Abstract
Glycosylphosphatidylinositol (GPI) enriches GPI-anchored proteins (GPI-AP) in lipid rafts by intimate interaction of its lipid moiety with sphingolipids and cholesterol. In addition to such lipid-lipid interactions, it has been reported that GPI may interact with protein moiety linked to GPI and affect protein conformations because GPI delipidation reduced immunoreactivities of protein. Here, we report that GPI-APs that have not undergone fatty acid remodeling exhibit reduced immunoreactivities in Western blotting, similar to delipidated proteins, compared with normal remodeled GPI-APs. In contrast, immunostaining in flow cytometry and immunoprecipitation did not show significant differences between remodeled and unremodeled GPI-APs. Moreover, detection with premixed primary/secondary antibody complexes or Fab fragments eliminated this difference in Western blotting. These results indicate that normally remodeled GPI enhanced oligomerization of GPI-APs and that inefficient oligomerization of unremodeled GPI-APs was responsible for reduced immunoreactivities. Moreover, the reduction in immunoreactivities of delipidated GPI-APs was most likely caused by the same effect. Finally, by chemical cross-linking of surface proteins in living cells and cell killing assay using a pore-forming bacterial toxin, we showed that enhanced oligomerization by GPI-remodeling occurs under a physiological membrane environment. Thus, this study clarifies the significance of GPI fatty acid remodeling in oligomerization of GPI-APs and provides useful information for technical studies of these cell components.
Keywords: PGAP3, lipid remodeling, glycosylphosphatidylinositol
Glycosylphosphatidylinositol (GPI) is widely conserved among eukaryotes from yeast to human. More than 100 different plasma membrane proteins are posttranslationally modified at their processed C termini with GPI, which serves to anchor proteins to cell surfaces (1). After the transfer of GPI to proteins in the endoplasmic reticulum (ER), the GPI moiety in GPI-anchored proteins (GPI-AP) is subjected to at least three rounds of structural remodeling, including inositol deacylation (2), glycochain remodeling (3), and fatty acid remodeling (4), that are catalyzed by post-GPI attachment to protein (PGAP) proteins. Inositol deacylation, the removal of an acyl chain, typically palmitate, from the inositol by PGAP1 in the ER, is critical for efficient transport of GPI-APs from the ER to the Golgi apparatus and for normal mammalian development (5). Glycochain remodeling, the removal of phosphoethanolamine from the second mannose of GPI by PGAP5 in the ER, is important for efficient sorting to and exit from the ER exit site (3). The third structural remodeling, fatty acid remodeling, occurs during the transport through the Golgi. Before attachment to proteins in the ER, GPI contains an unsaturated fatty acid, such as arachidonate (C20:4) at the sn-2 position in phosphatidylinositol (PI) moiety. The unsaturated chain is replaced with a saturated chain, commonly stearic acid (C18:0), for which at least two proteins, PGAP2 (4) and PGAP3, are required (Fig. 1) (6). PGAP3 is involved in removal of the sn-2 unsaturated fatty acid, because GPI-APs harboring an sn-2 unsaturated fatty acid are expressed on the surface of PGAP3-defective cells (6). Therefore, PGAP3 is considered to be a phospholipase A2 enzyme, albeit its enzymatic activity has not been directly proven. PGAP2 is involved in reacylation of the sn-2 position with a stearic acid. Thus, this fatty acid remodeling, which produces GPI-APs possessing two saturated fatty acid chains, is critical for accumulation of GPI-APs in lipid rafts where (glyco)sphingolipids and cholesterol are enriched in a liquid-ordered manner (7). Enrichment of GPI-APs in lipid rafts, owing to this lipid composition, is considered to be the most important function of GPI, as this enrichment caused by lipid-lipid interaction affects many biological behaviors of GPI-APs, such as signal transduction, interaction with other molecules, intracellular trafficking and localization, and secretion (8–10).
Fig. 1.
Processing of GPI-anchor by PGAP3 and PI-PLC. GPI-APs are subjected to fatty acid remodeling in the Golgi. An sn-2 unsaturated fatty acid (for example, C20:4) is cleaved by PGAP3 and substituted with a saturated chain (typically C18:0) by an unknown acyltransferase, remodeling GPI to two saturated long acyl chains. PI-PLC cleaves between phosphate and diacylglycerol in the phosphatidylinositol portion of GPI. The cleaved form becomes a soluble delipidated protein.
In addition to the above features of GPI, several reports have indicated another possibly important aspect of GPI, in which it might interact with its protein moiety, based on the unexpected facts that removal of the GPI lipid moieties, namely delipidation, by phospholipases, such as phospholipase C and GPI-specific phospholipase D, drastically reduces reactivities to antibodies (11–13). This phenomenon has been commonly observed in many GPI-APs, such as mammalian Thy-1, CD58, and CD59 and protozoan VSG, EP-, and GPEET-procyclins. The reduction in immunoreactivity is most likely due to conformational changes of proteins caused by altered interactions of the protein moiety with delipidated GPI and/or proximate surface circumstances (11). Thus, protein-GPI interactions may modulate the functions of these proteins.
Surprisingly, we found that such considerably reduced immunoreactivities were also observed in a wide range of GPI-APs in PGAP3-deficient cells, which have not undergone fatty acid remodeling and still carry two fatty acid chains. The differences between remodeled and unremodeled GPI-APs exist only in the composition of the sn-2 fatty acyl chain, either saturated or unsaturated, that is completely buried within the membrane. Also, unremodeled GPI is tethered to the surface membrane and not freely accessible to the attached protein. Thus, reduced immunoreactivities appeared unexplained by altered protein moiety interactions with cleaved GPI and/or proximate surface circumstances, as was explained in the case of delipidated GPI-APs. Moreover, it is not easy to explain why many GPI-APs with completely different peptides were similarly affected by the same delipidated GPI. Therefore, it was considered that the mechanism causing reduced immunoreactivities remained unclear. Here, we report that both a defect in remodeling and delipidation of GPI-APs lead to reduced immunoreactivities in Western blotting due to the reduced oligomerization during denaturation/renaturation, and that the nature of remodeled GPI to oligomerize is also conserved under physiological membrane conditions. Thus, through the studies of fatty acid remodeling of GPI-APs, an important characteristic of GPI to enhance oligomerization of GPI-APs is revealed here, which would affect many biological aspects, such as immunoreactivities, signal transduction, and stabilization of rafts.
