Abstract
Objectives
Symptomatic osteoarthritis (OA) is a common painful disease with limited treatment options. A rising number of OA patients have been treated with intraarticular injections of hyaluronic acid, including the high molecular weight hylan G-F 20, which is injected following arthrocentesis. This study investigated the effectiveness of hylan G-F 20 to lower coefficient of friction (COF) and prevent chondrocyte apoptosis in vitro.
Methods
A disc-on-disc bovine cartilage bearing was used to measure the static and kinetic COF when lubricated with hylan G-F 20, human synovial fluid (HSF) and phosphate buffered saline (PBS). Following friction testing, we stained paraffin embedded sections of these cartilage bearings for activated caspase-3, a marker of apoptosis.
Results
Bearings lubricated with hylan G-F 20 had kinetic COF values that were similar to bearings lubricated with PBS, but significantly higher than those lubricated with HSF. There were no significant differences in static COF values in bearings lubricated with hylan G-F 20 as compared to PBS or HSF. However, bearings lubricated with HSF had a significantly lower static COF values compared to bearings lubricated with PBS. The mean percentage of caspase-3 positive chondrocytes in the superficial and upper intermediate zones of bearings lubricated with hylan G-F 20 were significantly higher when compared to bearings lubricated with HSF or unloaded controls, but significantly lower than those lubricated with PBS.
Conclusion
These findings indicate that joint lubrication may prevent chondrocyte apoptosis by lowering the COF. Furthermore, removal of synovial fluid prior to hylan G-F 20 injection may be detrimental to cartilage health.
Key Indexing Terms: articular cartilage, chondrocyte, apoptosis, synovial fluid, hylan
Introduction
Osteoarthritis (OA) is a painful, debilitating disease in articular joints with large societal implications (1, 2). Currently, treatment options for patients with OA are severely limited, and no disease-modifying treatments, apart from total joint replacement, are available. Most patients are treated with nonsteroidal anti-inflammatory drugs or corticosteroid injections to relieve pain. Viscosupplementation, in which various forms of hyaluronic acid, including hylan G-F 20 (Synvisc, Genzyme Corporation), are administered via intraarticular injection, is becoming a popular alternative.
Synovial fluid is a blood plasma dialysate that contains lubricating components, including hyaluronic acid and lubricin, important to joint lubrication and hence chondroprotection to articular joints. Hyaluronic acid is thought to play a number of roles in synovial fluid, including shock absorption and lubrication, and is responsible for the viscoelastic behavior of synovial fluid (3). Joint lubrication occurs in both hydrodynamic and boundary modes (4). Hyaluronic acid is vital to hydrodynamic joint lubrication, which occurs when the fluid layer is wider than the surface asperities and is dominated by fluid mechanics, including viscosity (4). During periods of high load and low velocity, lubricin serves as the primary boundary lubricant in joints, particularly when cartilage is pressurized during gait. However, hyaluronic acid has also been shown to contribute to lubrication in the boundary mode, particularly in concert with lubricin (5–9).
Healthy articular cartilage is smooth and void of fissures or attachments (10). In addition to surface fibrillation and ultimately full thickness cartilage loss, osteoarthritic cartilage experiences a loss of chondrocytes via apoptosis (10–12). It is important to note that chondrocytes maintain various metabolic functions in articular cartilage, including the maintenance of the extracellular matrix (13, 14), and that OA pathogenesis is mediated in part by apoptotic mechanisms (15–17). Cartilage wear leading to OA and precocious joint failure has been reported in the absence of adequate joint lubrication in vivo (18–20), but the biological underpinnings of wear in response to mechanical mechanisms have not been established. Since articular cartilage has little capacity for renewal, preventing apoptosis via supplemental lubrication may be key to counteract the onset of OA and vital to cartilage preservation (21).
This study investigated the effectiveness of using hylan G-F 20 for lubricating cartilage bearings in the boundary mode to prevent chondrocyte apoptosis using a bovine in vitro disc-on-disc cartilage bearing (9, 22). We hypothesized that the coefficient of friction using hylan G-F 20 would be less than that of phosphate buffered saline (PBS) and equal to that of human synovial fluid (HSF). We also hypothesized that the percentage of cells stained for activated caspase-3, a marker of chondrocyte apoptosis, of the bearing lubricated with hylan G-F 20 will be less than that of PBS and equal to that of HSF.
Materials and Methods
Bovine Cartilage Preparation
To evaluate the ability of the lubricants to provide boundary lubrication and prevent chondrocyte apoptosis, we used a bovine cartilage disc-on-disc bearing system (9). Full thickness cartilage plug bearings 6 mm (small disc) and 12 mm (large disc) in diameter were cored from the approximate load bearing regions of femoral condyle of bovine stifle joints (N=5) collected within 2 hours of slaughter. Following harvest, the bearings were rinsed 3 times with cell culture media (DMEM/5% FBS) and cultured for 24 hours at 37°C. Testing was performed on the cultured plugs at room temperature.
Test Lubricants
Hylan G-F 20 (Synvisc, Genzyme Corp, Cambridge, MA) was kept at room temperature away from light until testing. During testing, it was directly applied to cartilage bearing surfaces from the product packaging using a 22 gauge needle. HSF was aspirated from knee joints of post-mortem donors with no history of joint disease within 12 hours of death (3 male donors, Ages 25–39, NDRI) or aspirated from patients undergoing total joint replacement were collected in the operating room and pooled together (N=12). All HSF was frozen and stored at −80°C until analysis and testing. Plasma protein levels in the synovial fluids were not measured (23), but synovial fluids visually contaminated with blood were not used. PBS served as a negative control. All lubricants were tested at room temperature.
