Abstract
The validation of the use of plasma plant sterols as a marker of cholesterol absorption is frail. Nevertheless, plant sterol concentrations are routinely used to describe treatment-induced changes in cholesterol absorption. Their use has also been advocated as a clinical tool to tailor cholesterol-lowering therapy. Prior to wider implementation, however, the validity of plant sterols as absorption markers needs solid evaluation. Therefore, we compared plasma plant sterol concentrations to gold-standard stable isotope-determined cholesterol absorption. Plasma campesterol/TC concentrations (camp/TC) were measured in a population of 175 mildly hypercholesterolemic individuals (age: 59.7 ± 5.6 years; BMI: 25.5 ± 2.9kg/m2; LDL-C: 4.01 ± 0.56 mmol/l). We compared cholesterol absorption according to the plasma dual-isotope method in subjects with the highest camp/TC concentrations (N = 41, camp/TC: 2.14 ± 0.68 μg/mg) and the lowest camp/TC concentrations (N = 39, camp/TC: 0.97 ± 0.22 μg/mg). Fractional cholesterol absorption did not differ between the groups (24 ± 12% versus 25 ± 16%, P = 0.60), nor was it associated with plasma camp/TC concentrations in the total population of 80 individuals (β = 0.13; P = 0.30, adjusted for BMI and plasma triglycerides). Our findings do not support a relation between plasma plant sterol concentrations and true cholesterol absorption and, therefore, do not favor the use of these sterols as markers of cholesterol absorption. This bears direct consequences for the interpretation of earlier studies, as well as for future studies targeting intestinal regulation of cholesterol metabolism.
Keywords: campesterol, stable isotopes, intestine, phytosterols
Human intestinal cholesterol absorption displays a large inter-individual variation, ranging from 20 to 80% (1, 2). Several techniques have been described to measure cholesterol absorption in man, each with their own strengths and weaknesses (reviewed in Refs. 3, 4). Generally, these methods are laborious and costly, as they require administration and analysis of isotopic tracers, thereby limiting their use in large-scale studies. For this purpose, more than two decades ago, plasma noncholesterol sterol concentrations were introduced as markers of cholesterol absorption and synthesis. The plant sterols campesterol and sitosterol and the cholesterol metabolite cholestanol were shown to be associated with cholesterol absorption (5, 6), whereas the cholesterol precursors lathosterol and desmosterol correlated with cholesterol synthesis (7, 8). The conception of cholesterol precursors as a reflection of the cholesterol synthesis pathway is intuitively plausible and has subsequently been corroborated by repetitive positive validation against various methods (7–11). Plant sterols derive strictly from the diet and share a high structural similarity with cholesterol. Nevertheless, their validity as markers of cholesterol absorption has been less well established, given that these absorption markers were initially validated against the cholesterol balance method in two relatively small study populations of 17 (5) and 63 (12) subjects. Subsequently, reported associations in larger study populations were not only weak but also have remained without additional prospective validation since. Notwithstanding, plasma plant sterol concentrations are currently used to describe cholesterol absorption in steady-state (13, 14) or intervention-induced changes in absorption (15–17). Finally, plasma plant sterol concentrations have also been suggested as a clinical tool to customize cholesterol-lowering treatment (18, 19). In our opinion, these markers warranted thorough assessment before such suggestions can be supported.
We revalidated plasma plant sterol concentrations as markers of cholesterol absorption in a population of 80 mildly hypercholesterolemic subjects. Our results do not favor their use as valid markers of human cholesterol absorption.
METHODS
Study design
Our cross-sectional study consisted of a cholesterol absorption measurement according to the plasma dual-isotope method (20) in 80 mildly hypercholesterolemic adults, who were selected for this measurement based on their plasma campesterol/TC concentrations. This study was approved by the Institutional Review Board of the Academic Medical Center, Amsterdam, the Netherlands. Each participant gave written informed consent.
Selection of subjects
Subjects who participated in previous studies at our department were invited for screening or were recruited via advertisements in local newspapers. Caucasian subjects 18 years of age or older with plasma low-density lipoprotein cholesterol (LDL-C) concentrations between 3.0 and 5.0 mmol/l were considered eligible if they did not meet the following exclusion criteria: use of statins or consumption of plant sterol- or stanol-enriched food products; a history of arterial disease, including unstable angina, myocardial infarction, transient ischemic attack, or a cerebrovascular accident; diabetes mellitus; thyroid illness; uncontrolled hypertension; familial hypercholesterolemia (FH), diagnosed either by genotyping or by WHO diagnostic criteria; plasma triglyceride (TG) concentrations > 3.0 mmol/l; body mass index (BMI) > 30 kg/m2; or excessive alcohol consumption. Women had to be postmenopausal without hormone-replacement therapy.
