Abstract
BACKGROUND
We used a multicenter retrospective cohort study design to evaluate whether human leukocyte antigen (HLA) antibody donor screening would reduce the risk of transfusion-related acute lung injury (TRALI) or possible TRALI.
STUDY DESIGN AND METHODS
In the Leukocyte Antibody Prevalence Study-II (LAPS-II), we evaluated pulmonary outcomes in recipients of 2596 plasma-rich blood components (transfusable plasma and plateletpheresis) sent to participating hospitals; half of the components were collected from anti-HLA–positive donors (study arm) and half from anti-HLA–negative donors (control arm) matched by sex, parity, and blood center. A staged medical record review process was used. Final recipient diagnosis was based on case review by a blinded expert panel of pulmonary or critical care physicians.
RESULTS
TRALI incidence was 0.59% (seven cases) in study arm recipients versus 0.16% (two cases) in control arm recipients for an odds ratio (OR) of 3.6 (95% confidence interval [CI], 0.7–17.4; p = 0.10). For possible TRALI cases (nine study arm, eight control arm), the OR was 1.2 (95% CI, 0.4–3.0; p = 0.81), and for TRALI and possible TRALI aggregated together, it was 1.7 (95% CI, 0.7–3.7; p = 0.24). Transfusion-associated circulatory overload incidence was identical in the two arms (1.17 and 1.22%, respectively; OR, 1.0; p = 1.0).
CONCLUSIONS
TRALI incidence in recipients of anti-HLA–positive components was relatively low for a look-back study (1 in 170) and was higher than in the control arm, but did not reach significance. Based on this trend, the data are consistent with the likelihood that TRALI risk is decreased by selecting high-volume plasma components for transfusion from donors at low risk of having HLA antibodies.
Donor-based risk reduction interventions for transfusion-related acute lung injury (TRALI) have been widely adopted in the United States and elsewhere in the past several years.1–5 These strategies are based on data indicating that TRALI has continued to be the leading cause of blood transfusion-related deaths in the United States,6 the consensus that approximately 80% of TRALI cases are mediated by donor human leukocyte antigen (HLA) antibodies,7 and surveillance system findings that TRALI cases are reduced by use of such risk reduction strategies.2,8,9 Thus, as of 2009, almost all US blood centers are transfusing plasma supplied primarily by male or never-pregnant female donors, and many centers are screening previously pregnant platelet (PLT) and/or plasma apheresis donors for HLA antibody.1,9
Most transfused components containing HLA antibody do not result in recipients developing TRALI. Reasons for this include transfusion to a recipient who does not have a cognate HLA antigen profile, as well as less established factors such as the titer and/or other undefined characteristics of the specific transfused HLA antibody or the existence of underlying predisposing recipient risk factors (first hit). Most current theories of TRALI pathogenesis agree that two hits are usually necessary for TRALI to occur: the first is a recipient condition that results in neutrophil priming and the second is the infusion of a biologic response modifier that activates primed neutrophils. The biologic response modifier can be an HLA antibody, an HNA antibody, or several candidate nonantibody substances, such as bioactive lipids.10–12 In terms of this model, HLA antibody–containing components may fail to cause TRALI in the absence of a first hit.
At least four studies have assessed outcomes in recipients of previously donated components from HLA antibody–positive donors who were implicated as causing TRALI in an index recipient.13–16 In aggregate, these studies detected five new cases of TRALI that were not reported to the blood bank when records of 171 patients were reviewed (2.9%). In addition, two lookback studies were reported on donors who were HLA antibody positive but had not been implicated in TRALI. In one of these studies, 62 HLA antibody–positive female donors gave a total of 211 blood components, and only one case of TRALI (which had previously been reported to the blood bank) was identified.17 In the second study, no TRALI cases were detected in 167 recipients of transfusions from four HLA antibody–positive donors or in 295 recipients from 12 HLA antibody–negative donors. However, this conclusion appeared to be based solely on review of transfusion service records.18 One additional study did not detect TRALI in any of 265 recipients of either HLA antibody–positive or HLA antibody–negative plateletpheresis units but did not clearly delineate the number of recipients in each group.19
Taken as a group, these lookback studies have significant limitations in that they used differing methods for HLA antibody screening, reviewing recipient outcome data and for diagnosing TRALI; they had small sample sizes considering the low incidence of TRALI; and most did not evaluate TRALI occurrence in recipients of control components using a blinded study design. Since it is well known that TRALI is both underrecognized and underre-ported,20 it is unclear if the findings in these studies can be generalized because methods for ascertainment were not robust.