EXPERIMENTAL PROCEDURES
Cells, plasmids, and antibodies
PGAP3 knock-out mice were created as previously described. Mouse embryonic fibroblasts (MEF) isolated from the mice (14) were cultured in DMEM high-glucose medium supplemented with 10% fetal calf serum (FCS). Mouse PGAP3 was cloned by PCR using primers (GTTGGTCGAGGTGTGACAGAATGGCCAAGC and CTTCTCTAGACACTCTCTCCCAGCCTCTTTAGTC) and subcloned into pLIB2-BSD retroviral vector, which was made from pLIB vector (Clontech Laboratories, Inc., Madison, WI) by modifying the multicloning site and inserting blasticidin resistance gene (BSD) driven by pgk promoter (15). Human placental alkaline phosphatase N-terminally tagged with signal sequence derived from CD59 and hemagglutinin peptide (HA-PLAP) was described previously (16). EGFP-Flag-CD59 was generated by tandem fusion of signal peptide derived from human growth hormone, enhanced green fluorescent protein (EGFP), Flag-tag, and CD59, and subcloned into pRCMV retroviral vector. Wild-type Chinese hamster ovary (CHO) cells (3B2A, GD3S-37) and PGAP2/PGAP3 double mutant (DM) CHO cells that stably expressed human decay accelerating factor (DAF) and CD59 have been previously described (6). These CHO cells were cultured in Ham's F-12 supplemented with 10% FCS. Monoclonal anti-CD59 (5H8, prepared in this lab), Thy-1 (G7, prepared in this lab), PLAP (8B6, Sigma-Aldrich, Inc., St. Louis, MO), urokinase-type plasminogen activator receptor (uPAR, 5D6, prepared in this lab), and DAF (IA10, prepared in this lab) were used for detection of GPI-APs. Additionally, anti-EGFP (Roche Applied Science, Indianapolis, IN), HA (HA7, Sigma-Aldrich), Flag (M2, Sigma-Aldrich) were used for tag peptide detection. Anti-Ribophorin-1, GAPDH, and Synthaxin-6 were used as quantitative controls. Fab fragments of G7 were prepared using the ImmunoPure Fab preparation kit containing immobilized papain (Pierce Biotechnology, Inc., Rockford, IL). Premixed antibodies were generated by incubating same amounts of primary and secondary antibodies that had been prepared by diluting the stock solutions (1:1000) in 5% skim milk containing TBS (20 mM Tris-Cl, pH 7.4, 150 mM NaCl) at room temperature for 2 h.
Extraction of proteins, phosphatidylinositol-specific phospholipase C treatment, and immunoblotting
Whole-cell lysates for Western blots were prepared using n-octyl-β-D-glucoside (OβG) buffer (20 mM Tris-Cl, pH 7.4, 150 mM NaCl, 1 mM EDTA, and 60 mM OβG) on ice for 30 min, then centrifuged at 4°C for 15 min to remove debris. For phosphatidylinositol-specific phospholipase C (PI-PLC) treatment, the lysate was incubated at 20°C for 1 h with 1 unit/ml of Bacillus cereus PI-PLC (Molecular Probes, Life Technologies, Grand Island, NY). For analysis of intracellular GPI-APs, cells were incubated at 10°C for 6 h or at 37°C for 30 min with PI-PLC, washed with PBS, and then lysed with OβG buffer. Samples were prepared with boiling or nonboiling treatment, and then subjected to SDS-PAGE or alkaline phosphatase (ALP) analyses. For blotting, samples were loaded onto SDS-PAGE and then transferred to PVDF or nitrocellulose membranes, and then probed with each antibody.
Immunoprecipitation of HA-PLAP and EGFP-Flag-CD59
Cells were lysed with OβG buffer. The lysates were incubated with 5H8 or 8B6 antibodies together with protein-G beads (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) in a cold room overnight. After washing the beads with TBS several times, bound proteins were eluted using 2× SDS sample buffer (4% SDS, 0.7M Tris-Cl, pH 6.8, 10% glycerol).
Flow cytometry for GPI-APs
Cells were harvested using trypsin/EDTA mixture (Sigma-Aldrich) or with 5 mM EDTA-containing PBS (for EGFP-Flag-CD59), and the surface expression of GPI-APs was determined by staining with 5H8, IA10, G7, or 8B6 antibodies, followed by flow cytometric analyses (CantII; BD Biosciences Co., Franklin Lakes, NJ). Control staining was obtained with isotype-matching antibodies or without primary antibodies, using cells that were PGAP3-restored PGAP3−/− MEF cells or cells equivalent to wild-type cells as a representative cell type; control staining obtained from one control cell type was used in all figures of the same experiments.
Measurement of ALP activity
ALP activity of HA-PLAP was measured using three methods. In the first method, cell extracts were prepared with OβG lysis buffer, and the lysate ALP activity measured using an SEAP assay kit (Clontech Laboratories). The second method involved a protein denaturation/renaturation experiment, in which cell lysis with Triton lysis buffer [100 mM Tris-Cl, pH 9.5, 100 mM NaCl, 5 mM MgCl2, 1% Triton X-100, and 1 Protease inhibitor cocktail without EDTA (Roche Applied Science)] with or without 2.5% SDS was performed, and then ALP activity was measured by addition of 0.25 mM CSPD (Roche Applied Science). And finally, ALP activity was measured on transferred membranes after SDS-PAGE using OβG lysis buffer, and ALP activity was measured by adding CDP-star according to manufacturer's instruction (GE Healthcare Bio-Sciences AB), a luminous substrate for ALP.
Detergent-resistant membrane fractionation
Cells were harvested from the plate by using PBS containing 2.5 mM EDTA and 0.5% BSA. After centrifugation, the cell pellet was resuspended in MBS-E [25 mM 2-(N-morpholino)ethanesulfonic acid, pH 6.5, 150 mM NaCl, and 5 mM EDTA] containing protease inhibitors supplemented with 1% TX-100, incubated for 20 min on ice, and homogenized by a potter-type Teflon homogenizer. The volume of lysis buffer was 25 times the weight of cell pellet (typically 25–30 million cells/ml lysis buffer). One milliliter of lysate was mixed with 1 ml of 80% sucrose in MBS-E, transferred to a centrifuge tube for SW41-Ti, overlaid with 7 ml of 30% and 2 ml of 5% sucrose in MBS-E, ultracentrifuged at 38,000 rpm for 16–18 h at 4°C, and fractionated from the top using Piston Gradient Fractionator (BioComp Systems) with each fraction of 1 ml (total 11 fractions). Aliquots of each fraction were mixed with 6 × sample buffer, without a reducing reagent, and applied to 5–20% gradient SDS-PAGE.
Chemical cross-linking of cell surface proteins
Cells cultured in 6-well plates were treated with 1 mM of cross-linking agent 3,3′-Dithiobis(sulfosuccinimidylpropionate) (DTSSP) dissolved in PBS for 30 min at room temperature. DTSSP contains amine-reactive NHS-ester at each end of a spacer arm 12.0 Å (8-atom). DTSSP-treated and nontreated cells were lysed in OβG lysis buffer. Samples for Western blotting were prepared under nonboiling, nonreducing conditions. The dimers and monomers were visualized using primary (G7 anti-Thy-1)/secondary antibody premix.
Treatment of aerolysin and viability assay
Nontoxic fluorescein-conjugated proaerolysin (FLAER) was used to analyze binding efficiency to the cell surface. Cells (1 × 106) were stained with 10−8 and 10−9 M of FLAER. For viability assay, 5 × 103 MEFs per well were cultured in 96-well plates. Prewarmed toxin (0.1 ml) was added to the wells and incubated for 3 h at 37°C. After removal of toxin, treated cells were further incubated for 24 h. The viable cells were assessed using WST-1 reagent (Roche). Absorption at 450 and 690 nm was measured, and percentage of viability was calculated as 100 × [(toxin-treated(A450-A650) − background(A450-A650)) / (nontreated(A450-A650) − background(A450-A650))]
RESULTS
Considerably weakened Western blotting reactivity of GPI-APs lacking fatty acid remodeling
Discrepancies were found in the expressions of unremodeled GPI-APs lacking fatty acid remodeling due to defective PGAP3 between immunoblotting of whole-cell lysate and flow cytometry that measured cell surface expression. In Western blot analyses, compared with PGAP3-recovered PGAP3-knockout (PGAP3−/−) MEFs, PGAP3−/− MEFs showed remarkably decreased intensities of Thy-1, CD59, and PLAP bands (Fig. 2A, left). However, in flow cytometry, the surface expression of these GPI-APs on PGAP3−/− MEFs was comparable with PGAP3-recovered MEFs (Fig. 2A, right). In addition to MEF, CHO cells stably expressing CD59 and DAF were employed to check whether this phenomenon was present in remodeling-deficient CHO mutant cells. Wild-type 3B2A parent cells and PGAP2/PGAP3 DM cells lacking fatty acid remodeling also showed discrepancies in CD59 and DAF expressions, similar to PGAP3−/− MEF (Fig. 2B). These results indicate that the immunoreactivity discrepancies between Western blot analyses and flow cytometry were general phenomena in unremodeled GPI-APs.
