Abstract
The two oxysterols, 27-hydroxycholesterol (27OH) and 24S-hydroxycholesterol (24OH), are both inhibitors of cholesterol synthesis and activators of the liver X receptor (LXR) in vitro. Their role as physiological regulators under in vivo conditions is controversial, however. In the present work, we utilized a previously described mouse model with overexpressed human sterol 27-hydroxylase (CYP27A1). The levels of 27OH were increased about 12-fold in the brain. The brain levels of HMG-CoA reductase mRNA and HMG-CoA synthase mRNA levels were increased. In accordance with increased cholesterol synthesis, most of the cholesterol precursors were also increased. The level of 24OH, the dominating oxysterol in the brain, was decreased by about 25%, most probably due to increased metabolism by CYP27A1. The LXR target genes were unaffected or slightly changed in a direction opposite to that expected for LXR activation. In the brain of Cyp27−/− mice, cholesterol synthesis was slightly increased, with increased levels of cholesterol precursors but normal mRNA levels of HMG-CoA reductase and HMG-CoA synthase. The mRNA levels corresponding to LXR target genes were not affected. The results are consistent with the possibility that both 24OH and 27OH are physiological suppressors of cholesterol synthesis in the brain. The results do not support the contention that 27OH is a general activator of LXR target genes in this organ.
Keywords: brain cholesterol homeostasis, liver X receptor activation, 27-hydroxycholesterol, 24S-hydroxycholesterol
Cholesterol has a remarkable capacity to regulate its own synthesis and metabolism (1). Treatment with cholesterol causes a suppressed synthesis, reduced absorption from the intestine, and increased rate of degradation into bile acids. The latter two effects are mediated by the nuclear liver X receptor (LXR) (2). Under in vitro conditions, side-chain oxidized oxysterols have a much higher capacity to downregulate cholesterol synthesis and to activate the LXR target genes than does cholesterol itself (as reviewed in 3). Because of this, oxysterols have been suggested to mediate, at least in part, the above effects of cholesterol. There are three different mechanisms by which the oxysterols may affect cholesterol-sensitive genes: 1) interaction with the sterol-regulatory element binding proteins (SREBP mechanism); 2) activation of the LXR mechanisms; and 3) effects on the degradation of specific enzymes, in particular HMG-CoA reductase (HMGCR).
The major oxysterols in the circulation of man and mouse are 24S-hydroxycholesterol (24OH) and 27-hydroxycholesterol (27OH) (3). 24OH is formed by the enzyme cholesterol 24S-hydroxylase (CYP46A1). This enzyme is located almost exclusively in the brain. There is a constant flux of 24OH from the brain across the blood-brain barrier into the circulation. This oxysterol is then taken up by the liver and converted into bile acids and conjugates of un-metabolized or partially metabolized 24OH (4). 27OH is formed by the enzyme sterol 27-hydroxylase (CYP27A1). This enzyme is present in almost all cells in the body. There is a constant flux of 27OH and metabolites of this oxysterol from extra-hepatic sources to the liver. In the liver, the 27-oxygenated steroids are converted into bile acids. There is a significant uptake of 27OH in the brain, where it is rapidly metabolized into a steroid acid, 7α-hydroxy-3-oxo-4-cholestenoic acid (5). This acid is also able to cross the blood-brain barrier and is rapidly eliminated from the brain and metabolized in the liver. There is a marked difference between the strong effects of the above side-chain oxidized oxysterols under in vitro conditions and the modest effects on cholesterol homeostasis observed in transgenic mouse models with increased or reduced levels of the above oxysterols (3, 6, 7). There are very few studies in which the in vivo effects of increased or decreased levels of oxysterols have been studied in detail.
Of the two oxysterols, 24OH is the most-efficient activator of LXR in vitro (8). In a recent study, we developed mice with an overexpression of human CYP46A1 having a production of 24OH four to seven times higher than wild-type mice (9). In contrast to the expectations, the overexpression had little or no effect on the LXR target genes. There was a significant upregulation of cholesterol synthesis in the brain, most probably a compensation for the consumption of cholesterol by the CYP46A1 enzyme. Similar findings were reported with a mouse model with a transient transfection of CYP46A1 in hippocampus and cortex (10).
27OH is a weaker activator of LXR than 24OH. On the other hand, it is present at higher levels than 24OH in plasma and almost all extra-cerebral tissues. It appears likely that the capacity of an oxysterol to inhibit cholesterol synthesis or function as activator of LXR is dependent upon the ratio between the oxysterol and cholesterol (7). When the ligand-binding domain of LXR is exposed to mixtures of cholesterol and a side-chain oxidized oxysterol, a 500- to 1,000-fold excess of cholesterol will prevent the binding of the oxysterol. The ratio between a side-chain oxidized oxysterol and cholesterol is higher than 1 per 1,000 in the brain, in cholesterol-loaded macrophages and possibly also in the lung (7). In these specific biological systems, variations in the oxysterol levels would be expected to lead to changes in the activation of the LXR systems and possibly also variations in the degree of suppression of cholesterol synthesis.
In the present work, we have studied the effect of an overexpression of the CYP27A1 enzyme on cholesterol synthesis and LXR-targeted genes in the brain of mice. These mice are known to have markedly increased levels of 27OH in plasma, liver, and lung but normal levels of cholesterol (11). A possible effect of the overexpression on LXR-targeted genes in these mice has not been studied previously. It is shown here that in similarity to the situation with overexpression of CYP46A1, there was no general stimulation of LXR-targeted genes in the brain of the CYP27A1 transgenic mice. Surprisingly, the overexpression led, however, to increased cholesterol synthesis in the brain. This increased synthesis was associated with markedly reduced levels of 24OH. In a previous work, we showed that a knockout of the Cyp27a1 gene causes a slight upregulation of cholesterol synthesis in mouse brain (12). This is confirmed here, and we also show that the knockout has little or no effect on the expression of a number of LXR target genes.
MATERIALS AND METHODS
Animals
CYP27A1-overexpressed mice generated using the β-actin promoter (11) were from our ongoing breeding colony in Huddinge University Hospital Animal Facility [founders were provided by Eran Leitersdorf (11)]. The mice were on a C57Bl/6 background. The colony was maintained by breeding heterozygous pairs. This breeding is supposed to generate both homozygotes and heterozygotes in addition to wild types. It was not possible to discriminate between homozygotes and heterozygotes with the use of copy number or levels of 27OH in serum or feces. The situation is the same as that in mice with an overexpression of CYP46 using a β-actin promoter (4). In such mice, it is also not possible to discriminate between homozygotes and heterozygotes with the use of copy numbers or levels of 24OH in circulation or feces. C57BL/6 mice were purchased from Charles River Laboratories, Germany, and used as a control group. Animals were housed under standard environmental conditions, with free access to food and water. Animals were genotyped by PCR with DNA extracted from ear pieces by a reagent from Viagen. Transgenes were detected by using primers specific to human CYP27A1.
