for a wide variety of plants, including many soft fruits such as strawberry (Govan et al., 2008), red raspberry (Fernández-Fernández et al., 2011), blackberry (Bassil et al., 2010), blueberry and cranberry (Bassil, 2012). Until now, no SSR markers have been tested or developed for R. arcticus. Graham et al. (2002) designed markers for red raspberry, and these have proven suitable for other Rubus species, including black raspberry (R. occidentalis) and blackberry (R. fruticosus agg.).
The aims of this study were to develop tools for the morphological identification of arctic bramble cultivars in the field, to test the applicability of red raspberry SSR markers for arctic bramble in order to find a simple and repeatable molecular method for identification, and give a first report of the problems that can be encountered with cultivar stability of arctic bramble. No previous studies are available on the genuineness of arctic bramble cultivars in field collections or on the development of SSR markers. A few previous studies are available on morphological differences of cultivars but these are not sufficiently simple for cultivar identification in field conditions.
MATERIALS AND METHODS
Morphological markers
A study on Rubus arcticus (arctic bramble) cultivars was carried out on three commercial farms in Finland, North-Savo region (62°56′–63°79′N, 26°86′–28°38′E). In all farms, the 1000-m2 arctic bramble field included buffer rows on each side of the experimental rows, and ten buffer plants at both ends of the rows to avoid border effects. The buffer plant was arctic bramble as a mixed population. Perpendicular to the rows, the field was divided into eight blocks. The experimental design was randomized complete block design (RCBD), with one replicate of all cultivars in each of the eight blocks, resulting in a total of 24 plants per cultivar (all farms included). The aim of the design was to exclude possible interference from downy mildew infestation, and to minimize the effects of compositional differences in the soil. In June 2006, the vegetatively propagated plants were planted approx. 30 cm apart in raised beds covered with plastic mulch with no physical barrier between the plants. Observations were made from 2007 to 2010. The six cultivars analysed were ‘Mesma’, ‘Muuruska’, ‘Pima’, ‘Mespi’, ‘Elpee’ and ‘Alli’. The cultivars ‘Mespi’ and ‘Mesma’ are selections from a strain from Piikkiö in south-west Finland and Maaninka in North-Savo (Ryynänen, 1972). The cultivar ‘Pima’ (TTA-163) is a cross between ‘Mespi’ and ‘Mesma’ (Ryynänen and Dalman, 1983). The cultivars ‘Elpee’ and ‘Marika’ originate from Konnevesi and Laukaa, respectively, both located in Middle Finland (Pirinen et al., 1998). The cultivar ‘Muuruska’ originates from Kiiminki in Ostrobothnia. The cultivar ‘Susanna’ (TTA-127), originating from Vimpeli (MTT, 2012), is also available for growers but is not described in the literature (Supplementary Data Fig. S1). There is also a new named cultivar ‘Alli’ (open-pollinated strain of unknown parents), which in previous literature is referred to as clone 12 B 14 (Hukkanen et al., 2008). The farmers handled the arctic bramble fields according to good agricultural practice. Morphological observations made from the flowers and fruits included: petal and sepal number, flower diameter, arrangement of petals, size of calyx in relation to corolla, fruit weight, yield and soluble sugar content. The sugar content of the fruits was analysed from the harvest of farm No. 1 in 2007 with a refractometer (Atago, Tokyo, Japan). Glucose was used as standard. Means of three technical replicates of eight samples, each representing fruits from one plant, were calculated. Observations made from the leaves included: width and height of the middle leaflet, shape of the base of terminal leaflet, shape of terminal leaflet and leaf margin serration. Differences in fingertip touch of the leaves were recorded.
In August 2011, the hairiness of the leaf epidermis was analysed to find an explanation for the clear differences observed in fingertip touch. The leaves were collected from the Kuopio Research Garden of the University of Eastern Finland and from one farm. Four morphologically typical leaves were selected from each cultivar and a disc was taken from each leaf, resulting in four replicates. The leaf discs were analysed with an XL30 ESEM-TMP scanning electron microscope (Fei Company, Eindhoven, the Netherlands). Leaf hairiness was recorded from the central parts of the leaves, excluding the hairs from 0·2 mm of the leaf margin because all arctic bramble cultivars have dense hairiness in the leaf margins. The hairs were counted, and the mean values were used for statistical analysis.
Statistical analyses were made with IBM SPSS Statistics 19. Analysis of variance for RCBD was used to identify differences in the characteristics of the different cultivars at the 5 % risk level for each farm and year separately. Tukey's test was used to identify the cultivars that differed in each characteristic at the 5 % risk level.
Molecular markers
The applicability of SSR markers previously developed for red raspberry (Graham et al., 2002) was tested with eight arctic bramble cultivars, i.e. ‘Alli’, ‘Mesma’, ‘Muuruska’, ‘Pima’, ‘Mespi’, ‘Elpee’, ‘Marika’ and ‘Susanna’, hybrid arctic bramble (R. arcticus nothosubsp. stellarcticus) cultivars ‘Beata’ (TTA-143) and ‘Sofia’ (TTA-147), and with a number of undefined open-pollinated arctic bramble and hybrid arctic bramble seedlings. The samples used were from: field and in vitro culture collections in the Kuopio Research Garden of the University of Eastern Finland from 2009 and 2010; in vitro culture collection of Agrifood Research Finland MTT Laukaa from 2011; frozen leaf samples from the commercial nursery Biotaimi Ltd from 2006; research fields of farmers in the North-Savo region from 2009; and dried leaf samples from Agrifood Research Finland MTT Sotkamo and from Savo Vocational College Muuruvesi, both from 1995.
