Abstract
The role of top-down control in visual search has been a subject of much debate. Recent research has focused on whether attentional and oculomotor capture by irrelevant salient distractors can be modulated through top-down control, and if so, whether top-down control can be rapidly initiated based on current task goals. In the present study, participants searched for a unique shape in an array containing otherwise homogeneous shapes. A cue prior to each trial indicated the probability that an irrelevant color singleton distractor would appear on that trial. Initial saccades were less likely to land on the target and participants took longer to initiate a saccade to the target when a color distractor was present than when it was absent; this cost was greatly reduced on trials in which the probability that a distractor would appear was high, as compared to when the probability was low. These results suggest that top-down control can modulate oculomotor capture in visual search, even in a singleton search task in which distractors are known to readily capture both attention and the eyes. Furthermore, the results show that top-down distractor suppression mechanisms can be initiated quickly in anticipation of irrelevant salient distractors and can be adjusted on a trial-by-trial basis.
Keywords: Oculomotor capture, Cognitive control, Automaticity, Precuing
Visual attention selects salient or goal-related visual input for access to awareness, memory, and action. Voluntary (top-down) deployments of attention depend on current behavioral goals; however, stimulus-driven (bottom-up) attentional capture by salient objects (e.g., those that exhibit high local feature contrast) can occur even when those objects are not relevant to the current task. The degree to which voluntary attentional control can attenuate stimulus-driven attentional capture by a salient sensory event has been the subject of lively debate.
The attentional and oculomotor systems are closely linked; indeed, shifts of attention are thought to directly precede eye movements (e.g., Hoffman & Subramaniam, 1995). Several studies have demonstrated that irrelevant distractors that would normally capture attention can also elicit saccades to their location (e.g., Theeuwes, de Vries, & Godijn, 2003; Theeuwes, Kramer, Hahn, & Irwin, 1998). The debate on whether top-down control can modulate involuntary capture by irrelevant distractors (e.g., Bacon & Egeth, 1994) has been extended to the oculomotor domain. It has been argued that oculomotor capture occurs automatically, regardless of the current task goals (Theeuwes, Kramer, Hahn, Irwin, & Zelinsky, 1999), especially when saccades are initiated rapidly (Mulckhuyse, van Zoest, & Theeuwes, 2008). However, there is some evidence that oculomotor capture is contingent on top-down set. For example, Wu and Remington (2003) found that oculomotor capture was reduced or eliminated when participants were forced to use a feature search mode (i.e., search for a specific target feature) rather than singleton detection mode (i.e., search for the salient item).
Recent studies (Geyer, Müller, & Krummenacher, 2008; Müller, Geyer, Zehetleitner, & Krummenacher, 2009) examined suppression of capture by varying the frequency of distractor presence in search displays between blocks and varying participants’ experience with distractor-present trials. They found that learning to suppress distractors plays an important role in reducing attentional and oculomotor capture. Furthermore, they showed that distractor interference was weaker in blocks with high rather than with low distractor probability, suggesting that incentive to suppress distractors (presumably greater in high-probability blocks) affects the degree to which those distractors interfere with search. They concluded that attentional and oculomotor capture by salient color singleton distractors can be attenuated in visual search via top-down control (see also Sayim, Grubert, Herzog, & Krummenacher, 2010).
However, some questions remain about the nature of this suppression process. Blocked designs introduce various complexities. For example, a distractor-present trial may automatically recruit cognitive control mechanisms on the following trial (Kerns, Cohen, Macdonald, Cho, Stenger, & Carter, 2004). Consecutive distractor-present trials necessarily occur far more frequently in blocks with high distractor probability than in blocks with low distractor probability, giving rise to the possibility of intertrial priming effects. The presence or absence of a distractor on consecutive trials is just one intertrial contingency; it is difficult to account for all possible intertrial contingencies in a blocked design with varying probabilities of specific trial types (cf. Mayr, Awh, & Laurey, 2003). Because these intertrial contingencies are not equated across probability conditions, their contribution to overall performance is greater in high-probability conditions. Thus, it becomes difficult to disentangle intertrial effects from effects due solely to probability manipulations.