Fig. 2.
Discrepancies in GPI-APs expression between flow cytometry and immunoblotting in PGAP3-deficient cells. A: PGAP3-deficient mouse embryonic fibroblast (PGAP3−/− MEF) cells, stably expressing either HA-PLAP, CD59, or EGFP-Flag-CD59, were infected with retrovirus vector-based mock (Vec) or PGAP3 (PGAP3) virions; GPI-AP expressions analyzed by Western blotting using whole-cell lysate or flow cytometry (left and right panels, respectively). Geometric means of surface expressions in flow cytometry are indicated in the right side as relative percentages when those in wild-type cells are presumed as 100%. B: Wild-type parent (3B2A) and derivative PGAP2/PGAP3 DM CHO cells stably expressing human CD59 and DAF were analyzed as in (A). Gray shadows, staining with indicated antibodies; dotted lines, control staining with isotype-matching antibodies; Syntaxin-6 and Ribophorin-1, quantitative controls; loading samples for Western blotting prepared under nonreducing boiling conditions.
Intracellular pools of GPI-APs do not account for different estimates by Western blotting
As flow cytometry measures only surface GPI-APs, one possible explanation for the above discrepancies was that wild-type cells possessed much larger intracellular pools of GPI-APs than the cell surfaces. To evaluate the amounts of intracellular GPI-APs, MEF cells expressing HA-tagged PLAP (HA-PLAP) were incubated with PI-PLC to remove surface GPI-APs at 37°C or at 10°C, the latter blocking exocytosis and endocytosis. Both cell preparations showed significantly decreased surface expression of PLAP (Fig. 3A), indicating effective PI-PLC activity. Subsequently, intracellular PLAP analyses by Western blotting (Fig. 3B) showed that PI-PLC treatment strikingly decreased PLAP amounts in whole-cell lysates as well as surface expression. This was considered to indicate that the majority of PLAP existed on cell surfaces. In addition, it appeared that intracellular pools were small enough to exclude the possibility that the immunoreactivity discrepancies between Western blot and flow cytometric analyses came from differing intracellular pools of remodeled and unremodeled GPI-APs. This was also confirmed by PGAP2/PGAP3 DM CHO cells. PI-PLC removed more than 90% of DAF, uPAR, and CD59 expressed on the cell surface at 10°C and even more at 37°C, as determined by flow cytometry (Fig. 3C). The amounts of remaining GPI-APs after PI-PLC treatment in whole-cell lysates correlated best with those of surface expression detected by flow cytometry (Fig. 3D), indicating that CHO cells also had much smaller GPI-AP intracellular pools than on the surface.
Fig. 3.
Estimation of intracellular pools of GPI-APs. Intracellular GPI-AP pools estimated in MEF (A and B) and CHO (C and D) cells. A: Removal efficiencies of surface GPI-APs confirmed by flow cytometry. MEF cells (see Fig. 2) were incubated with PI-PLC at 10°C for 6 h or at 37°C for 30 min to prevent protein trafficking, then stained with anti-PLAP antibody. B: After cleavage of surface GPI-APs by PI-PLC, loading samples were prepared from whole-cell lysates under nonreducing boiling conditions, and intracellular HA-PLAP pools were estimated by Western blotting with anti-PLAP antibody. C and D: Wild-type parent (GD3S-C37) and derivative PGAP2/PGAP3 DM CHO cells stably expressing CD59 and DAF were treated with PI-PLC as in (A) and stained with antibodies against DAF, uPAR, and CD59 (C); loading samples were prepared as in (B), and intracellular GPI-AP pools were estimated by Western blotting using respective antibodies (D). Gray shadows, PI-PLC-treated cells; solid lines, PI-PLC-untreated cells; dotted lines, staining with isotype-matching control antibody; Syntaxin-6 and Caveolin-1, quantitative controls.
Weakened signals of unremodeled GPI-APs in Western blotting were most likely due to their weak reactivities to antibodies
The suspicion was that a technical issue still remained, such that unremodeled GPI-APs might have been lost during the experimental procedure. Thus, the presence of GPI-APs was confirmed in each step of Western blot analyses. Analysis by fluorescence laser scanner confirmed that similar amounts of remodeled and unremodeled EGFP-Flag-CD59 proteins were loaded into polyacrylamide gels and transferred to nitrocellulose membranes, albeit immunodetection of EGFP-Flag-CD59 on the membrane with anti-CD59 antibody exhibited significant differences between the gels and membranes (Fig. 4A). Consistent with surface expressions of HA-PLAP in flow cytometry (Fig. 4B), anti-HA antibody detected similar amounts of HA-PLAPs by Western blotting, contrary to the response of anti-PLAP antibodies that showed big differences between remodeled and unremodeled samples (Fig. 4C). Measuring PLAP enzymatic activities in whole-cell lysates from both cells also confirmed that similar amounts of PLAP were present (Fig. 4D). Thus, all results obtained here were comparable, except for Western blot analyses with anti-GPI-AP antibodies, suggesting that the discrepancy with Western blotting was most likely produced by differences in antibody immunoreactivities against remodeled and unremodeled GPI-APs.
Fig. 4.
Immunoblotting with anti-GPI-AP antibodies versus immunoblotting with anti-HA antibody, flow cytometry, and enzymatic activities. A: MEF cells (see Fig. 2) were lysed, and whole-cell lysates prepared under nonreducing boiling conditions were applied to SDS-PAGE. EGFP fluorescence in gel and on transferred membrane (left and middle panels, respectively) were detected using fluorescence laser scanner; blotting membrane was then probed against 5H8 anti-CD59 antibody (right panel); nitrocellulose membrane was used instead of PVDF membrane due to high fluorescent background. The same MEF cells were used for flow cytometry, Western blotting, and enzyme assay (B–D, respectively). B: Cells were analyzed with anti-PLAP antibody in flow cytometry. Gray shadows, staining with 8B6 antibody; dotted lines, staining with isotype-matching control antibody. Numbers indicate the ratio of the geomeans. C: Loading samples were prepared from whole-cell lysates under nonreducing boiling conditions. HA-PLAP were detected with 8B6 anti-PLAP and HA7 anti-HA antibodies in Western blotting. GAPDH, quantitative control. D: ALP activities of HA-PLAP in whole-cell lysates were measured.
Inefficient oligomerization, not altered conformation of unremodeled GPI-APs, was most likely responsible for weak signals in Western blotting
It has been reported that delipidation of GPI from GPI-APs by phospholipases induces conformational changes of proteins linked to GPI and results in the drastically decreased reactivities to antibodies (11, 12). By analogy to these GPI-cleaved proteins, low immunoreactivities of unremodeled GPI-APs might be caused by altered conformations of the protein moieties. In Western blot analyses, GPI-APs were denatured by SDS plus boiling and then renatured to some extent during transfer onto membranes. It could be interpreted that the remodeled and unremodeled GPIs affected renaturation of proteins to different extents by interacting differently with the protein moiety during the denaturation/renaturation cycle.