Cyp27a1−/− mice on a C57BL/6 background were also used in the study (12). The knock-out and wild-type controls were generated from heterozygotes as described previously (12). Mice were housed under standard environmental conditions with free access to food and water. At the age of 6 weeks, the knock-out mice were fed a diet containing 0.05% cholic acid for 4 months before euthanasia. This substitution compensates for the reduced bile acid synthesis in these mice (13).
The mice were euthanized by cervical dislocation, and trunk blood was collected. All the mice were euthanized at the same time in the morning. The brain was dissected out. In the study with the overexpressed mice, the whole brain was used. In the study with the Cyp27a1−/− mice, cortex as well as hippocampus were collected. The brain pieces were put in liquid nitrogen and stored at −70°C until analyzed.
Ethical considerations
All experimental procedures in this study were in compliance with National Institutes of Health Guide for Care and Use of Laboratory Animals, the European Communities Council Directive of 24 November 1986 (86/609/EEC), and approved by the Southern Stockholm Research Animal Ethics Committee. The experiments were performed, when possible, by examiners blinded to the genotypes or treatments of the mice.
Lipid extraction and analysis
Livers and brains were extracted according to the Folch method. Folch solution (chloroform-methanol 2:1,v/v), 3 and 10 ml, was added to about 100 mg liver tissue and half of a brain, or a hippocampus or a cortex from half of a brain, respectively. After 24 h at room temperature, extracts were transferred to new vials and evaporated under argon. After evaporation, the extracts were redissolved in 1 ml Folch solution and stored at −20°C until required.
Sterols and oxysterols were measured in the Folch extract by GC-MS after alkaline hydrolysis using deuterium-labeled internal standards as previously described (14, 15).
RNA preparation and real-time PCR
Total RNA was prepared using TRIzol reagent (Invitrogen; Carlsbad, CA) according to the manufacturer's protocol. RNA (1 µg) was transcribed into cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems; Carlsbad, CA). The cDNA obtained was diluted 1:10 in RNase-free H2O. Real-time PCR was performed with 5 µl cDNA and 12.5 µl SYBRGreen Mastermix (Applied Biosystems). The forward and reverse primers are shown in Table 1. All values were normalized to hypoxanthine phosphoribosyltransferase mRNA concentrations for hepatic analyses.
TABLE 1.
Sequence of forward and reverse primers used for real-time PCR
| Gene | Sequence (5′–3′) |
| Hprt Fwd | GGT GAA AAG GAC CTC TCG AAG TG |
| Hprt Rev | ATA GTC AAG GGC ATA TCC AAC AAC A |
| Hmgcr Fwd | CCG GCA ACA ACA AGA TCT GTG |
| Hmgcr Rev | ATG TAC AGG ATG GCG ATG CA |
| Hmgcs Fwd | CTC TGT CTA TGG TTC CCT GGC T |
| Hmgcs Rev | TCC AAT CCT CTT CCC TGC C |
| Abca1 Fwd | CCC AGA GCA AAA AGC GAC TC |
| Abca1 Rev | GGT CAT CAT CAC TTT GGT CCT TG |
| Abcg1 Fwd | GCTGAAGAGGACTCCGCCT |
| Abcg1 Rev | GAGGATGCAGAACTGGGTGAG |
| Srebp1c Fwd | GGAGCCATGGATTGCACATT |
| Srebp1c Rev | GGCCCGGGAAGTCACTGT |
| Srebp2 Fwd | GCG TTC TGG AGA CCA TGG A |
| Srebp2 Rev | ACA AAG TTG CTC TGA AAA CAA ATC |
| Apo E Fwd | GCA GGC GGA GAT CTT CCA |
| Apo E Rev | CCA CTG GCG ATG CAT GTC |
| Cyp7b1 Fwd | CCT CTT TCC TCC ACT CAT ACA CAA |
| Cyp7b1 Rev | GAA CCG ATC GAA CCT AAA TTC CTT |
| Cyp27a1Fwd | GCC TTG CAC AAG GAA GTG ACT |
| Cyp27a1 Rev | CGC AGG GTC TCC TTA ATC ACA |
| Lxrα Fw | GCTCTGCCTACATCGTGGTCA |
| Lxrα Rev | TGCGCTCAGGCTCATCCT |
| Fas Fwd | GGCATCATTGGGCACTCCTT |
| Fas Rev | GCTGCAAGCACAGCCTCTCT |
| Cyp46a1 Fwd | AACCATCTGGCATTCACAGTGA |
| Cyp46a1 Rev | GGAACCGACAACCTCATCCA |
Statistics
Gene expression data are expressed as mean ± range as described by Livak and Schmittgen (16). Sterol determinations are expressed as mean ± SEM. Statistical comparisons were performed using the unpaired Students t-test.
RESULTS
Plasma, brain, and liver levels of oxysterols and cholesterol in CYP27A1 transgenic mice and controls
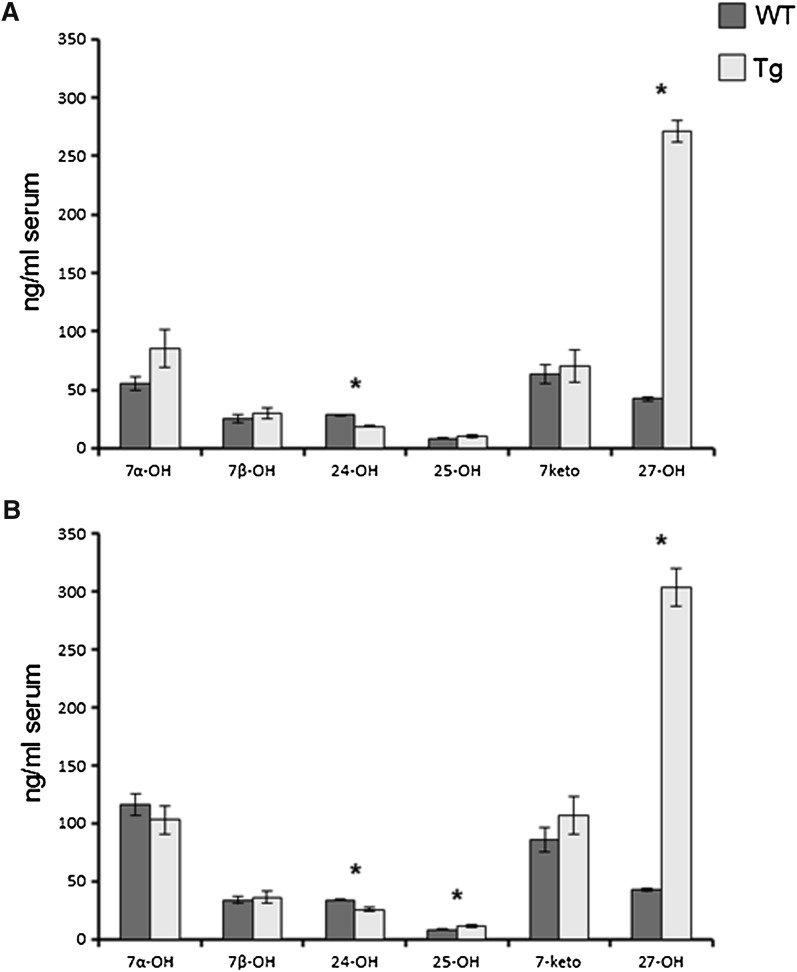
In accordance with the previous publication (11), the plasma levels of 27OH were 5- to 6-fold higher in the transgenic mice than in the controls (P < 0.001, Fig. 1). There was no significant difference between male and female mice. In accordance with the previous findings, the levels of 24OH were about 30% lower in the transgenic mice (P < 0.001 in both genders), possibly due to increased metabolism. The levels of 25-hydroxycholesterol (25OH) were significantly higher in the female transgenic mice than in the controls (P < 0.05). This may be due to the fact that CYP27A1 has some 25-hydroxylase activity (17). There were no significant differences in levels of the other oxysterols measured (7 α-, 7β-, and 7-oxo cholesterol) between the two groups of mice (P > 0.05).
