Abstract
The role of macrophage lipoprotein lipase (LpL) in the development of atherosclerosis and adiposity was examined in macrophage LpL knockout (MLpLKO) mice. MLpLKO mice were generated using cre-loxP gene targeting. Loss of LpL in macrophages did not alter plasma LpL activity or lipoprotein levels. Incubation of apolipoprotein E (ApoE)-deficient β-VLDL with peritoneal macrophages from ApoE knockout mice lacking macrophage LpL (MLpLKO/ApoEKO) led to less cholesteryl ester formation than that found with ApoEKO macrophages. MLpLKO/ApoEKO macrophages had reduced intracellular triglyceride levels, with decreased CD36 and carnitine palmitoyltransferase-1 mRNA levels compared with ApoEKO macrophages, when incubated with VLDL. Although both MLpLKO/ApoEKO and ApoEKO mice developed comparable hypercholesterolemia in response to feeding with a Western-type diet for 12 weeks, atherosclerosis was less in MLpLKO/ApoEKO mice. Epididymal fat mass and gene expression levels associated with inflammation did not differ between the two groups. In conclusion, macrophage LpL plays an important role in the development of atherosclerosis but not adiposity.
Keywords: apolipoprotein E, foam cell, CD36, knockout mice
Atherosclerosis and obesity are associated with chronic inflammation (1, 2). In addition, both processes are associated with and likely driven by macrophage infiltration and activation within tissues. The transformation of recruited monocytes into cholesteryl ester-laden macrophages, or foam cells, is central in the development of atherosclerotic lesions. Uptake of modified lipoproteins via scavenger receptors is one proposed mechanism by which foam cells are formed in vivo. The accumulation of excess free cholesterol in macrophages also contributes to atherosclerosis by stimulating tumor necrosis factor (TNF)-α and interleukin (IL)-6 production, which triggers endoplasmic reticulum (ER) stress and apoptosis (3). Moreover, fatty acids (FA), especially saturated FA, have been implicated in the promotion of atherosclerosis via the stimulation of proinflammatory cytokine production and ER stress through the activation of toll-like receptor (TLR)2, TLR4, and fatty acid-binding protein 4 (FABP4) in macrophages (4, 5).
Obesity in humans and mice leads to macrophage accumulation within adipose (2), a process that has been postulated to lead to inflammation and insulin resistance. There appears to be a paracrine interaction between adipocytes and macrophages that controls inflammation in obese adipose tissue, and TNF-α appears to be a major macrophage-derived paracrine mediator of inflammation in adipose tissue (2, 6, 7). TNF-α produced by macrophages induces lipolysis and releases FA from adipocytes, which in turn augments inflammatory changes within macrophages and leads to a further increase in TNF-α production. Thus, FA and TNF-α derived from adipocytes and macrophages, respectively, control the paracrine loop that aggravates inflammation within obese adipose tissue.
Lipoprotein lipase (LpL) is synthesized by parenchymal cells in muscle and adipose, binds to the capillary endothelium by interacting with heparan sulfate proteoglycans and glycosylphosphatidylinositol-anchored HDL-binding protein 1 (GPIHBP1) (8), and hydrolyzes triglyceride (TG)-rich chylomicrons and VLDL to liberate FA, produce chylomicron remnants and LDL, and create surface lipids that transfer to HDL (9, 10). We have previously demonstrated that mice overexpressing human LpL in a variety of tissues, such as the heart, skeletal muscle, adipose, and aorta, are protected against atherosclerosis by the reduction of atherogenic lipoproteins in the LDL receptor-deficient background (11) or by the increase of HDL in the apolipoprotein E (ApoE)-deficient background (12). Furthermore, pharmaceutical interventions that increase LpL expression reduce atherosclerosis (13, 14). Thus, through its actions on circulating lipoproteins, LpL is generally viewed as an antiatherogenic enzyme. However, none of these actions are thought to involve macrophage LpL.
Macrophage LpL is thought to increase macrophage inflammation capacity and promote atherosclerosis. LpL can act as a molecular bridge between proteoglycans and lipoprotein receptors, such as remnant-like particles and LDL receptors, to induce the retention and/or uptake of atherogenic lipoproteins by cells within the vascular wall (15, 16). Macrophage LpL disruption through the transplantation of bone marrow or fetal liver cells from LpL-deficient mice into C57BL/6 mice decreases atherosclerotic lesion development (17, 18). These techniques are powerful tools for the study of macrophage regulation of plasma lipoprotein metabolism and in vivo atherogenesis process (19). However, graft rejection or irradiation of recipient mice may influence the experimental outcomes. Indeed, the findings regarding plasma lipoproteins in two macrophage LpL-deficient mouse models generated by two different laboratories were dissimilar (17, 18).
The role of LpL in adipose macrophage biology has not been explored. In one model, the LpL knockout mice rescued by a transgenic expression of LpL under the control of a muscle creatine kinase promoter had LpL deficiency in both adipocytes and macrophages. These mice have diminished weight gain and fat mass on an ob/ob background (20). However, the specific role of macrophage LpL, which infiltrates obese adipose tissue, remains unclear. Similarly, the importance of macrophage LpL in adipose inflammation due to a high-fat diet is unknown.
In the present study, we generated macrophage LpL knockout (MLpLKO) mice using cre/loxP gene targeting and determined the role of macrophage LpL in plasma lipoprotein metabolism, atherosclerosis, and adiposity.
METHODS
Mice and diets
Mice heterozygous for the floxed LpL allele (referred to as LpL+/f; f denotes the floxed allele) on a 129/Sv background were generated as described elsewhere (21). LpL+/f mice, which were backcrossed four times into the C57B/6 background, were cross-bred with transgenic mice on a C57B/6 background, in which Cre recombinase was expressed in macrophages under the control of the lysozyme promoter Lys-Cre (Jackson Laboratory) (22). LpL+/f mice carrying one copy of the Lys-Cre transgene were then mated with LpL+/f mice lacking Cre to generate MLpLKO mice and littermate wild-type (WT) controls [LpLf/f (fLpL), Lys-Cre (CRE), and LpL+/+ (WT)]. Thereafter, MLpLKO mice were cross-bred with ApoE knockout mice (ApoEKO) to generate MLpLKO/ApoEKO mice. Littermate ApoEKO mice were used as controls. Genotyping was performed via PCR using genomic DNA isolated from the tail tip (21, 22). Three diets were used: i) a normal chow diet containing 4.8% (w/w) fat and 25.1% (w/w) protein (CE-2, Japan CLEA); ii) a Western-type diet (WTD) containing 21% (w/w) fat and 0.21% (w/w) cholesterol (D12079B, Research Diets Inc., NJ); and iii) a high-fat diet (HFD) containing 23.6% (w/w) fat (D12451, Research Diets). The WTD was used to induce obesity and atherosclerotic lesions in MLpLKO/ApoEKO mice, and the HFD was utilized to induce obesity in MLpLKO mice. Animal care and experimental procedures were performed according to the regulations of Jichi Medical University.
Northern blot analysis
Total RNA was extracted with TRIZOL reagent (Invitrogen). Ten micrograms of RNA were subjected to electrophoresis on a 1% agarose gel containing formamide, and then transferred onto a nylon filter (Hybond N; Amersham Bioscience). A murine LpL cDNA probe was radiolabeled with [α-32P]deoxy-CTP. Following a 2 h prehybridization period, Northern blots were hybridized with the probe in a Rapid-hyb buffer (Amersham Biosciences) for 1 h at 65°C (23).
Real-time PCR
One microgram of total RNA was reverse-transcribed with a high-capacity cDNA reverse transcriptase kit (Applied Biosystems). All reactions were done in triplicate, and relative amounts of mRNA were calculated using a standard curve or comparative CT method on a 7300 Real-Time PCR system (Applied Biosystems), according to the manufacturer's protocol. Mouse β-actin mRNA was used as the invariant control. The primer-probe sets for real-time PCR are listed in Table 1.
TABLE 1.