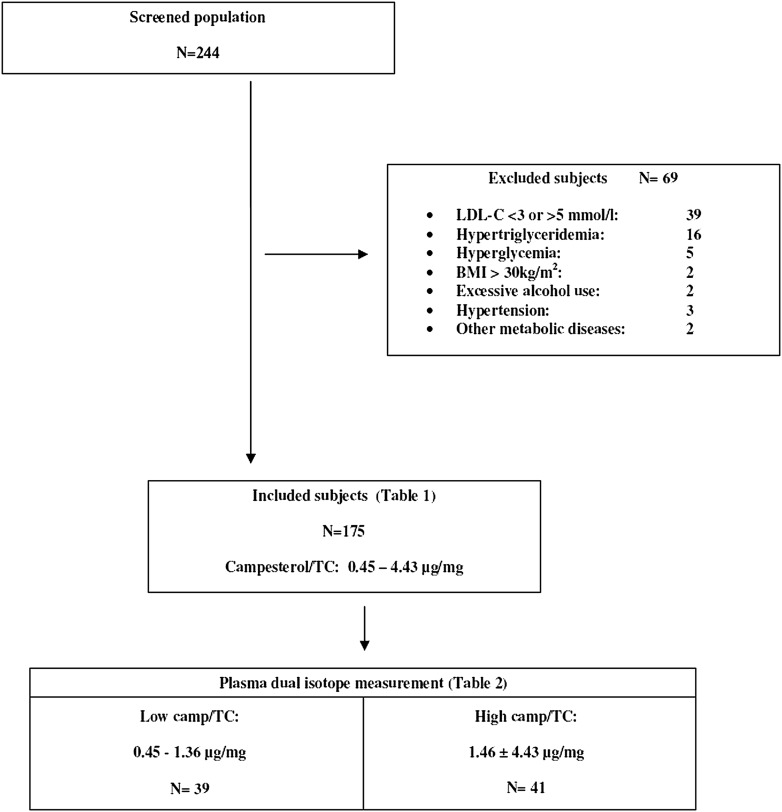
According to our power calculation described below, we set out to include at least 160 mildly hypercholesterolemic subjects in whom plasma noncholesterol sterol concentrations were measured. These subjects were stratified according to their plasma cholesterol-adjusted campesterol (campesterol/TC) concentrations. Participants were invited for the cholesterol absorption measurement, starting with those with the highest and the lowest campesterol/TC concentrations, respectively. Inclusion was ceased after the first 80 subjects with a successfully performed plasma dual-isotope measurement. Study design and the selection of participants are outlined in Fig. 1.
Fig. 1.
Study flowchart.
Measurements of cholesterol absorption
Cholesterol absorption was measured according to the plasma stable dual-isotope method (2). In short, after an overnight fast, blood was drawn (T = 0 h) and subjects received an intravenous dose of 30 mg [3,4- 13C2] cholesterol (Isotec, Miamisbury, OH) dissolved in 22 ml 10% Liposyn III (Hospira Inc., Lake Forest, IL) over a period of 20 min. The 13C2-cholesterol was prepared for injection by dissolving the tracer in warm USP ethanol into a clear solution under sterile conditions. Thereafter, the isotope/ethanol mixture was added to 10% Liposyn III for a total administrable volume of 22 ml. Immediately after infusion of the intravenous tracer dose, subjects ingested 50 mg of [25,26,26,26,27,27,27-D7] cholesterol (Cambridge Isotope Laboratories, Andover, MA), administered in a stomach-soluble gelatin capsule together with a standardized breakfast, consisting of 2 slices of whole wheat bread with 15 g of margarine, 40 g cheese, 150 ml semi-skimmed milk, and coffee with a small slice of gingerbread. This breakfast contained 50 g carbohydrate, 27 g protein, 15 g fat, 440 kcal, and 84 mg cholesterol (including 50 mg of the tracer). Subjects refrained from food for 4 h following the test meal to allow for complete gastric emptying. Participants maintained their usual dietary pattern as well as physical activity and kept a dietary record throughout the study. Additional blood samples were collected at 24, 48, and 72 h postadministration of the tracers.
Plasma was isolated by centrifugation and stored at −80°C, for subsequent cholesterol extraction and measurement of the tracer enrichments. Fractional cholesterol absorption (FCA) was calculated as the plasma ratio of D7 and 13C-cholesterol enrichment at T = 72 h divided by the ratio D7 / 13C-cholesterol species administered to the subjects (2). These enrichments were measured by means of GCMS, as described below. Dietary intake was calculated using a Dutch nutrition database program (www.dieetinzicht.nl). Plant sterol intake was calculated through the USDA National Nutrient Database for Standard Reference (ndb.nal.usda.gov).
Analytic procedures
Fasted plasma TC, HDL-C, and TG concentrations were measured using standard automated methods; LDL-C concentrations were calculated using the Friedewald formula (20). Plasma noncholesterol sterol concentrations were analyzed from nonsaponifiable plasma material with GCMS, as previously described (21). Plasma enrichments of D7-cholesterol and 13C2-cholesterol were measured by GCMS (Center for Liver, Digestive and Metabolic Diseases, University of Groningen). Plasma free cholesterol was extracted with ethanol/acetone (1:1, v/v) for analysis of isotopic enrichment by GCMS. Cholesterol derivatives were separated with a 5975C InertXL EI/CI MSD (Agilent Technologies) in the electron ionization mode using a ZB5ms column [30 m(l) × 0.25 mm (ID) × 0.25 μm (film) (Phenomenex)]. Ions monitored were m/z 368–375 corresponding to the M0–M7 mass isotopomers. The measured fractional isotopomer distribution was corrected for the fractional distribution due to natural abundance of 13C and 2H by multiple linear regression as described by Lee et al. (22) to obtain excess fractional distribution of mass isotopomers resulting from isotope dilution and incorporation of the infused tracer. In this approach, M7 represented the orally administered label. The day-to-day coefficient of variance for the ratio 12C/13C was 0.06%.
Statistical analyses
Sample size.
Assuming a common standard deviation of cholesterol absorption of 10%, a sample size of 10 subjects in each group has more than 80% power to detect a difference in means of 20%. An additional power calculation showed that this sample size of 20 would have 80% power to detect a significant and clinically acceptable correlation (R = 0.60) between two continuous variables, such as plasma plant sterol concentrations and cholesterol absorption rates. However, as plasma plant sterol levels were previously validated in a population of 17 (5) and 63 (12) subjects, we chose to validate these markers in an equally large population of 80 subjects; i.e., 40 subjects with high plasma campesterol/TC concentrations versus 40 with low campesterol/TC concentrations.
Study outcomes.
Normally distributed data are presented as means and standard deviations (SD). Skewed data were log-transformed prior to testing and are presented as median with the range. Differences in parameters between subjects with high and low campesterol/TC concentrations were analyzed with independent sample t-tests. Associations between plasma plant sterol concentrations and cholesterol absorption in the entire study group were analyzed by means of multiple regression analysis, with corrections for BMI and triglyceride concentrations, as those differed between subjects with high and low campesterol/TC concentrations. Analyses were performed by use of SPSS 15.0 for Windows software (SPSS Inc., Chicago, IL).