Enzyme-linked Immunoabsorbant Assay (ELISA) of HSF
An ELISA using anti-lubricin monoclonal antibody 9G3 was designed and validated. High binding 96-well plates were coated overnight with purified human lubricin in 0.1 M NaH2PO4,Na2HPO4 buffer, pH 6.5, at a final concentration of 10 µg/ml. The plates were washed and blocked with 5% milk in phosphate buffered saline and Tween 20 (PBST) for 2 hours at room temperature (RT). The plate was subsequently washed with PBST. HSF test samples were added to the plate at a 1:50 dilution, then 9G3 was subsequently added at a 1:5000 dilution, and the plate was incubated for 1 hour at RT. After a wash with PBST, goat anti-mouse IgG was added to the plate at 1:2000 dilution and incubated for 1 hour at RT. The plate was then washed, and TMB single solution (Invitrogen) was added. 1M HCL was added 30 minutes later to stop the reaction which was read at 450nm.
Friction and Wear Testing in Bovine Bearings
Prior to testing, the average total cartilage thickness for each bearing pair was calculated (2.84 ± 0.38 mm) from caliper measurements at four regions locations about the circumference of both the small and large cartilage bearings. Small bearing diameters (5.45 ±0.28 mm) were also measured using calipers.
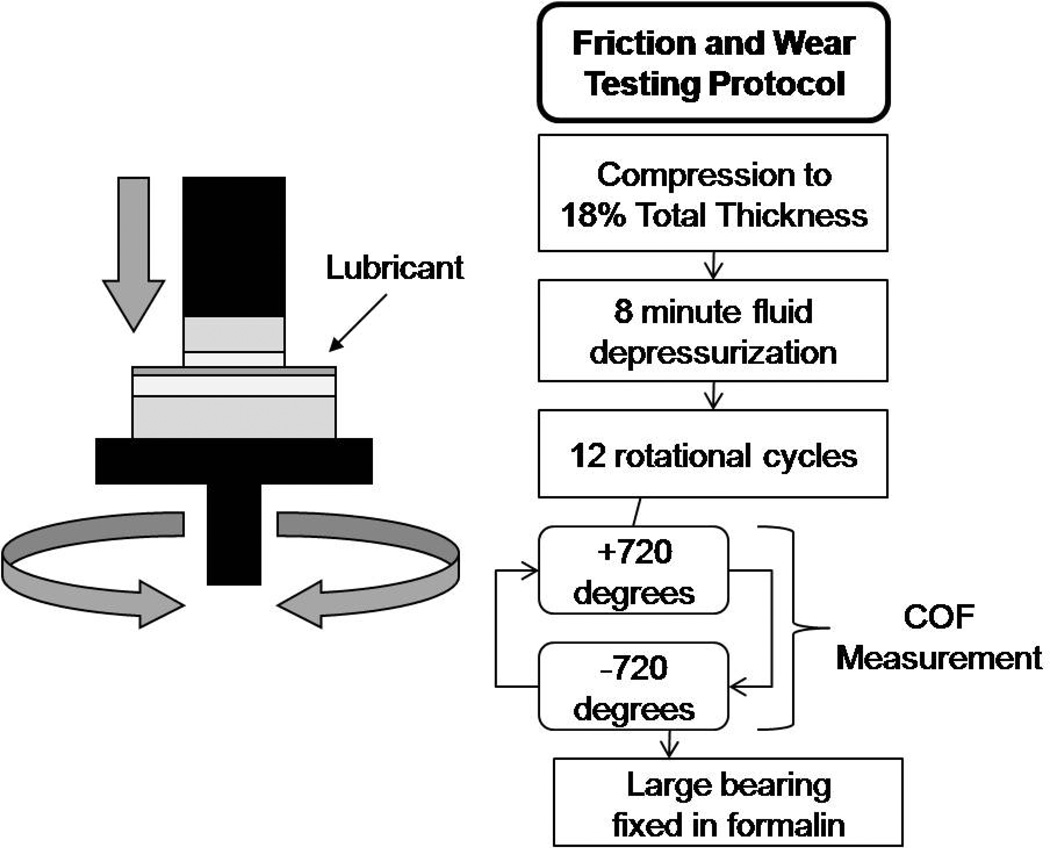
Cartilage bearings were loaded in an disc-on-disc configuration using a material testing system (EnduraTEC 3200; Bose Corporation, Eden Prairie, MN, USA), which was programmed to apply an axial strain while axial rotations were prescribed to the bearing (Figure 1). This testing paradigm was adapted from Schmidt et al to accommodate cell culture following friction and wear testing (22). The maximum ranges of the load, torque and displacement transducers of the test system were ±22 N, ±0.7 Nmm, and ±6.5 mm, respectively. The cartilage bearings were fixed to the testing platform with cyanoacrylate glue, which was applied to the bony surface of the bearing plugs and allowed to dry completely before testing. During this time, cell culture media was added between the joint surfaces to prevent cartilage desiccation. Prior to testing, cell culture media was then rinsed off of the cartilage bearing surfaces three times with PBS. Test lubricant, either PBS, hylan G-F 20 or HSF, was applied between the bearing surfaces (N=8 for all groups). The bearings were axially loaded to 18% of the mean total cartilage thickness, and held at that displacement for 8 minutes to allow fluid depressurization (9, 22, 24). The large disc was then rotated in torsion +2 revolutions and reset −2 revolutions at an effective velocity of 0.3 mm/s (9) for 12 continuous cycles. Unloaded control discs, 12mm in diameter, were kept in cell culture media during testing. All tests were performed between 48 and 72 hours of harvest, individual bearings were only tested with a single lubricant, and bearings taken from each knee were tested using each test lubricant.
Figure 1. Schematic diagram of friction and wear testing system and a flow chart of experimental protocol.