Fig. 1.
The effect of overexpression of human CYP27A1 on the serum levels of oxysterols in adult male (a) and female (b) mice. n = 7–8 in each experiment with each gender. Data are presented as mean ± SEM.* P < 0.05.
As reported previously (11) the levels of 27OH in the liver were increased by about 4-fold in both the male and female mice with overexpressed CYP27A1 (results not shown).
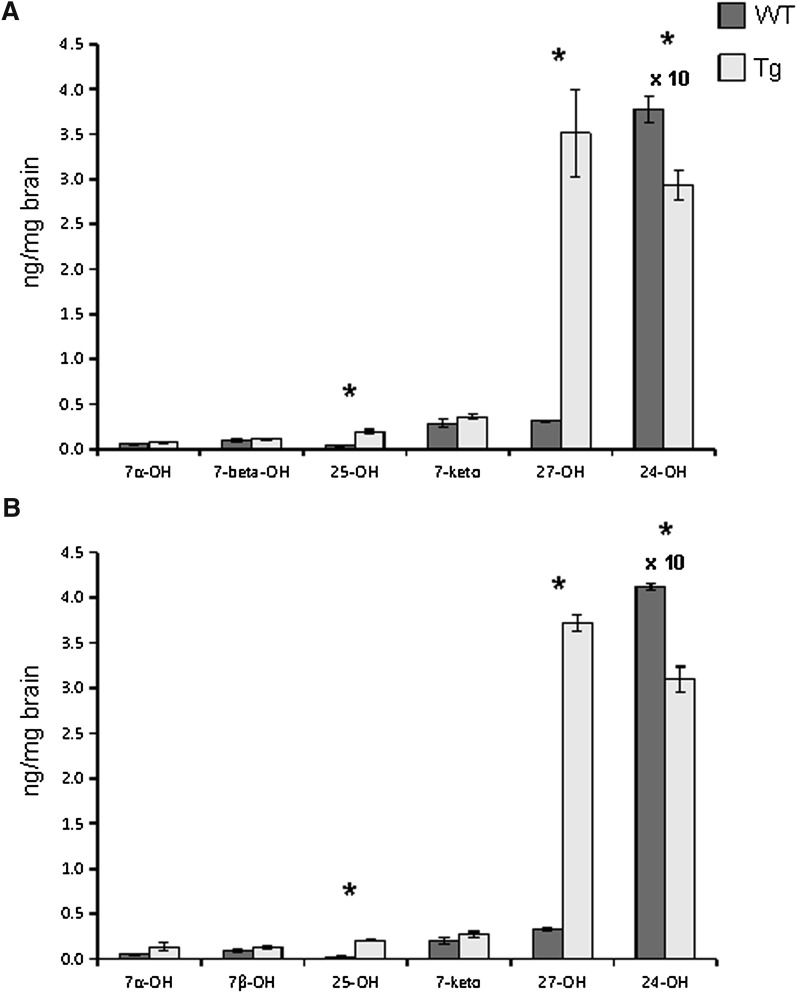
As shown in Fig. 2, the levels of 27OH were about 11-fold higher in the brain of the transgenic mice than in the controls. The levels of 25OH were 5- to 10-fold higher in the brain of the transgenic mice, reflecting the fact that human CYP27A1 has some 25-hydroxylase activity (17). The levels of the dominating oxysterol in the brain, 24OH, were significantly reduced, by about 25%, in the brain of the transgenic mice (P < 0.001 in both males and females).
Fig. 2.
The effect of overexpression of human CYP27A1 on the levels of oxysterols in the adult brain of male (a) and female (b) mice. n = 7–8 in each experiment with each gender. Data are presented as mean ± SEM.* P < 0.05.
Effects of the overexpression of CYP27A1 on oxysterols, cholesterol, and cholesterol precursors in the brain
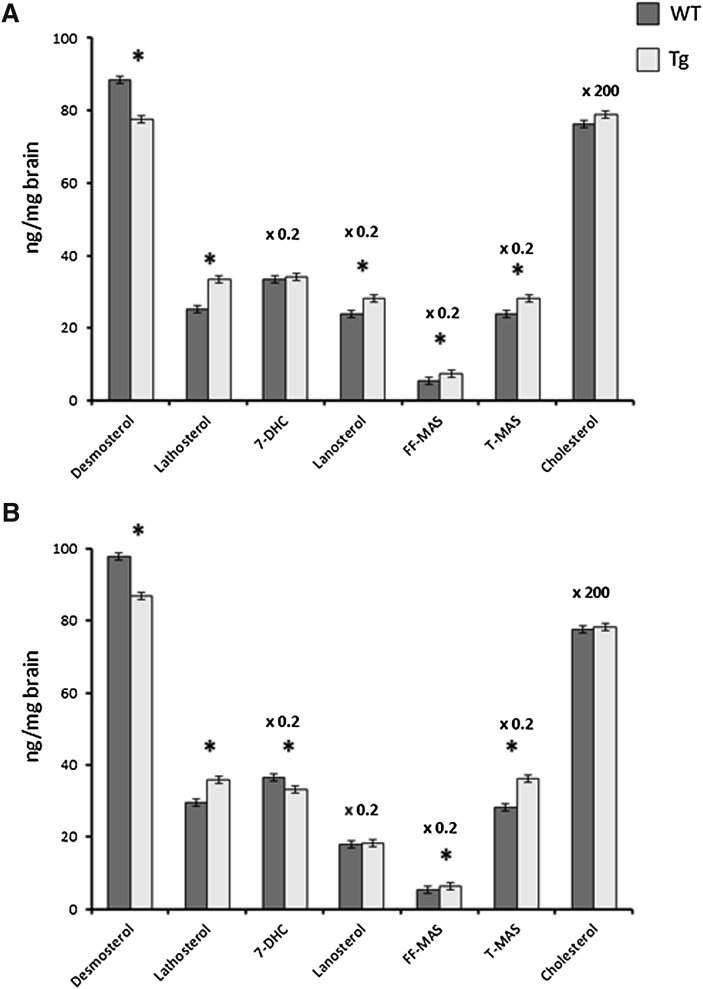
The overexpression had no significant effect on the levels of cholesterol in the brain of the male or female mice (P > 0.05, Fig. 3). Most of the cholesterol precursors measured [lathosterol, lanosterol, follicular fluid meiosis-activating sterol (FF-MAS), testis meiosis-activating sterol (T-MAS)] were increased, suggesting an increased synthesis. In the case of lathosterol, FF-MAS, and T-MAS, the increase was highly significant in both males and females (P < 0.001). It is noteworthy that the levels of desmosterol were decreased rather than increased (P < 0.01 in both males and females).