RESULTS
Baseline characteristics
Of 244 subjects, 175 met the inclusion and exclusion criteria (Table 1). Plasma campesterol/TC concentrations ranged from 0.45 to 4.43 μg/mg; sitosterol/TC from 0.31 to 2.95 μg/mg; and cholestanol/TC from 0.31 to 2.55 μg/mg. Lathosterol/TC levels varied from 0.32 to 2.75 μg/mg. We found significant negative correlations between plasma absorption markers on the one hand and plasma lathosterol/TC concentrations on the other (R = −0.39 for campesterol/TC; R = −0.42 for sitosterol/TC; and R = −0.39 for cholestanol/TC; P < 0.001 for all). Plasma noncholesterol sterol concentrations were significantly associated with BMI (R = −0.25, P = 0.001 for campesterol/TC; R = −0.30, P < 0.001 for sitosterol/TC; R = −0.150, P = 0.048 for cholestanol/TC; and R = 0.24, P = 0.001 for lathosterol/TC) and with plasma triglyceride concentrations (R = −0.19, P = 0.014 for campesterol/TC; R = −0.27, P < 0.001 for sitosterol/TC; R = −0.194, P = 0.010 for cholestanol/TC; and R = 0.28, P < 0.001 for lathosterol/TC).
TABLE 1.
Mildly hypercholesterolemic study population (N = 175)
| Characteristic | Value |
| Age, years | 59.7 ± 5.6 |
| Male/ female, n | 119 / 56 |
| BMI, kg/m2 | 25.5 ± 2.9 |
| Total cholesterol, mmol/l | 6.12 ± 0.69 |
| LDL-cholesterol, mmol/l | 4.01 ± 0.56 |
| HDL-cholesterol, mmol/l | 1.61 ± 0.43 |
| Triglycerides, mmol/l | 1.03 [0.29–2.72] |
| Campesterol, mg/dl | 0.90 ± 0.44 |
| Sitosterol, mg/dl | 0.62 ± 0.30 |
| Cholestanol, mg/dl | 0.86 ± 0.27 |
| Lathosterol, mg/dl | 0.74 ± 0.30 |
| Campesterol/TC, μg/mg | 1.51 ± 0.70 |
| Sitosterol/TC, μg/mg | 1.08 ± 0.51 |
| Cholestanol/TC, μg/mg | 1.40 ± 0.40 |
| Lathosterol/TC, μg/mg | 1.22 ± 0.49 |
Data are presented as means ± SD, median [range] or number (n).
Plasma dual-isotope method
Population characteristics and plasma noncholesterol sterol levels.
Thirty-nine subjects with low campesterol/TC concentrations and 41 subjects with high concentrations were subjected to the plasma dual-isotope measurement (Table 2). By definition, plasma noncholesterol sterol concentrations differed significantly between the two groups (Table 2, P < 0.001 for all). Subjects with low campesterol/TC concentrations exhibited significantly higher BMI values compared with subjects with high campesterol/TC concentrations (27.0 ± 3.3 kg/m2 versus 24.4 ± 2.2 kg/m2, P < 0.001), as well as significantly higher plasma triglyceride levels (1.14 [0.52–2.67] mmol/l versus 0.89 [0.29–1.82] mmol/l, P = 0.006). There were no differences in dietary cholesterol or plant sterol intake. There were no significant differences between men and women (data not shown).
TABLE 2.
Study population plasma dual-isotope measurement (N = 80)
| Characteristic | Low camp/TC N = 39 | High camp/TC N = 41 |
| Age, years | 59.1 ± 5.7 | 59.9 ± 5.6 |
| Male/female, n | 27/12 | 29/12 |
| BMI, kg/m2 | 27.0 ± 3.3 | 24.4 ± 2.2* |
| Total cholesterol, mmol/l | 6.14 ± 0.77 | 6.23 ± 0.69 |
| LDL-cholesterol, mmol/l | 4.01 ± 0.61 | 4.11 ± 0.57 |
| HDL-cholesterol, mmol/l | 1.56 ± 0.37 | 1.69 ± 0.43 |
| Triglycerides, mmol/l | 1.14 [0.52–2.67] | 0.89 [0.29–1.82]** |
| Glucose, mmol/l | 5.13 ± 0.50 | 5.08 ± 0.35 |
| Campesterol, mg/dl | 0.56 ± 0.14 | 1.19 ± 0.37* |
| Sitosterol, mg/dl | 0.41 ± 0.14 | 0.87 ± 0.31* |
| Cholestanol, mg/dl | 0.76 ± 0.17 | 0.97 ± 0.21* |
| Lathosterol, mg/dl | 0.90 ± 0.34 | 0.55 ± 0.20* |
| Campesterol/TC, μg/mg | 0.97 ± 0.22 | 2.14 ± 0.68* |
| Sitosterol/TC, μg/mg | 0.74 ± 0.23 | 1.58 ± 0.52* |
| Cholestanol/TC, μg/mg | 1.28 ± 0.24 | 1.68 ± 0.35* |
| Lathosterol/TC, μg/mg | 1.53 ± 0.59 | 0.92 ± 0.30* |
| Daily cholesterol intake (mg) | 247 ± 100 | 215 ± 42 |
| Daily plant sterol intake (mg) | 125 ± 47 | 123 ± 36 |
Data are presented as means ± SD, median [range] or number (n). Analyses were performed with independent sample t-tests. Triglyceride data were skewed and log-transformed prior to testing; however, untransformed medians and range are presented. *P < 0.001; **P = 0.05.
Plasma markers of absorption were negatively associated with lathosterol/TC concentrations (β = −0.49, P < 0.001 for campesterol/TC; β = −0.52, P < 0.001 for sitosterol/TC; and β = −0.58, P < 0.001 for cholestanol/TC) in the entire group of 80 study subjects.