DNA was extracted from fresh, frozen or dried plant samples (leaves or overwintering buds). The samples were homogenized with FastPrep® FP120 (Qbiogene Inc., Carlsbad, CA, USA) in extraction tubes containing ceramic sphere and sea sand, and DNA was extracted with the CTAB method according to Doyle and Doyle (1990). Primer pair nos. 26, 126, 157, 223, 262, 277 and 280, developed for raspberry SSR loci (Graham et al., 2002), were initially tested with 24 DNA samples extracted from arctic bramble cultivars and open-pollinated arctic bramble and hybrid arctic bramble seedlings at Agrifood Research Finland MTT. The PCR reactions were performed in a 10-μL reaction mixture containing 20–30 ng template DNA, 75 mm Tris-HCl (pH 9·0), 2 mm MgCl2, 50 mm KCl, 20 mm (NH4)2SO4, 0·2 µm of each forward and reverse primer, 200 µm dNTP and 0·5 U DNA polymerase (Biotools, B & M Labs, S.A., Spain). Amplification was carried out in 96-well plates in a PTC-100 Programmable Thermal Controller (MJ Research Inc., Bio-Rad Laboratories, Hercules, CA, USA). The PCR reaction consisted of 5 min denaturation at 94 °C, followed by 35 cycles of 30 s at 94 °C, 45 s at 50 °C, 1 min at 72 °C and 7 min final extension at 72 °C. Labelled (Hex, Tet or Fam) amplification products were detected by capillary electrophoresis using a MegaBACE 1000 DNA sequencer with ET400-R as size standard (GE Healthcare, Little Chalfont, UK).
Of all primer pairs, no. 126 (Forward: FAM-5′CCTGCATTTTTCTGTATTTTGG3′; Reverse: 5′TCAGTTTTCTTCCCACGGTTA3′) was able to distinguish between all eight arctic bramble cultivars and was thus selected for further studies carried out at the University of Eastern Finland. In total, 78 DNA samples were analysed, representing 28 different accessions. The PCR reaction mixture consisted of 1 × DreamTaqTM Green PCR Master Mix (Fermentas International Inc., Burlington, ON, Canada), 0·2 mm of both primers and 17–22 ng genomic DNA in a total volume of 25 µL. Amplification was carried out with a PTC-100TM Programmable Thermal Controller using the following programme: initial denaturation for 3 min at 95 °C, followed by 35 cycles of 30 s at 95 °C, 30 s at 50 °C, and 1 min at 72 °C, and final extension for 5 min at 72 °C. The PCR products were diluted 1 : 10 and mixed with loading solution (70 % formamide, 1 mm EDTA) and ET400-R size standard. Fragments were separated with capillary electrophoresis (MegaBACE 750).
RESULTS
Morphological markers for arctic bramble cultivar identification
In the studies carried out on the farms in 2007, we observed that arctic bramble rhizomes were spreading vigorously, the shoots emerging from the neighbouring plant openings. Therefore, particular attention was paid to collect data that genuinely represented each cultivar.
Flowers
The flower parameters measured (petal and sepal number, flower diameter, arrangement of petals, size of calyx in relation to corolla) did not reveal any significant differences between the cultivars (results not shown). In fact, considerable variation was observed in the petal and sepal numbers within the cultivars. For example, in the cultivar ‘Alli’ the petal number varied from six to 11. Other parameters either varied greatly within a cultivar, e.g. petal and sepal numbers and flower diameter, or were the same for all cultivars, e.g. the size of calyx was smaller than corolla in all cultivars. The arrangement of the petals appeared to be more dependent on the weather and the age of the opened flower than on the cultivar. According to the results, flower parameters are not recommended for the identification of arctic bramble.
Leaflets
The length, width and length-to-width ratio of the middle leaflet, together with leaf margin serration, were found to be suitable parameters for cultivar identification. Middle leaflets of the cultivars ‘Muuruska’ and ‘Mesma’ had length-to-width ratios of 1·84–1·89, whereas the ratio in the other cultivars varied from 1·26 to 1·78 (Table 1). Visually this was shown as narrow middle leaflets typical for ‘Muuruska’ and ‘Mesma’ (Table 2). The middle leaflets in cultivars ‘Elpee’, ‘Pima’, ‘Mespi’ and ‘Alli’ were clearly broader. On farm No. 1, the length of the middle leaflet of ‘Alli’ was 50–52, mm whereas in the other wide-leaflet cultivars it varied from 39 to 46 mm, being significantly shorter than in ‘Alli’. The middle leaflets of ‘Alli’ were also broader than in the other cultivars, the difference being statistically significant on farm nos. 1 and 3 in 2008. Although the leaves on farm no. 3 were generally smaller, the length-to-width ratio remained the same. The cultivars could thus be divided into two groups according to the characteristics of the middle leaflet; cultivars with narrow middle leaflets included ‘Muuruska’ and ‘Mesma’, and cultivars with broad middle leaflets included ‘Alli’, ‘Pima’, ‘Mespi’ and ‘Elpee’.
Table 1.