Fig. 3.
The effect of overexpression of human CYP27A1 on the levels of cholesterol and cholesterol precursors in the adult brain of male (a) and female (b) mice. n = 7–8 in each experiment with each gender. Data are presented as mean ± SEM.* P < 0.05.
Effects of the overexpression of CYP27A1 on mRNA levels in the brain
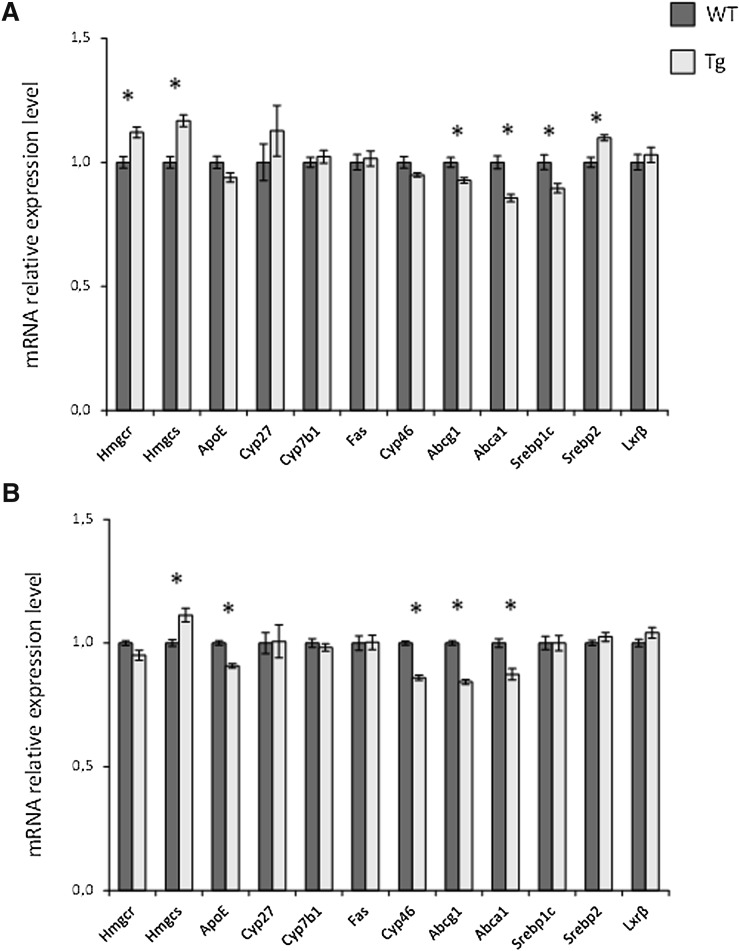
The mRNA levels of Hmgcr were increased in the brain of the male CYP27A1 transgenic mice, suggesting increased synthesis (P = 0.001, Fig. 4a). The mRNA levels of Hmgcs were increased in the brain of both male and female CYP27A1 transgenic mice (P < 0.001 and <0.01, respectively). In the brain of the male CYP27A1 transgenic mice, Srebp2 mRNA was significantly increased (P < 0.01, Fig. 4a). If 27OH is of importance as an activator of LXR target genes, the expression of these genes would be expected to increase. This was not observed, and, in contrast, expression of some of the LXR target genes was slightly decreased; for example Abcg1 and Abca1 expression levels were decreased in both male and female transgenic mice. Cyp46a1 mRNA levels were slightly decreased in the brain of female mice.
Fig. 4.
The effect of overexpression of human CYP27A1 on mRNA levels in the adult brain of male (a) and female (b) mice. n = 7–8 in each experiment with each gender. Data are presented as mean ± SEM.* P < 0.05.
Effects of a knockout of Cyp27a1
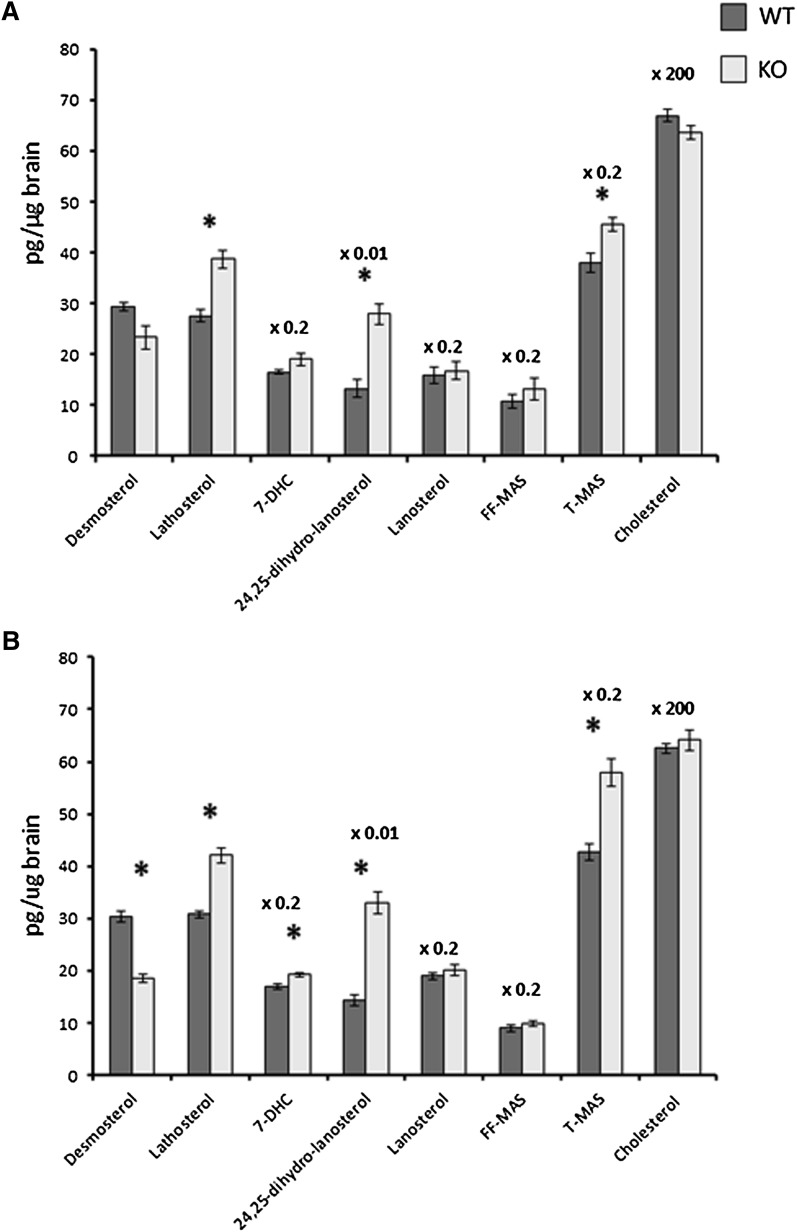
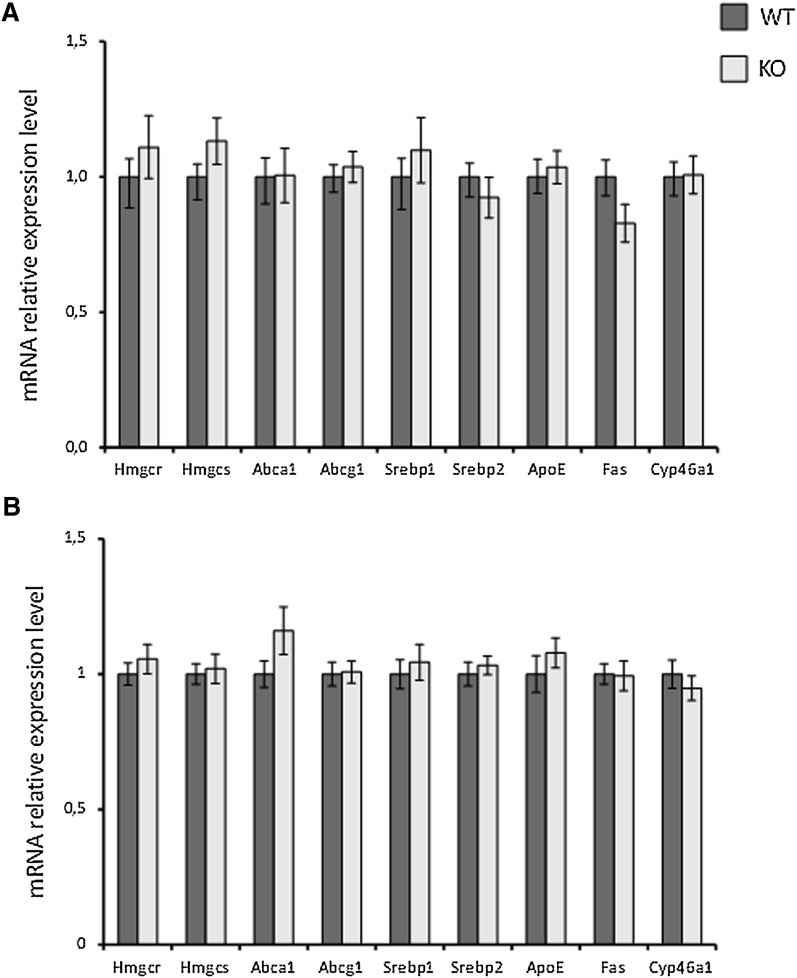
In a previous work, we showed that a knockout of cyp27a1 causes a slight increase of cholesterol synthesis in the brain as judged by increased levels of lathosterol, lanosterol, 24,25-dihydrocholesterol, and 7-dehydrocholesterol (12). Essentially the same results were obtained here in cortex from Cyp27a1−/−mice treated with cholic acid (Fig. 5). The increase in the levels of lathosterol was highly significant (P = 0.001 and P < 0.001 for males and females, respectively). In contrast to the other cholesterol precursors, the levels of desmosterol were decreased rather than increased. In accordance with the previous work (12), the mRNA levels of Hmgcr and Hmgcs were not increased in the cortex of the Cyp27−/− mice (P > 0.05). As shown in Fig. 6, the effect of the knockout had no significant effect on the LXR target genes. The situation was the same in the hippocampus as in the cortex (results not shown). To study the possibility that the knockout of Cyp27a1 may affect the levels of 24OH in the brain, we measured these levels in the cortex of male and female controls and Cyp27a1−/− mice. The male controls had brain levels of 24OH of 51 ± 1 ng/mg and the male Cyp27−/− mice 51 ± 2 ng/mg (mean ± SEM, n = 6 and 5, respectively). The corresponding figures for the brain levels of 24OH in the female mice were 47 ± 2 ng/mg and 47 ± 1 ng/mg, respectively (n = 6 and 6, respectively).
Fig. 5.
The effect of a knockout of Cyp27a1 on the levels of cholesterol and cholesterol precursors in the cortex of the brain of adult male (a) and female (b) mice. n = 5–6 in each experiment with each gender. Data are presented as mean ± SEM.* P < 0.05.
Fig. 6.
The effect of a knockout of Cyp27a1 on mRNA levels in the brain cortex of male (a) and female (b) mice. n = 5 in each experiment with each gender. Data are presented as mean ± SEM.
DISCUSSION
Effects of the overexpression on cholesterol synthesis
The increased mRNA levels of Hmgcr and Hmgcs in the brain of the CYP27A1 transgenic mice suggest increased synthesis of cholesterol. In accordance with this, most of the cholesterol precursors in the brain were also increased. The increased cholesterol synthesis was associated with a marked reduction in the content of 24OH, by about 25%. It seems likely that the reduction in the level of this oxysterol, known to be an efficient suppressor of cholesterol synthesis in vitro (18, 19), is the explanation for the increased cholesterol synthesis. The increase in the level of the inhibiting oxysterol, 27OH, cannot compensate for this. It should be pointed out that even in the brain of the CYP27A1 transgenic mice, the level of 27OH is less than 10% of the level of 24OH. In wild-type mice, the level of 27OH is less than 1% of the level of 24OH (Fig. 2).
The reason for the decreased levels of 24OH is likely to be increased metabolism by the overexpressed CYP27A1 enzyme, and this enzyme is required for the metabolism of 24OH (4). Decreased levels of 24OH were observed not only in the brain but also in the circulation. It should be emphasized that in spite of the decreased levels of 24OH, there must be an increased metabolism of cholesterol to 24OH to explain the fact that the cholesterol levels were not changed in spite of the increased rate of synthesis.