Primers for real-time PCR
| Primer | Forward | Reverse | Probe |
| β-actin | CGATGCCCTGAGGCTCTTT | TGGATGCCACAGGATTCCA | CCAGCCTTCCTTCTT |
| CPT-1 | GAACCCCAACATCCCCAAAC | TCCTGGCATTCTCCTGGAAT | CACCAGGCTACAGTGG |
| TLR4 | AGCTTCAATGGTGCCATCATT | CCAGGTGCTGCAGCTCTTCT | TGAGTGCCAATTTCATGG |
| FABP4 | CCGCAGACGACAGGAAGG | AGGGCCCCGCCATCT | AAGAGCATCATAACCC |
| TNF-α | AGGGATGAGAAGTTCCCAAATG | TGTGAGGGTCTGGGCCATA | CCTCCCTCTCATCAGTT |
| CHOP | CATCCCCAGGAAACGAAGAG | GCTAGGGACGCAGGGTCAA | AAGAATCAAAAACCTTCACTACT |
| Arginase1 | CATGGGCAACCTGTGTCCTT | TCCTGGTACATCTGGGAACTTTC | CTCCTGAAGGAACTGAAA |
| iNOS | ATCCTGCAAAAGCAG | GCAAACCCAAGGTCTACGTTCA | GAGCACGCTGAGTACCTCATTG |
| LpLa | Mm00434764_m1 | ||
| CD36a | Mm01135198_ml | ||
| ACOX1a | Mm00443579_m1 | ||
| MCADa | Mm01323360_g1 | ||
| F4/80a | Mm00802529_m1 | ||
| CD11ca | Mm00498698_m1 | ||
| CD206a | Mm00485148_m1 | ||
| Fizz1a | Mm00445109_m1 | ||
| MCP-1a | Mm00441242_m1 | ||
| IL6a | Mm00446190_m1 | ||
| CD68a | Mm00839636_g1 |
Supplied by Applied Biosystems.
Plasma lipids, lipoproteins, and glucose
Blood was collected from mice that were fasted for 16 h. Total cholesterol (TC) and TG levels in plasma were determined enzymatically via kits (Determiner TC 555 and Determiner TG 555, Kyowa Medex, Tokyo). HPLC analyses of plasma were performed as previously described (24). Blood glucose was determined via a FreeStyle blood glucose monitoring system (NIPRO).
Peritoneal macrophages
Peritoneal macrophages were obtained three days after a 1 ml intraperitoneal injection of 3% thioglycollate broth. Macrophages were plated on 12-well plates, and cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS) and antibiotics for 3 h. Thereafter, cells were washed with PBS, and if not stated otherwise, the adherent macrophages were maintained in DMEM supplemented with 10% (v/v) FBS and antibiotics.
LpL activity
Postheparin plasma was analyzed for LpL activity as previously described (25). Fasted mice were injected with 300 U/kg of heparin intravenously, and plasma was obtained 10 min later and assayed in triplicate for LpL activity. LpL activity in tissue homogenates and in media of cultured macrophages treated with heparin (10 U/ml) was measured, as previously described by Hocquette et al. (26).
Cholesteryl ester formation assay
Blood was collected from ApoEKO mice that were fasted for 16 h after being fed a WTD for over two weeks. β-VLDL was isolated by density gradient ultracentrifugation. Following a 24 h incubation period in medium containing 5 mg/ml of lipoprotein-deficient serum (LPDS), macrophages from both ApoEKO and MLpLKO/ApoEKO mice were incubated with either 5 or 10 μg of ApoE-deficient β-VLDL, 5 mg/ml of LPDS, and [1-14C] oleate-albumin complex at 37°C for 24 h. Cholesteryl ester formation was determined as previously described (12).
VLDL uptake
VLDL was isolated from plasma of normolipidemic volunteers fasted overnight. Macrophages were incubated with 100 μg of VLDL in DMEM containing 5% FBS for 6 h in a Lab-Tek II Chamber slide system (Thermo Scientific Inc.). After washing with 4% paraformaldehyde, macrophages were stained with Oil red O. Macrophages were also incubated with 100 μg of VLDL in 12 well plates for 6 h. Thereafter, cells were washed twice with PBS, and lipids were extracted with hexane/isopropanol (3:2, v/v). The organic phase was evaporated to dryness under flowing nitrogen, redissolved in isopropanol, and then cholesterol and TG levels were enzymatically determined. Cellular proteins were dissolved in 0.1N NaOH and assessed with a BCA kit. Total RNA was also extracted, and the genes associated with intracellular FA metabolism, proinflammatory cytokines, and ER stress were quantified by real-time PCR.
Diet-induced atherosclerosis
At 14 weeks of age, ApoEKO and MLpLKO/ApoEKO male mice were fed a WTD for 12 weeks. Blood was collected, and plasma lipids and lipoproteins were determined. Mice were euthanized after 12 weeks, and atherosclerosis was determined. Atheromatous plaques in the aorta were visualized by staining with Sudan IV, and the luminal side of the stained aorta was photographed (12). Image capture and analysis were performed with Adobe Photoshop 6 image analysis software. The extent of atherosclerosis was expressed as the percentage of surface area of the entire aorta covered by lesions and designated as the en face surface lesion area (27). The cross-sectional lesion area was evaluated according to the modified method of Paigen et al. (28). In brief, the heart was perfused with saline containing 4% (w/v) formalin and fixed for more than 48 h in the same solution. The basal half of the heart was embedded in Tissue-Tek OCT compound (Sakura Finetek Co., Tokyo), and serial sections were embedded in Cryostat (6 μm thick), as previously described (12). Four sections, each separated by 60 μm, were used to evaluate the lesions; two at the end of the aortic sinus and two at the junctional site of sinus and ascending. Sections were stained with Oil red O and counterstained with hematoxylin. Cross-sections of aortic roots were incubated with primary antibodies for mouse MOMA-2 (1:600; Accurate Chemical and Scientific) overnight at 4°C to investigate macrophage infiltration in the atherosclerotic lesion. After washing, sections were incubated with biotinylated anti-rat antibody for 1 h at room temperature, and then with avidin-biotin peroxidase complex (Vector Labs) for 30 min. Lastly, sections were developed with 3,3′-diaminobenzidine tetrahydrochloride (DAB) (Sigma), and counterstained with hematoxylin.
Histology
The adipose tissue of mice fed a WTD for 12 weeks was fixed with 10% neutral-buffered formalin, embedded in paraffin, and sections were stained with hematoxylin and eosin. Sections were photographed and the average size of lipid droplet from each mouse was determined by analyzing 200 cells using ImageJ software (National Institutes of Health).
Statistics
Data were presented as means ± S.E. Student t- test was used to compare the mean values between two groups, and the one way ANOVA (ANOVA) was used for multiple comparisons. When ANOVA results were statistically significant (i.e., P < 0.05), then individual comparisons were made with the Tukey posthoc test.