Therefore, the question of whether unremodeled PLAP was more sensitive to denaturation by SDS and exhibited more inefficient renaturation after removal of SDS than did remodeled PLAP was examined by monitoring PLAP enzymatic activities. Contrary to expectations, PLAP activities from samples treated with various SDS concentrations and denaturation/renaturation did not show any significant differences between remodeled and unremodeled PLAPs (Fig. 5A). These results indicated that differences in conformational changes were small under these conditions, consistent with the fact that both PLAPs were similarly recognized by antibodies in flow cytometry (Figs. 2A and 4B). Similar results were obtained when urea was used instead of SDS (data not shown).
Fig. 5.
Conformational change-independent reduction of immunoreactivities in unremodeled GPI-APs. A: Comparison of ALP activities in PGAP3−/− MEF cells restored with PGAP3 or mock (gray and dark gray bars, respectively) after denaturation or renaturation (left and right panels, respectively). Whole-cell lysates were prepared using Triton lysis buffer (100 mM Tris-Cl, pH 9.5, 100 mM NaCl, 5 mM MgCl2, and 1% Triton X-100). For denaturation, SDS was added to lysate at indicated SDS percentage; for renaturation, whole-cell lysates were prepared with Triton lysis buffer containing 2.5% of SDS, then diluted to indicated SDS percentage with Triton lysis buffer without SDS. Activities were measured with 0.25 mM of CSPD, an ALP substrate. B–D: Loading samples prepared from whole-cell lysates under nonboiling (on ice, 15 min) or boiling (95°C, 3 min) nonreducing conditions; immunoblotting with anti-HA (B) and anti-PLAP (C) antibodies. C: Short exposure (bottom panel) and long exposure (top panel). D: Blotted PVDF membrane was incubated with CDP-star to measure ALP activities. Single asterisk represents partially denatured monomeric HA-PLAP due to the lack of boiling. This incomplete denaturation is most likely responsible for the broad bands. Double asterisks represent completely denatured monomeric HA-PLAP (also shown in Figs. 2–4). Triple asterisks represent enzymatically active dimeric HA-PLAP. The absolute amount of dimeric HA-PLAP was much less than monomer; therefore, HA7 anti-HA antibody barely detected the dimeric form (B), whereas anti-PLAP antibody detected the dimeric form much more strongly than monomer, most likely due to high avidity (not affinity) by divalent conjugation (C). E: Surface expression of HA-PLAP detected by anti-HA antibody in PGAP3−/− MEF cells restored with PGAP3 or mock. Gray shadows, staining with HA7 antibody; dotted lines, staining with isotype-matching control antibody. Numbers indicate the ratio of the geomeans. F: Loading samples were prepared from whole-cell lysates of MEFs under nonreducing, nonboiling conditions, and then fluorescence intensities in SDS-PAGE were measured using fluorescence laser scanner (top panel); EGFP-Flag-CD59 was immunoblotted by anti-EGFP, anti-Flag, and anti-CD59 antibodies, and then cell lysates in (B–E) were prepared using OβG buffer.
Next, the effect of boiling was examined using samples prepared from whole-cell lysates, under boiling or nonboiling conditions in the presence of SDS and absence of a reducing reagent, and subjected to Western blotting. It was confirmed by detection with anti-HA antibody that similar amounts of remodeled and unremodeled HA-PLAPs were loaded to PVDF membrane, with boiled samples reproducing the bands of mass shown in Figs. 2A and 4C (Fig. 5C, double asterisks), although nonboiling treatments produced proteins that moved more slowly and yielded broader bands than boiling, which were thought to be partially denatured (Fig. 5B, single and double asterisks, respectively). Antibody against PLAP was then applied to a membrane prepared under the same condition as in Fig. 5B. Surprisingly, nonboiled samples exhibited much stronger intensities than did boiled samples (Fig. 5C, triple and double asterisks, respectively), and the apparent molecular masses of the intense bands did not coincide with those detected by HA7 antibody (Fig. 5B, C, single asterisk). Judging from these sizes, the intense bands corresponded to dimeric PLAP. As PLAP is known to be functional only as a homodimer (17), the enzymatic activities of HA-PLAP blotted on membranes were measured using CDP-star, a luminous substrate for PLAP. As predicted, the intense bands observed completely coincided with bands revealed by CDP-star (Fig. 5C, D, triple asterisks). This functional dimeric PLAP was barely detectable with HA7 antibody (Fig. 5B), although the same antibody normally recognized HA-PLAP in flow cytometry (Fig. 5E). This indicates that SDS was strong enough to convert the majority of the dimer to monomer even under nonboiling conditions. Furthermore, this indicates that the very minor population of dimeric HA-PLAP (Fig. 5C, triple asterisks) was very efficiently detected with anti-PLAP antibody compared with major monomeric HA-PLAP (single asterisk) in the same lanes. The intensities of monomeric HA-PLAP bands produced under nonboiling conditions were weaker and almost invisible compared with those produced under boiling (Fig. 5C, double asterisks), most likely due to the broadness revealed by anti-HA antibody (Fig. 5B). Importantly, no differences in dimeric HA-PLAP band intensities were observed between the remodeled and unremodeled HA-PLAPs (Fig. 5C, triple asterisks, lower panel), indicating no differences in sensitivity to SDS and consistent with results in Fig. 5A. Thus, remodeled and unremodeled HA-PLAPs showed different signal intensities only after denaturation/renaturation cycles in Western blotting. These results suggested the hypothesis that antibody affinities against GPI-APs were not very high and, therefore, that dimerization or oligomerization of GPI-APs might be critical for efficient detection by allowing multivalent binding with antibodies, increasing these avidities. This also suggested that remodeled GPI could have induced oligomerization more efficiently than unremodeled GPI during renaturation that occurred when SDS was removed from monomeric GPI-APs. Boiling in the presence of SDS might have caused complete monomerization, but during transfer from electrophoresis gels to the blotting membranes, some monomers might have oligomerized due to GPI hydrophobic interactions. In addition, the efficiency of oligomerization might have been higher in remodeled GPI than in unremodeled GPI, by analogy with the fact that saturated long acyl chains of remodeled GPI associate with each other more densely and tightly than the bending unremodeled GPI. This effect would have led to GPI-AP enrichment in lipid rafts in the cell membrane. This hypothesis was supported by detection of EGFP-Flag-CD59, a chimeric protein in which EGFP, Flag-tag, and CD59 were tandemly fused (Fig. 5F). Similar amounts of remodeled and unremodeled EGFP-Flag-CD59, confirmed by fluoroscanning of loaded gels, were detected with three different antibodies against EGFP, Flag-tag, and CD59 in Western blot analyses. Antibodies against EGFP and Flag also showed large differences in signal intensities, as did an antibody against CD59, clearly indicating that these differences did not arise from the specific protein-GPI interactions, such as between CD59 and GPI, and that the present hypothesis was reasonable. This hypothesis was proven more directly using premixed primary/secondary antibody complexes to increase their avidities, overcoming the supposed low affinity of the primary antibody by increasing the chances for multivalent binding. In fact, premixed primary/secondary antibody complexes strongly increased the faint intensity of unremodeled HA-PLAP, moderately increased that of remodeled HA-PLAP (Fig. 6A), and eventually equalized the intensities of remodeled and unremodeled GPI-APs, such as HA-PLAP, Thy-1, and EGFP-Flag-CD59 (Fig. 6A–D). Moreover, monovalent Fab fragments produced weak but similar intensities in remodeled and unremodeled Thy-1 (Fig. 6C). Thus, equalization of band intensities by premixed primary/secondary antibody complexes and Fab fragments clearly indicates that inefficient oligomerization of unremodeled GPI, with relatively low antibody affinities but not altered GPI-AP conformations, was responsible for the remarkably weak signals observed in Western blot analyses.