Cholesterol is a substrate for CYP27A1. A minor fraction of cholesterol in the brain of the CYP27A1 transgenic mice may therefore be metabolized into 27OH that can cross the blood-brain barrier and be eliminated from the brain. If such a mechanism is active, the increased consumption of cholesterol could be expected to result in a compensatory increase in the synthesis. The normal expression of Cyp27a1 in the brain is very low, however, and even after an overexpression, it seems less likely that such a metabolic pathway could be of major importance. In man, almost all 27OH in cerebrospinal fluid originates from the circulation and not from the brain (20). Under normal conditions, very little 27OH is thus formed from brain cholesterol.
There was a clear gender difference in the response to the overexpression of CYP27A1.Thus, the degree of stimulation of cholesterol synthesis was higher in the brain of the male than in the brain of the female CYP27A1 transgenic mice. The mechanism behind this difference is unknown at the present state of knowledge.
In a previous work, we showed that a knockout of the Cyp27a1 gene causes a slight but significant upregulation of cholesterol synthesis, as judged by increased levels of cholesterol precursors (12). These findings were confirmed in the present work, using cortex and hippocampus instead of whole brain. The most likely explanation for the increased cholesterol synthesis is that the normal flux of 27OH through the brain has a slight inhibitory effect on cholesterol synthesis. This slight inhibition is not reflected in the mRNA levels of Hmgcr and Hmgcs.
The Cyp27a1−/− mice investigated here were treated with cholic acid to compensate for the deficient bile acid synthesis (13). The deficiency may lead to reduced absorption of fat and fat-soluble vitamins. Although less likely, the possibility must be considered that cholic acid in itself may have some effect on cholesterol synthesis in the brain. This possibility was, however, excluded in our previous investigation, demonstrating that Cyp27a1−/− mice on a normal chow diet without cholic acid supplementation also had increased synthesis of cholesterol in the brain (12).
There are two pathways for synthesis of cholesterol from lanosterol, one involving lathosterol and 7-dehydrocholesterol as intermediates (the Kandutsch-Russel pathway) and one involving 5,7,24-cholestatrien-3β-ol and desmosterol as intermediates (the Bloch pathway). It is noteworthy that in both mouse models studied here, the level of lathosterol was increased, whereas the level of desmosterol was decreased. Thus, there seems to be a channelling from the Bloch pathway to the Kandutsch-Russel pathway under the conditions employed. Evidence has been presented that neurons and astrocytes preferentially utilize the Kandutsch-Russel and the Block pathway, respectively (21). Our data do not, however, allow firm conclusions about the cellular origin of the increased cholesterol synthesis.
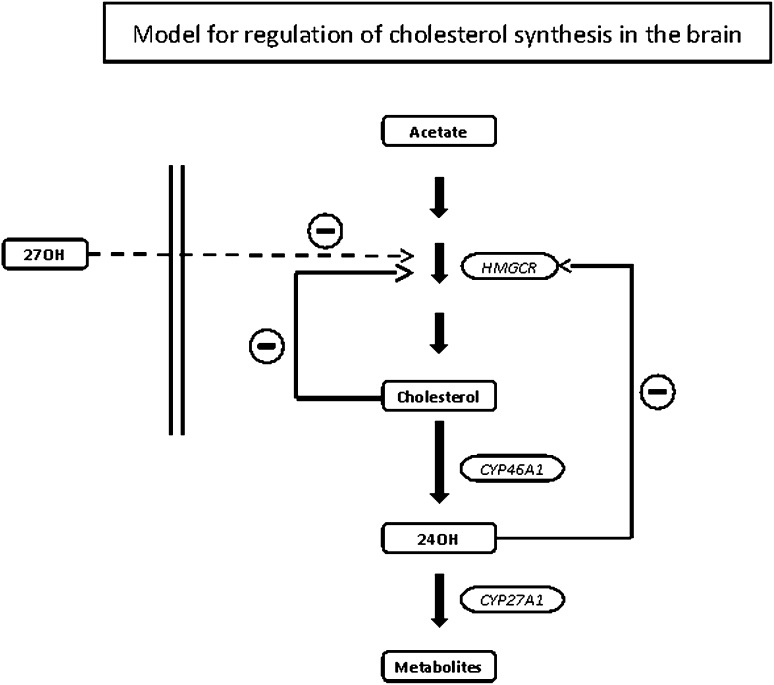
Theoretical model for regulation of cholesterol homeostasis in the brain
The most important players in the regulation of cholesterol homeostasis in the brain appear to be HMGCR, catalyzing the rate-limiting step in cholesterol synthesis, and CYP46A1, catalyzing cholesterol metabolism into 24OH. Similar to the situation in the liver, HMGCR in the brain is subject to a sophisticated regulation at both the transcriptional and the posttranscriptional levels. A tendency to an increase of the cholesterol pool will result in a decreased rate of synthesis of cholesterol by the SREBP mechanism. A tendency to a decrease of the cholesterol pool will have the opposite effect. Due to this, the size of the cholesterol pool will not change. In contrast to HMGCR, CYP46A1 is subject to very little regulation at the transcriptional level (22). Neither cholesterol nor side-chain oxidized oxysterols have a significant effect on transcription (22). Availability of substrate cholesterol seems to be the most-important regulatory factor under normal conditions.
Knocking out the cyp46 mechanism for elimination of cholesterol results in a reduction of cholesterol synthesis in the brain by about 40% without affecting cholesterol levels (23). Inhibition of this mechanism by the drug voriconazole also causes decreased cholesterol synthesis with unchanged levels of cholesterol (24). Overexpression of CYP46A1 in mice results in increased consumption of brain cholesterol with a compensatory increase in the synthesis (9, 10). This compensation leads to unchanged levels of cholesterol in the brain.
The markedly reduced levels of 24OH in the brain of mice with overexpressed CYP27A1 may explain the increased synthesis of cholesterol. 24OH is known to be an efficient inhibitor of cholesterol synthesis in vitro (18, 19), and given the present results and the relatively high levels of 24OH in relation to cholesterol in the brain, it seems likely that 24OH is a physiological suppressor of cholesterol synthesis.
The finding of increased cholesterol synthesis in the brain of the Cyp27a1−/− mice suggests that 27OH may also be a physiological suppressor of cholesterol synthesis. In view of the very low levels of 27OH in the brain in relation to 24OH, it seems likely that it is the flux of 27OH into the brain that is the regulator, rather than the concentrations. It should be emphasized that in humans, the flux of 27OH into the brain, about 5 mg/24 h (24), is similar to the flux of 24OH out of the brain, which is about 6 mg/24 h (25, 26). In spite of this high flux, the levels of 27OH in the brain measured post mortem are markedly lower than the levels of 24OH (25).
The rate of flux of 27OH into the brain is dependent upon the integrity of the blood-brain barrier (20), and a defective barrier can thus be expected to lead to a higher flux into the brain. It is known that apoE deficiency, as well as apoE deficiency, together with expression of the human apoE4 isoform, leads to a compromised blood-brain barrier (27). If 27OH is a suppressor of cholesterol synthesis in the brain, apoE deficiency would be expected to lead to reduced synthesis. This has been reported to be the case in one study (28), but not in another (29).