RESULTS
Characterization of MLpLKO mice
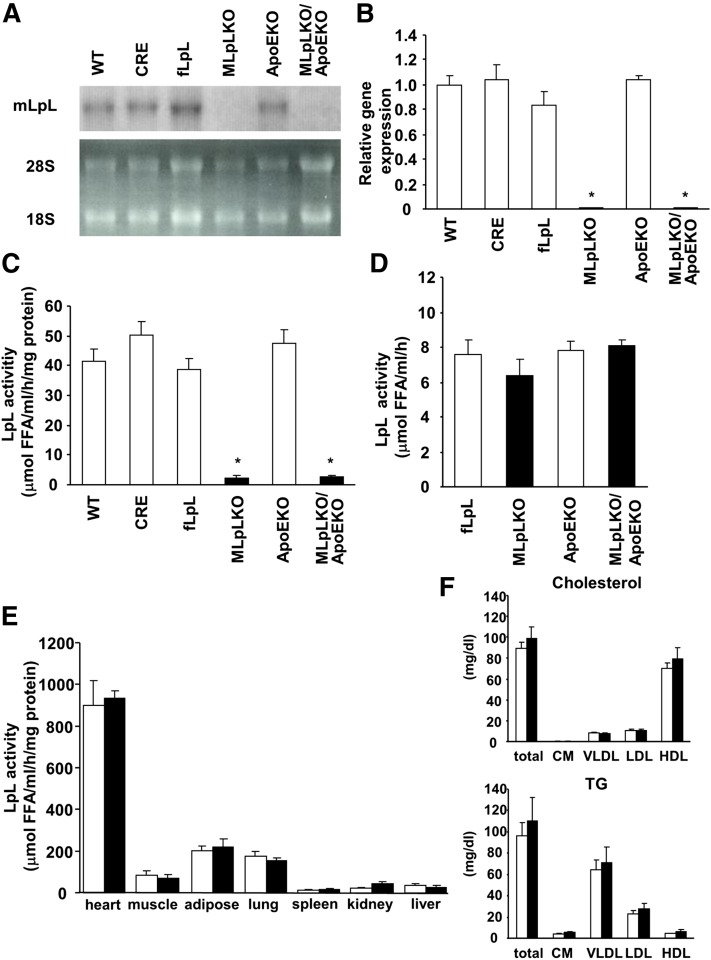
Peritoneal macrophages from MLpLKO mice had significantly less LpL mRNA than WT, CRE, and fLpL mice (Fig. 1A, B). LpL activity in macrophages was also barely detectable in MLpLKO mice (Fig. 1C). In contrast, postheparin plasma LpL activity was not reduced in MLpLKO mice compared with control fLpL mice (Fig. 1D). The ApoEKO background did not influence the macrophage and plasma LpL. Like MLpLKO mice, MLpLKO/ApoEKO mice had much reduced macrophage LpL, but not plasma LpL. Furthermore, macrophage LpL deficiency did not influence LpL activity in heart, muscle, adipose tissue, or lung in the ApoEKO mice (Fig. 1E).
Fig. 1.
Tissue LpL expression in MLpLKO mice. (A) Ten micrograms of total macrophage RNA was subjected to Northern blot analysis. Murine LpL cDNA was used as the probe. (B) LpL mRNA in macrophage was determined by real-time PCR (n = 3–4 in each group). (C) LpL activity in media of cultured macrophages treated with heparin (10 U/ml) was determined in triplicate. WT, CRE, fLpL, and MLpLKO, n = 6–7; ApoEKO and MLpLKO/ApoEKO, n = 3. (D) Postheparin plasma LpL activity (n = 7–8 in each group). (E) Tissues from ApoEKO (open bars, n = 4) and MLpLKO/ApoEKO (filled bars, n = 6) were homogenized and assayed for LpL activity in triplicate. (F) HPLC lipoprotein profiles of MLpLKO mice show distribution of cholesterol and TG in plasma lipoproteins. fLpL (open bars, n = 8) and MLpLKO (filled bars, n = 8) mice were fed a regular chow diet. Values are expressed as means ± SE. *P < 0.001, MLpLKO versus WT, CRE, fLpL, and ApoEKO, or MLpLKO/ApoEKO versus WT, CRE, fLpL, and ApoEKO.
Plasma lipids and lipoproteins were determined in MLpLKO mice fed a regular chow diet. Plasma TC, TG, lipoprotein, and glucose levels were not significantly different between MLpLKO and fLpL mice (Table 2 and Fig. 1F). MLpLKO/ApoEKO mice had significantly higher plasma TC levels than MLpLKO mice. There were no significant differences in plasma TC and TG levels between MLpLKO/ApoEKO and ApoEKO mice (Table 3). We also assessed plasma lipids in MLpLKO/ApoEKO and ApoEKO mice fed a WTD diet for 12 weeks. The WTD induced a 2.2- and 2.3-fold increase in plasma TC in ApoEKO and MLpLKO/ApoEKO mice, respectively, with no significant differences between the two groups. Therefore, macrophage LpL did not influence plasma lipoprotein metabolism in WT and ApoEKO mice, which have defective lipoprotein metabolism.
TABLE 2.
Plasma lipids, blood glucose, and body weights of fLPL and MLpLKO mice
| N | TC (mg/dl) | TG (mg/dl) | Glucose (mg/dl) | Body Weight (g) | |
| fLpL | 11 | 96 ± 3 | 132 ± 13 | 49 ± 2 | 24.6 ± 0.6 |
| MLpLKO | 11 | 95 ± 4 | 144 ± 22 | 51 ± 2 | 23.9 ± 0.6 |
Values are expressed as means ± SE.
TABLE 3.
Plasma lipid levels of ApoEKO and MLpLKO/ApoEKO mice fed a WTD
| Mice | N | Prior to a WTD | 12 weeks on a WTD | |
| ApoEKO | 15 | TC (mg/dl) | 632 ± 68 | 1384 ± 113 |
| TG (mg/dl) | 137 ± 18 | 146 ± 16 | ||
| MLpLKO/ApoEKO | 17 | TC (mg/dl) | 638 ± 48 | 1497 ± 97 |
| TG (mg/dl) | 145 ± 15 | 177 ± 19 |
Values are expressed as means ± SE.
Cholesteryl ester formation in mouse peritoneal macrophages
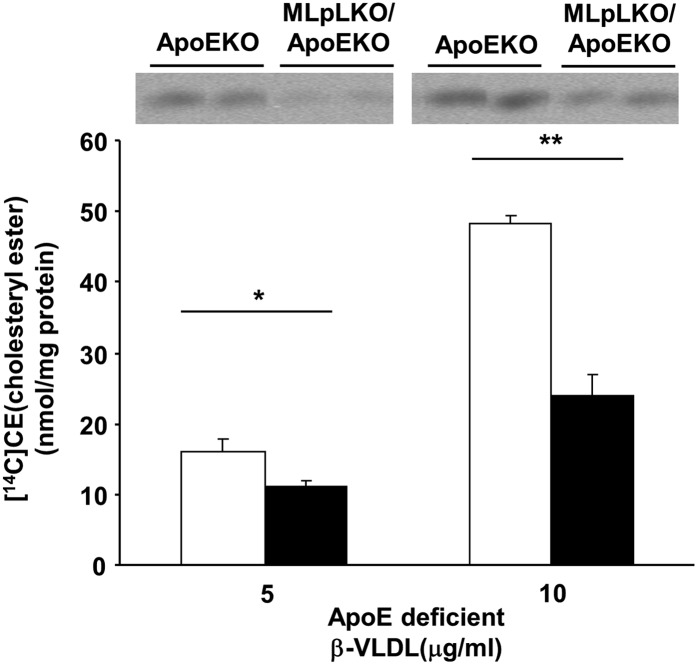
β-VLDL was isolated from ApoEKO mice fed a WTD for over two weeks, and it was used to stimulate cholesteryl ester formation in peritoneal macrophages. Cholesteryl ester foam cell formation by MLpLKO/ApoEKO macrophages incubated with 5 μg of ApoE-deficient β-VLDL was 31% less than that by ApoEKO macrophages, and the reduction with 10 μg of β-VLDL was 51% less (Fig. 2).
Fig. 2.
Cholesteryl ester formation stimulated via ApoE-deficient β-VLDL. β−VLDL was obtained from ApoEKO mice that had been fed a WTD for over two weeks. Thioglycollate-elicited peritoneal macrophages were prepared from ApoEKO (open bars, n = 10) and MLpLKO/ApoEKO mice (filled bars, n = 10). Following LPDS treatment for 24 h, cells were incubated with either 5 or 10 μg of ApoE-deficient β-VLDL for 24 h, and then cholesteryl ester formation was determined. Values are expressed as means ± SE. *P < 0.05, **P < 0.01.