Size and shape of the middle leaflet of arctic bramble cultivars, indicating length-to-width ratio to be a good identification parameter
| Cultivar | Length (mm) |
Width (mm) |
Length-to-width ratio |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Farm 1 |
Farm 2 |
Farm 3 | Farm 1 |
Farm 2 |
Farm 3 | Farm 1 |
Farm 2 |
Farm 3 | |||||||
| 2008 | 2009 | 2008 | 2009 | 2008 | 2008 | 2009 | 2008 | 2009 | 2008 | 2008 | 2009 | 2008 | 2009 | 2008 | |
| ‘Alli’ | 52a | 50a | 52a | 53a | 45a | 39a | 36a | 38a | 39a | 34a | 1·35a | 1·37a | 1·38ab | 1·38a | 1·32a |
| ‘Mesma’ | 48ab | 47ab | 47ab | 50a | 42ab | 27bc | 27b | 25bc | 29b | 23b | 1·84b | 1·77b | 1·89c | 1·78b | 1·84b |
| ‘Muuruska’ | 44bc | 43 | 39ab | 24b | 23 | 21b | 1·86b | 1·89 | 1·85b | ||||||
| ‘Pima’ | 45 | 46ab | 39 | 32. | 34a | 29 | 1·41 | 1·34a | 1·35 | ||||||
| ‘Mespi’ | 46bc | 40 | 44b | 43 | 38 | 34e | 27 | 35a | 32 | 22 | 1·37a | 1·51 | 1·26a | 1·34 | 1·78 |
| ‘Elpee’ | 42c | 44b | 39ab | 29cd | 29c | 25bc | 1·48a | 1·51b | 1·58c | ||||||
The results are displayed from three different farms from one or two years. Observations were made from three leaves of each of the eight replicate plants; when one or more of the replicates had died they were excluded from the statistical analyses and are only displayed as an average. Values followed by the same letter are not significantly different from each other at P < 0·05 (Tukey's test).
Table 2.
All arctic bramble cultivars could be distinguished from each other based on three characteristics: shape of middle leaflet (length-to-width ratio), shape of serration and fingertip touch of leaf surface – these identification parameters are also easy to use in the field
| Cultivar | Shape of middle leaflet | Middle leaflet | Serration | Leaf margin serration | Fingertip touch of leaf surface |
|---|---|---|---|---|---|
| ‘Alli’ | Wide |  |
Siruate |
|
Leathery |
| ‘Mesma’ | Narrow |  |
Double-serrate |
|
Velvety |
| ‘Muuruska’ | Narrow |
|
Dentate |
|
Leathery |
| ‘Pima’ | Wide |

|
Incised |
|
Neither velvety nor leathery |
| ‘Mespi’ | Wide |

|
Siruate |
|
Neither velvety nor leathery |
| ‘Elpee’ | Wide |

|
Crenate |
|
Neither velvety nor leathery |
The shape of the leaf margin serrate was also a good parameter for cultivar identification (Table 2). The cultivar ‘Elpee’ has crenate serration, which could also be double crenate in the lower part of the leaflet. The cultivar ‘Mespi’ has siruate serration, as does the cultivar ‘Alli’. The siruate serration in ‘Mespi’ is uniform, whereas the serration in ‘Alli’ varies from siruate to double-double-serrate. The cultivar ‘Muuruska’ has dentate serration and the cultivar ‘Mesma’ double-serrate serration. The cultivar ‘Pima’ has incised leaf margins. Together, the length-to-width ratio and the serration of the leaflets were able to differentiate between all cultivars, except for ‘Alli’ and ‘Mespi’.
Some differences were also observed in the folding of the leaflets and the visibility of the secondary veins. The cultivars ‘Mespi’ and ‘Muuruska’ had smooth leaflets, whereas the cultivar ‘Pima’ had strong folding in the leaflets.
Hairiness of leaf epidermis
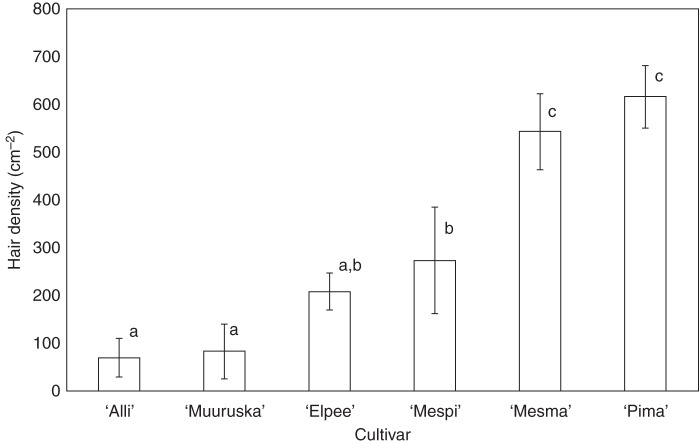
Fingertip touch was used as a practical identification tool for the cultivars. All trainees over the years were able to adopt this method, which was applicable both in open field and in tunnel cultivation. This experience raised the hypothesis of differences in the surface properties such as hairiness of the leaves. The leaf hair measurements confirmed this to be the case (Fig. 1). The leaves of cultivars ‘Alli’ and ‘Muuruska’, which were shiny and felt leathery, had on average 71 and 85 hairs cm−2, respectively. The difference in fingertip touch was particularly clear between the cultivars ‘Alli’ and ‘Mesma’, the latter feeling velvety soft and having as many as 544 hairs cm−2. Fingertip touch was particularly suited to distinguish between ‘Mesma’ and ‘Alli’ in the early summer when leaf shape and margin serration differences were not yet clearly observable. Although the cultivar ‘Pima’ had over 617 hairs cm−2 the fingertip touch was not as velvety as in ‘Mesma’. Scanning electron microscopy revealed that the leaf hairs of ‘Pima’ were more delicate than those of the other cultivars (Fig. 2). The leaves of the cultivars ‘Elpee’ and ‘Mespi’ had 209 and 274 hairs cm−2, respectively, were not shiny and leathery, and the fingertip feeling was not as velvety as in the cultivar ‘Mesma’ or not as leathery as in ‘Alli’.
Fig. 1.