Furthermore, the flexibility of this suppression process remains unexplored. Lien, Ruthruff, and Johnston (2010) observed contingent capture effects using trial-by-trial cuing of a target feature, suggesting that top-down control settings can be initiated rapidly. However, it is unknown whether observers can use foreknowledge about a salient distractor to suppress capture by that distractor. Distractor suppression in general is difficult, because the contents of working memory are known to guide attention (Downing, 2000). As a result, attention may be directed toward items that observers are attempting to suppress (as in the “attentional white bear effect”; Tsal & Makovski, 2006). Therefore, distractor suppression may not be as flexible as other top-down attentional sets. However, suppression of salient distractors may be a special case, because observers may try to ignore local feature contrast, or “salience,” rather than a specific feature or location. Because the concept of “salience” is more abstract than the concept of a specific feature (e.g., the color red), the working memory representation of salience might not be as robust. Thus, attention might not be automatically directed toward the salient distractor, allowing efficient top-down salient distractor suppression sets to be rapidly initiated.
In the present study, we examine the role of top-down control and suppression of oculomotor capture by cuing distractor probability on a trial-by-trial basis. This design controls for intertrial effects by equating the frequency of intertrial contingencies across probability conditions. The target is always defined as a unique shape, and the physical properties of the stimuli in distractor-present and distractor-absent trials are equivalent in the different probability conditions. Therefore, any behavioral changes that occur following different cues reflect more or less emphasis on distractor suppression—a cognitive set for ignoring.
We asked participants to make a rapid eye movement to a shape singleton target and to ignore color singleton distractors when they occurred. Irrelevant salient distractors are known to capture attention when the target feature is defined only by its status as a singleton (e.g., Bacon & Egeth, 1994, Exp. 1; Theeuwes, 1991); therefore, we expected the singleton distractor to interfere with task performance by increasing errors, slowing latencies, or both. We reasoned that participants would have more incentive to prepare distractor suppression mechanisms when a cue indicated that it was highly likely that a distractor would be present on an upcoming trial. In contrast, when a distractor was unlikely to occur, they would be more likely to rely on the bottom-up salience of the target singleton to capture their eyes, because in the absence of a distractor, the target would be the most salient item. Thus, if top-down control can be rapidly initiated to suppress oculomotor capture by irrelevant distractors, we would expect to see weaker evidence for oculomotor capture on distractor-present trials when a distractor was likely to occur than when a distractor was unlikely to occur. If, however, the most salient stimulus influences eye movements to the same degree, regardless of the observer’s goals, then the magnitude of capture by the distractor should be unaffected by distractor probability.
Method
Participants
A group of 12 Johns Hopkins University students and community members (7 female, 5 male) at least 18 years of age, with normal or corrected-to-normal vision, were paid to participate in one session lasting 1–2 h. The experimental protocol was approved by the Johns Hopkins Homewood Institutional Review Board, and participants provided written informed consent.
Apparatus
All stimuli were displayed on a Dell Dimension 4100 computer with a Dell P780 monitor. The viewing distance was 42 cm. Stimulus presentation and data analysis were performed using programs written in MATLAB (The Mathworks) and using Psychophysics Toolbox software (Brainard, 1997).
Eye movements were recorded using an EyeLink I eyetracker. A chinrest was used for head stabilization. Right eye position was sampled at 250 Hz. Fixation was monitored, and a trial began only when the participant fixated the center of the screen; if the eye moved, the participant was instructed to fixate the center, and the trial started over. An eye movement was classified as a saccade if its acceleration exceeded 9500°/sec2 or if its velocity exceeded 35°/s and stayed above 35°/s for at least three consecutive samples. Saccade endpoints were defined as the time after an eye movement when the velocity of the saccade fell below 35°/s for three consecutive samples. Eyetracker calibration was performed at the beginning of each block.