Test lubricant was applied between the bearing surfaces, and the bearings were axially loaded to 18% of the mean total cartilage thickness, and held at that displacement for 8 minutes to allow for fluid depressurization. The large disc was then rotated in torsion +2 revolutions and reset −2 revolutions at an effective velocity of 0.3 mm/s for 12 continuous cycles. Axial load and torque were measured throughout the testing protocol.
Coefficient of Friction Determination
The static COF (a measure of the stick-slip condition) and the kinetic COF (a measure of the equilibrium COF) were calculated using Equation 1 (22).
| (1) |
To calculate the static COF, the absolute maximum torque that occurred within the first 10 degrees of rotation and the equilibrium axial force following the 8 minute depressurization period were substituted into Equation 1. To calculate the kinetic COF, the average torque observed during the last 720 degrees of rotation and the equilibrium axial force were used.
Activated Caspase-3 Staining and Quantification
To test the efficacy of each lubricant to provided chondroprotection during frictional testing, we stained paraffin-embedded sections of each large cartilage bearing disc with an antibody specific for activated caspase-3, which stains chondrocytes primed for apoptosis. Immediately following testing, cartilage discs were fixed in 10% buffered formalin. The unloaded control discs were also fixed in formalin at the time of testing. The discs were paraffin embedded and cut vertically at the center of the disc into thin sections of full cartilage thickness (250 µm). Sections were heated to 600°C for 30 min, deparaffinized and hydrated in xylene and alcohol. Rabbit polyclonal antibody against active caspase-3 (cat#ab13847, Abcam, Cambridge, MA) at 1:50 dilution was added to slides at 40°C overnight according to VectaStain procedures. Following the addition of biotinylated secondary antibody solution and 3,3'-diaminobenzidine (DAB), slides were counterstained with 0.5% Methyl green and cover slip slides fixed with Permount mounting media (Fisher Scientific, Waltham, MA). Apoptosis quantification was performed at 20X magnification for cells in the superficial and intermediate zones along the articular surface where loading occurred at 3 areas of interest. Images were captured at 20X with Image-Pro Plus software (Media Cyberkinetics, Bethesda, MD), and the percentage of apoptotic cells was determined by counting the number of cells positive activated caspase-3 and the total number of cells for 3 distinct 100µm zones representing areas of the superficial and upper intermediate zones: Zone A (articular surface-100µm deep), Zone B (100–200µm deep), Zone C (200–300µm deep). Total percentage of apoptotic cells refers to the mean across all 3 zones.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) Staining
To confirm apoptosis in the bovine cartilage discs, we performed a TUNEL assay on the large bovine cartilage disc sections using an ApopTag Plus Peroxidase In Situ Apoptosis Kit (Millipore, Billerica, MA). Sections were heated at 60°C for 30 minutes, deparaffinized in 3 changes of xylene and serial ethanol, then pretreated with proteinase K (20µg/ml) for 15 minutes at room temperature, quenched endogenous peroxidase in 3% hydrogen peroxide for 5 minutes, and incubated with equilibration buffer for 30 seconds. Excess liquid was tapped off and the sections were with terminal deoxynucleotidyl transferase enzyme at 37 °C for 1 hour in a humidified chamber. After the reaction was stopped, sections were washed 3 times in PBS, and incubated with anti-digoxigenin conjugate for 30 minutes at room temperature and washed in PBS. Peroxidase substrate was applied to sections, which were stained for 4min, washed in deionized H2O, and counterstained with 0.5%methyl green. Sections were washed in deionized dH2O again, dehydrated in xylene 3 times, and then mounted with Permount. Images were captured at 20X with Image-Pro Plus software.
Statistical Analysis
Comparisons were made between the COF values for the different test lubricants (PBS, hylan G-F 20, HSF) and unloaded control specimens using Kruskal-Wallis One Way Analysis of Variance on Ranks with Dunn’s multiple comparison post hoc tests using the Unistat Statistical Package (Unistat Ltd, London, England). The tests were run separately for the static and kinetic COF values. The percentage of apoptotic cells measured in Zone A, Zone, B and Zone C of histological sections were compared using a repeated measures two-way analysis of variance with Holm-Sidak multiple comparison post hoc tests using Sigmaplot software (Systat Software Inc, Chicago, IL). Pearson correlation coefficients between the static COF and the percentage of apoptotic cells in Zone A and correlations between kinetic COF and the percentage of apoptotic cells in Zone A were performed, and a linear regression was fitted to each plot, using Sigmaplot software. Goodness of fit and significance of the correlation are reported. For all analyses, statistical significance was set at α = 0.05 a priori and the two-tailed p-value is reported. All values are presented as the mean ± standard deviation.