Metabolic effects of macrophage LpL deficiency
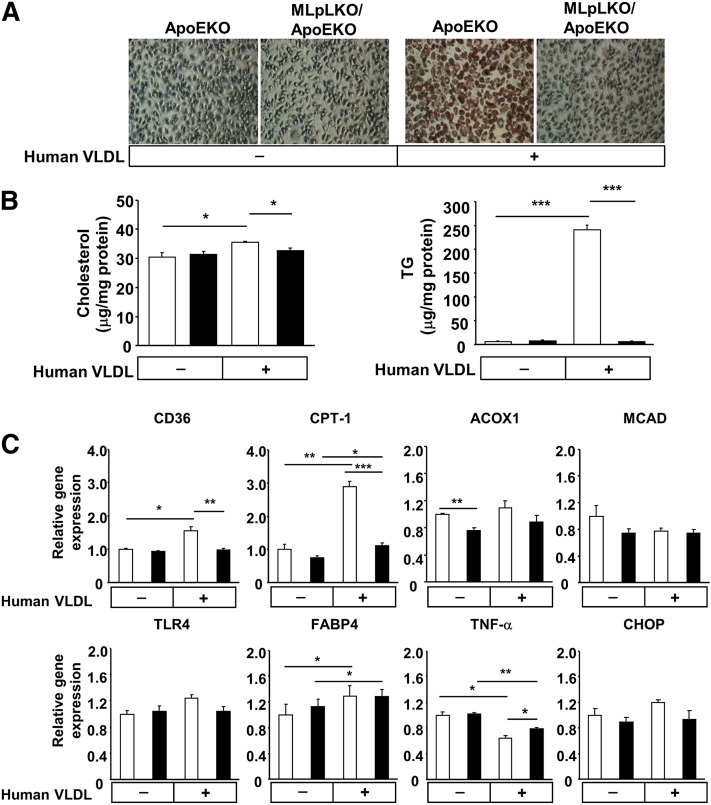
To investigate the consequences of macrophage LpL deficiency on intracellular lipid metabolism, MLpLKO/ApoEKO macrophages were incubated with VLDL isolated from normolipidemic human volunteers. Oil red O staining demonstrated that VLDL loading resulted in substantial amounts of intracellular lipid droplets in ApoEKO macrophages but not in MLpLKO/ApoEKO macrophages (Fig. 3A). At baseline, cholesterol and TG concentrations were comparable in macrophages from ApoEKO and MLpLKO/ApoEKO mice (Fig. 3B). VLDL loading resulted in a greater increase of TG in ApoEKO than in MLpLKO/ApoEKO macrophages (P < 0.001). Cholesterol levels were marginally but significantly decreased in MLpLKO/ApoEKO compared with ApoEKO macrophages (P < 0.05). These observations indicate that the hydrolysis of VLDL via macrophage LpL can control intracellular TG accumulation even in the absence of ApoE.
Fig. 3.
VLDL-induced lipid accumulation in macrophages. VLDL was obtained from normolipidemic volunteers. Macrophages from ApoEKO and MLpLKO/ApoEKO mice were cultivated in DMEM and 5% FBS in the absence (control) or presence of VLDL (100 μg/ml) for 6 h. (A) Intracellular neutral lipids were stained with Oil red O. (B) Intracellular cholesterol and TG concentrations were measured enzymatically (ApoEKO, n = 5; MLpLKO/ApoEKO, n = 5). (C) Gene expression levels involved in FA metabolism. Total RNA was extracted from ApoEKO (open bars, n = 4) and MLpLKO/ApoEKO (closed bars, n = 4) macrophages, which were cultivated in DMEM and 5% FBS in the absence (control) or presence of VLDL (100 μg/ml) for 6 h. Gene expression levels were determined by real-time PCR. Values are expressed as means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001.
We then assessed whether changes in lipid uptake altered the expressions of genes involved in intracellular FA metabolism, inflammation, and ER stress. In the absence of exogenous lipid loading, gene expression of CD36, TLR4, FABP4, and carnitine palmitoyltransferase-1 (CPT-1) did not differ between ApoEKO and MLpLKO/ApoEKO macrophages. VLDL loading approximately doubled CD36 mRNA in ApoEKO macrophages, but it led to no changes in MLpLKO/ApoEKO macrophages (Fig. 3C), which resulted in a 45% reduction of CD36 mRNA in MLpLKO/ApoEKO macrophages compared with ApoEKO macrophages (P < 0.01) Although both macrophages had increases in CPT-1 in response to VLDL loading, ApoEKO macrophages had a 2.9-fold increase in CPT-1, whereas MLpLKO/ApoEKO macrophages had only a 1.5-fold increase (P < 0.05). Unlike CD36 and CPT-1, VLDL loading did not lead to a significant difference in TLR4, ACOX1, MCAD, or FABP4 between the two macrophages.
Inflammation is well documented to alter atherosclerosis development. Surprisingly, VLDL loading significantly reduced TNF-α expression in both LpL-expressing and LpL-knockout macrophages; however, ApoEKO macrophages had lower TNF-α expression than MLpLKO/ApoEKO macrophages. Lipid loading did not lead to ER stress; CHOP did not differ between the two types of macrophages irrespective of the presence of VLDL. These data complement recent studies by Spann et al. showing reduced inflammation in foam cells from mice fed a high-cholesterol diet (29).
Diet-induced atherosclerosis
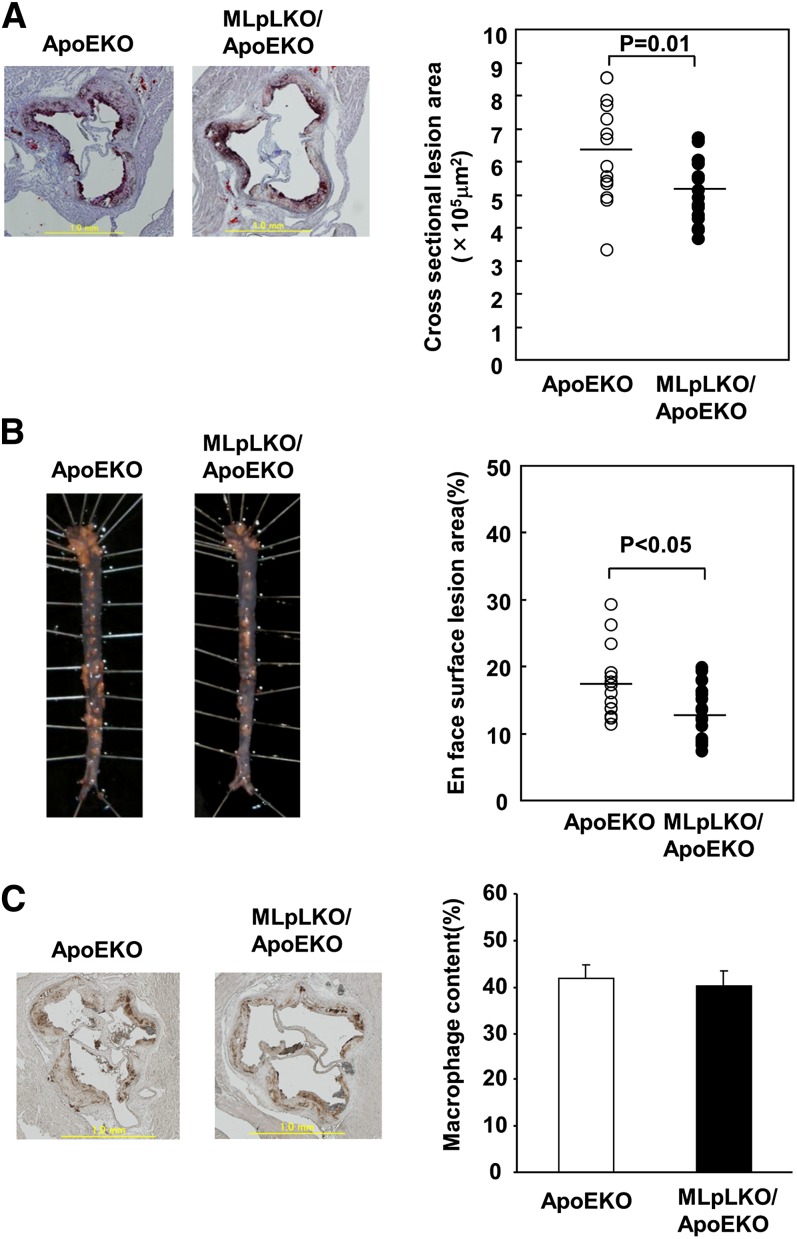
Following 12 weeks on a WTD, atherosclerosis was evaluated by two methods: cross-sectional analysis of aortic roots and the en face surface lesion area of the aorta. Cross-sectional lesion areas of MLpLKO/ApoEKO mice were significantly smaller than those of ApoEKO mice by 19.3% (505 ± 24 × 103 μm2 versus 626 ± 30 × 103 μm2, P = 0.01) (Fig. 4A). The en face surface lesion areas of MLpLKO/ApoEKO mice were also smaller than those of ApoEKO mice by 20.6% (13.5 ± 1.0% versus 17.0 ± 1.4%, P < 0.05) (Fig. 4B). Macrophage content in cross-sectional lesion areas stained with MOMA-2 was also decreased in MLpLKO/ApoEKO mice in proportion to the reduction of atherosclerotic lesion area. The ratio of macrophage-infiltrating area to atherosclerotic lesion area was not significantly different between the two genotypes (Fig. 4C). Thus, LpL-deficient macrophages prevent lesion development without altering macrophage content in ApoEKO mice.