Hair density in the leaf epidermis of arctic bramble varied markedly between the cultivars. This explains the difference in the fingertip touch, which varies from leathery in cultivars ‘Alli’ and ‘Muuruska’ to soft velvety in cultivar ‘Mesma’. The values are means of four replicates ± s.d. Values followed by the same letter are not significantly different at P < 0·05 (Tukey's test).
Fig. 2.
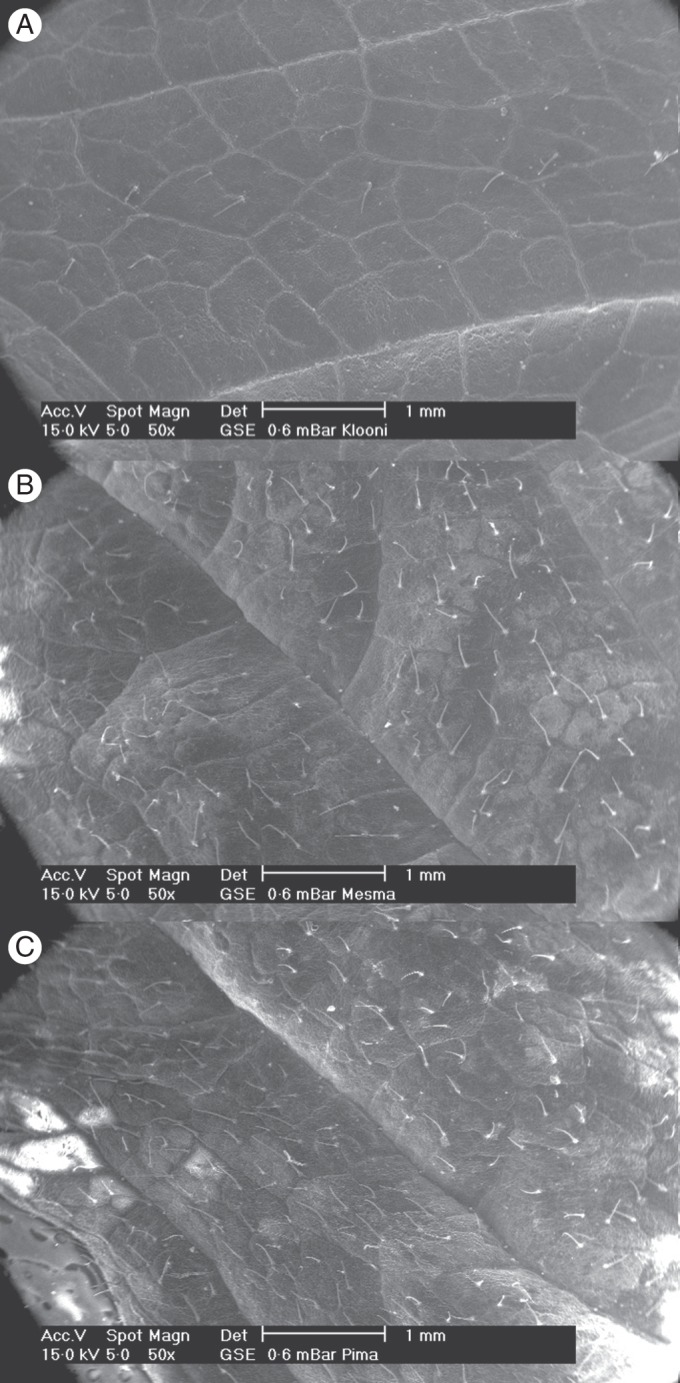
Scanning electron micrographs showing differences in leaf epidermis hairiness and in the thickness of the hairs between the arctic bramble cultivars. Cultivar ‘Alli’ (A) had the lowest and ‘Mesma’ (B) and ‘Pima’ (C) the highest hair density. The images also suggested that hair thickness might partially explain the fingertip touch, in particular between ‘Mesma’ (velvety) and ‘Pima’.
In conclusion from the morphological markers, the length-to-width ratio of the middle leaflet, leaf margin serration and the hairiness of the leaves together could distinguish between all arctic bramble cultivars (Table 2). These parameters have the benefit of being easily learned and also being observed under field conditions.
When identifying arctic bramble cultivars based on the leaves, it is important to examine all the leaves when selecting those for closer observation. The youngest, newly opened leaves are rarely typical of the cultivar. The first leaves are often smaller and located in the lower, shady parts of the plant. Therefore, identification based on the leaves can be made reliably only by an experienced observer. The easiest evaluation period is in the beginning of July and in the beginning of harvest in mid-July, as also recommended by UPOV (2003, 2012). Later during the season leaf senescence may interfere with identification.
Fruits and yield
Yield varied greatly between the years and per plant, ranging from 6·13 to 64·88 g per plant (‘Muuruska’ in 2007 and ‘Mesma’ in 2008, respectively) (Table 3). The largest fruits were in cultivars ‘Mesma’ (2007 and 2008) and ‘Mespi’ (2009). The only significant difference was that cultivar ‘Mesma’ had larger fruits than cultivar ‘Muuruska’. The soluble sugar content of the fruits varied from 12·3 to 14·4 g soluble solids/100 g of juice (Table 4). Cultivars ‘Pima’ and ‘Mespi’ had significantly higher soluble solid content than cultivar ‘Mesma’.
Table 3.