Results
Coefficient of Friction
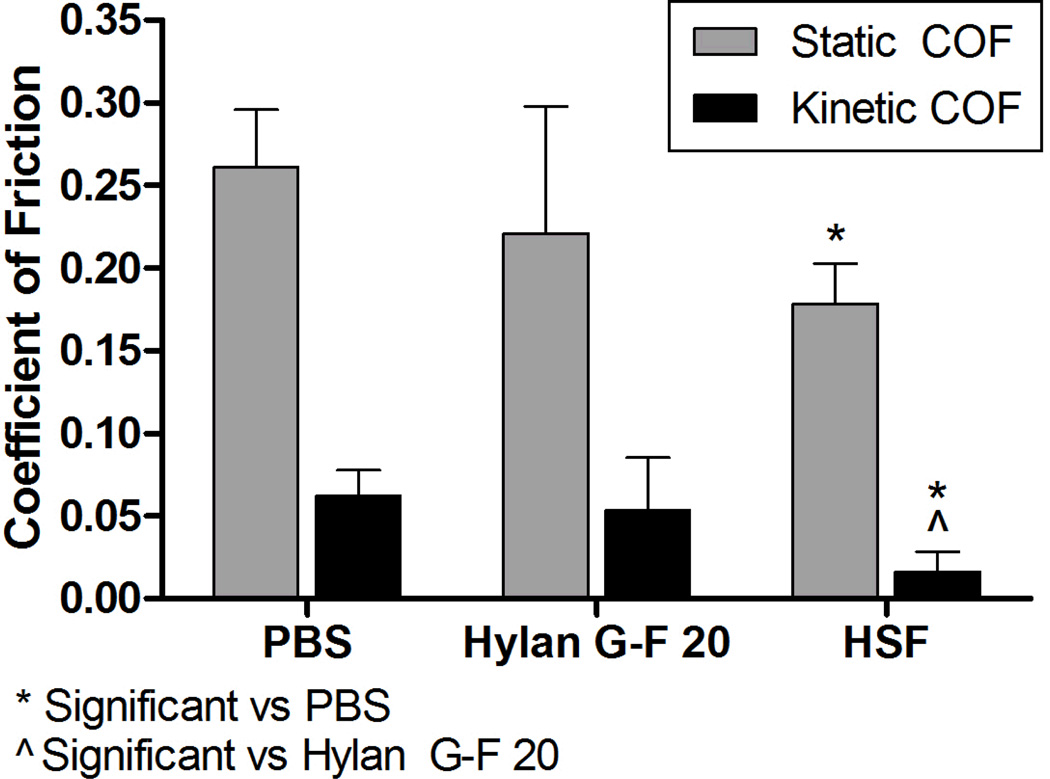
The mean static COF was significantly lower for HSF lubricated bearings compared to PBS lubricated bearings (p=0.006) (Figure 2). Hylan G-F 20 lubricated bearings had a mean static COF that was not significantly lower than PBS (p=0.18) or significantly higher than HSF (p=0.67). However, the mean kinetic COF values were significantly lower for the bearings lubricated with HSF when compared to those lubricated with hylan G-F 20 (p=0.022) or PBS (p=0.003). There was no difference between hylan G-F 20 and PBS. There was no difference between COF from sources of HSF (static COF p=0.070 and kinetic COF p=0.086), and lubricin concentrations were similar (288 ±77.7 µg/ml for post-mortem HSF and 416 µg/ml for pooled total joint replacement HSF). The equilibrium axial load was 2.11 ±0.92 N, which corresponds to 0.09 ±0.03 MPa, and the maximum load applied to the bearings was 13.51 ±5.3 N, which corresponds to 0.58 ±0.23 MPa.
Figure 2. COF of bovine osteochondral bearings.
Bearings were tested with test lubricants, either PBS, hylan G-F 20 or HSF following the protocol outlined in Figure 1. Lubrication with HSF had the lowest mean static and kinetic COF values. Bearings lubricated with hylan G-F 20 had no significant difference compared to either group in static COF, but had a significantly higher kinetic COF compared to HSF. *p<0.05
Caspase-3 and TUNEL
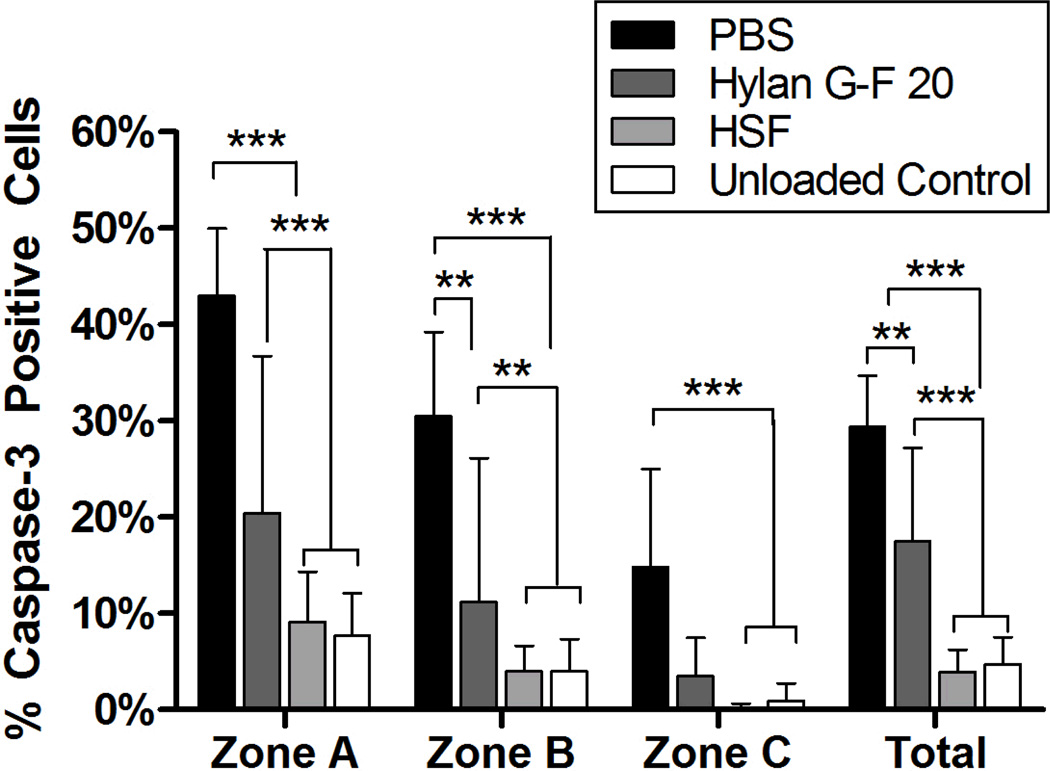
A significant interaction (p<0.001) was observed between the lubricant treatment group and zone. Hylan G-F 20 lubricated bearings had significantly higher mean percentage of apoptotic cells in zone A, zone B, and when pooled over all 3 zones as compared to HSF lubricated bearings (Zone A p<0.001, Zone B p=0.006, total p<0.001) and unloaded controls (Zone A p<0.001, Zone B p=0.004, total p<0.001), but there was no difference between the groups in Zone C (Figure 3). A significant increase in the mean percentage of cells staining positive for activated caspase-3 was observed in bearings lubricated with PBS compared to bearings lubricated with HSF (Zone A p<0.001, Zone B p<0.001, Zone C p=0.003, total p<0.001), hylan G-F 20 (Zone A p<0.001, Zone B p=0.005, total p=0.001) and unloaded bearings (Zone A p<0.001, Zone B p<0.001, Zone C p=0.005, total p<0.001). We also observed significant differences between the zones in bearings lubricated with PBS and Hylan G-F 20. For these bearings, Zone A had a significantly higher mean percentage of apoptotic cells in Zone A compared to Zone B (PBS p<0.001, hylan G-F 20 p=0.004), and in Zone B compared to Zone C (PBS p<0.001, hylan G-F 20 p=0.009). HSF also showed a significant difference between Zone A and Zone C (p=0.028). Cells in representative osteochondral plugs staining positive for activated caspase-3 were also TUNEL positive (Figure 4).