Fig. 4.
Effects of a WTD on atherosclerosis in MLpLKO/ApoEKO mice. Cross-sectional lesion area in the aortic sinus (A) and en face surface lesion area of the aorta (B) from ApoEKO (n = 15) and MLpLKO/ApoEKO (n = 17) mice fed a WTD for 12 weeks. Fatty streak lesion areas were determined, as described in Methods. Mean values are indicated by horizontal lines. (C) Macrophages in atherosclerotic lesion areas of ApoEKO (n = 6) and MLpLKO/ApoEKO (n = 6) mice stained with rat monoclonal antibody MOMA-2, followed by biotinylated goat anti-rat IgG. Values are expressed as means ± SE.
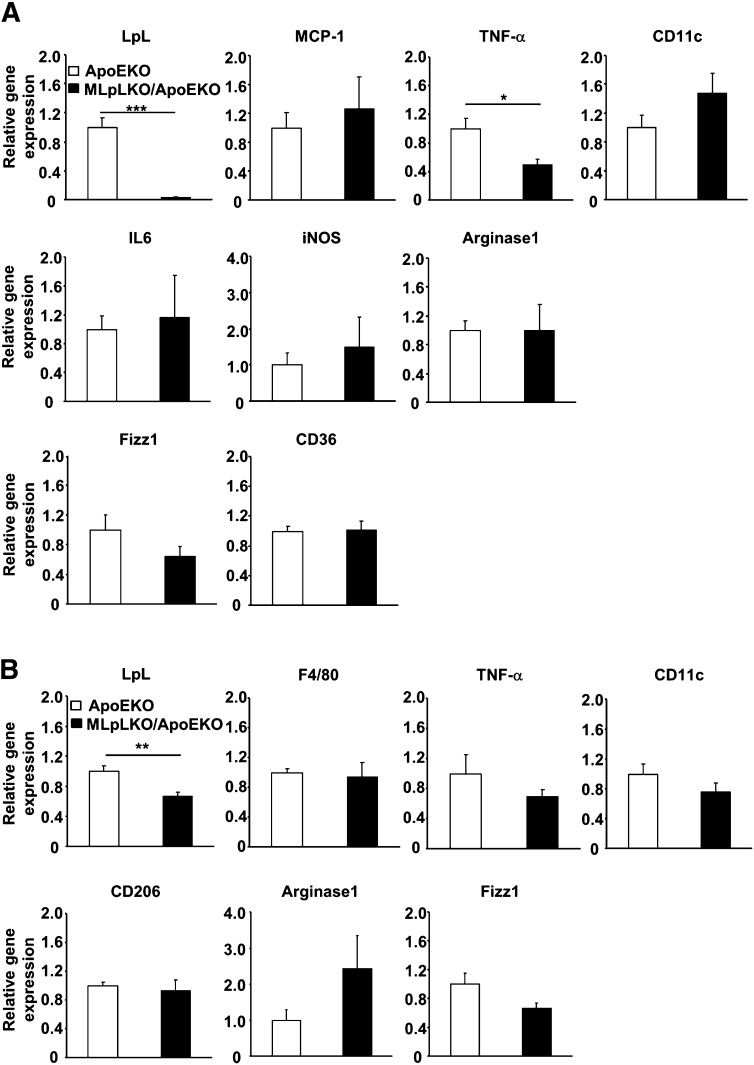
Peritoneal macrophages were isolated from MLpLKO/ApoEKO and ApoEKO mice fed a WTD for 12 weeks, and gene expression levels of inflammation markers were assessed. Although there were no significant differences in MCP-1, IL6, iNOS, or CD36, TNFα was decreased by 50% (P < 0.01) in MLpLKO/ApoEKO macrophages compared with ApoEKO macrophages (Fig. 5A). Moreover, mRNA was extracted from whole aorta in mice fed a WTD for 12 weeks, and gene expression levels were assessed. Although LpL was significantly decreased in the aortas from MLpLKO/ApoEKO mice, the expression levels of inflammation markers did not differ between ApoEKO and MLpLKO/ApoEKO mice (Fig. 5B). So the effects of diet and/or its secondary actions in vivo affect macrophages differently than was found with in vitro VLDL treatment.
Fig. 5.
Gene expression levels in peritoneal macrophage and aorta from mice fed a WTD. Total RNA was extracted from thioglycollate-elicited peritoneal macrophage (A) and aorta (B) from ApoEKO (open bars, n = 5) and MLpLKO/ApoEKO (filled bars, n = 5) mice fed a WTD for 12 weeks. Gene expression levels were determined by real-time PCR. Values are expressed as means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001.
Diet-induced obesity
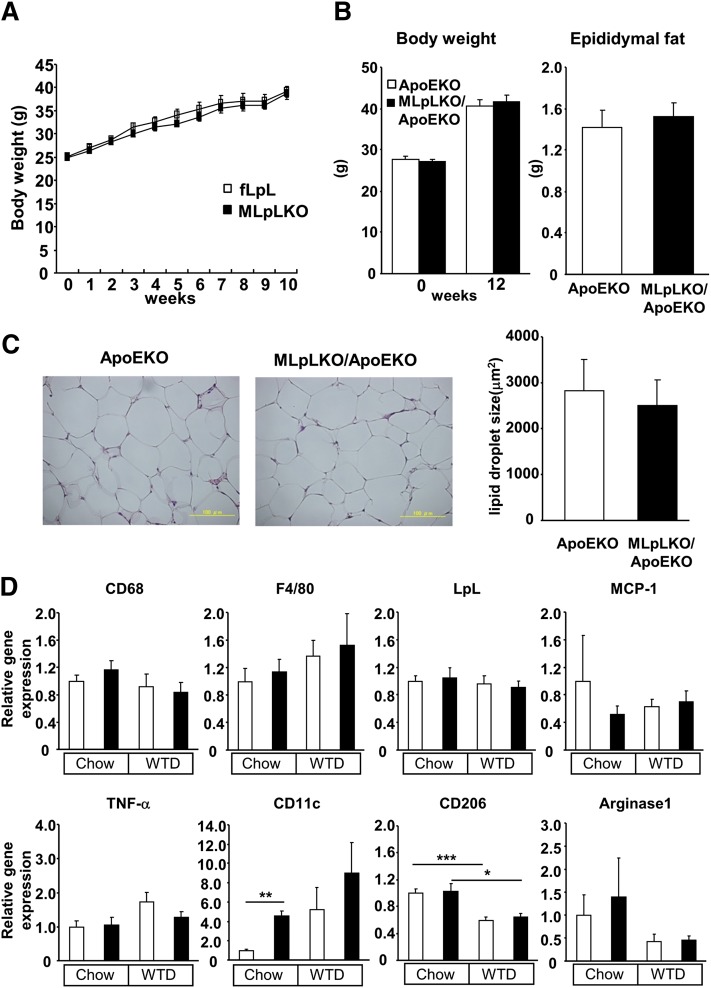
As there are substantial amounts of LpL in adipose tissue where macrophages infiltrate during the development of obesity, we assessed the consequence of macrophage LpL deficiency on adiposity. At 9 weeks of age, male MLpLKO mice were fed a HFD for 10 weeks and weighed weekly. Both MLpLKO and control fLpL mice gained weight at similar rate over the entire observation period (Fig. 6A). Moreover, neither body weight nor epididymal fat mass was significantly different between ApoEKO and MLpLKO/ApoEKO mice fed a WTD for 12 weeks (Fig. 6B). The size of lipid droplet in adipocytes did not differ between MLpLKO/ApoEKO and ApoEKO mice (Fig. 6C).