If the flux of 27OH into the brain inhibits cholesterol synthesis, it is evident that this inhibition cannot counteract the positive effect of the overexpression on cholesterol synthesis in the present mouse model. The positive effect is that the increased metabolism of 24OH by CYP27A1 leads to reduced levels of 24OH, which is therefore less potent to inhibit cholesterol synthesis. The situation is similar in our mouse model with increased CYP46A1 activity in the brain (9). The overexpression caused increased cholesterol synthesis in spite of the increased brain levels of 24OH.
Figure 7 summarizes a model for regulation of cholesterol synthesis in the brain that is consistent with all the present data as well as data from previous in vivo and in vitro studies (9, 10, 12, 18, 19, 22–24). Further studies are needed, however, to confirm this model. It should be emphasized that the present results are based on stable animal models under steady-state conditions. Thus, acute changes in the production or concentration of the oxysterols may give different results. It should also be emphasized that the present results were obtained with whole brain, and we cannot exclude the possibility that the situation may be somewhat different in some small compartments in the brain.
Fig. 7.
Theoretical model for the regulation of cholesterol homeostasis in the brain.
Effects on LXR-targeted genes
Similar to the situation in mice with an overexpression of CYP46A1 (9), the overexpression of CYP27A1 led to moderate changes only in the expression of LXR-targeted genes. (cf. Fig. 4). Instead of an expected increase, some of the LXR target genes were slightly decreased by the overexpression. The biological significance of the small decrease is difficult to evaluate, however.
We have considered the possibility that the effects on the LXR-targeted genes that were opposite those expected (Abcg1, Abca1, Srebpc1, Apoe; Fig. 4) could be due to a sulfated form of 27OH. Thus, it has been reported that the sulfate of 25OH is an antagonist to a normal oxysterol-dependent activation of the LXR (30). If increased levels of 27OH in the circulation lead to increased levels of the sulfate of 27OH, this could explain that the expression of some of the LXR-targeted genes changed in a direction opposite to that expected. In a separate study, an assay of the sulfate of 27OH was developed (A. Jure, unpublished observations). The fraction of the total level of 27OH that was sulfated in the mice with an overexpression of CYP27A1 was, however, about the same as in the wild-type (A. Jure, unpublished observations). Thus, it is seems less likely that 27OH sulfate is of importance for the effects observed.
The possibility must also be considered that the slightly decreased expression of some of the LXR target genes is a consequence of a decreased expression of LXR itself. LXRβ is the dominating form of LXR present in the brain, but the mRNA corresponding to this factor was not decreased as a consequence of the overexpression.
A third explanation for the small decrease in mRNA level of some LXR target genes (Abcg1, Abca1, Srebp1c, Apoe; Fig. 4) is the decrease in the pool of 24OH. 24OH is an efficient activator of LXR, and a decreased pool of this oxysterol would be expected to reduce the activation of LXR. Such a reduction may compensate for a potential activation due to the increased levels of 27OH in the brain of our mouse model and result in unchanged or reduced expression of the LXR target genes. On the other hand, we have shown that in our CYP46A1 overexpressing mice with brain levels of 24OH increased by a factor of about 2, expression of the LXR target genes was not increased (9). If the levels of 24OH are of importance for expression of the above LXR target genes in the brain, the levels are important for the basal expression only.
To summarize, our results do not favor the hypothesis that 27OH is a general activator of LXR in the brain. The levels of 24OH in the brain may be of some importance for the expression of some of the LXR target genes under basal conditions. The results are consistent with the possibility that both 24OH and 27OH have a suppressive effect on cholesterol synthesis in this organ. The effects are moderate, however, and it is evident that the side-chain oxidized oxysterols studied are not important master regulators of cholesterol homeostasis in the brain.
Acknowledgments
The skillful technical assistance of Inger Moberg is gratefully acknowledged.
Footnotes
Abbreviations:
- CYP46A1
- cholesterol 24S-hydroxylase
- CYP27A1
- sterol 27-hydroxylase
- FF-MAS
- follicular fluid meiosis-activating sterol
- HMGCR
- HMG CoA reductase
- LXR
- liver X receptor
- 24OH
- 24S-hydroxycholesterol
- 27OH
- 27-hydroxycholesterol
- 25OH
- 25-hydroxycholesterol
- SREBP
- sterol-regulatory element binding protein
- T-MAS
- testis meiosis-activating sterol
This work was supported by grants from the Swedish Science Council, Brain Power, the Swedish Alzheimer´s Foundation, and Hjärnfonden.
REFERENCES
- 1.Goldstein J. L., DeBose-Boyd R. A., Brown M. S. 2006. Protein sensors for membrane sterols. Cell. 124: 35–46. [DOI] [PubMed] [Google Scholar]
- 2.Willy P. J., Umesono K., Ong E. S., Evans R. M., Heyman R. A., Mangelsdorf D. J. 1995. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 9: 1033–1045. [DOI] [PubMed] [Google Scholar]
- 3.Björkhem I., Diczfalusy U. 2002. Oxysterols: friends, foes, or just fellow passengers? Arterioscler. Thromb. Vasc. Biol. 22: 734–742. [DOI] [PubMed] [Google Scholar]
- 4.Bjorkhem I., Andersson U., Ellis E., Alvelius G., Ellegard L., Diczfalusy U., Sjovall J., Einarsson C. 2001. From brain to bile. Evidence that conjugation and omega-hydroxylation are important for elimination of 24S-hydroxycholesterol (cerebrosterol) in humans. J. Biol. Chem. 276: 37004–37010. [DOI] [PubMed] [Google Scholar]
- 5.Meaney S., Heverin M., Panzenboeck U., Ekström L., Axelson M., Andersson U., Diczfalusy U., Pikuleva I., Wahren J., Björkhem I. 2007. Novel route for the elimination of brain oxysterols across the blood-brain barrier: conversion into 7-alpha-hydroxy-3-oxo-4-cholestenoic acid. J. Lipid Res. 48: 944–951. [DOI] [PubMed] [Google Scholar]