Fig. 6.
Restoration of immunoreactivities of unremodeled GPI-APs by premixed antibody complexes. A: Immunoblotting against HA-PLAP by sequential incubations with primary and secondary antibodies (left) or by single-step incubation with premixed primary/secondary antibody complexes (right). Signals from two membranes were measured under same conditions. B: Samples from three independent experiments analyzed by sequential and single-step incubation methods (top and middle, respectively) as described in (A) and Syntaxin6, quantitative control (bottom). C: Thy-1 was immunoblotted by two methods as described in (A) (first and second panels from top) and by peroxidase-conjugated Fab fragments of anti-Thy-1 antibody (third panel from top); and Ribophorin1, quantitative control (bottom panel). D: EGFP-Flag-CD59 immunoblotted by two methods as described in (A) (top and middle panels) using anti-CD59 antibody. Ribophorin1, quantitative control (bottom panel). For single-step incubation, the same amounts of 1:1000 diluted primary and secondary antibodies were premixed at room temperature for 2 h; loading samples were prepared from whole-cell lysates under boiling (A and B) and nonboiling (C and D), nonreducing conditions.
Strongly reduced immunoreactivity on blotted membranes of PI-PLC-treated GPI-APs was most likely due to inability to oligomerize and not due to the altered conformation of delipidated proteins
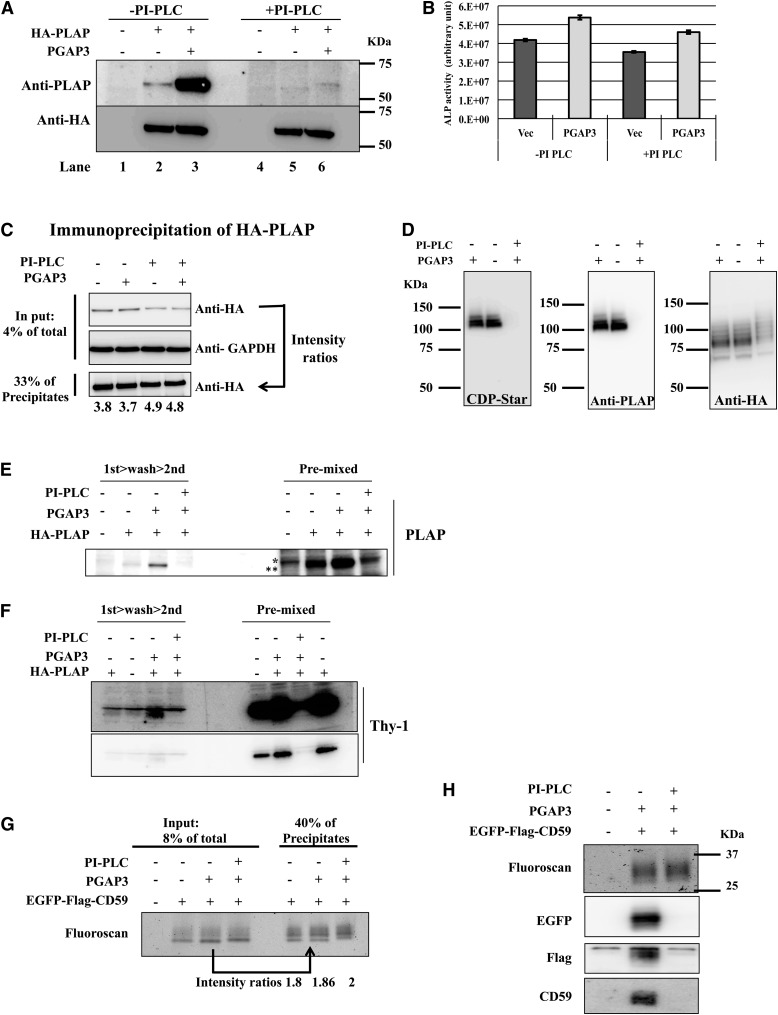
Finally, the possible causes of severely reduced immunoreactivities of delipidated GPI-AP produced by PI-PLC were examined in terms of protein conformational changes caused by interaction with delipidated GPI, as reported previously (11), or decreased oligomerization similar to unremodeled GPI-APs, as proposed here. Western blot analyses of HA-PLAP after PI-PLC treatment showed drastically reduced immunoreactivities, even more markedly than unremodeled HA-PLAP (Fig. 7A). The molecular weight of delipidated HA-PLAP appeared to be similar to nontreated HA-PLAP, because the molecular mass of the lipid portion eliminated by PI-PLC is less than 1 kDa and much smaller than that of HA-PLAP. Next, the enzymatic activity of HA-PLAP and the immunoprecipitation efficiencies were examined to determine whether GPI cleavage affected protein conformations. PI-PLC treatment did not significantly affect enzymatic activity (Fig. 7B) or the efficiency of immunoprecipitation by anti-PLAP antibody, among remodeled, unremodeled, and PI-PLC-treated HA-PLAPs, evaluated using anti-HA antibody (Fig. 7C). EGFP-Flag-CD59 also did not show significant differences in immunoprecipitation efficiency using anti-CD59 antibody among remodeled, unremodeled, and PI-PLC-treated ones, which was also evaluated in antibody-irrelevant fluoroscanning images of EGFP-Flag-CD59 within the polyacrylamide gel (Fig. 7G, upper panel). These results clearly indicate that immunoreactivities of delipidated and unremodeled GPI-APs were not reduced if they were not denatured. On the other hand, the sensitivity of delipidated HA-PLAP to SDS was greatly increased under nonboiling conditions, based on clear decreases in dimeric HA-PLAP that was detected by both enzymatic activities of PLAP on the blotting membrane (Fig. 7D, left) and in its immunoreactivities to anti-PLAP antibody (Fig. 7D, middle), compared with remodeled and unremodeled HA-PLAPs as explained in Fig. 5. This was most likely due to complete monomerization that was accelerated by the lack of hydrophobic interactions among delipidated GPIs. Nor could premixed primary/secondary antibody complexes restore immunoreactivity in either HA-PLAP or Thy-1, being different from unremodeled forms that were shown in Fig. 6 (Fig. 7E, F). This inability of premixed antibody complexes to restore the immunoreactivities of delipidated GPI-APs was due to inability to form oligomers and not due to altered conformations of GPI-cleaved proteins during a denaturation/renaturation cycle. The immunoreactivities of not only anti-CD59 but also anti-EGFP and anti-Flag antibodies were also strongly reduced in delipidated EGFP-Flag-CD59, an effect that could not be explained by conformational changes caused by specific interactions of CD59 with delipidated GPI (Fig. 7H).
Fig. 7.