Fig. 6.
Body weights and epididymal fat mass. (A) Nine-week-old male MLpLKO (filled squares, n = 11) and control fLpL (open squares, n = 8) mice were fed a HFD for 10 weeks and weighed weekly. (B) At 14 weeks of age, ApoEKO (n = 15) and MLpLKO/ApoEKO (n = 17) mice maintained on a chow diet were switched to a WTD for 12 weeks. Body weights and epididymal fat mass were determined at the end of the WTD feeding. (C) Histology of epididymal adipose tissue. The average size of lipid droplet was determined in ApoEKO (n = 5) and MLpLKO/ApoEKO (n = 5) mice fed a WTD for 12 weeks. (D) Gene expression levels in adipose tissue of mice fed a WTD for 12 weeks beginning at age 14 weeks. Identically aged mice maintained on chow were used as controls. Total RNA was extracted from epididymal fat in ApoEKO (open bars, chow; n = 5, WTD; n = 5–8) and MLpLKO/ApoEKO (filled bars, chow; n = 5, WTD; n = 5–8) mice, and gene expression levels were determined by real-time PCR. *P < 0.05, **P < 0.01, ***P < 0.001.
On regular chow diet, LpL mRNA levels in adipose tissue did not differ between the ApoEKO and MLpLKO/ApoEKO mice, despite the similar expression levels of macrophage markers, specifically CD68 and F4/80, in adipose tissue (Fig. 6D). CD11c, a marker of M1 macrophage, was increased by 4.6-fold in adipose tissue from MLpLKO/ApoEKO mice compared with ApoEKO adipose tissue. Significant differences in TNF-α or MCP-1 expression levels were not observed between the two types of mice. When mice were challenged with a WTD for 12 weeks, F4/80 and CD11c mRNA levels were markedly increased in both adipose tissues, but no significant differences were found between the lines. In contrast, the WTD significantly reduced CD206 in both types of adipose tissue, with no significant difference between two groups.
DISCUSSION
In the present study, we generated a macrophage LpL knockout mouse using cre-loxP gene targeting. The following important physiological roles of macrophage LpL were observed in this mouse model: i) macrophage LpL did not influence postheparin plasma LpL activity or lipoprotein metabolism; ii) macrophage LpL deficiency led to a small decrease in cholesterol ester foam cell formation and diet-induced atherosclerosis; iii) intracellular TG accumulation was reduced in association with decreased CD36 expression; and iv) a deletion of macrophage LpL did not influence LpL activity or inflammation in adipose tissue.
Cre-loxP gene targeting resulted in a complete deletion of LpL in macrophages. MLpLKO mice demonstrated normal plasma LpL activity and lipoprotein profiles. Furthermore, MLpLKO/ApoEKO mice had a similar level of plasma lipids as ApoEKO mice, a model of defective lipoprotein metabolism, when fed a regular chow diet or WTD. The effects of macrophage LpL on plasma lipoproteins are controversial (17, 18, 30). Eck et al. reported that macrophage LpL deletion via bone marrow transplantation (BMT) in WT mice reduced plasma cholesterol, whereas TG levels were increased eight weeks after BMT (18). Conversely, consistent with our findings, Babaev et al. reported that macrophage LpL did not affect plasma LpL activity or lipoproteins in mice with a WT or LDL receptor knockout background (17, 30). Furthermore, despite the previous studies demonstrating that macrophage LpL knockout mice generated with bone marrow or fetal liver cell transplantation have a reduced LpL activity in the heart, spleen, or lung, where macrophages were present, MLpLKO mice in the present study had normal LpL activity in these tissues. The reason for this discrepancy is unclear, but it might be related to differences in technologies, specifically the use of transplanted LpL-knockout fetal liver cells and bone marrow versus cre-loxP to generate macrophage LpL deficiency.
We did not find differences in LpL in the lung. Some lung LpL is thought to be synthesized in other tissues and then trapped within the lung due to the high level expression of GPIHBP1. In fact, Young and his colleagues showed that mice with a total body deletion of LpL rescued with the expression only in the skeletal muscle had readily detectable LpL protein in the lung despite low LpL transcription levels (31).
ApoE is the most important ligand for receptor-mediated lipoprotein uptake. LpL also functions as a ligand to promote lipoprotein binding to the LDL receptor, LDL receptor-associated protein, and extracellular proteoglycans (32–34). In the present study, MLpLKO mice on an ApoEKO background were fed a WTD for 12 weeks to elucidate the in vivo consequences of macrophage LpL deficiency on atherosclerosis. Foam cell formation stimulated via ApoE-deficient β-VLDL was significantly reduced in MLpLKO/ApoEKO macrophages compared with ApoEKO macrophages. This suggested that macrophage LpL plays an important role in the development of atherosclerosis. We found that the cross-sectional lesion areas of the aortic root were significantly reduced by 19.3% in MLpL/ApoEKO versus ApoEKO mice, when challenged with a WTD for 12 weeks. The en face aortic lesion areas were also significantly decreased by 20.6% in MLpLKO/ApoEKO compared with ApoEKO mice. These observations are in agreement with the findings of Babaev et al. and Eck et al., who demonstrated that macrophage LpLKO mice generated via bone marrow or fetal liver cell transplantation had less diet-induced atherosclerosis on a WT or LDL receptor knockout background (17, 18, 30). It should be noted that the consequences of a macrophage LpL deficiency on attenuating atherosclerosis were more moderate in an ApoE or LDLR knockout background compared with WT.
VLDL loading resulted in a marked TG accumulation in ApoEKO macrophages, whereas this effect was completely prevented in LpL-deficient macrophages. The accumulation of cholesterol from VLDL was very small in ApoEKO macrophages. VLDL-induced lipid accumulation occurs via three different mechanisms: i) the uptake of whole VLDL particles; ii) the uptake of VLDL remnants resulting from VLDL-TG lipolysis; and iii) the uptake of FA produced by VLDL-TG lipolysis. Since cholesterol accumulation through VLDL loading was much smaller than that of TG in ApoEKO macrophages, the uptake of FA generated from VLDL lipolysis via LpL enzymatic activity could be the most important of the three mechanisms in the setting of ApoE deficiency.
Surprisingly, VLDL loading reduced TNF-α expression in both ApoEKO and MLpLKO/ApoEKO macrophages. The effect of VLDL lipolysis on proinflammatory cytokine production is controversial (35, 36). Saraswathi et al. reported that VLDL induces proinflammatory genes, including TNF-α and MCP-1, in macrophages (36), whereas Li et al. revealed no such effects of VLDL on TNF-α expression (35). The reason for this discrepancy is unclear; however, the FA composition of TG from VLDL, unsaturated versus saturated FA (36), might influence proinflammatory cytokine production in macrophages. In addition, both the amount of lipoproteins and LpL and the location of the lipolysis (in medium or on the cell surface) might alter the relative exposure of the cells to LpL-produced lipids.