Yield in arctic bramble was highly fluctuating as seen from the yield results from farm 1 from three consecutive years
| Cultivar | 2007 |
2008 |
2009 |
|||
|---|---|---|---|---|---|---|
| Berry size (g) | Yield (g per plant) | Berry size (g) | Yield (g per plant) | Berry size (g) | Yield (g per plant) | |
| ‘Alli’ | 1·00ab | 14·45ab | 0·63a | 60·88a | 0·61a | 18·25a |
| ‘Mesma’ | 1·32a | 45·00bc | 0·97d | 64·88a | 0·87a | 17·75a |
| ‘Muuruska’ | 0·81b | 6·13a | 0·68ab | 12·00bc | ||
| ‘Pima’ | 0·78ab | 57·13c | 0·75abc | 40·25ab | 0·58 | 15·00 |
| ‘Mespi’ | 0·98ab | 61·63c | 0·86bcd | 34·25abc | 0·90 | 18·29 |
| ‘Elpee’ | 0·96ab | 30·88abc | 0·90cd | 39·63ab | 0·85a | 11·88ab |
Values followed by the same letter are not significantly different from each other at P < 0·05 (Tukey's test). When plants died during the study the statistical analysis could not be done for the RCDB design of the study but the averages are included in the table.
Table 4.
The soluble solid content of the berries from farm 1 in 2007
| Cultivar | Soluble sugar content (g soluble solids/100 g of juice) |
|---|---|
| ‘Alli’ | 13·5ab |
| ‘Mesma’ | 12·3a |
| ‘Muuruska’ | 13·3ab |
| ‘Pima’ | 14·0b |
| ‘Mespi’ | 14·4b |
| ‘Elpee’ | 12·8ab |
Values followed by the same letter are not significantly different from each other at P < 0·05 (Tukey's test).
The fruits of cultivar ‘Alli’ were uniformly ruby red, while in nature and in other cultivars ripe fruits commonly vary in colour from pale green to ruby red, and ripeness is not evaluated by colour but by the translucency and softness of the fruit surface. Yield and fruit properties are important commercially but for cultivar identification in the field they are not as useful as leaf parameters.
Molecular markers for arctic bramble cultivar identification
The applicability of seven SSR primer pairs developed for red raspberry was examined for their ability to differentiate between eight arctic bramble cultivars. All tested SSR primers gave amplification products in arctic bramble and hybrid arctic bramble accessions (Table 5). However, as the primers were designed for red raspberry they might contain some mismatches when used in arctic bramble. It was thus necessary to lower the annealing temperature to 50 °C.
Table 5.
SSR primers developed for red raspberry (Graham et al., 2002) were able to amplify fragments from arctic bramble cultivars and open-pollinated arctic bramble and hybrid arctic bramble seedlings
| SSR primer | Size range (bp) | Number of alleles |
|---|---|---|
| 26 | 99–112 | 5 |
| 126 | 123–177 | 15 |
| 157 | 177–195 | 3 |
| 223 | 130–142 | 5 |
| 262 | 190–208 | 6 |
| 277 | 205–218 | 6 |
| 280 | 209–234 | 10 |
The fragment sizes, which were comparable with those in red raspberry or slightly shorter, ranged from 99 to 234 bp, and the number of polymorphic alleles varied from three to 15 per locus.
Although all markers were polymorphic (Table 5), SSR marker no. 126 was the only one that could make a distinction between all arctic bramble cultivars (Table 6A) and was thus selected for further studies. To confirm the reliability of the marker for cultivar identification, several samples of the same cultivar from different sources were analysed (Table 6A). In addition, the marker was tested with various open-pollinated hybrid arctic bramble and arctic bramble seedlings, and was shown to differentiate also between the cultivars and seedlings (Table 7). There was a pattern of stutter bands of rather high intensity in all samples. Nevertheless, the results were easy to interpret and the repeatability of the method was good, as the same results were obtained in two different laboratories despite the slightly different protocols used. The DNA extracted both from the leaves and from overwintering buds was of sufficient quality for SSR amplification. Stability of the marker was also confirmed by analysing leaf samples of ‘Mespi’, ‘Muuruska’, ‘Pima’ and ‘Elpee’ collected in 1995. The results showed that allele lengths of these cultivars had remained identical for over 15 years.
Table 6.
(A) SSR primer pair no. 126 (Graham et al., 2002) is applicable for arctic bramble cultivar identification – shown are the sizes (bp) of alleles characteristic to each cultivar; n, number of individual plants analysed. (B) Alleles in arctic bramble plants originated from various collections of ‘Alli’, ‘Marika’, ‘Mespi’ and ‘Pima’ cultivars
| (A) Cultivar SSR alleles (bp) |
n | (B) Diverging alleles from cultivar collections |
|||
|---|---|---|---|---|---|
| ‘Alli’ | 126, 175 | 13 | 126, 177 | ||
| ‘Elpee’ | 135, 157 | 5 | |||
| ‘Marika’ | 159, 163 | 4 | 123, 175 | 150, 175 | 175, 175 |
| ‘Mesma’ | 146, 148 | 7 | |||
| ‘Mespi’ | 126, 173 | 6 | 146, 173 | ||
| ‘Muuruska’ | 135, 165 | 6 | |||
| ‘Pima’ | 126, 135 | 9 | 123, 126 | 131, 175 | |
| ‘Susanna’ | 175, 175 | 1 | |||
Table 7.