Plasma noncholesterol sterols and cholesterol absorption.
Mean fractional cholesterol absorption as measured by the plasma dual-isotope method was 24% ± 14, ranging from 1 to 73%. As average cholesterol absorption was lower compared with the literature [i.e., 24% instead of 40 to 50% (1, 2)], we evaluated whether this difference might be due to lower bioavailability of the oral D7-cholesterol tracer caused by our method of administration. We could not substantiate this (see supplementary data).
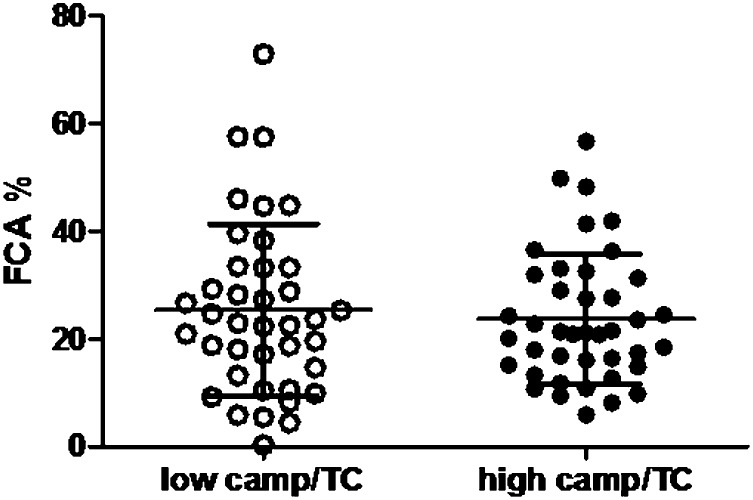
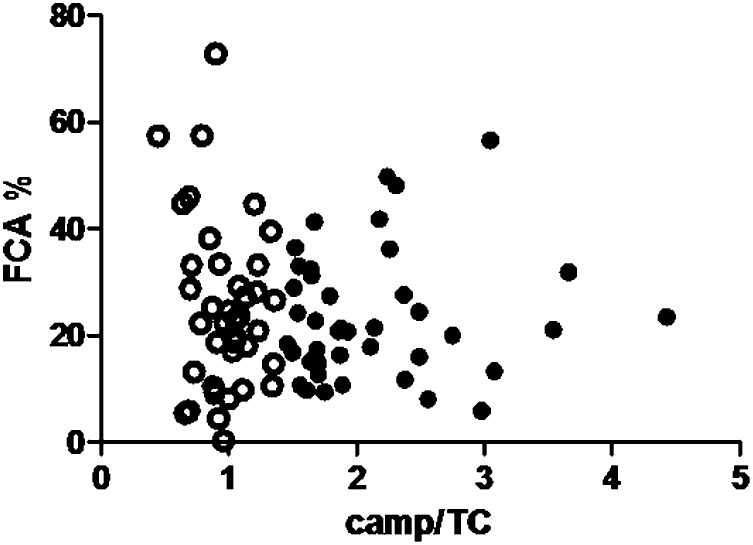
Measured FCA did not differ significantly between subjects with high and low campesterol/TC concentrations (Fig. 2). There were no significant differences between men and women (data not shown). Measured FCA was not associated with plasma campesterol/TC concentrations: β = 0.13, P = 0.30, adjusted for BMI and plasma triglyceride concentrations (Fig. 3). This lack of association with cholesterol absorption was also observed for plasma sitosterol/TC concentrations (β = 0.08, P = 0.52). Plasma cholestanol/TC concentrations were significantly associated with measured FCA (β = 0.26, P = 0.035), as well as plasma lathosterol/TC concentrations (β = −0.30, P = 0.016). These associations were similar when absolute plant sterol concentrations were used (β = 0.25, P = 0.14 for campesterol; β = 0.17, P = 0.17 for sitosterol; β = 0.48, P < 0.001 for cholestanol; and β = −0.19, P = 0.12 for lathosterol). No significant associations were found when campesterol/lathosterol concentrations were used (data not shown).
Fig. 2.
No difference in FCA between high and low absorbers. Cholesterol absorption was 25 ± 16% in 39 subjects with low campesterol/TC levels (open circles) and 24 ± 12% in 41 subjects with high campesterol/TC concentrations (filled circles).
Fig. 3.
No correlation between plasma campesterol/TC concentrations and cholesterol absorption (β = 0.10, P = 0.40 unadjusted and β = 0.13, P = 0.30 after adjustment for BMI and triglycerides). Subjects with low plasma campesterol/TC concentrations are represented by the open circles, those with high plasma campesterol/TC concentrations as filled circles.
DISCUSSION
The present study demonstrates that plasma plant sterol concentrations are not associated with measured cholesterol absorption in a population of 80 mildly hypercholesterolemic subjects. These markers were unable to distinguish between “high and low cholesterol absorbers,” as we found comparable FCA rates in individuals despite 3-fold varying plasma campesterol/TC concentrations. We chose to select “high and low absorbers” based on cholesterol-adjusted plant sterol levels and campesterol in particular, since this sterol is more readily absorbed (16). In addition, we also evaluated associations between cholesterol absorption and other cholesterol-adjusted noncholesterol sterols, including cholestanol concentrations and lathosterol/campesterol ratios, as well as absolute values. Plasma cholestanol/TC and lathosterol/TC concentrations were significantly associated with measured FCA.