Figure 3. Activated caspase-3 in superficial and upper intermediate zone chondrocytes.
Bearings lubricated with hylan G-F 20 had a significantly higher percentage of cells positive for activated caspase-3 cells in Zone A, Zone B and across all zones compared to unloaded control bearings or bearings lubricated with HSF, indicating that hylan GF-20 is less chondroprotective than HSF. Bearings lubricated with PBS had a significantly higher percentage of apoptotic cells in all zones compared to all other groups. **p<0.01, ***p<0.001
Figure 4. Bovine osteochondral bearings stained for activated caspase-3 (top) and TUNEL (bottom).
Cells positive for activated caspase-3 and TUNEL are brown. Negative cells are stained blue. We observed more apoptotic cells in the PBS and hylan G-F 20 lubricated bearings compared to HSF lubricated bearings and unloaded controls.
Correlation between static and kinetic COF and percentage of apoptotic cells
We observed a positive correlation between static COF values and the percentage of apoptotic cells (p=0.007) and between kinetic COF values and the percentage of apoptotic cells (p=0.015). Figure 5 shows HSF data points clustered near the origin, indicating that low COF values correspond with low percentage of apoptotic cells. In contrast, the PBS data points clustered further up the regression line, indicating that high COF values correspond with high percentage of apoptotic cells. The data points for the bearings lubricated with hylan G-F 20 fell between the high and low clusters, indicating that hylan G-F 20 possesses better chondroprotective and lubricating ability as compared to PBS.
Figure 5. Correlation between static COF (left) and kinetic COF (right) and percentage of activated caspase-3 positive cells in Zone A of the superficial zone.
We observed a significant correlation between coefficient of friction and percentage of apoptotic cells in Zone A of the cartilage bearings. We observed trends for each lubricant, with the HSF lubricated bearings showing clustering towards the origin, indicating low COF and low percentage of apoptotic cells. In contrast, PBS lubricated bearings clustered in the upper right hand section of the plot, indicating high COF and high percentage of apoptotic cells. Hylan G-F 20 lubricated bearings fell between the two extremes, showing that it has some lubricating and chondroprotective abilities compared to PBS. Note that the x-axis differs between graphs for better visualization of the data points.
Discussion
We observed a significantly higher mean percentage of caspase-3 positive and a significantly higher mean kinetic COF in bearings lubricated with hylan G-F 20 compared to those lubricated with HSF. While these findings suggest that hylan G-F 20 was able to prevent apoptosis with more efficiency than PBS, apoptosis was more prevalent compared to the HSF treated bearings. Furthermore hylan G-F 20 was unable to significantly lower static or kinetic COF values compared to PBS under these testing conditions. These results suggest that hylan G-F 20 itself may be insufficient as a boundary lubricant in joints, and that this viscosupplement does not provide the same degree of chondroprotection to superficial zone and upper middle zone chondrocytes as native human synovial fluid under these boundary lubrication conditions. The significant correlations between static and kinetic COF and the percentage of apoptotic cells provide further evidence that elevated friction in this bearing system results in an increase in the percentage of apoptotic cells in the superficial 100µm of the bovine cartilage explant bearing.
Apoptotic cell death in vivo is mediated by cell-matrix interactions (25, 26), growth factor and cytokine signaling (27), and tissue injury. Cartilage explants and cultured chondrocytes have been used to study apoptosis in response to hydrostatic pressure (28), shear stress and strain (29, 30), and mechanical injury (31, 32). In our study, the elevated friction leading to apoptosis is likely due to an increase in shear stress. Currently, the mechanopathway relating mechanical stress via friction and apoptosis in chondrocytes has not been established but is under investigation.
In this study we observed a zonal dependence on the mean percentage of apoptotic cells, especially in bearings lubricated with PBS and hylan G-F 20, which exhibited higher percentages of apoptosis compared to bearings lubricated with HSF and unloaded control bearings. These findings indicate that the bulk of the apoptotic response occurs in the uppermost 300 µm of the cartilage where the shear forces and deformation are greatest (30, 33) and the significant correlation between COF and apoptosis indicates that an increase in friction is associated with apoptosis. The zonal differences also suggest that the collagen architecture in the superficial zone can absorb most of the shear stress and protect the deeper zones from deformation, and hence protecting the deeper chondrocytes from apoptosis in the early stages.