SSR marker 126 was tested for hybrid arctic bramble cultivars, ‘Beata’ and ‘Sofia’, and a number of undefined open-pollinated hybrid arctic bramble and arctic bramble seedlings; the marker was able to differentiate the arctic bramble cultivars (Table 6A) from all studied accessions; n is the number of accessions having the same alleles
| Accessions (n) | Alleles (bp) |
|---|---|
| ‘Beata’, ‘Sofia’ | 123, 141 |
| Open-pollinated seedling of hybrid arctic brambles (2) | 123, 145 |
| Open-pollinated seedling of hybrid arctic bramble | 123, 147 |
| Open-pollinated seedling of hybrid arctic bramble | 123, 175 |
| Open-pollinated seedling of hybrid arctic brambles (3) | 131, 175 |
| Open-pollinated seedling of arctic bramble | 122, 131 |
| Open-pollinated seedling of arctic bramble | 126, 126 |
| Open-pollinated seedling of arctic brambles (5) | 126, 157 |
| Open-pollinated seedling of arctic bramble | 135, 148 |
| Open-pollinated seedling of arctic bramble | 157, 157 |
| Open-pollinated seedling of arctic bramble | 164, 164 |
| Open-pollinated seedling of arctic bramble | 166, 168 |
In autumn 2010, the late-developing fruits were not harvested in the Kuopio Research Garden of the University of Eastern Finland. In the following spring, seeds from those fruits germinated abundantly in the containers, which were originally set up to grow just one cultivar per container. This observation prompted us to carry out a small-scale study to evaluate the genuineness of the known cultivars in some arctic bramble collections. As demonstrated by the SSR markers in Table 6B, four of the eight cultivars showed at least partial replacement by (unknown) hybrids. In addition, SSR marker analysis revealed a discrepancy in the origin of the cultivar ‘Pima’. In the literature it is claimed that ‘Pima’ is a cross between ‘Mespi’ and ‘Mesma’ (Ryynänen and Dalman, 1983). According to SSR results ‘Pima’ and ‘Mespi’ share one allele, but there is no common allele between ‘Pima’ and ‘Mesma’ (Table 6A). Thus ‘Mesma’ cannot be the parent of ‘Pima’. Also, in an earlier analysis with AFLP markers, the close relationship between ‘Pima’ and ‘Mespi’ was clearly shown, but there was a greater genetic distance between ‘Pima’ and ‘Mesma’ (Lindqvist-Kreuze et al., 2003). This is an unsettling outcome, which should have direct consequences for how genuineness is maintained in cultivars of arctic bramble and similar types of plants.
DISCUSSION
Maintenance of cultivar purity is challenging in arctic bramble and similar plants that show vigorous spreading of rhizomes and effective germination of seeds produced after obligatory cross-pollination. In arctic bramble, all germinating seeds result from fertilization of the mother plant (original cultivar) with pollen from a plant of a different self-incompatibility group. Thus, the new plant is always a hybrid with only half of its genome from the original arctic bramble cultivar. We discovered loss of cultivar purity in the field collections of arctic bramble and conclude that fruits not harvested from the plants are the most probable sources for this. It is also possible that birds and other small animals that eat the fruits carry the seeds between the plots. It is common practice in field collections that different cultivars are grown in relatively close proximity. The foreign alleles found in this study in SSR analyses can have their origin in other arctic bramble cultivars or clones or other Rubus species grown nearby. This is the first time that purity problems have been reported for arctic bramble, the threat being even greater as arctic bramble crosses freely with, for example, R. arcticus ssp. × stellarcticus or R. arcticus ssp. stellatus (Larsson, 1969). Possible cross-pollination and resulting hybrid plant development must therefore be prevented. Pollination cages should be used (AEGIS, 2010), or the plants should be isolated from potential pollinators (Reed et al., 2004). Care should also be taken to collect all fruits from the plants to prevent hybrid seedlings from growing in the cultivar-specific pots. This applies to all plants, which share the characteristics with arctic bramble of being obligate out-breeders.
Problems in cultivar purity have been reported previously in gene banks of open-pollinating rye species (Chebotar et al., 2003). The ex situ collections of lettuce were shown to contain 10 % of non-authentic cultivars (van de Wouw et al., 2011). Recently, SSR fingerprinting has revealed severe discrepancies in the identities of black raspberry cultivars (Dossett et al., 2012). In holly (genus Ilex) nurseries, loss of purity has been a widespread problem (Graf, 2010). As it is likely that cultivar purity problems are greater in nurseries than in gene banks or other collections, practical methods should be available for cultivar identification in nurseries and orchards.
The best morphological parameters for arctic bramble cultivar identification in the field turned out to be the length-to-width ratio of the middle leaflet, leaf serration and hairiness of the leaf epidermis sensed by fingertip touch. The shape of the middle leaflet has been described for several arctic bramble cultivars (Ryynänen, 1972; Ryynänen and Dalman, 1983; Pirinen et al., 1998), but not for the purpose of distinguishing between cultivars. Our results are consistent with those reported previously. The length-to-width ratio of the middle leaflet has not been used as an identification tool; instead, verbal description of the middle leaflet has been given, or the length and width have been provided separately. Leaf serration or hairiness has not been described previously for any arctic bramble cultivar. The morphological parameters selected by us have the benefit of being stable in various environments (different farms), as compared with characteristics such as leaf size, flowering and yield, which can be greatly affected by light, nutrients and water. From the yield results it can be concluded that cultivar ‘Muuruska’ cannot be recommended for commercial production due to low yield.
In the morphological identification of arctic bramble cultivars we used UPOV (2003, 2012) guidelines for raspberry and strawberry as references, as there are no guidelines for arctic bramble. The plant material in our study included 24 healthy, vegetatively propagated plants of each variety, whereas the minimum number of plants required by UPOV is ten for raspberry (UPOV, 2003) and 20 for strawberry (UPOV, 2012). The arctic bramble plants were monitored during four consecutive growing cycles, instead of two required by UPOV (2003, 2012). According to UPOV (2003, 2012) guidelines, the tests should normally be conducted at one location. We followed the parameters on three farms to verify the results, thus being in compliance also with the guidelines for distinctness (UPOV, 2008). Overall, our studies fulfil well the requirements of the UPOV guidelines. From UPOV guidelines for strawberry we found middle leaflet length-to-width ratio and middle leaflet serration to be useful also in arctic bramble. From the guidelines for raspberry we did not find any parameters as useful for arctic bramble.