As part of the Retrovirus Epidemiology Donor Study-II (REDS-II), we previously conducted the Leukocyte Antibody Prevalence Study-I (LAPS-I), which identified 1032 whole blood and apheresis donors with HLA antibody.21 Since LAPS-I was conducted before the implementation of TRALI risk reduction policies, donations from these HLA antibody–positive donors were transfused to recipients. Given the limitations of previous studies noted above and the recognition that the recent implementation of TRALI risk reduction strategies will reduce the possibility of conducting future large-scale TRALI lookback studies, we designed a large multicenter retrospective cohort study termed the Leukocyte Antibody Prevalence Study-II (LAPS-II). Based on surveillance data showing a higher TRALI risk from transfused plasma components, LAPS-II was confined to high-plasma-volume components (fresh-frozen plasma and other transfusable plasma manufactured from whole blood or apheresis as well as plateletpheresis components).2,8,9
LAPS-II was designed to evaluate a primary endpoint of combined incidence of TRALI and possible TRALI in study recipients of at least one HLA antibody–positive high-plasma-volume component versus control recipients of at least one HLA antibody–negative high-plasma-volume component. Secondary endpoints were the association of HLA antibody class, HLA antibody specificity, HLA antibody screening test signal strength, and concomitant HNA antibody on TRALI occurrence.
MATERIALS AND METHODS
LAPS-II was conducted as part of the National Heart, Lung, and Blood Institute's (NHLBI) REDS-II program. The REDS-II coordinating center (Westat, Rockville, MD) and five REDS-II blood centers participated in LAPS-II; these included the American Red Cross Southern Region (Douglasville, GA); BloodCenter of Wisconsin (Milwaukee, WI); Blood Centers of the Pacific (San Francisco, CA); Hoxworth Blood Center, University of Cincinnati Academic Health Center (Cincinnati, OH); and the Institute for Transfusion Medicine (Pittsburgh, PA). Each blood center recruited a set of participating hospitals in its region. In total, 42 hospitals participated; these hospitals received approximately 50% of the high-plasma-volume components issued by the five blood centers (51% of transfusable plasma and 48% of plateletpheresis).
Donor HLA antibody status was obtained from the previously conducted LAPS-I study, which determined the prevalence of HLA and neutrophil antibodies in whole blood and apheresis donors enrolled from November 2006 through May 2007.21,22 In LAPS-I, screening tests for HLA Class I and Class II antibodies were performed on an ethylenediaminetetraacetate plasma sample using multi-antigen bead kits (LabScreen LSM12, LabScreen Mixed, One Lambda, Canoga Park, CA). The version of the manufacturer's package insert available at the time indicated that the test cutoff either be set at a normalized background ratio (NBG) of 2.2 or that the cutoff be independently established by the individual testing laboratory. LAPS-I cutoff values were determined by calculating the mean plus three standard deviations of the natural log-transformed distribution of NBG values in the enrolled cohort of 1138 nontransfused male blood donors; assay cutoffs were thereby set at NBG values of greater than 10.8 for Class I and greater than 6.9 for Class II. For purposes of selecting control donors for LAPS-II, HLA antibody–negative donors were defined as donors with NBG values of less than 2.2. Further testing to determine the specificity of HLA antibodies was performed using the One Lambda LS1A04 or LS2A01 single-antigen bead assays; the LAPS-I assay cutoffs for a positive result were set at a median fluorescence intensity of greater than 2500 for Class I and greater than 1500 for Class II.23
Components targeted for LAPS-II investigation were selected as follows. Each blood center performed a record search to trace all high-plasma-volume components donated either at the time of enrollment (designated as index components), subsequent to the index donation but before December 2008, or within the 2 years before the index donation by LAPS-I HLA antibody–positive donors (Fig. 1). Components distributed to participating hospitals were eligible for inclusion in the study. Based on sample size calculations (see below), 1298 HLA antibody–positive components were selected. The initial intent was to divide these equally among the five blood centers; however, since one center had only 103 eligible HLA antibody–positive components, the remainder were divided among the other four centers such that each of these centers was assigned 299 HLA antibody–positive components. Because the number of HLA antibody–positive components available at each of these four centers exceeded this required number, a sampling scheme was utilized. All index components (those donations that tested as HLA antibody positive) and subsequent components (donated after the date of a positive HLA antibody result) were included and the remainder of each center's quota was then filled by components from previous donations. A similar process was followed for the sample of 1298 matched HLA antibody–negative components; matching was performed by donor sex, donor parity in female donors, blood center, and timing of donation (index, subsequent, or previous).