Immunoblotting-specific reduction of immunoreactivities in delipidated GPI-APs. Whole-cell lysates prepared from MEF cells (see Fig. 2) were incubated with or without 1 unit/ml of PI-PLC from B. cereus prior to following experiments. A: Loading samples were prepared from whole-cell lysates under nonreducing boiling conditions. HA-PLAP was detected by anti-PLAP and anti-HA antibodies (top and bottom panels, respectively). B: ALP activities were measured in whole-cell lysates. C: Immunoprecipitation of HA-PLAP. Input (top and middle panels, 4% of total) and output (bottom panel, 33% of precipitates) obtained after immunoprecipitation. Proteins were immunoprecipitated from whole-cell lysates with 8B6 anti-PLAP antibody followed by protein G beads, then probed with HA7 and anti-GAPDH antibody as a control. Loading samples were prepared under nonreducing boiling conditions. The ratios of band intensities in bottom panel (output) to those in top panel (input) are shown at the bottom. D: ALP activity and immunoblotting of HA-PLAP. Loading samples were prepared from whole-cell lysates under nonreducing, nonboiling conditions. ALP activity (left), immunoblotting with anti-PLAP antibody (middle), and immunoblotting of anti-HA antibody (right). Note that the intense bands in the left and middle panels represent dimeric functional HA-PLAPs, whereas the bands in the right panel represent partially denatured monomeric HA-PLAPs, as explained in the text and Fig. 5 legend. E and F: Immunoblotting of whole-cell lysates by sequential and single-step incubations as described in Fig. 6A (left and right, respectively) with anti-PLAP (E) and anti-Thy-1 (F) antibodies. E: Single asterisk, nonspecific bands (upper); double asterisks, specific bands (lower). F: Long exposure (top) and short exposure (bottom). Note that Thy-1 is an endogenous protein. G: EGFP-Flag-CD59 was immunoprecipitated with 5H8 anti-CD59 antibody plus protein G beads. Loading samples (8% of total lysates for input and 40% of the precipitates for output) were prepared under nonreducing, nonboiling conditions and applied to SDS-PAGE. Fluorescence was measured by fluorescence laser scanner, and the ratios of band intensities in output to those in input were calculated and are shown at the bottom. H: Immunoreactivity of EGFP-Flag-CD59 after incubation with or without PI-PLC was analyzed as described in Fig. 5F.
Fatty acid remodeling affects cellular dynamics of GPI-APs through the oligomerization
We previously reported that fatty acid remodeling in CHO cells is critical for raft association of GPI-APs (6), based on the enrichment in a detergent-resistant membrane (DRM) fraction (cold 1% TritonX-100) representing lipid rafts. Because we used a normal sequential probing procedure in Western blotting, which resulted in the decreased intensities of GPI-AP bands selectively in DRM of PGAP3-deficient cells, we reevaluated it using anti-HA antibody or premixed primary/secondary antibody complexes. HA-PLAP was fractionated by low-speed centrifugation following solubilization with a cold buffer containing 1% TritonX-100. Western blotting with anti-PLAP antibody showed very weak signal of unremodeled HA-PLAP in DRM fraction (6). In contrast, blotting with anti-HA drastically strengthened the signal, resulting in intensities comparable with remodeled HA-PLAP (Fig. 8A). Not much but a significant amount of unremodeled HA-PLAP was separated into a detergent-soluble fraction, which was confirmed by fractionation with density-gradient ultracentrifugation (Fig. 8B). Thy-1 showed more prominent change in distribution between remodeled and unremodeled Thy-1 (Fig. 8C). More than half of unremodeled Thy-1 was solubilized, whereas majority of remodeled Thy-1 was in DRM, which clearly indicated that fatty acid remodeling is critical for enrichment of GPI-APs in rafts. The different ratios of DRM to the detergent-soluble fraction between HA-PLAP and Thy-1 were most likely due to the tendency of the protein moiety to form dimer/oligomer, as PLAP is known to form very stable dimer but Thy-1 is not (17).
Fig. 8.
Fatty acid remodeling accelerates oligomerization of GPI-APs under physiological membrane environments. PGAP3−/− MEF cells restored with PGAP3 or mock that expressed HA-PLAP (A and B) and HA-Thy1 (C) were suspended in lysis buffer containing 1% TritonX-100, and then separated into detergent-resistant membrane (DRM) fraction representing lipid rafts and detergent-soluble (Soluble) fraction representing nonrafts by low-speed centrifugation (A and C) or by sucrose density-gradient ultracentrifugation (B). A: HA-PLAP expressed was detected by anti-PLAP antibody (third panel from top) and anti-HA antibody (bottom panel). B: HA-PLAP in each fraction after ultracentrifugation was detected by HA7 anti-HA antibody. C: Thy1 was detected using premixed G7 anti-Thy-1 and HRP-conjugated anti-Rat IgG antibodies complex (bottom panel). Loading samples prepared under nonreducing boiling conditions. Cav1, caveolin1; TfR, transferrin receptor; Flotillin and caveolin1 were used as detergent-resistant membrane (DRM) markers, and transferrin receptor was used as detergent-soluble fraction marker. D: Cells expressing HA-Thy1 were treated with 1 mM of cross-linking reagent DTSSP for 30 min at room temperature. Loading samples were prepared from whole-cell lysate under nonreducing, nonboiling conditions. Three independent experiments are shown. Single and double asterisks indicate monomeric and dimeric HA-Thy1 or endogenous Thy1; long exposure (top); short exposure (bottom). E: The ratios of dimers to monomers were quantitatively calculated. Statistical analysis was done with Student-t-test (P = 0.014). F: Cells were stained with 10−8 M or 10−9 M of nontoxic FITC-conjugated proaerolysin (FLAER). Bold line, PGAP3-restored PGAP3−/− MEF cells; continuous line, empty vector integrated PGAP3−/− MEF cells; dotted line, no staining cells as a control. G: Viabilities of MEFs after 24 h cultivation with fresh medium following treatment with indicated concentrations of aerolysin (nM) for 3 h. The cell number at starting point was regarded as 100%. PGAP3, PGAP3-restored PGAP3−/− MEF cells; Vec, empty vector integrated PGAP3−/− MEF cells.
DRM assay is thought to be somewhat artificial, although many researchers use this assay to analyze the affinity of proteins to lipid rafts. To study the significance of GPI-anchor in terms of oligomerization of GPI-APs under physiological conditions, we used a cross-linker reagent that directly proves physical proximity. Cross-linking of surface proteins in living cells by membrane-impermeable cross-linker 3,3′-Dithiobis[sulfosuccinimidyl propionate] (DTSSP), whose spacer length is 12 Å, revealed that significantly more amount of dimeric (and trimeric) Thy-1 is present in PGAP3-restored PGAP3−/− MEF than in PGAP3−/− MEF (Fig. 8D, E), indicating the nature of remodeled GPI to oligomerize is seen under physiological membrane environment.
To further ensure the biological significance of oligomerization through the remodeling of GPI, we performed a cell-killing assay using aerolysin, a pore-forming bacterial toxin. It is known that monomeric proaerolysins bind to single GPI-APs and heptamerize to make lethal pores after the activation by protease-mediated cleavage (18). The binding efficiency of the toxin to surface GPI-APs was similar between PGAP3-deficient and PGAP3-sufficient cells (Fig. 8F), whereas cell-killing ability was significantly weaker in PGAP3-deficient MEF (Fig. 8G), most likely due to the slower oligomerization. These results further support our interpretation that discrepancy between different immunoreactivities in Western blotting and flow cytometry reflects different degrees of oligomerization of GPI-APs in the plasma membrane due to fatty acid compositions.
DISCUSSION
What are the merits of employing GPI for anchoring proteins to the cell membrane? Cell surfaces of protozoa are densely covered with GPI-APs, but cell surfaces of mammals are not so densely covered. GPI biosynthesis is essential for the survival of yeast and protozoa but not essential for mammalian cells, albeit it is essential for mammalian embryo development. Thus, GPI might have different significance in different organisms, but as GPI has been conserved through eukaryotic evolution, some important aspects should be retained. One such conserved fundamental GPI property is based upon the structural characteristic that the GPI moieties of almost all surface GPI-APs contain two saturated long acyl chains, such as ceramide, or two saturated fatty acid chains, which endow GPIs with high affinity for the liquid-ordered phase. In most organisms, the majority of PI, a source of GPI, has an sn-2 unsaturated fatty acid; therefore, it appears reasonable that lipid remodeling is evolutionarily conserved, although the mechanisms might differ among organisms. Fatty acid remodeling by the PGAP proteins in mammalian cells guarantees this property by replacement of unsaturated chains with saturated ones. Thus, defective PGAP3 activity leads to impaired enrichment of GPI-APs in the detergent-insoluble membrane fraction corresponding to lipid rafts (6) as well as to altered immune responses in PGAP3-knockout mice (14). Therefore, study of differences in behaviors of remodeled and unremodeled GPI-APs is very important for understanding the underlying basis of GPI.