Gene expression analysis provided some insight into how LpL deficiency alters macrophage function and in turn atherosclerosis. Exposure of ApoEKO macrophages to VLDL increased CD36 mRNA levels, but MLpLKO/ApoEKO macrophages did not increase CD36 mRNA after VLDL treatment. CD36 has many functions, including as a scavenger receptor for oxidatively modified LDL in macrophages, and some (37, 38), but not all (39) studies suggest that loss of CD36 reduces atherosclerosis. CD36 is also a receptor/transporter for long-chain FA in adipose tissue, heart, and skeletal muscle (40). CD36 is downstream of peroxisome proliferator-activated receptors (PPAR), especially the anti-inflammatory PPARγ (41), and its increased expression in LpL-expressing macrophages is likely due to PPAR activation. Thus, LpL production of FA and greater expression of CD36 in macrophages appeared to facilitate FA uptake from VLDL. This would be expected to supply the cells with a lipid source of substrate for energy generation. Macrophage phagocytosis demands a large amount of ATP and plays an essential role in host defense. Chandak et al. have recently reported that a deficiency in adipose triglyceride lipase, a rate limiting enzyme involved in the hydrolysis of lipid droplet-associated TG, in macrophages resulted in a decrease in CPT-1 expression and impaired phagocytosis (42). Others have also previously reported that macrophage LpL acts on TG-rich lipoproteins to produce FA, which is an important source of energy for phagocytosis (43). Thus, it is possible that reduced availability of FA leads to decreased macrophage phagocytosis in MLpLKO mice. Macrophage phagocytosis has been shown to enhance or suppress the development of atherosclerosis, depending on the context (44, 45).
Obesity is associated with chronic inflammation of adipose tissue, where macrophages are a major source of proinflammatory mediators (2, 7). However, the role of macrophage LpL on the development of obesity is still unclear. We found that body weight increased at a similar rate in fLpL and MLpLKO mice on a HFD for 10 weeks. Moreover, body and gonadal fat pad weights did not differ between ApoEKO and MLpLKO/ApoEKO mice after 12 weeks on a WTD. Despite the comparable macrophage infiltration into adipose tissue, as determined by CD68 or F4/80 expression levels, LpL activity and its mRNA expression in adipose tissue did not differ between ApoEKO and MLpLKO/ApoEKO mice. MCP-1 and TNF-α expression levels were not lower in the adipose tissue of MLpLKO/ApoEKO mice. These data suggest that macrophage LpL in adipose tissue is not a major modulator of adiposity.
In conclusion, we generated mice with a macrophage deletion of LpL and demonstrated a role of macrophage LpL in the development of atherosclerosis. Because the area occupied by macrophages in lesions from mice with or without macrophage LpL expression was identical, LpL effects on lesion size and macrophage content are commensurate. Studies in vitro suggest that the primary role of LpL is to affect macrophage lipid uptake, perhaps by creating remnant lipoproteins that can interact with cell surface lipoprotein receptors (46). Furthermore, our data demonstrate that macrophage LpL is not involved in the inflammatory response of adipose to a high-fat diet. Our studies further illustrate the physiological functions of LpL in various tissues and add to the perception that reduced LpL in macrophages, but not muscle, may be desirable.
Footnotes
Abbreviations:
- ApoEKO
- ApoE knockout
- ATGL
- adipose triglyceride lipase
- BMT
- bone marrow transplantation
- CHOP
- C/EBP homologous protein
- CPT-1
- carnitine palmitoyltransferase-1
- ER
- endoplasmic reticulum
- FABP4
- fatty acid binding protein 4
- FIZZ1
- found in inflammatory zone 1
- fLpL
- floxed LpL
- HFD
- high-fat diet
- IL6
- interleukin 6
- iNOS
- inducible nitric oxide synthase
- LPDS
- lipoprotein-deficient serum
- MCAD
- medium-chain acyl-CoA dehydrogenase
- MLpLKO
- macrophage LpL knockout
- TC
- total cholesterol
- TG
- triglyceride
- TLR4
- toll-like receptor 4
- TNF-α
- tumor necrosis factor-α
- WTD
- Western-type diet
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education and Takeda Science Foundation (H.Y.) and by National Institutes of Health Grant HL-45095 (I.J.G.).
REFERENCES
- 1.Libby P., Okamoto Y., Rocha V. Z., Folco E. 2010. Inflammation in atherosclerosis: transition from theory to practice. Circ. J. 74: 213–220. [DOI] [PubMed] [Google Scholar]
- 2.Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., Ferrante A. W., Jr 2003. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112: 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y., Schwabe R. F., DeVries-Seimon T., Yao P. M., Gerbod-Giannone M. C., Tall A. R., Davis R. J., Flavell R., Brenner D. A., Tabas I. 2005. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-alpha and interleukin-6: model of NF-kappaB- and map kinase-dependent inflammation in advanced atherosclerosis. J. Biol. Chem. 280: 21763–21772. [DOI] [PubMed] [Google Scholar]
- 4.Erbay E., Babaev V. R., Mayers J. R., Makowski L., Charles K. N., Snitow M. E., Fazio S., Wiest M. M., Watkins S. M., Linton M. F., et al. 2009. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 15: 1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fessler M. B., Rudel L. L., Brown J. M. 2009. Toll-like receptor signaling links dietary fatty acids to the metabolic syndrome. Curr. Opin. Lipidol. 20: 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suganami T., Nishida J., Ogawa Y. 2005. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler. Thromb. Vasc. Biol. 25: 2062–2068. [DOI] [PubMed] [Google Scholar]
- 7.Xu H., Barnes G. T., Yang Q., Tan G., Yang D., Chou C. J., Sole J., Nichols A., Ross J. S., Tartaglia L. A., et al. 2003. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112: 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voss C. V., Davies B. S., Tat S., Gin P., Fong L. G., Pelletier C., Mottler C. D., Bensadoun A., Beigneux A. P., Young S. G. 2011. Mutations in lipoprotein lipase that block binding to the endothelial cell transporter GPIHBP1. Proc. Natl. Acad. Sci. USA. 108: 7980–7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberg I. J. 1996. Lipoprotein lipase and lipolysis: central roles in lipoprotein metabolism and atherogenesis. J. Lipid Res. 37: 693–707. [PubMed] [Google Scholar]
- 10.Santamarina-Fojo S., Dugi K. A. 1994. Structure, function and role of lipoprotein lipase in lipoprotein metabolism. Curr. Opin. Lipidol. 5: 117–125. [DOI] [PubMed] [Google Scholar]
- 11.Shimada M., Ishibashi S., Inaba T., Yagyu H., Harada K., Osuga J. I., Ohashi K., Yazaki Y., Yamada N. 1996. Suppression of diet-induced atherosclerosis in low density lipoprotein receptor knockout mice overexpressing lipoprotein lipase. Proc. Natl. Acad. Sci. USA. 93: 7242–7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yagyu H., Ishibashi S., Chen Z., Osuga J., Okazaki M., Perrey S., Kitamine T., Shimada M., Ohashi K., Harada K., et al. 1999. Overexpressed lipoprotein lipase protects against atherosclerosis in apolipoprotein E knockout mice. J. Lipid Res. 40: 1677–1685. [PubMed] [Google Scholar]
- 13.Tsutsumi K., Inoue Y., Shima A., Iwasaki K., Kawamura M., Murase T. 1993. The novel compound NO-1886 increases lipoprotein lipase activity with resulting elevation of high density lipoprotein cholesterol, and long-term administration inhibits atherogenesis in the coronary arteries of rats with experimental atherosclerosis. J. Clin. Invest. 92: 411–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsutsumi K., Inoue Y., Shima A., Murase T. 1995. Correction of hypertriglyceridemia with low high-density lipoprotein cholesterol by the novel compound NO-1886, a lipoprotein lipase-promoting agent, in STZ-induced diabetic rats. Diabetes. 44: 414–417. [DOI] [PubMed] [Google Scholar]
- 15.Beisiegel U. 1996. New aspects on the role of plasma lipases in lipoprotein catabolism and atherosclerosis. Atherosclerosis. 124: 1–8. [DOI] [PubMed] [Google Scholar]