Stimuli
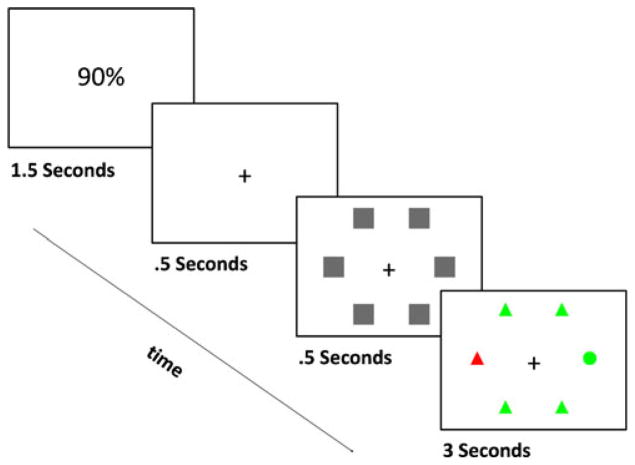
Each stimulus display consisted of six shapes (five circles and one triangle or five triangles and one circle), each subtending approximately 1.6° of visual angle (see Fig. 1). The shapes were arranged in a circular array surrounding fixation with a radius of 8.83° of visual angle. The shapes were regularly spaced at locations 30°, 90°, 150°, 210°, 270°, and 330° (polar angle) from vertical. On each trial, five identical shapes (circles or triangles) were placed randomly in five of the six possible locations on the screen. A singleton shape (whichever was not used for the other five shapes) was placed at the remaining location.
Fig. 1.
An example of a distractor-present trial. Each trial began with a cue indicating distractor probability, followed by a fixation cross. Placeholders then appeared, and these were removed to reveal a display containing one unique target shape, along with five homogeneous nontarget shapes. Participants were required to initiate a saccade toward the unique shape in the display (in this case, the circle). The display remained on the screen for 3 s, regardless of the latency of the participant’s response. All shapes were colored green by default, but on some proportion of trials (10% or 90%, depending on the cue), one of the nontargets was colored red (a distractor-present trial, as shown here). The background was black
The experiment included both no-distractor trials (in which all six shapes were rendered in green) and distractor trials (in which the color of one of the nontarget shapes was rendered in red; these colors were not equiluminant). Consequently, the target and distractor stimuli never appeared at the same location. All shapes appeared on a black background. Prior to each trial, participants were presented with a cue indicating the probability that a distractor would be present on the upcoming trial. This cue was either “10%” or “90%,” appearing in gray at the center of the screen.
Design and procedure
At the start of each trial, participants pressed the space bar to indicate that they were fixating on a gray dot presented at the center of the screen. If their eyes deviated from fixation prior to the onset of the target display, they were required to repeat this process. Then, a probability cue appeared at the center of the screen for 1,500 ms, followed by a gray fixation cross at the center of the screen. After 500 ms, square gray placeholders appeared at the six locations of the upcoming display items, also for 500 ms. The placeholders were then removed to reveal the six shapes. Participants were instructed to move their eyes to the unique shape in the display as quickly and accurately as they could. The shapes remained on the screen for 3 s. Feedback was provided by a tone following the participant’s response. If the participant failed to respond within 3 s, a message appeared on the screen with an instruction to respond more quickly. To assess accuracy, the screen was divided into six equal wedges, each projecting from the center at a 60° angle and centered on one of the six possible target locations. A trial was considered correct when the first saccade landed within the wedge centered on the target.
Participants were trained with 12 trials containing no distractors, followed by two blocks of 30 trials each (counterbalanced across participants) containing either “10%” or “90%” distractor probability cues. These cues were reliable: 90% of the trials following a “90%” cue included a distractor, and the remaining 10% were distractor-absent trials (and similarly for “10%” cue trials). The cues were held constant within each training block. Participants then completed five blocks of 100 trials each, and the cue preceding each trial was selected randomly to be either “10%” or “90%.” For some participants, not all five blocks were completed, due to fatigue, discomfort, or technical problems; however, each participant completed a minimum of two blocks (2 participants completed two blocks, 2 completed three blocks, 5 completed four blocks, and 3 completed five blocks). Participants received a score at the end of each block based on a combination of the speed and accuracy of their responses, and monetary rewards were given at the end of the experiment based on these scores.