Hylan G-F 20 is administered to patients who do not respond well to non-pharmacological treatments, such as weight loss and physical therapy, or simple analgesics. Hylan G-F 20 is an FDA approved high molecular weight (average 6000 kDa) hyaluronan product with two cross-linked components that originate from chicken combs (34). Either 3 weekly doses of 2 mL or a single 6 mL dose is administered to patients diagnosed with OA (34, 35). Prior to injection, arthrocentesis is advised, which may remove important components of the synovial fluid, including lubricin, which is vital to boundary lubrication (19, 21, 36). Therefore, replacement of native synovial fluid with hylan G-F 20 alone could possibly have proximal detrimental effects on cell survival.
There are a number of limitations associated with this model of evaluating the efficacy of hylan G-F 20 to prevent friction and apoptosis. The viscosity of hylan G-F 20 is much higher than synovial fluid, causing interstitial fluid depressurization of cartilage to occur at a lower rate than when the bearings are lubricated with HSF or PBS. Hylan G-F 20 is an elastoviscous fluid with elasticity (storage modulus G') of 111 ±13 Pa at 2.5 Hz and a viscosity (loss modulus G'') of 25 ±2 Pa (34). Normal synovial fluid exhibits a storage modulus (G’) of approximately 19.3 ±3 Pa and a loss modulus of 10 ±1 Pa (37, 38). This thixotropic behavior may be preventing the cartilage bearings from achieving proper asperity contact with one another, by creating a thick fluid layer, and not allowing lubrication to occur truly in the boundary mode, and deflating static COF. After the large disc rotated, the fluid was displaced by motion and kinetic COF is significantly higher for hylan G-F 20 lubricated bearings compared to HSF lubricated bearings.
Furthermore, the test protocol used in our study was adapted from the methods cited (9). However, the technique was modified to permit subsequent culture of cartilage explant bearings. A disc of articular cartilage was used, in lieu of an annulus, as the upper bearing surface in order to prevent additional mechanical disruption during osteochondral plug harvest (9, 22). The cited methods require cartilage explants to be held while mechanically stressed without culture medium for ~2.5hr (9, 22). By shortening the testing procedure to approximately 20 minutes, we were able to collect data about boundary lubrication, since the entraining velocity and load are similar to these previous methods (9, 22), while preserving the cellular viability. While the duration of loading was shorter in our method, it still approximates zero-interstitial pore pressure at the beginning of oscillation. Previous studies have shown that after 8 minutes 85% of pore pressure was likely dissipated and the bearing surfaces were close to the equilibrium COF. It’s also important to note that each measurement represents an independent pair of cartilage bearings, as each was tested only a single time in order to observe histological data linked to particular lubricants. The wear protocol following the decompression period was also extended to 12 cycles, as opposed to 2 (6, 22).
Numerous studies have tested the outcome of hylan G-F 20 injection compared to other intraarticular HA treatments, including NSAID and corticosteroid therapies (39). The mode of action of hyaluronan injection in decreasing joint pain in OA affected joints remains unclear. Some studies have reported that loss of viscosity due to HA depletion may play a role in OA progression, although that finding has been challenged (40, 41). Adding a cross-linked HA, such as hylan G-F 20, to SF reinforces non-Newtonian behavior, which is characteristic of healthy synovial fluid (42). Alternate hypotheses, including biosynthetic-chondroprotective effects, anti-inflammatory effects, and analgesic effects due to protective action on nociceptive nerve endings, have been alternatively proposed (41).
In spite of studies showing safety and efficacy in treating OA pain as a clinical end point, the prevention of further cartilage damage following HA injection has not been established, although a delay of total knee replacement has been demonstrated in some patients with severe OA (43). The present study informs us that joint lubrication is a complex phenomenon and chondroprotection requires more than a low COF but is also related to the prevention of chondrocyte apoptosis. Our results suggest that the resident normal synovial fluid of a weight bearing joint should not be removed in the evaluation and treatment of the symptomatic large joint.
Many studies have indicated a possible synergistic effect of combining HA and lubricin in both the prevention of secondary OA in animal studies (44), as well as decreases in the COF in in vitro cartilage bearings (6, 9) and latex-on-glass bearings (8). HA of various lower molecular weights and concentrations have been shown to lower static and kinetic COF in uncultured bearings (6, 9), but the ability of these molecules to prevent chondrocyte apoptosis was not investigated. Based on their ability to lower COF in these studies, there may be value in combining HA and lubricin to develop a therapeutic for patients with OA or other degenerative joint diseases (6, 9).
This study suggests that the use of hylan G-F 20 following arthrocentesis may not adequately protect cartilage from mechanical wear due to increased friction or biological wear that occurs due to chondrocyte apoptosis. This study also determined that an increase in the COF of articular cartilage is correlated with an increase in chondrocyte apoptosis.
Acknowledgements
The authors would like to thank Koosha Aslani for assistance with the testing system. We acknowledge use of synovial fluid provided by the National Disease Research Interchange (NDRI), with support from NIH grants U42 RR006042, P20-RR024284, RO1-AR049199, RO1-AR050180, and R21-AR055937.