Our findings are in stark contrast to current clinical practice, in which plasma plant sterol concentrations are used as reliable markers of cholesterol absorption. This was first suggested by a study of 17 individuals from two families with familial hypertriglyceridemia (5). A significant association was found between plasma campesterol/TC concentrations and fractional cholesterol absorption rates (R = 0.73). A similarly high correlation was found in 37 siblings of low and high absorbers (23). However, these were both relatively small-scaled studies in related individuals, which might have strengthened the observed associations, as it was shown that plasma plant sterol concentrations are heritable to a certain degree (24). Evaluation of these markers in larger populations of unrelated individuals yielded significantly weaker correlations: R = 0.32 for plasma cholestanol levels in 61 middle-aged men (6), and R = 0.53, P = 0.01 for plasma cholestanol in a population of men with both high and low glucose concentrations (25). Although plasma plant sterols were measured, associations with measured cholesterol absorption were not reported. Similar correlations were found in 63 middle-aged men (R = 0.42 for campesterol and R = 0.30 for sitosterol) (12), as well as in a combined population of 22 lean and obese subjects (R = 0.48 for campesterol and R = 0.44 for sitosterol) (26). In other words, merely 10 to 25% of cholesterol absorption could be explained by plasma plant sterol concentrations in these populations. Despite the modest associations, plasma plant sterol concentrations have been used in numerous publications as a measure for FCA ever since. For example, in the past two years, 37 studies used plasma plant sterols as indirect markers of cholesterol absorption (pubmed.gov).
Negative studies are scarce and only concern specific, small-sized populations: serum plant sterol levels did not reflect cholesterol absorption in mildly hypercholesterolemic males consuming plant stanol-enriched food products (27), in postmenopausal women with coronary artery disease (28), or in children with Smith-Lemli-Opitz syndrome (29). We report the first adequately powered, negative study in a randomly selected population of middle-aged, mildly hypercholesterolemic but otherwise healthy subjects. Baseline characteristics of our subjects were similar to previous study populations in which positive associations between plasma plant sterols and cholesterol absorption were found (6, 12). This also applies to ingested dietary plant sterols (30), the observed range of plant sterol concentrations (12, 23, 24, 31), as well as their negative correlations with plasma lathosterol levels, BMI, and triglyceride levels (6, 32–35). Measured cholesterol absorption rates ranged from 1 to 73%, which is in line with literature (reviewed in Ref. 36), although mean cholesterol absorption was lower as compared with other studies; FCA averaged 24% instead of 40 to 50%. Of note, the lower mean cholesterol absorption could not be attributed to lower bioavailability of the oral D7-cholesterol tracer, caused by the method of administration (see supplementary data). Furthermore, it is probably not due to population-specific characteristics, as we found similar FCA rates in another population of 15 mildly hypercholesterolemic Dutch men (unpublished data). Measured FCA was associated with plasma cholestanol/TC concentrations and negatively with plasma lathosterol/TC concentrations, which is also in line with previous findings (6, 25).
Despite the aforementioned affirmative findings, a sample size large enough to detect a clinically relevant association, and the fact that our study was designed to answer the specific question whether cholesterol absorption differs between subjects with high and low plasma campesterol/TC concentrations, we failed to demonstrate any association with measured cholesterol absorption. This absence of a direct correlation between plasma plant sterol levels and cholesterol absorption may not be surprising when the metabolic routes of the different sterols are compared. Despite their high degree of structural similarity (37), there are numerous differences in the metabolic fate of plant sterols compared with cholesterol within the human body. Plant sterols are not endogenously synthesized and derive strictly from the diet. They are absorbed via the Niemann-Pick C1 Like 1 transporter in the proximal small intestine, but they are a very poor substrate for ACAT2 and, hence, are not incorporated in chylomicrons. The resulting accumulation of the plant sterols in the enterocyte activates efflux into the lumen via the ABCG5/G8 heterodimer transporter (38). Instead of following the chylomicron route, plant sterols may be transported to the blood via ABCA1 (39–41). The small amounts that reach the liver are more rapidly secreted into the bile compared with cholesterol, probably due to a low plasma and hepatic esterification rate (42, 43). This complex, but efficient efflux machinery results in plasma plant sterol levels that are approximately 200-fold lower compared with cholesterol in normal individuals (8, 43). These low levels are not only determined by intestinal absorption but also, or possibly even more, by hepatic clearance. For instance, a sitosterolemia patient, characterized by 100-fold elevated plasma plant sterol concentrations due to a mutation in the ABCG5/G8 transporter, exhibited completely normalized plasma plant sterol levels after liver transplantation, despite a persistent intestinal defective ABCG5/G8 transporter (44). Clearly, all steps involved in plant sterol transport may control their plasma concentrations and considering the fact that kinetic parameters for both NPC1L1 and ABCG5/G8 differ between plant sterol and cholesterol, good correlations between plant sterol and cholesterol absorption and metabolic turnover are not to be expected.
To investigate potential relations between activity of plant sterol transporter metabolism and their plasma levels, we considered several factors contributing to variations in plasma plant sterol concentrations, as recently reviewed (31). These include dietary intake of plant sterols, gender, polymorphisms in ABCG5/G8, presence of metabolic syndrome, and apoE phenotypes. Dietary intake of plant sterols did not differ between the high and low campesterol/TC groups and none of our subjects consumed plant sterol-enriched food products, although the relation between dietary intake of plant sterols and serum plant sterol concentrations is not very strong (45–49). Furthermore, there were no differences in gender distribution between the high and low campesterol/TC group. The ABCG8 D19H variant has been associated with decreased plasma plant sterol concentrations, possibly mediated by a gain of function of the ABCG8 transporter (21, 50). As each of our subjects had participated in a previous study in which we investigated associations between five nonsynonymous polymorphisms in ABCG5/G8 and plasma sterol concentrations (21), we are able to state that we found no differences in distribution of these variants between the high and low campesterol/TC groups (data not shown), although our current study population was too small for a genetic association study. We did not find any relation between these variants and measured cholesterol absorption, and to the best of our knowledge, there are no reports showing hampered cholesterol absorption in subjects carrying this phenotype. We cannot rule out the possibility that a skewed distribution of apoE phenotypes between the high and low campesterol/TC group might have influenced our results. We did not genotype subjects for apoE phenotype, as reports on the associations between apoE variants and cholesterol absorption have been inconsistent (2, 31, 51), the prevalence of the apoE4 genotype in the Netherlands is low (52), and apoE polymorphisms also affect serum cholesterol and triglyceride concentrations (53), thereby complicating the implication of certain associations with plasma plant sterols. Finally, although none of our subjects had type 2 diabetes or met the clinical criteria for metabolic syndrome, subjects with low plasma campesterol/TC concentrations did express higher BMI and plasma triglyceride concentrations in our study, which is in line with the literature (6, 32–35). We corrected our results for this difference. Moreover, significant weight loss over a period of two years has been shown to significantly alter FCA rates and plasma plant sterol concentrations (54), illustrating that both cholesterol absorption and plant sterol concentrations are dynamic parameters, influenced by modifiable patient characteristics, such as obesity or insulin resistance. At present, plasma plant sterols are often handled as stable parameters in a given individual (24), often with unjustified disregard toward possible dynamic confounders. Lack of consensus throughout the literature regarding the correct use of plant sterols as markers of cholesterol absorption further underlines the scientific inconclusiveness. This was recently acknowledged by the founders of these markers who, following review of the literature on this subject, stated that “it is worth using several instead of only one absorption sterol marker for making conclusions of altered absorption of cholesterol, and even then, the presence of at least some absolute measurement is valuable” (13).