Results
All saccade latencies longer than 1,500 ms or shorter than 100 ms were removed from all analyses (2.1% of all trials). All error trials were removed from the initial saccade latency analysis (17.7%).
Distractor probability and saccade error rate
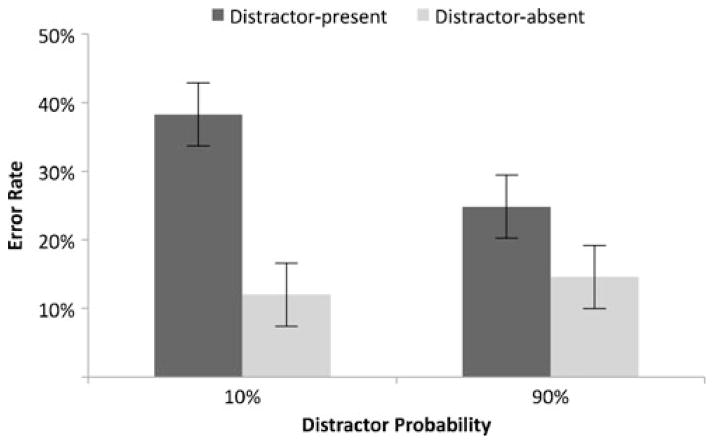
A 2×2 ANOVA was conducted to examine the effect of distractor probability (10% or 90%) and distractor presence (present or absent) on the saccade error rate (see Fig. 2). The saccade error rate (the percentage of initial saccades that went to a nontarget) was higher on distractor-present (32.2%) than on distractor-absent (13.3%) trials, F(1, 11) = 20.48, p < .001. The main effect of cue type was also significant, F(1, 11) = 5.83, p < .05, with more errors on low (i.e., 10%) distractor probability trials (25.2%) than on high (i.e., 90%) distractor probability trials (19.7%). The interaction between cue type and distractor presence was significant, F(1, 11) = 9.63, p < .05. This interaction was driven by differences in accuracy on distractor-present trials; there were more errors on distractor-present trials in the low-probability condition (38%) than in the high-probability condition (25%), t(11) = 3.19, p < .01. The difference in error rates between the low- and high-probability conditions for distractor-absent trials was not significant, t(11) = 1.08, p > .05. These results indicate that the participants’ knowledge of the probability that a distractor would be present on the upcoming trial affected oculomotor capture. Specifically, they made fewer incorrect saccades on distractor-present trials when that distractor was expected to occur, as compared to when it was unexpected.
Fig. 2.
Saccade error rates in different distractor probability conditions. The cost of a distractor increased as distractor probability decreased. Error bars represent a within-subjects error term (Loftus & Masson, 1994)
Error saccades went directly to the distractor 43.7% of the time. The likelihood of an incorrect saccade going to the distractor (rather than to another nontarget item) was not affected by distractor probability, F(1, 11) < 1.
Distractor probability and target acquisition time
We conducted a 2×2 ANOVA examining the effects of distractor presence and distractor probability on target acquisition time (defined as the time at which the participants’ gaze landed inside the target wedge; these saccades landed on average within 1.28° of visual angle of the target shape). This measure included any time taken to look at nontarget locations when the first eye movement was not directly to the target (i.e., error trials). Participants found the target more quickly on distractor-absent trials (459 ms) than on distractor-present trials (493 ms), F(1, 11) = 14.087, p < .01. There was no main effect of distractor probability, F(1, 11) < 1, but the interaction was significant, F(1, 11) = 12.44, p < .01 (see Fig. 3). The effect of distractor probability on distractor-absent trials (434 vs. 485 ms for low and high distractor probability, respectively) was significant, t(11) = 2.99, p < .05. If the interaction was driven only by distractor-absent trials, it could be argued that observers were not reducing capture, but instead were simply being more cautious following high-distractor-probability cues. However, this was not the case; the difference on distractor-present trials (511 ms vs. 476 ms) was also significant, t(11) = 2.3, p < .05, with reduced interference from the distractor on high-probability as compared to low-probability trials.
Fig. 3.