- 6.Björkhem I. 2002. Do oxysterols control cholesterol homeostasis? J. Clin. Invest. 110: 725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Björkhem I. 2009. Are side-chain oxidized oxysterols regulators also in vivo? J. Lipid Res. 50 (Suppl.): 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janowski B. A., Grogan M. J., Jones S. A., Wisely G. B., Kliewer S. A., Corey E. J., Mangelsdorf D. J. 1999. Structural requirements of ligands for the oxysterol liver X receptors LXRalpha and LXRbeta. Proc. Natl. Acad. Sci. USA. 96: 266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shafaati M., Olin M., Bavner A., Pettersson H., Rozell B., Meaney S., Parini P., Bjorkhem I. 2011. Enhanced production of 24S-hydroxycholesterol is not sufficient to drive liver X receptor target genes in vivo. J. Intern. Med. 270: 377–387. [DOI] [PubMed] [Google Scholar]
- 10.Hudry E., Van Dam D., Kulik W., De Deyn P. P., Stet F. S., Ahouansou O., Benraiss A., Delacourte A., Bougneres P., Aubourg P., et al. 2010. Adeno-associated virus gene therapy with cholesterol 24-hydroxylase reduces the amyloid pathology before or after the onset of amyloid plaques in mouse models of Alzheimer's disease. Mol. Ther. 18: 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meir K., Kitsberg D., Alkalay I., Szafer F., Rosen H., Shpitzen S., Avi L. B., Staels B., Fievet C., Meiner V., et al. 2002. Human sterol 27-hydroxylase (CYP27) overexpressor transgenic mouse model. Evidence against 27-hydroxycholesterol as a critical regulator of cholesterol homeostasis. J. Biol. Chem. 277: 34036–34041. [DOI] [PubMed] [Google Scholar]
- 12.Båvner A., Shafaati M., Hansson M., Olin M., Shpitzen S., Meiner V., Leitersdorf E., Bjorkhem I. 2010. On the mechanism of accumulation of cholestanol in the brain of mice with a disruption of sterol 27-hydroxylase. J. Lipid Res. 51: 2722–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Repa J. J., Lund E. G., Horton J. D., Leitersdorf E., Russell D. W., Dietschy J. M., Turley S. D. 2000. Disruption of the sterol 27-hydroxylase gene in mice results in hepatomegaly and hypertriglyceridemia. Reversal by cholic acid feeding. J. Biol. Chem. 275: 39685–39692. [DOI] [PubMed] [Google Scholar]
- 14.Acimovic J., Lovgren-Sandblom A., Monostory K., Rozman D., Golicnik M., Lutjohann D., Bjorkhem I. 2009. Combined gas chromatographic/mass spectrometric analysis of cholesterol precursors and plant sterols in cultured cells. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877: 2081–2086. [DOI] [PubMed] [Google Scholar]
- 15.Dzeletovic S., Breuer O., Lund E., Diczfalusy U. 1995. Determination of cholesterol oxidation products in human plasma by isotope dilution-mass spectrometry. Anal. Biochem. 225: 73–80. [DOI] [PubMed] [Google Scholar]
- 16.Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 17.Lund E., Bjorkhem I., Furster C., Wikvall K. 1993. 24-, 25- and 27-hydroxylation of cholesterol by a purified preparation of 27-hydroxylase from pig liver. Biochim. Biophys. Acta. 1166: 177–182. [DOI] [PubMed] [Google Scholar]
- 18.Schroepfer G. J., Jr 2000. Oxysterols: modulators of cholesterol metabolism and other processes. Physiol. Rev. 80: 361–554. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y., Muneton S., Sjovall J., Jovanovic J. N., Griffiths W. J. 2008. The effect of 24S-hydroxycholesterol on cholesterol homeostasis in neurons: quantitative changes to the cortical neuron proteome. J. Proteome Res. 7: 1606–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leoni V., Masterman T., Patel P., Meaney S., Diczfalusy U., Björkhem I. 2003. Side chain oxidized oxysterols in cerebrospinal fluid and the integrity of blood-brain and blood-cerebrospinal fluid barriers. J. Lipid Res. 44: 793–799. [DOI] [PubMed] [Google Scholar]
- 21.Nieweg K., Schaller H., Pfrieger F. W. 2009. Marked differences in cholesterol synthesis between neurons and glial cells from postnatal rats. J. Neurochem. 109: 125–134. [DOI] [PubMed] [Google Scholar]
- 22.Ohyama Y., Meaney S., Heverin M., Ekstrom L., Brafman A., Shafir M., Andersson U., Olin M., Eggertsen G., Diczfalusy U., et al. 2006. Studies on the transcriptional regulation of cholesterol 24-hydroxylase (CYP46A1): marked insensitivity toward different regulatory axes. J. Biol. Chem. 281: 3810–3820. [DOI] [PubMed] [Google Scholar]
- 23.Lund E. G., Xie C., Kotti T., Turley S. D., Dietschy J. M., Russell D. W. 2003. Knockout of the cholesterol 24-hydroxylase gene in mice reveals a brain-specific mechanism of cholesterol turnover. J. Biol. Chem. 278: 22980–22988. [DOI] [PubMed] [Google Scholar]
- 24.Shafaati M., Mast N., Beck O., Nayef R., Heo G. Y., Bjorkhem-Bergman L., Lutjohann D., Bjorkhem I., Pikuleva I. A. 2010. The antifungal drug voriconazole is an efficient inhibitor of brain cholesterol 24S-hydroxylase in vitro and in vivo. J. Lipid Res. 51: 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heverin M., Meaney S., Lutjohann D., Diczfalusy U., Wahren J., Bjorkhem I. 2005. Crossing the barrier: net flux of 27-hydroxycholesterol into the human brain. J. Lipid Res. 46: 1047–1052. [DOI] [PubMed] [Google Scholar]
- 26.Lütjohann D., Breuer O., Ahlborg G., Nennesmo I., Siden A., Diczfalusy U., Björkhem I. 1996. Cholesterol homeostasis in human brain: evidence for an age-dependent flux of 24S-hydroxycholesterol from the brain into the circulation. Proc. Natl. Acad. Sci. USA. 93: 9799–9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bell R. D., Winkler E. A., Singh I., Sagare A. P., Deane R., Wu Z., Holtzman D. M., Betsholtz C., Armulik A., Sallstrom J., et al. 2012. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 485: 512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenner A. M., Lim W. L., Ng M. P., Wenk M. R., Shui G., Sharman M. J., Gandy S. E., Martins R. N. 2010. The effect of APOE genotype on brain levels of oxysterols in young and old human APOE epsilon2, epsilon3 and epsilon4 knock-in mice. Neuroscience. 169: 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jansen P. J., Lutjohann D., Thelen K. M., von Bergmann K., van Leuven F., Ramaekers F. C., Monique M. 2009. Absence of ApoE upregulates murine brain ApoD and ABCA1 levels, but does not affect brain sterol levels, while human ApoE3 and human ApoE4 upregulate brain cholesterol precursor levels. J. Alzheimers Dis. 18: 319–329. [DOI] [PubMed] [Google Scholar]
- 30.Xu L., Bai Q., Rodriguez-Agudo D., Hylemon P. B., Heuman D. M., Pandak W. M., Ren S. 2010. Regulation of hepatocyte lipid metabolism and inflammatory response by 25-hydroxycholesterol and 25-hydroxycholesterol-3-sulfate. Lipids. 45: 821–832. [DOI] [PubMed] [Google Scholar]