In conclusion, plasma plant sterol concentrations do not reflect cholesterol absorption in a relatively large population of mildly hypercholesterolemic subjects. Difficulties in the interpretation and correct use of plasma plant sterol concentrations, as well as differences between cholesterol and plant sterol metabolism, disqualify their current use as reliable markers of cholesterol absorption.
Supplementary Material
Acknowledgments
The authors kindly acknowledge all study participants, Elsa Hartemink for clinical assistance at the research facility and Guus Duchateau at Unilever who supported the D7-cholesterol bioavailability experiment. The authors thank Dr. D. Lutjohann for his critical appraisal of our manuscript and corresponding discussions.
Footnotes
Abbreviations:
- BMI
- body mass index
- camp/TC
- campesterol/TC
- FCA
- fractional cholesterol absorption
- FH
- familial hypercholesterolemia
- LDL-C
- LDL cholesterol
- TC
- total cholesterol
- TG
- triglyceride
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures and two tables.
REFERENCES
- 1.Grundy S. M. 1983. Absorption and metabolism of dietary cholesterol. Annu. Rev. Nutr. 3: 71–96. [DOI] [PubMed] [Google Scholar]
- 2.Bosner M. S., Lange L. G., Stenson W. F., Ostlund R. E., Jr 1999. Percent cholesterol absorption in normal women and men quantified with dual stable isotopic tracers and negative ion mass spectrometry. J. Lipid Res. 40: 302–308. [PubMed] [Google Scholar]
- 3.Matthan N. R., Lichtenstein A. H. 2004. Approaches to measuring cholesterol absorption in humans. Atherosclerosis. 174: 197–205. [DOI] [PubMed] [Google Scholar]
- 4.Mackay D., Jones P. J. 2011. Evaluation of methods for the determination of cholesterol absorption and synthesis in humans. Atherosclerosis. 218: 253–262. [DOI] [PubMed] [Google Scholar]
- 5.Tilvis R. S., Miettinen T. A. 1986. Serum plant sterols and their relation to cholesterol absorption. Am. J. Clin. Nutr. 43: 92–97. [DOI] [PubMed] [Google Scholar]
- 6.Miettinen T. A., Tilvis R. S., Kesäniemi Y. A. 1989. Serum cholestanol and plant sterol levels in relation to cholesterol metabolism in middle-aged men. Metabolism. 38: 136–140. [DOI] [PubMed] [Google Scholar]
- 7.Bjorkhem I., Miettinen T., Reihnér E., Ewerth S., Angelin B., Einarsson K. 1987. Correlation between serum levels of some cholesterol precursors and activity of HMG-CoA reductase in human liver. J. Lipid Res. 28: 1137–1143. [PubMed] [Google Scholar]
- 8.Kempen H. J., Glatz J. F., Gevers Leuven J. A., van der Voort H. A., Katan M. B. 1988. Serum lathosterol is an indicator of whole-body cholesterol synthesis in humans. J. Lipid Res. 29: 1149–1155. [PubMed] [Google Scholar]
- 9.Vuoristo M., Miettinen T. A. 1986. Serum cholesterol precursor sterols in coeliac disease: effects of gluten free diet and cholestyramine. Gut. 27: 1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthan N. R., Raeini-Sarjaz M., Lichtenstein A. H., Ausman L. M., Jones P. J. 2000. Deuterium uptake and plasma cholesterol precursor levels correspond as methods for measurement of endogenous cholesterol synthesis in hypercholesterolemic women. Lipids. 35: 1037–1044. [DOI] [PubMed] [Google Scholar]
- 11.Jones P. J., Pappu A. S., Illingworth D. R., Leitch C. A. 1992. Correspondence between plasma mevalonic acid levels and deuterium uptake in measuring human cholesterol synthesis. Eur. J. Clin. Invest. 22: 609–613. [DOI] [PubMed] [Google Scholar]
- 12.Miettinen T. A., Tilvis R. S., Kesäniemi Y. A. 1990. Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am. J. Epidemiol. 131: 20–31. [DOI] [PubMed] [Google Scholar]
- 13.Miettinen T. A., Gylling H., Nissinen M. J. 2011. The role of serum non-cholesterol sterols as surrogate markers of absolute cholesterol synthesis and absorption. Nutr. Metab. Cardiovasc. Dis. 21: 765–769. [DOI] [PubMed] [Google Scholar]
- 14.Paramsothy P., Knopp R. H., Kahn S. E., Retzlaff B. M., Fish B., Ma L., Ostlund R. E., Jr 2011. Plasma sterol evidence for decreased absorption and increased synthesis of cholesterol in insulin resistance and obesity. Am. J. Clin. Nutr. 94: 1182–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthan N. R., Pencina M., LaRocque J. M., Jacques P. F., D'Agostino R. B., Schaefer E. J., Lichtenstein A. H. 2009. Alterations in cholesterol absorption/synthesis markers characterize Framingham offspring study participants with CHD. J. Lipid Res. 50: 1927–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Himbergen T. M., Matthan N. R., Resteghini N. A., Otokozawa S., Ai M., Stein E. A., Jones P. H., Schaefer E. J. 2009. Comparison of the effects of maximal dose atorvastatin and rosuvastatin therapy on cholesterol synthesis and absorption markers. J. Lipid Res. 50: 730–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Descamps O. S., De Sutter J., Guillaume M., Missault L. 2011. Where does the interplay between cholesterol absorption and synthesis in the context of statin and/or ezetimibe treatment stand today? Atherosclerosis. 217: 308–321. [DOI] [PubMed] [Google Scholar]