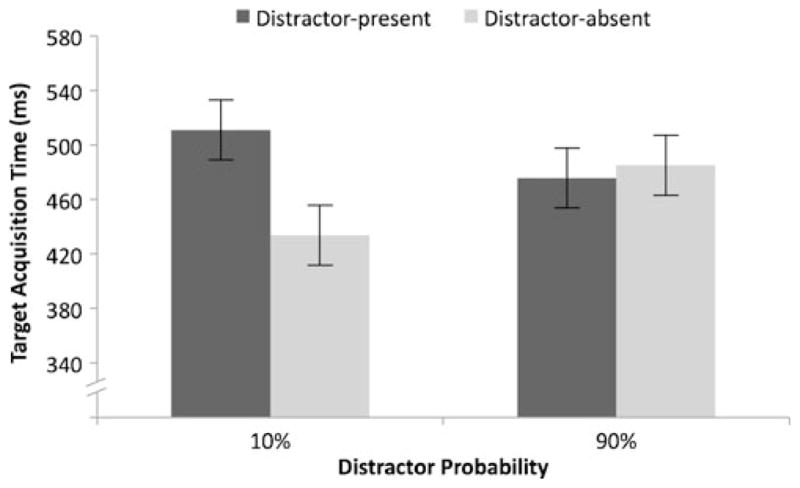
Target acquisition time in different distractor probability conditions. The cost of a distractor increased as distractor probability decreased. Error bars represent a within-subjects error term (Loftus & Masson, 1994)
These data suggest that adjustments in control settings provide a strategic benefit; the reduction in incorrect eye movements following a “90%” cue allows observers to find the target more quickly. However, initiating these top-down suppression mechanisms slows target acquisition when no distractor appears. Therefore, increased top-down control can reduce distractor interference, thereby speeding target acquisition, but it slows target acquisition in the absence of a distractor.
Distractor probability and initial saccade latency
We also analyzed the latency to initiate the first saccade on correct trials (saccadic response time, or SRT). Responses were slower on distractor-present trials (395 ms) than on distractor-absent trials (373 ms), F(1, 11) = 12.6, p < .001. The main effect of cue type was not significant, F(1, 11) = 2.72, p > .05, but there was a significant interaction between cue type and distractor presence, F(1, 11) = 9.14, p < .05. This interaction was driven mainly by differences between low- and high-probability distractor-absent trials (362 vs. 385 ms), t(11) = 3.42, p < .01. There was no effect of distractor probability on distractor-present trials, t(11) < 1. This suggests that differences in error rate were not due to a slowing of SRTs in the high-probability condition on distractor-present trials.
SRT was on average faster on incorrect trials (336 ms) than on correct trials (378 ms), F(1, 11) = 12.43, p < .01. Saccades that went directly to the distractor were faster (298 ms) than incorrect saccades that went elsewhere (367 ms), F(1, 11) = 13.82, p < .01.
Discussion
Foreknowledge of the probability that a salient distractor would appear affected the behavioral cost of that distractor. Oculomotor capture was reduced and participants found the target more quickly on distractor-present trials when distractors were likely, suggesting that participants could voluntarily and flexibly alter top-down attentional control settings rapidly—within the 2.5 s between the onset of the cue and the onset of the target display. Thus, our results suggest that observers can rapidly adopt a top-down cognitive set to ignore salient, irrelevant distractors in their environment, thereby reducing interference from those distractors and allowing observers to more quickly locate a target. This suggests that capture by a salient singleton is not an automatic, all-or-none process, but rather that it is cognitively penetrable by top-down attentional control settings. This conclusion is consistent with the notion that top-down control can modulate the effect of irrelevant salient singletons (e.g., Bacon & Egeth, 1994; Geyer et al., 2008) and inconsistent with accounts that exclude top-down control during singleton search (e.g., Theeuwes, 1991; Theeuwes et al., 1998).
Because of the trial-by-trial cuing design, all participants had equivalent experience with distractor-present trials (50% of all trials) prior to each trial. Therefore, these results can only be attributable to adjustments of top-down cognitive control, rather than to implicit adjustments based on lower-level habituation of the orienting response (cf. Sokolov, 1975) or to intertrial effects (e.g., Mayr et al., 2003). Our data support the notion that the implementation of top-down attentional control settings is not constrained by frequently changing task demands (e.g., Lien et al., 2010).