References
- 1.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: A first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20(10):739–744. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 2.Maetzel A, Li LC, Pencharz J, Tomlinson G, Bombardier C, Pro CHA. The economic burden associated with osteoarthritis, rheumatoid arthritis, and hypertension: a comparative study. Ann Rheum Dis. 2004;63(4):395–401. doi: 10.1136/ard.2003.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swann DA, Radin EL, Nazimiec M, Weisser PA, Curran N, Lewinnek G. Role of Hyaluronic-Acid in Joint Lubrication. Ann Rheum Dis. 1974;33(4):318–326. doi: 10.1136/ard.33.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gleghorn JP, Bonassar LJ. Lubrication mode analysis of articular cartilage using Stribeck surfaces. J Biomech. 2008;41(9):1910–1918. doi: 10.1016/j.jbiomech.2008.03.043. [DOI] [PubMed] [Google Scholar]
- 5.Jay GD, Harris DA, Cha CJ. Boundary lubrication by lubricin is mediated by O-linked beta(1–3)Gal-GalNAc oligosaccharides. Glycoconjugate J. 2001;18(10):807–815. doi: 10.1023/a:1021159619373. [DOI] [PubMed] [Google Scholar]
- 6.Kwiecinski JJ, Dorosz SG, Ludwig TE, Abubacker S, Cowman MK, Schmidt TA. The effect of molecular weight on hyaluronan's cartilage boundary lubricating ability - alone and in combination with proteoglycan 4. Osteoarthritis Cartilage. 2011 doi: 10.1016/j.joca.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 7.Tadmor R, Chen N, Israelachvili JN. Thin film rheology and lubricity of hyaluronic acid solutions at a normal physiological concentration. J Biomed Mater Res. 2002;61(4):514–523. doi: 10.1002/jbm.10215. [DOI] [PubMed] [Google Scholar]
- 8.Jay GD, Lane BP, Sokoloff L. Characterization of a bovine synovial fluid lubricating factor. III. The interaction with hyaluronic acid. Connect Tissue Res. 1992;28(4):245–255. doi: 10.3109/03008209209016818. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt TA, Gastelum NS, Nguyen QT, Schumacher BL, Sah RL. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum. 2007;56(3):882–891. doi: 10.1002/art.22446. [DOI] [PubMed] [Google Scholar]
- 10.Lorenz H, Richter W. Osteoarthritis: cellular and molecular changes in degenerating cartilage. Progress in histochemistry and cytochemistry. 2006;40(3):135–163. doi: 10.1016/j.proghi.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Goggs R, Carter SD, Schulze-Tanzil G, Shakibaei M, Mobasheri A. Apoptosis and the loss of chondrocyte survival signals contribute to articular cartilage degradation in osteoarthritis. Vet J. 2003;166(2):140–158. doi: 10.1016/s1090-0233(02)00331-3. [DOI] [PubMed] [Google Scholar]
- 12.Goldring MB. The role of the chondrocyte in osteoarthritis. Arthritis Rheum. 2000;43(9):1916–1926. doi: 10.1002/1529-0131(200009)43:9<1916::AID-ANR2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 13.Kuhn K, D'Lima DD, Hashimoto S, Lotz M. Cell death in cartilage. Osteoarthritis Cartilage. 2004;12(1):1–16. doi: 10.1016/j.joca.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Thomas CM, Fuller CJ, Whittles CE, Sharif M. Chondrocyte death by apoptosis is associated with cartilage matrix degradation. Osteoarthritis and cartilage / OARS. Osteoarthritis Research Society. 2007;15(1):27–34. doi: 10.1016/j.joca.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 15.D'Lima D, Hermida J, Hashimoto S, Colwell C, Lotz M. Caspase inhibitors reduce severity of cartilage lesions in experimental osteoarthritis. Arthritis Rheum. 2006;54(6):1814–1821. doi: 10.1002/art.21874. [DOI] [PubMed] [Google Scholar]
- 16.Blanco FJ, Guitian R, Vazquez-Martul E, de Toro FJ, Galdo F. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis and rheumatism. 1998;41(2):284–289. doi: 10.1002/1529-0131(199802)41:2<284::AID-ART12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 17.Sharif M, Whitehouse A, Sharman P, Perry M, Adams M. Increased apoptosis in human osteoarthritic cartilage corresponds to reduced cell density and expression of caspase-3. Arthritis and rheumatism. 2004;50(2):507–515. doi: 10.1002/art.20020. [DOI] [PubMed] [Google Scholar]
- 18.Marcelino J, Carpten JD, Suwairi WM, Gutierrez OM, Schwartz S, Robbins C, et al. CACP, encoding a secreted proteoglycan, is mutated in camptodactyly-arthropathy-coxa vara-pericarditis syndrome. Nature genetics. 1999;23(3):319–322. doi: 10.1038/15496. [DOI] [PubMed] [Google Scholar]
- 19.Jay GD, Torres JR, Rhee DK, Helminen HJ, Hytinnen MM, Cha CJ, et al. Association between friction and wear in diarthrodial joints lacking lubricin. Arthritis and rheumatism. 2007;56(11):3662–3669. doi: 10.1002/art.22974. PMCID: 2688668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drewniak EI, Jay GD, Fleming BC, Zhang L, Warman ML, Crisco JJ. Cyclic loading increases friction and changes cartilage surface integrity in lubricin mutant mouse knees. Arthritis and rheumatism. 2011 doi: 10.1002/art.33337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhee DK, Marcelino J, Baker M, Gong Y, Smits P, Lefebvre V, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115(3):622–631. doi: 10.1172/JCI200522263. PMCID: 548698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt TA, Sah RL. Effect of synovial fluid on boundary lubrication of articular cartilage. Osteoarthritis Cartilage. 2007;15(1):35–47. doi: 10.1016/j.joca.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Kushner I, Somerville JA. Permeability of human synovial membrane to plasma proteins. Relationship to molecular size and inflammation. Arthritis Rheum. 1971;14(5):560–570. doi: 10.1002/art.1780140503. [DOI] [PubMed] [Google Scholar]