For the molecular-level identification of the arctic bramble cultivars, SSR analysis was chosen because of its advantages over other techniques. The UPOV has published guidelines for molecular marker selection and determined the most important criteria for the markers (UPOV, 2010). SSR marker no. 126 (Graham et al., 2002), tested in this study for arctic bramble, met the UPOV criteria: it has high discriminatory power; the capillary electrophoresis results are easy to score; and the method was shown to be reproducible between two laboratories. Repeatability over time was also proven by the analysis of ‘Elpee’, ‘Pima’, ‘Muuruska’ and ‘Mespi’ samples collected more than 15 years ago, as the allele lengths had remained unaltered.
The morphological and molecular identification methods are complementary. The morphological parameters are the most important practical identification tools, being particularly useful for farmers when cultivar identification is needed in the field during the growing season. The identification can guide the need for replanting in the case that one or some of the cultivars have taken over and the cultivar ratio has become unfavourable for pollination efficacy and fruit yield. Morphological identification tools are also needed in field collections of cultivars in assessing the genuineness of the plants. Morphological characteristics can be applied only during the growing season and may, to some extent, be affected by environmental factors. It is therefore important that an alternative identification method based on molecular markers is available for use at any time independent of the growth stage of the plant.
In conclusion, arctic bramble was used here as an example of a plant which needs particular care to maintain cultivar purity. The distinctive characteristics of this plant are self-incompatibility, insect pollination, easy germination of seeds and vigorous rhizome growth. These features call for growth in pots for physical isolation, as well as prevention of pollination or seed germination, or careful removal of developing seedlings. As yet, such measures have not been recommended for arctic bramble. For the verification of cultivar identity, both morphological and molecular methods were developed for arctic bramble. The most useful morphological characteristics were the length-to-width ratio of the middle leaflet and leaf margin serration. A more unconventional but useful parameter was fingertip touch of the leaf, shown to be due to different hairiness of the leaves. A molecular SSR marker originally developed for red raspberry and shown to be applicable for some other Rubus species was suitable also for arctic bramble. These tools, practices and precautions together should improve the identification of arctic bramble cultivars and their maintenance and serve as guides for other plants with similar biology.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
This work was supported by the Finnish Cultural Foundation, Alma and Jussi Jalkanen Foundation (grant no. 65102013); August Johannes and Aino Tiura Foundation (grant no. 560); European Agricultural Guidance and Guarantee Fund (EAGGF) project no. 23456; and Development Program for Mainland Finland (EAFRD) project no. 1999. We express our thanks to Arto Koistinen for his help with scanning electron microscopy, and to Anne Ukkonen for her assistance with the measurement of soluble solids.
LITERATURE CITED
- Aaltonen M, Antonius K, Karhu S, et al. Suomen kansallisten kasvivarojen pitkäaikaissäilytysohjeet, Hedelmä- ja marjakasvit. Maa- ja elintarviketalous. 2006;89:134–138. [Google Scholar]
- AEGIS. Template for the preparation of operational genebank manuals. 2010 http://aegis.cgiar.org/fileadmin/www.aegis.org/FOR_WEB_FINAL/Template_final240910.pdf. (accessed 18 April 2012) [Google Scholar]
- Bassil NV. Microsatellite markers: valuable in Vaccinium L. International Journal of Fruit Science. 2012;12:288–293. [Google Scholar]
- Bassil NV, Muminova M, Njuguna W. Microsatellite-based fingerprinting of western blackberries from plants, IQF berries and puree. Acta Horticulturae. 2010;859:73–80. [Google Scholar]
- Chebotar S, Röder M, Korzum V, Saal B, Weber W, Börner A. Molecular studies on genetic integrity of open pollinating species rye (Secale cereale L.) after long-term genebank maintenance. Theoretical and Applied Genetics. 2003;107:1469–1476. doi: 10.1007/s00122-003-1366-1. [DOI] [PubMed] [Google Scholar]
- Dossett M, Bassil NV, Finn CE. SSR fingerprinting of black raspberry cultivars shows discrepancies in identification. Acta Horticulturae. 2012;946:49–53. [Google Scholar]
- Ervi L, Hanioja P, Kivinen E. Mesimarjan (Rubus arcticus L.) marjontaa koskevia tutkimuksia. Acta Agriculturae Fenniae. 1955;83:93–112. [Google Scholar]
- FAO. The second Report on the state of the world's plant genetic resources. Rome: Food and Agriculture Organisation of the United Nations. 2010 http://www.fao.org/docrep/013/i1500e/Finland.pdf. (accessed 18 April 2012). [Google Scholar]
- Fernández-Fernández F, Antanaviciute L, Govan CL, Sargent DJ. Development of a multiplexed microsatellite set for fingerprinting red raspberry (Rubus idaeus) germplasm and its transferability to other Rubus species. Journal of Berry Research. 2011;1:177–187. [Google Scholar]