Establishment of top-down distractor suppression
While there were clear changes in behavior following the different probability cues, it is unclear whether these changes were a result of explicit strategy adjustments on the part of the observer; they could instead be attributable to implicit adjustments in top-down control settings. For example, when the probability of incompatible trials in a Stroop (1935) task increases, interference is reduced, indicating that observers increase cognitive control in response to more frequent interference (Logan & Zbrodoff, 1979). This effect persists even when the observer is unaware of those changes in probability, suggesting that control can be implemented as a result of implicit learning of environmental contingencies (Blais, Harris, Guerrero, & Bunge, 2010). Furthermore, implicit changes in control settings can be elicited from a cue, though those implicit changes typically require a very short cue–target stimulus onset asynchrony (e.g., Risko, Blais, Stolz, & Besner, 2008).
We observed no interaction between block number and distractor presence,1 indicating that the magnitude of capture did not change over the course of the experiment. These data suggest that participants were able to use the cues to enact top-down control almost immediately, consistent with an explicit understanding of the cues. However, implicit item-specific adjustments in control can also be rapidly initiated (Jacoby, Lindsay, & Hessels, 2003). We cannot rule out the possibility that the cues in our experiment elicited rapid, implicit adjustments in top-down control settings. Further research is needed to clarify the roles of explicit and implicit processing in top-down distractor suppression.
The mechanisms underlying top-down distractor suppression
Ludwig, Ranson, and Gilchrist (2008) used an accumulator model to describe two ways in which an irrelevant salient distractor can disrupt eye movements. The distractor can accumulate enough evidence to cross the response threshold first, attracting the eyes (direct capture), and it can increase noise in the accumulation of evidence from the remaining items, resulting in an eye movement directed toward a nonsalient, nontarget item (indirect capture). In the present study, we found that both direct and indirect capture were reduced following high-distractor-probability cues, suggesting that both types of capture can be modulated via top-down control.
A slightly modified accumulator model can account for top-down control in our task. Because a higher standard of evidence would result in fewer erroneous saccades influenced by the presence of a distractor, an increase in response threshold would reduce both direct and indirect capture (assuming within-trial noise; e.g., Ratcliff & Rouder, 1998). Participants might adjust their response threshold more or less, depending on whether they anticipate the presence of a salient irrelevant distractor on the upcoming trial. This account fits well with the slowing of responses we observed on distractor-absent trials in the 90% condition as compared to the 10% condition. A threshold adjustment account would also predict slower responses on distractor-present trials following a “90%” cue; an increased response threshold would lead to reduced errors by allowing more time for evidence to accumulate, thus representing a speed–accuracy trade-off. We found no difference in SRTs for the 10% and 90% conditions on distractor-present trials. However, we did find that error saccades occurred more frequently in the 10% condition than in the 90% condition on distractor-present trials. This suggests that participants were actively filtering the distractor, either by reducing the rate of accumulation of evidence in favor of the distractor or by reducing the baseline of evidence in favor of the distractor, such that more evidence had to be accumulated in order for it to cross the response threshold (effectively increasing the response threshold for the distractor only; cf. Ludwig et al., 2008). Because we found reductions in both direct and indirect capture, a change in the rate of accumulation of evidence is a more plausible account of our data.
The absence of a difference in SRTs for the two probability conditions on distractor-present trials suggests that evidence accumulation in favor of the target itself is not affected by the distractor probability cue. Instead, it appears that top-down control in this task is implemented by a combination of adjusting the response threshold and filtering the incoming signal from the irrelevant distractor by reducing the rate of accumulation of evidence in favor of that distractor.