- 16.Ishibashi S., Yamada N., Shimano H., Mori N., Mokuno H., Gotohda T., Kawakami M., Murase T., Takaku F. 1990. Apolipoprotein E and lipoprotein lipase secreted from human monocyte-derived macrophages modulate very low density lipoprotein uptake. J. Biol. Chem. 265: 3040–3047. [PubMed] [Google Scholar]
- 17.Babaev V. R., Fazio S., Gleaves L. A., Carter K. J., Semenkovich C. F., Linton M. F. 1999. Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in vivo. J. Clin. Invest. 103: 1697–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Eck M., Zimmermann R., Groot P. H., Zechner R., Van Berkel T. J. 2000. Role of macrophage-derived lipoprotein lipase in lipoprotein metabolism and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 20: E53–E62. [DOI] [PubMed] [Google Scholar]
- 19.Linton M. F., Fazio S. 1999. Macrophages, lipoprotein metabolism, and atherosclerosis: insights from murine bone marrow transplantation studies. Curr. Opin. Lipidol. 10: 97–105. [DOI] [PubMed] [Google Scholar]
- 20.Weinstock P. H., Levak-Frank S., Hudgins L. C., Radner H., Friedman J. M., Zechner R., Breslow J. L. 1997. Lipoprotein lipase controls fatty acid entry into adipose tissue, but fat mass is preserved by endogenous synthesis in mice deficient in adipose tissue lipoprotein lipase. Proc. Natl. Acad. Sci. USA. 94: 10261–10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Augustus A., Yagyu H., Haemmerle G., Bensadoun A., Vikramadithyan R. K., Park S. Y., Kim J. K., Zechner R., Goldberg I. J. 2004. Cardiac-specific knock-out of lipoprotein lipase alters plasma lipoprotein triglyceride metabolism and cardiac gene expression. J. Biol. Chem. 279: 25050–25057. [DOI] [PubMed] [Google Scholar]
- 22.Clausen B. E., Burkhardt C., Reith W., Renkawitz R., Forster I. 1999. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8: 265–277. [DOI] [PubMed] [Google Scholar]
- 23.Yagyu H., Kitamine T., Osuga J., Tozawa R., Chen Z., Kaji Y., Oka T., Perrey S., Tamura Y., Ohashi K., et al. 2000. Absence of ACAT-1 attenuates atherosclerosis but causes dry eye and cutaneous xanthomatosis in mice with congenital hyperlipidemia. J. Biol. Chem. 275: 21324–21330. [DOI] [PubMed] [Google Scholar]
- 24.Usui S., Hara Y., Hosaki S., Okazaki M. 2002. A new on-line dual enzymatic method for simultaneous quantification of cholesterol and triglycerides in lipoproteins by HPLC. J. Lipid Res. 43: 805–814. [PubMed] [Google Scholar]
- 25.Yagyu H., Chen G., Yokoyama M., Hirata K., Augustus A., Kako Y., Seo T., Hu Y., Lutz E. P., Merkel M., et al. 2003. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J. Clin. Invest. 111: 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hocquette J. F., Graulet B., Olivecrona T. 1998. Lipoprotein lipase activity and mRNA levels in bovine tissues. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 121: 201–212. [DOI] [PubMed] [Google Scholar]
- 27.Tangirala R. K., Rubin E. M., Palinski W. 1995. Quantitation of atherosclerosis in murine models: correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. J. Lipid Res. 36: 2320–2328. [PubMed] [Google Scholar]
- 28.Paigen B., Morrow A., Holmes P. A., Mitchell D., Williams R. A. 1987. Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis. 68: 231–240. [DOI] [PubMed] [Google Scholar]
- 29.Spann N. J., Garmire L. X., McDonald J. G., Myers D. S., Milne S. B., Shibata N., Reichart D., Fox J. N., Shaked I., Heudobler D., et al. 2012. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 151: 138–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Babaev V. R., Patel M. B., Semenkovich C. F., Fazio S., Linton M. F. 2000. Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in low density lipoprotein receptor-deficient mice. J. Biol. Chem. 275: 26293–26299. [DOI] [PubMed] [Google Scholar]
- 31.Olafsen T., Young S. G., Davies B. S., Beigneux A. P., Kenanova V. E., Voss C., Young G., Wong K. P., Barnes R. H., 2nd, Tu Y., et al. 2010. Unexpected expression pattern for glycosylphosphatidylinositol-anchored HDL-binding protein 1 (GPIHBP1) in mouse tissues revealed by positron emission tomography scanning. J. Biol. Chem. 285: 39239–39248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beisiegel U., Weber W., Bengtsson-Olivecrona G. 1991. Lipoprotein lipase enhances the binding of chylomicrons to low density lipoprotein receptor-related protein. Proc. Natl. Acad. Sci. USA. 88: 8342–8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rumsey S. C., Obunike J. C., Arad Y., Deckelbaum R. J., Goldberg I. J. 1992. Lipoprotein lipase-mediated uptake and degradation of low density lipoproteins by fibroblasts and macrophages. J. Clin. Invest. 90: 1504–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tabas I., Li Y., Brocia R. W., Xu S. W., Swenson T. L., Williams K. J. 1993. Lipoprotein lipase and sphingomyelinase synergistically enhance the association of atherogenic lipoproteins with smooth muscle cells and extracellular matrix. A possible mechanism for low density lipoprotein and lipoprotein(a) retention and macrophage foam cell formation. J. Biol. Chem. 268: 20419–20432. [PubMed] [Google Scholar]
- 35.Li L., Renier G. 2007. Adipocyte-derived lipoprotein lipase induces macrophage activation and monocyte adhesion: role of fatty acids. Obesity (Silver Spring). 15: 2595–2604. [DOI] [PubMed] [Google Scholar]
- 36.Saraswathi V., Hasty A. H. 2006. The role of lipolysis in mediating the proinflammatory effects of very low density lipoproteins in mouse peritoneal macrophages. J. Lipid Res. 47: 1406–1415. [DOI] [PubMed] [Google Scholar]
- 37.Febbraio M., Podrez E. A., Smith J. D., Hajjar D. P., Hazen S. L., Hoff H. F., Sharma K., Silverstein R. L. 2000. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J. Clin. Invest. 105: 1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marleau S., Harb D., Bujold K., Avallone R., Iken K., Wang Y., Demers A., Sirois M. G., Febbraio M., Silverstein R. L., et al. 2005. EP 80317, a ligand of the CD36 scavenger receptor, protects apolipoprotein E-deficient mice from developing atherosclerotic lesions. FASEB J. 19: 1869–1871. [DOI] [PubMed] [Google Scholar]
- 39.Moore K. J., Kunjathoor V. V., Koehn S. L., Manning J. J., Tseng A. A., Silver J. M., McKee M., Freeman M. W. 2005. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J. Clin. Invest. 115: 2192–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coburn C. T., Knapp F. F., Jr, Febbraio M., Beets A. L., Silverstein R. L., Abumrad N. A. 2000. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J. Biol. Chem. 275: 32523–32529. [DOI] [PubMed] [Google Scholar]
- 41.Ricote M., Welch J. S., Glass C. K. 2000. Regulation of macrophage gene expression by the peroxisome proliferator-activated receptor-gamma. Horm. Res. 54: 275–280. [DOI] [PubMed] [Google Scholar]
- 42.Chandak P. G., Radovic B., Aflaki E., Kolb D., Buchebner M., Frohlich E., Magnes C., Sinner F., Haemmerle G., Zechner R., et al. 2010. Efficient phagocytosis requires triacylglycerol hydrolysis by adipose triglyceride lipase. J. Biol. Chem. 285: 20192–20201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin B., Loike J. D., Kako Y., Weinstock P. H., Breslow J. L., Silverstein S. C., Goldberg I. J. 1997. Lipoprotein lipase regulates Fc receptor-mediated phagocytosis by macrophages maintained in glucose-deficient medium. J. Clin. Invest. 100: 649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schrijvers D. M., De Meyer G. R., Herman A. G., Martinet W. 2007. Phagocytosis in atherosclerosis: molecular mechanisms and implications for plaque progression and stability. Cardiovasc. Res. 73: 470–480. [DOI] [PubMed] [Google Scholar]
- 45.Thorp E., Cui D., Schrijvers D. M., Kuriakose G., Tabas I. 2008. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe-/- mice. Arterioscler. Thromb. Vasc. Biol. 28: 1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ostlund-Lindqvist A. M., Gustafson S., Lindqvist P., Witztum J. L., Little J. A. 1983. Uptake and degradation of human chylomicrons by macrophages in culture. Role of lipoprotein lipase. Arteriosclerosis. 3: 433–440. [DOI] [PubMed] [Google Scholar]