Kukulansky et al. employed antibodies that recognize various epitopes of Thy-1 to evaluate the reactivities against delipidated Thy-1. In their report, all monoclonal antibodies, 30-H12 and HO-13-4 recognizing the Thy-1.2 allelic determinant and 31-11, 42-21, and G7 recognizing nonpolymorphic determinant of Thy-1, showed reduced immunoreactivities on Western blotting, as well as did polyclonal antibodies against Thy1 (13). Therefore, they considered that the phenomenon could be explained by conformational change of delipidated Thy-1. Because the delipidation by PI-PLC released GPI-APs from cell surface, they could not investigate whether delipidated GPI-APs on the plasma membrane could be recognized normally by antibodies. Although we have not used various antibodies that recognize different epitopes of one GPI-AP, all antibodies against GPI-APs used here showed similar phenomenon on Western blotting when unremodeled GPI-APs were used as antigens. Nevertheless, these antibodies normally recognized unremodeled GPI-APs in flow cytometry, which eventually led to a hypothesis different from theirs, i.e., that reduced oligomerization could be responsible for the reduced immunoreactivities.
The major findings in the present study are: i) GPI-APs in PGAP3-deficient cells, which have not undergone fatty acid remodeling but still carry two lipid chains, were found to demonstrate considerable reductions in immunoreactivities in Western blotting; ii) reduced immunoreactivities of unremodeled GPI-APs and delipidated GPI-APs in Western blotting were most likely not due to altered protein conformations that have been proposed as a mechanism for the effects of delipidated GPI-APs; iii) remarkable losses in oligomerization during denaturation/renaturation cycles were responsible for immunoreactivity reductions in unremodeled GPI-APs, implying that spontaneous oligomerization is a fundamental property of remodeled GPI; and most importantly, iv) the nature of spontaneous oligomerization through the remodeled GPI, which was revealed by in vitro experiments, is conserved under physiological membrane environment. In addition to these finding, we reconfirm that fatty acid remodeling is critical for the enrichment of GPI-APs in lipid rafts. Cross-linking of surface proteins gives more solid evidence to prove remodeled GPI-dependent oligomerization of GPI-APs under physiological membrane environment, because of the very short spacer length (DTSSP has a 12 Å spacer) than in the DRM assay, which reflects accumulation of GPI-APs in liquid-ordered phase by the interaction with other specific lipids but not direct protein-protein binding. The cell-killing assay using aerolysin also indicated that remodeled GPI accelerates heptamerization of GPI-bound aerolysin, although we cannot eliminate the possibility that remodeled GPI accelerates activation of aerolysin by increasing the chance to meet with the protease. Thus, these results support our interpretation that the discrepancy between different immunoreactivities in Western blotting and flow cytometry reflects different degrees of oligomerization of GPI-APs in the plasma membrane due to fatty acid compositions.
These findings revealed certain significant aspects of GPI, in that remodeled GPI intrinsically accelerated GPI-AP oligomerization, which is considered critical for stabilizing and expanding lipid rafts, activation of signaling pathways, and trafficking of GPI-APs to the apical side of polarized cells (8–10, 19). Suzuki et al. used advanced single-molecule fluorescent imaging to show that, in resting cells, virtually all GPI-APs are mobile and continually forming transient homodimers through both GPI-GPI and protein-protein interactions of GPI-APs and that the lack of interaction through GPI-GPI shortened the lifetime of homodimers by 2.5 times (20). These transient rafts containing homodimeric GPI-APs are most likely one of the basic units that form larger and more stable rafts capable of signaling (20). Sengupta et al. have also reported using a combination of pair-correlation and photoactivated localization microscopy to show that, in the steady-state, GPI-APs are organized into clusters with radii less than 60 nm and containing about two GPI-APs with relatively high local density, indicating that they may form transient dimers (21). These reports are consistent with the present findings that remodeled GPI efficiently formed homodimer/oligomers by GPI-GPI interactions, and these reports have clarified this important nature of remodeled GPI. This is in addition to GPI's ability to form liquid-ordered phases by interactions with sphingolipids and cholesterol, as reported previously (6).
Most GPI-APs are considered to exist as monomers outside of lipid rafts and to move freely on the plasma membrane during staining with antibodies in flow cytometry, which allows the divalent conjugation of one antibody with two GPI-APs, even if GPI-APs exist as monomers at the beginning. This results in high avidity binding of antibody with GPI-APs, which is the reason why antibodies against GPI-APs in flow cytometry did not show significant differences in immunoreactivities between remodeled and unremodeled GPI. The situation with Western blotting is different, because GPI-APs are immobilized on PVDF membrane. Unless GPI-APs are dimerized before immobilization on the membrane, a single antibody would not be able to make divalent binding to GPI-APs. The majority of unremodeled GPI-APs were bound with antibodies by monovalent interactions because of low efficiency to form oligomers, whereas remodeled GPI-APs efficiently oligomerized, most likely during the transfer to PVDF membrane. Premix of first/second antibodies allows formation of big complex/aggregation of antibodies, because second antibodies are polyclonal. The big antibody complex can make multivalent binding to proteins immobilized separately. A single first antibody cannot make divalent binding to single monomeric protein. Thus, using a premix of first/second antibodies overcomes decreased oligomerization of unremodeled GPI-APs in PGAP3−/− MEF. Unremodeled and delipidated GPI-APs also produced different results in terms of enhancement of band signals by premixed first/second antibody complex in Western blotting. The reason remains unclear, but our speculation is that unremodeled GPI-APs do not efficiently oligomerize, yet some of them formed oligomers through hydrophobic interactions with GPI during renaturation, since unremodeled GPI-APs still carried two fatty acid chains. In contrast, delipidated GPI-APs completely lost lipid moiety, and thus, oligomerization may not occur during renaturation. Alternatively, conformational change particular to delipidated GPI-APs as previously reported (13) might be responsible for complete loss of restoration by premixed first/second antibody complex, although our results (Fig. 8C, G, H) seem inconsistent with this explanation.
This study also points out the need for caution in comparing the amounts of proteins harboring different types of GPI, such as in remodeled, unremodeled, or delipidated forms, or even harboring transmembrane domains instead of GPI. Proteins with remodeled GPI might have produced stronger intensities than others in Western blot analyses and, therefore, might have been overestimated. This technical problem was overcome by employing preformed primary/secondary antibody complexes. G7 anti-Thy-1, 5H8 anti-CD59, and 8B6 anti-PLAP exhibited remarkably enhanced sensitivities with preformed primary/secondary antibody complexes. It was reported that 8B6 binds to hPLAP with an affinity constant of 5 × 109 LM−1 when used in RIA (22). Because PLAP forms stable dimers, this affinity constant seems to represent avidity, indicating that the real affinity is relatively low. On the other hand, all antibodies except for HA7 showed decreased immunoreactivities when unremodeled GPI-APs were used as antigens. Therefore, it was not certain that the affinities of antibodies against GPI-APs were generally lower than the average of other antibodies against non-GPI-anchored proteins. Possibly, it might be more valid to state that GPI-APs possess high avidities for antibodies through oligomerization.