A closer look at the data suggests that top-down control affects the processing of the distractor at an early stage. Saccades directed toward the distractor were much faster than saccades directed toward other nontargets or toward the target, suggesting that instances of direct capture, which were subject to top-down modulation, were the result of a stimulus-driven process. In a separate analysis, we conducted a split-half analysis of all trials based on SRT. Looking at the faster half only (59% of error saccades directed to the distractor, mean error SRT = 267 ms), there was still an effect of probability on distractor-present trials; there were more errors in the 10% condition (53%) than in the 90% condition (35%), t(11) = 3.02, p < .05. Because even the fastest instances of capture were affected by distractor probability, it appears that participants were able to use top-down control early in their search to suppress involuntary, stimulus-driven error saccades.
Conclusions
The data presented here reveal that precues can rapidly and flexibly initiate a cognitive set for ignoring a distractor, effectively suppressing interference from salient distractors. Although the presence of an irrelevant salient stimulus still carried a cost, even in the high-distractor-probability condition, oculomotor capture was significantly attenuated when distractors could be anticipated. Thus, participants could deliberately ignore the most salient object in the display with little warning, based solely on contingent preparation.
Acknowledgments
The authors acknowledge the following funding sources: NRSA Institutional Training Grant T32 EY07143-14 from the Wilmer Eye Institute (to J.M.), ONR Grant N000141010278 (to H. E.E.), NIH Grant R01-DA013165 (to S.Y.), and NIH Grant R01-EY019039 (to V.S.). Portions of this work were presented at the 15th Annual Workshop on Object Perception, Attention, and Memory and at the 8th Annual Meeting of the Vision Sciences Society.
Footnotes
Because not all participants completed all five blocks, we conducted a 2 × 2 ANOVA on block number and distractor presence (with accuracy as the dependent variable) for the first two blocks for all 12 participants, and another ANOVA for the first four blocks with the 8 participants who completed at least that many blocks. There was no interaction between distractor presence and block for either the first two blocks, F(1, 11) < 1, or the first four blocks, F(1, 7) = 1.65, p > .1.
Contributor Information
Jeff Moher, Email: jmoher1@jhu.edu, Psychological and Brain Sciences, Johns Hopkins University, 3400 N. Charles St., Ames Hall, Baltimore, MD 21218, USA.
Jared Abrams, Department of Psychology, New York University, 6 Washington Place, New York, NY 10003, USA.
Howard E. Egeth, Psychological and Brain Sciences, Johns Hopkins University, 3400 N. Charles St., Ames Hall, Baltimore, MD 21218, USA
Steven Yantis, Psychological and Brain Sciences, Johns Hopkins University, 3400 N. Charles St., Ames Hall, Baltimore, MD 21218, USA.
Veit Stuphorn, Psychological and Brain Sciences, Johns Hopkins University, 3400 N. Charles St., Ames Hall, Baltimore, MD 21218, USA.
References
- Bacon W, Egeth HE. Overriding stimulus-driven attentional capture. Perception & Psychophysics. 1994;55:485–496. doi: 10.3758/bf03205306. [DOI] [PubMed] [Google Scholar]
- Blais C, Harris MB, Guerrero JV, Bunge SA. Rethinking the role of automaticity in cognitive control. The Quarterly Journal of Experimental Psychology. 2010 doi: 10.1080/17470211003775234. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Downing PE. Interactions between visual working memory and selective attention. Psychological Science. 2000;11:467–473. doi: 10.1111/1467-9280.00290. [DOI] [PubMed] [Google Scholar]
- Geyer T, Müller H, Krummenacher J. Expectancies modulate attentional capture by salient color singletons. Vision Research. 2008;48:1315–1326. doi: 10.1016/j.visres.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Hoffman J, Subramaniam B. The role of visual attention in saccadic eye movements. Perception & Psychophysics. 1995;57:787–795. doi: 10.3758/bf03206794. [DOI] [PubMed] [Google Scholar]