- 18.Miettinen T. A., Gylling H. 2005. Effect of statins on noncholesterol sterol levels: implications for use of plant stanols and sterols. Am. J. Cardiol. 96(1A): 40D–46D. [DOI] [PubMed] [Google Scholar]
- 19.Hoenig M. R., Rolfe B. E., Campbell J. H. 2006. Cholestanol: a serum marker to guide LDL cholesterol-lowering therapy. Atherosclerosis. 184: 247–254. [DOI] [PubMed] [Google Scholar]
- 20.Friedewald W. T., Levy R. I., Fredrickson D. S. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18: 499–502. [PubMed] [Google Scholar]
- 21.Jakulj L., Vissers M. N., Tanck M. W., Hutten B. A., Stellaard F., Kastelein J. J., Dallinga-Thie G. M. 2010. ABCG5/G8 polymorphisms and markers of cholesterol metabolism: systematic review and meta-analysis. J. Lipid Res. 51: 3016–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee W. N., Byerley L. O., Bergner E. A., Edmond J. 1991. Mass isotopomer analysis: theoretical and practical considerations. Biol. Mass Spectrom. 20: 451–458. [DOI] [PubMed] [Google Scholar]
- 23.Gylling H., Miettinen T. A. 2002. Inheritance of cholesterol metabolism of probands with high or low cholesterol absorption. J. Lipid Res. 43: 1472–1476. [DOI] [PubMed] [Google Scholar]
- 24.Berge K. E., von Bergmann K., Lutjohann D., Guerra R., Grundy S. M., Hobbs H. H., Cohen J. C. 2002. Heritability of plasma noncholesterol sterols and relationship to DNA sequence polymorphism in ABCG5 and ABCG8. J. Lipid Res. 43: 486–494. [PubMed] [Google Scholar]
- 25.Stranberg T. E., Salomaa V., Vanhanen H., Miettinen T. A. 1996. Associations of fasting blood glucose with cholesterol absorption and synthesis in nondiabetic middle-aged men. Diabetes. 45: 755–761. [DOI] [PubMed] [Google Scholar]
- 26.Miettinen T. A., Gylling H. 2000. Cholesterol absorption efficiency and sterol metabolism in obesity. Atherosclerosis. 153: 241–248. [DOI] [PubMed] [Google Scholar]
- 27.Gremaud G., Dalan E., Piguet C., Baumgartner M., Ballabeni P., Decarli B., Leser M. E., Berger A., Fay L. B. 2002. Effects of non-esterified stanols in a liquid emulsion on cholesterol absorption and synthesis in hypercholesterolemic men. Eur. J. Nutr. 41: 54–60. [DOI] [PubMed] [Google Scholar]
- 28.Gylling G., Hallikainen M., Rajaratnam R. A., Simonen P., Pihlajamäki J., Laakso M., Miettinen T. A. 2009. The metabolism of plant sterols is disturbed in postmenopausal women with coronary artery disease. Metabolism. 58: 401–407. [DOI] [PubMed] [Google Scholar]
- 29.Merkens L. S., Jordan J. M., Penfield J. A., Lütjohann D., Connor W. E., Steiner R. D. 2009. Plasma plant sterol levels do not reflect cholesterol absorption in children with Smith-Lemli-Opitz syndrome. J. Pediatr. 154: 557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ostlund R. E., Jr 2002. Phytosterols in human nutrition. Annu. Rev. Nutr. 22: 533–549. [DOI] [PubMed] [Google Scholar]
- 31.Chan Y. M., Varady K. A., Lin Y., Trautwein E., Mensink R. P., Plat J., Jones P. J. 2006. Plasma concentrations of plant sterols: physiology and relationship with coronary heart disease. Nutr. Rev. 64: 385–402. [DOI] [PubMed] [Google Scholar]
- 32.Ooi E. M., Ng T. W., Chan D. C., Watts G. F. 2009. Plasma markers of cholesterol homeostasis in metabolic syndrome subjects with or without type-2 diabetes. Diabetes Res. Clin. Pract. 85: 310–316. [DOI] [PubMed] [Google Scholar]
- 33.Pihlajamäki J., Gylling H., Miettinen T. A., Laakso M. 2004. Insulin resistance is associated with increased cholesterol synthesis and decreased cholesterol absorption in normoglycemic men. J. Lipid Res. 45: 507–512. [DOI] [PubMed] [Google Scholar]
- 34.Miettinen T. A., Strandberg T. E., Gylling H. 2000. Noncholesterol sterols and cholesterol lowering by long-term simvastatin treatment in coronary patients: relation to basal serum cholestanol. Arterioscler. Thromb. Vasc. Biol. 20: 1340–1346. [DOI] [PubMed] [Google Scholar]
- 35.Pinedo S., Vissers M. N., von Bergmann K., Elharchaoui K., Lütjohann D., Luben R., Wareham N. J., Kastelein J. J., Khaw K. T., Boekholdt S. M. 2007. Plasma levels of plant sterols and the risk of coronary artery disease: the prospective EPIC-Norfolk Population Study. J. Lipid Res. 48: 139–144. [DOI] [PubMed] [Google Scholar]