- Govan CL, Simpson DW, Johnson AW, Tobutt KR, Sargent DJ. A reliable multiplexed microsatellite set for genotyping Fragaria and its use in a survey of 60 F.×ananassa cultivars. Molecular Breeding. 2008;22:649–661. [Google Scholar]
- Graf W. Key for the main assortment of Ilex aquifolium cultivars in Central Europe based on leaf morphology. Acta Horticulturae. 2010;885:123–130. [Google Scholar]
- Graham J, Smith K, Woodhead M, Russell J. Development and use of simple sequence repeat SSR markers in Rubus species. Molecular Ecology Notes. 2002;2:250–252. [Google Scholar]
- Häkkinen S, Kokko H, Kärenlampi S. Sugars and organic acids in clones and cultivars of arctic bramble and hybrid. Sensory evaluation of juices and jellies. Agricultural Science in Finland. 1995;4:385–395. [Google Scholar]
- Hukkanen A, Kostamo K, Kärenlampi S, Kokko H. Impact of agrochemicals on Peronospora sparsa and phenolic profiles in three Rubus arcticus cultivars. Journal of Agricultural and Food Chemistry. 2008;56:1008–1016. doi: 10.1021/jf072973p. [DOI] [PubMed] [Google Scholar]
- Hulten E. The Circumpolar plants. II Dicotyledons. Kungliga Svenska Vetenskasakademiens handlingar. 1967;13:164–165. [Google Scholar]
- IPGRI International Plant Genetic Resources Institute. Report of a Working Group on Medical and Aromatic Plants. First Meeting, 12–14 September 2002. 2002 http://www.ecpgr.cgiar.org/fileadmin/bioversity/publications/pdfs/984_Report_of_a_working_group_on_medicinal_and_aromatic_plants.pdf. (accessed 23 March 2012) [Google Scholar]
- Kallio H, Laine M, Huopalahti R. Aroma of the berries of the hybrid Rubus stellatus Sm.×R. arcticus L. Reports of Kevo Subarctic Research Station. 1980;16:17–22. [Google Scholar]
- Larsson G. Experimental taxonomy as a base for breeding in northern rubi. Hereditas. 1969;63:283–351. [Google Scholar]
- Lindqvist-Kreuze H, Koponen H, Valkonen J. Genetic diversity of arctic bramble (Rubus arcticus L. subsp. arcticus) as measured by amplified fragment length polymorphism. Canadian Journal of Botany. 2003;81:805–813. [Google Scholar]
- MTT. 2012 https://portal.mtt.fi/portal/pls/portal/!TAI_MTT.TAI_MTT_RP_TUOTELUETTELO.report. (accessed 11 September 2012; in Finnish) [Google Scholar]
- Pirinen H, Dalman P, Kärenlampi S, Tammisola J, Kokko H. Description of three new arctic bramble cultivars and proposal for cultivar identification. Agricultural and Food Science in Finland. 1998;7:455–468. [Google Scholar]
- Reed B, Engelmann F, Dulloo M, Engels J. Technical guidelines for the management of field and in vitro germplasm collection. IPGRI Handbooks for Genebanks No. 7. 2004 http://www.sgrp.cgiar.org/sites/default/files/Field_InVitroGuidelines.pdf. (accessed 14 May 2012) [Google Scholar]
- Ryynänen A. Arctic bramble (Rubus arcticus L.) a new cultivated plant. Annales Agriculturae Fenniae. 1972;11:170–1723. [Google Scholar]
- Ryynänen A. Rubus arcticus L. and its cultivation. Annales Agriculturae Fenniae. 1973;12:1–76. [Google Scholar]
- Ryynänen A, Dalman P. A new variety of arctic bramble ‘Pima'. Annales Agriculturae Fenniae. 1983;22:1–7. [Google Scholar]
- Saastamoinen S. Mesimarja (Rubus arcticus L.) Suomessa. Annales Zoologicae Botanicae Society Fenniae Vanamo. 1930;13:356–414. [Google Scholar]
- SGSV Data Portal. 2010 http://www.nordgen.org/sgsv. (accessed 18 March 2012) [Google Scholar]
- Tammisola J. Incompatibility classes and fruit set in natural populations of arctic bramble (Rubus arcticus L.) in Finland. Journal of Agricultural Science in Finland. 1988;60:323–446. [Google Scholar]
- UPOV. Guidelines for the conduct of tests for distinctness, uniformity and stability, Raspberry (Rubus idaeus L.) 2003 http://www.upov.int/edocs/tgdocs/en/tg043.pdf. (accessed 14 May 2012) [Google Scholar]
- UPOV. Examining distinctness. 2008 http://www.upov.int/export/sites/upov/en/publications/tgp/documents/tgp_9_1.pdf. (accessed 15 May 2012) [Google Scholar]
- UPOV. Guidelines for DNA-Profiling: molecular marker selection and database construction (“BMT Guidelines”) 2010 http://www.upov.int/edocs/mdocs/upov/en/tc_edc/2006/bmt_guidelines_proj_4.pdf. (accessed 14 May 2012) [Google Scholar]
- UPOV. Guidelines for the conduct of tests for distinctness, uniformity and stability, Strawberry (Fragaria L.) 2012 http://www.upov.int/edocs/tgdocs/en/tg022.pdf. (accessed 14 May 2012) [Google Scholar]
- USDA-ARS National Genetic Resource Program. NCGR-Corvallis Rubus Catalog. Germplasm Resources Information Network (GRIN) database. Rubus arcticus. 2011 http://www.ars-grin.gov/cgi-bin/npgs/swish/accboth?si=0&query=rubus+arcticus&x=0&y=0&btnG=Go!&filter=0&as_sitesearch=ars.usda.gov&ie=&output=xml_no_dtd&client=usda&lr=&proxystylesheet=ARS&oe= (accessed 21 March 2012) [Google Scholar]
- Vool E, Karp K, Starast M, Paal T, Luik A. Growth habit of arctic bramble (Rubus arcticus) within Kaansoo Conservation area in Estonia. Baltic Forestry. 2011;17:170–178. [Google Scholar]
- van de Wouw M, van Treuren R, van Hintum T. Authenticity of old cultivars in genebank collections: a case study on lettuce. Crop Science. 2011;51:736–746. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.