- 24.Park S, Costa KD, Ateshian GA. Microscale frictional response of bovine articular cartilage from atomic force microscopy. J Biomech. 2004;37(11):1679–1687. doi: 10.1016/j.jbiomech.2004.02.017. PMCID: 2809665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mobasheri A, Carter SD, Martin-Vasallo P, Shakibaei M. Integrins and stretch activated ion channels; putative components of functional cell surface mechanoreceptors in articular chondrocytes. Cell biology international. 2002;26(1):1–18. doi: 10.1006/cbir.2001.0826. [DOI] [PubMed] [Google Scholar]
- 26.Pulai JI, Del Carlo M, Jr, Loeser RF. The alpha5beta1 integrin provides matrix survival signals for normal and osteoarthritic human articular chondrocytes in vitro. Arthritis and rheumatism. 2002;46(6):1528–1535. doi: 10.1002/art.10334. [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Armada MJ, Carames B, Lires-Dean M, Cillero-Pastor B, Ruiz-Romero C, Galdo F, et al. Cytokines, tumor necrosis factor-alpha and interleukin-1beta, differentially regulate apoptosis in osteoarthritis cultured human chondrocytes. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2006;14(7):660–669. doi: 10.1016/j.joca.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Islam N, Haqqi TM, Jepsen KJ, Kraay M, Welter JF, Goldberg VM, et al. Hydrostatic pressure induces apoptosis in human chondrocytes from osteoarthritic cartilage through up-regulation of tumor necrosis factor-alpha, inducible nitric oxide synthase, p53, c-myc, and bax-alpha, and suppression of bcl-2. Journal of cellular biochemistry. 2002;87(3):266–278. doi: 10.1002/jcb.10317. [DOI] [PubMed] [Google Scholar]
- 29.Lee MS, Trindade MC, Ikenoue T, Goodman SB, Schurman DJ, Smith RL. Regulation of nitric oxide and bcl-2 expression by shear stress in human osteoarthritic chondrocytes in vitro. Journal of cellular biochemistry. 2003;90(1):80–86. doi: 10.1002/jcb.10611. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto S, Nishiyama T, Hayashi S, Fujishiro T, Takebe K, Kanzaki N, et al. Role of p53 in human chondrocyte apoptosis in response to shear strain. Arthritis and rheumatism. 2009;60(8):2340–2349. doi: 10.1002/art.24706. [DOI] [PubMed] [Google Scholar]
- 31.D'Lima DD, Hashimoto S, Chen PC, Colwell CW, Jr, Lotz MK. Human chondrocyte apoptosis in response to mechanical injury. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2001;9(8):712–719. doi: 10.1053/joca.2001.0468. [DOI] [PubMed] [Google Scholar]
- 32.Dang AC, Warren AP, Kim HT. Beneficial effects of intra-articular caspase inhibition therapy following osteochondral injury. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2006;14(6):526–532. doi: 10.1016/j.joca.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Wong BL, Bae WC, Gratz KR, Sah RL. Shear deformation kinematics during cartilage articulation: effect of lubrication, degeneration, and stress relaxation. Molecular & cellular biomechanics : MCB. 2008;5(3):197–206. PMCID: 2847289. [PMC free article] [PubMed] [Google Scholar]
- 34.Frampton JE. Hylan G-F 20 single-injection formulation. Drugs Aging. 2010;27(1):77–85. doi: 10.2165/11203900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 35.Migliore A, Giovannangeli F, Granata M, Lagana B. Hylan g-f 20: review of its safety and efficacy in the management of joint pain in osteoarthritis. Clinical medicine insights Arthritis and musculoskeletal disorders. 2010;3:55–68. PMCID: 2998981. [PMC free article] [PubMed] [Google Scholar]
- 36.Hashimoto S, Ochs RL, Komiya S, Lotz M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis and rheumatism. 1998;41(9):1632–1638. doi: 10.1002/1529-0131(199809)41:9<1632::AID-ART14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 37.Mazzucco D, McKinley G, Scott RD, Spector M. Rheology of joint fluid in total knee arthroplasty patients. J Orthop Res. 2002;20(6):1157–1163. doi: 10.1016/S0736-0266(02)00050-5. [DOI] [PubMed] [Google Scholar]
- 38.Balazs E. The physical properties of synovial fluid and the special role of hyaluronic acid. In: A H, editor. Disorders of the knee. 2nd ed. Philadelphia: JB Lippincott; 1982. pp. 61–74. [Google Scholar]
- 39.Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2) doi: 10.1002/14651858.CD005321.pub2. CD005321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fam H, Bryant JT, Kontopoulou M. Rheological properties of synovial fluids. Biorheology. 2007;44(2):59–74. [PubMed] [Google Scholar]
- 41.Dunn S, Kolomytkin OV, Marino AA. Pathophysiology of osteoarthritis: evidence against the viscoelastic theory. Pathobiology : journal of immunopathology, molecular and cellular biology. 2009;76(6):322–328. doi: 10.1159/000245898. [DOI] [PubMed] [Google Scholar]
- 42.Mathieu P, Conrozier T, Vignon E, Rozand Y, Rinaudo M. Rheologic behavior of osteoarthritic synovial fluid after addition of hyaluronic acid: a pilot study. Clinical orthopaedics and related research. 2009;467(11):3002–3009. doi: 10.1007/s11999-009-0867-x. PMCID: 2758976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waddell DD, Bricker DC. Total knee replacement delayed with Hylan G-F 20 use in patients with grade IV osteoarthritis. Journal of managed care pharmacy : JMCP. 2007;13(2):113–121. doi: 10.18553/jmcp.2007.13.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teeple E, Elsaid KA, Jay GD, Zhang L, Badger GJ, Akelman M, et al. Effects of Supplemental Intra-articular Lubricin and Hyaluronic Acid on the Progression of Posttraumatic Arthritis in the Anterior Cruciate Ligament-Deficient Rat Knee. Am J Sports Med. 2010 doi: 10.1177/0363546510378088. [DOI] [PMC free article] [PubMed] [Google Scholar]