In summary, the present findings advance the comprehension of the significance of GPI as well as techniques for their detection. Besides its required presence for enrichment of GPI-APs in lipid rafts, GPI was shown here to be implicated in the dimerization/oligomerization of GPI-APs, thus enhancing their immunoreactivities.
Acknowledgments
The authors thank Drs. Yoshiko Murakami and Morihisa Fujita for useful advice and discussions; Yoshiki Yamaguchi at RIKEN for passionate discussions; and all members of the Kinoshita laboratory for technical and spiritual support.
Footnotes
Abbreviations:
- ALP
- alkaline phosphatase
- CHO
- Chinese hamster ovary
- DAF
- decay accelerating factor
- DM
- double mutant
- DRM
- detergent-resistant membrane
- EGFP
- enhanced green fluorescent protein
- GPI
- glycosylphosphatidylinositol
- GPI-AP
- GP-anchored protein
- HA-PLAP
- human placental alkaline phosphatase N-terminally tagged with signal sequence derived from CD59 and hemagglutinin peptide
- MEF
- mouse embryonic fibroblast
- OβG
- n-octyl-β-D-glucoside
- PGAP
- post-GPI attachment to protein
- PI
- phosphatidylinositol
- PI-PLC
- phosphatidylinositol-specific phospholipase C
- PLAP
- placental alkaline phosphatase
- uPAR
- urokinase-type plasminogen activator receptor
This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. J.S. was supported by Grants-in-aid for Scientific Research from the Ichiro Kanehara Foundation and from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
REFERENCES
- 1.Orlean P., Menon A. K. 2007. Thematic review series: lipid posttranslational modifications. GPI anchoring of protein in yeast and mammalian cells, or: how we learned to stop worrying and love glycophospholipids. J. Lipid Res. 48: 993–1011. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka S., Maeda Y., Tashima Y., Kinoshita T. 2004. Inositol deacylation of glycosylphosphatidylinositol-anchored proteins is mediated by mammalian PGAP1 and yeast Bst1p. J. Biol. Chem. 279: 14256–14263. [DOI] [PubMed] [Google Scholar]
- 3.Fujita M., Maeda Y., Ra M., Yamaguchi Y., Taguchi R., Kinoshita T. 2009. GPI glycan remodeling by PGAP5 regulates transport of GPI-anchored proteins from the ER to the Golgi. Cell. 139: 352–365. [DOI] [PubMed] [Google Scholar]
- 4.Tashima Y., Taguchi R., Murata C., Ashida H., Kinoshita T., Maeda Y. 2006. PGAP2 is essential for correct processing and stable expression of GPI-anchored proteins. Mol. Biol. Cell. 17: 1410–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ueda Y., Yamaguchi R., Ikawa M., Okabe M., Morii E., Maeda Y., Kinoshita T. 2007. PGAP1 knock-out mice show otocephaly and male infertility. J. Biol. Chem. 282: 30373–30380. [DOI] [PubMed] [Google Scholar]
- 6.Maeda Y., Tashima Y., Houjou T., Fujita M., Yoko-o T., Jigami Y., Taguchi R., Kinoshita T. 2007. Fatty acid remodeling of GPI-anchored proteins is required for their raft association. Mol. Biol. Cell. 18: 1497–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schroeder R., London E., Brown D. 1994. Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc. Natl. Acad. Sci. USA. 91: 12130–12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown D. A., Rose J. K. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 68: 533–544. [DOI] [PubMed] [Google Scholar]
- 9.Paladino S., Sarnataro D., Pillich R., Tivodar S., Nitsch L., Zurzolo C. 2004. Protein oligomerization modulates raft partitioning and apical sorting of GPI-anchored proteins. J. Cell Biol. 167: 699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tansey M. G., Baloh R. H., Milbrandt J., Johnson E. M., Jr 2000. GFRalpha-mediated localization of RET to lipid rafts is required for effective downstream signaling, differentiation, and neuronal survival. Neuron. 25: 611–623. [DOI] [PubMed] [Google Scholar]
- 11.Barboni E., Rivero B. P., George A. J., Martin S. R., Renoup D. V., Hounsell E. F., Barber P. C., Morris R. J. 1995. The glycophosphatidylinositol anchor affects the conformation of Thy-1 protein. J. Cell Sci. 108: 487–497. [DOI] [PubMed] [Google Scholar]
- 12.Bütikofer P., Malherbe T., Boschung M., Roditi I. 2001. GPI-anchored proteins: now you see ‘em, now you don't. FASEB J. 15: 545–548. [DOI] [PubMed] [Google Scholar]
- 13.Kukulansky T., Abramovitch S., Hollander N. 1999. Cleavage of the glycosylphosphatidylinositol anchor affects the reactivity of thy-1 with antibodies. J. Immunol. 162: 5993–5997. [PubMed] [Google Scholar]
- 14.Murakami H., Wang Y., Hasuwa H., Maeda Y., Kinoshita T., Murakami Y. 2012. Enhanced response of T lymphocytes from Pgap3 knockout mouse: insight into roles of fatty acid remodeling of GPI anchored proteins. Biochem. Biophys. Res. Commun. 417: 1235–1241. [DOI] [PubMed] [Google Scholar]
- 15.Fujita M., Watanabe R., Jaensch N., Romanova-Michaelides M., Satoh T., Kato M., Riezman H., Yamaguchi Y., Maeda Y., Kinoshita T. 2011. Sorting of GPI-anchored proteins into ER exit sites by p24 proteins is dependent on remodeled GPI. J. Cell Biol. 194: 61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murakami Y., Kanzawa N., Saito K., Krawitz P. M., Mundlos S., Robinson P. N., Karadimitris A., Maeda Y., Kinoshita T. 2012. Mechanism for release of alkaline phosphatase caused by glycosylphosphatidylinositol deficiency in patients with hyperphosphatasia mental retardation syndrome. J. Biol. Chem. 287: 6318–6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoylaerts M. F., Manes T., Millan J. L. 1997. Mammalian alkaline phosphatases are allosteric enzymes. J. Biol. Chem. 272: 22781–22787. [DOI] [PubMed] [Google Scholar]
- 18.Abrami L., Fivaz M., van der Goot F. G. 2000. Adventures of a pore-forming toxin at the target cell surface. Trends Microbiol. 8: 168–172. [DOI] [PubMed] [Google Scholar]
- 19.Simons K., Toomre D. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1: 31–39. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki K. G., Kasai R. S., Hirosawa K. M., Nemoto Y. L., Ishibashi M., Miwa Y., Fujiwara T. K., Kusumi A. 2012. Transient GPI-anchored protein homodimers are units for raft organization and function. Nat. Chem. Biol. 8: 774–783. [DOI] [PubMed] [Google Scholar]
- 21.Sengupta P., Jovanovic-Talisman T., Skoko D., Renz M., Veatch S. L., Lippincott-Schwartz J. 2011. Probing protein heterogeneity in the plasma membrane using PALM and pair correlation analysis. Nat. Methods. 8: 969–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durbin H., Tucker D. F., Milligan E. M., Bobrow L. G., Warne P. H., Pookim Y. L., Bodmer W. F. 1988. Production of monoclonal antibodies to placental alkaline phosphatase: preliminary characterisation includes identification of one antibody reactive with routinely fixed histological preparations. Int. J. Cancer Suppl. 2: 50–58. [DOI] [PubMed] [Google Scholar]