- 36.Pouteau E., Piguet-Welsch C., Berger A., Fay L. B. 2003. Determination of cholesterol absorption in humans: from radiolabel to stable isotope studies. Isotopes Environ. Health Stud. 39: 247–257. [DOI] [PubMed] [Google Scholar]
- 37.Vuoristo M., Miettinen T. A. 1994. Absorption, metabolism, and serum concentrations of cholesterol in vegetarians: effects of cholesterol feeding. Am. J. Clin. Nutr. 59: 1325–1331. [DOI] [PubMed] [Google Scholar]
- 38.Igel M., Giesa U., Lutjohann D., von Bergmann K. 2003. Comparison of the intestinal uptake of cholesterol, plant sterols and stanols in mice. J. Lipid Res. 44: 533–538. [DOI] [PubMed] [Google Scholar]
- 39.Field F. J., Born E., Mathur S. N. 2004. LXR/RXR ligand activation enhances basolateral efflux of beta-sitosterol in CaCo-2 cells. J. Lipid Res. 45: 905–913. [DOI] [PubMed] [Google Scholar]
- 40.Plösch T., Bloks V. W., Terasawa Y., Berdy S., Siegler K., Van der Sluijs F., Kema I. P., Groen A. K., Shan B., Kuipers F., et al. 2004. Sitosterolemia in ABC-transporter G5-deficient mice is aggravated on activation of the liver-X receptor. Gastroenterology. 126: 290–300. [DOI] [PubMed] [Google Scholar]
- 41.Relas H., Gylling H., Miettinen T. A. 2001. Fate of intravenously administered squalene and plant sterols in human subjects. J. Lipid Res. 42: 988–994. [PubMed] [Google Scholar]
- 42.Tavani D. M., Nes W. R., Billheimer J. T. 1982. The sterol substrate specificity of acyl CoA: cholesterol acyltransferase from rat liver. J. Lipid Res. 23: 774–781. [PubMed] [Google Scholar]
- 43.Salen G., Shore V., Tint G. S., Forte T., Shefer S., Horak I., Horak E., Dayal B., Nguyen L., Batta A. K. 1989. Increased sitosterol absorption, decreased removal, and expanded body pools compensate for reduced cholesterol synthesis in sitosterolemia with xanthomatosis. J. Lipid Res. 30: 1319–1330. [PubMed] [Google Scholar]
- 44.Glueck C. J., Speirs J., Tracy T., Streicher P., Illig E., Vandegrift J. 1991. Relationships of serum plant sterols (phytosterols) and cholesterol in 595 hypercholesterolemic subjects, and familial aggregation of phytosterols, cholesterol, and premature coronary heart disease in hyperphytosterolemic probands and their first-degree relatives. Metabolism. 40: 842–848. [DOI] [PubMed] [Google Scholar]
- 45.Miettinen T. A., Klett E. L., Gylling H., Isoniemi H., Patel S. B. 2006. Liver transplantation in a patient with sitosterolemia and cirrhosis. Gastroenterology. 130: 542–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vanhanen H. T., Miettinen T. A. 1992. Effects of unsaturated and saturated dietary plant sterols on their serum contents. Clin. Chim. Acta. 205: 97–107. [DOI] [PubMed] [Google Scholar]
- 47.Naumann E., Plat J., Mensink R. P. 2003. Changes in serum concentrations of non-cholesterol sterols and lipoproteins in healthy subjects do not depend on the ratio of plant sterols to stanols in the diet. J. Nutr. 133: 2741–2747. [DOI] [PubMed] [Google Scholar]
- 48.Escurriol V., Cofán M., Moreno-Iribas C., Larrañaga N., Martínez C., Navarro C., Rodríguez L., González C. A., Corella D., Ros E. 2010. Phytosterol plasma concentrations and coronary heart disease in the prospective Spanish EPIC cohort. J. Lipid Res. 51: 618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salen G., Ahrens E. H., Jr, Grundy S. M. 1970. Metabolism of beta-sitosterol in man. J. Clin. Invest. 49: 952–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teupser D., Baber R., Ceglarek U., Scholz M., Illig T., Gieger C., Holdt L. M., Leichtle A., Greiser K. H., Huster D., et al. 2010. Genetic regulation of serum phytosterol levels and risk of coronary artery disease. Circ. Cardiovasc. Genet. 3: 331–339. [DOI] [PubMed] [Google Scholar]
- 51.Kempen H. J., de Knijff P., Boomsma D. I., van der Voort H. A., Gevers Leuven J. A., Havekes L. 1991. Plasma levels of lathosterol and phytosterols in relation to age, sex, anthropometric parameters, plasma lipids, and apolipoprotein E phenotype, in 160 Dutch families. Metabolism. 40: 604–611. [DOI] [PubMed] [Google Scholar]
- 52.Klasen E. C., Smit M., de Kniff P., Gevers Leuven J., Kempen-Voogd R., Havekes L. 1987. Apolipoprotein E phenotype and gene distribution in The Netherlands. Hum. Hered. 37: 340–344. [DOI] [PubMed] [Google Scholar]
- 53.Wu K., Bowman R., Welch A. A., Luben R. N., Wareham N., Khaw K. T., Bingham S. A. 2007. Apolipoprotein E polymorphisms, dietary fat and fibre, and serum lipids: the EPIC Norfolk study. Eur. Heart J. 28: 2930–2936. [DOI] [PubMed] [Google Scholar]
- 54.Simonen P., Gylling H., Howard A. N., Miettinen T. A. 2000. Introducing a new component of the metabolic syndrome: low cholesterol absorption. Am. J. Clin. Nutr. 72: 82–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.