- Jacoby L, Lindsay D, Hessels S. Item-specific control of automatic processes: Stroop process dissociations. Psychonomic Bulletin & Review. 2003;10:638–644. doi: 10.3758/bf03196526. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, Macdonald AW, Cho RY, Stenger A, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Lien M-C, Ruthruff E, Johnston JC. Attentional capture with rapidly changing attentional control settings. Journal of Experimental Psychology: Human Perception and Performance. 2010;36:1–16. doi: 10.1037/a0015875. [DOI] [PubMed] [Google Scholar]
- Loftus GR, Masson M. Using confidence intervals in within-subject designs. Psychonomic Bulletin & Review. 1994;1:476–490. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- Logan GD, Zbrodoff NJ. When it helps to be misled: Facilitative effects of increasing the frequency of conflicting stimuli in a Stroop-like task. Memory & Cognition. 1979;7:166–174. [Google Scholar]
- Ludwig CJH, Ranson A, Gilchrist ID. Oculomotor capture by transient events: A comparison of abrupt onsets, offsets, motion, and flicker. Journal of Vision. 2008;8(14):11:1–16. doi: 10.1167/8.14.11. [DOI] [PubMed] [Google Scholar]
- Mayr U, Awh E, Laurey P. Conflict adaptation effects in the absence of executive control. Nature Neuroscience. 2003;6:1–3. doi: 10.1038/nn1051. [DOI] [PubMed] [Google Scholar]
- Mulckhuyse M, van Zoest W, Theeuwes J. Capture of the eyes by relevant and irrelevant onsets. Experimental Brain Research. 2008;186:225–235. doi: 10.1007/s00221-007-1226-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller HJ, Geyer T, Zehetleitner M, Krummenacher J. Attentional capture by salient color singleton distractors is modulated by top-down dimensional set. Journal of Experimental Psychology: Human Perception and Performance. 2009;35:1–16. doi: 10.1037/0096-1523.35.1.1. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, Rouder J. Modeling response times for two-choice decisions. Psychological Science. 1998;9:347–356. [Google Scholar]
- Risko EF, Blais C, Stolz JA, Besner D. Covert orienting: A compound-cue account of the proportion cued effect. Psychonomic Bulletin & Review. 2008;15:123–127. doi: 10.3758/PBR.15.1.123. [DOI] [PubMed] [Google Scholar]
- Sayim B, Grubert A, Herzog MH, Krummenacher J. Display probability modulates attentional capture by onset distractors. Journal of Vision. 2010;10:1–8. doi: 10.1167/10.3.10. [DOI] [PubMed] [Google Scholar]
- Sokolov EN. The neuronal mechanisms of the orienting reflex. In: Sokolov EN, Vinogradova OS, editors. Neuronal mechanisms of the orienting reflex. Hillsdale: Erlbaum; 1975. pp. 217–235. [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. doi: 10.1037/0096-3445.121.1.15. [DOI] [Google Scholar]
- Theeuwes J. Cross-dimensional perceptual selectivity. Perception & Psychophysics. 1991;50:184–193. doi: 10.3758/bf03212219. [DOI] [PubMed] [Google Scholar]
- Theeuwes J, Kramer A, Hahn S, Irwin D. Our eyes do not always go where we want them to go: Capture of the eyes by new objects. Psychological Science. 1998;9:379–385. [Google Scholar]
- Theeuwes J, Kramer A, Hahn S, Irwin D, Zelinsky GJ. Influence of attentional capture on oculomotor control. Journal of Experimental Psychology: Human Perception and Performance. 1999;25:1595–1608. doi: 10.1037//0096-1523.25.6.1595. [DOI] [PubMed] [Google Scholar]
- Theeuwes J, de Vries GJ, Godijn R. Attentional and oculomotor capture with static singletons. Perception & Psychophysics. 2003;65:735–746. doi: 10.3758/bf03194810. [DOI] [PubMed] [Google Scholar]
- Tsal Y, Makovski T. The attentional white bear phenomenon: The mandatory allocation of attention to expected distractor locations. Journal of Experimental Psychology: Human Perception and Performance. 2006;32:351–363. doi: 10.1037/0096-1523.32.2.351. [DOI] [PubMed] [Google Scholar]
- Wu SC, Remington R. Characteristics of covert and overt visual orienting: Evidence from attentional and oculomotor capture. Journal of Experimental Psychology: Human Perception and Performance. 2003;29:1050–1067. doi: 10.1037/0096-1523.29.5.1050. [DOI] [PubMed] [Google Scholar]