Fig. 1.
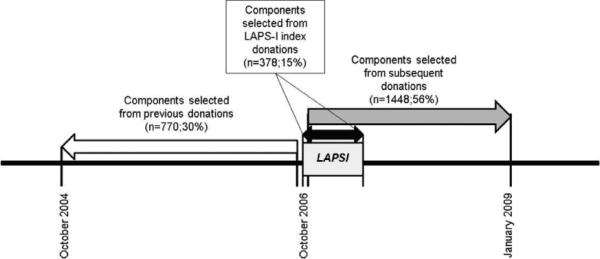
LAPS-II timeline. Components in the control arm were matched by the donor's sex, parity, center, and time period to the components in the study arm.
Recipient data from the hospital admission in which the LAPS-II component was transfused were extracted from medical, radiographic, and transfusion service electronic and/or paper records. Data extraction was performed by dedicated study personnel who were blinded to study versus control arm status of the LAPS-II components. Before data collection, LAPS-II data coordinators attended a training course conducted by personnel at University of California San Francisco (UCSF) who had previously employed similar data extraction procedures as part of a prospective TRALI incidence study conducted under the auspices of an NHLBI sponsored Specialized Centers of Clinically Oriented Research (SCCOR).24
Given the large number of components and recipients to be studied, the retrospective medical review protocol relied on a sequential method of data collection based on halting data collection when a specific criterion required for the diagnosis of TRALI (according to the commonly accepted Canadian Consensus Conference definition) had not been met.25 This scheme is illustrated in Fig. 2. Initial data extraction involved completion of a short data form that collected information pertaining to recipient sex, recipient ABO/Rh type, recipient diagnoses (up to 10 ICD-9 codes), whether a chest X-ray had been performed before and within 24 hours after transfusion of the LAPS-II component, and whether a transfusion reaction had been reported to the hospital transfusion service. If a posttransfusion chest X-ray had been performed, the verbatim pre- and posttransfusion X-ray reports were provided to the principal investigator at each blood center who evaluated whether the findings were compatible with bilateral pulmonary edema. If not, data collection was halted. If yes, more extensive chart review was conducted with the next triage criteria being the presence of hypoxemia within 6 hours of transfusion of the LAPS-II component (defined as a PaO2/FiO2 ratio of <300 or an oxygen saturation of <90% by pulse oximetry). If hypoxemia was present, a detailed extended data collection form was completed. Data elements included recipient demographics, recipient medical history and clinical findings, whether a diagnosis of TRALI or adult respiratory distress syndrome (ARDS) had been made clinically, presence of acute lung injury (ALI) risk factors, sequential chest X-ray information, congestive heart failure variables, fluid overload variables (including other components transfused and estimated intraoperative blood loss), arterial blood gases, complete blood counts, and other laboratory information. These data were then reviewed by a single critical care physician blinded to the HLA status of the LAPS-II component; this step was employed to reduce the workload of the expert panel (see below). This physician determined whether there was evidence of new or worsening bilateral pulmonary edema that could be compatible with TRALI. If so, then a third data collection activity was conducted. In this step, local study personnel prepared a detailed sequential (minute-by-minute) description of events proximate to the time of hypoxemia and transfusion of the LAPS-II component along with a summary of the patient's illness before transfusion, the posttransfusion clinical course, and a complete transfusion history. Available digital images of all chest X-rays were obtained and, in combination with the other extracted data, were included in a PowerPoint case summary file provided to members of a blinded expert adjudication panel.
Fig. 2.
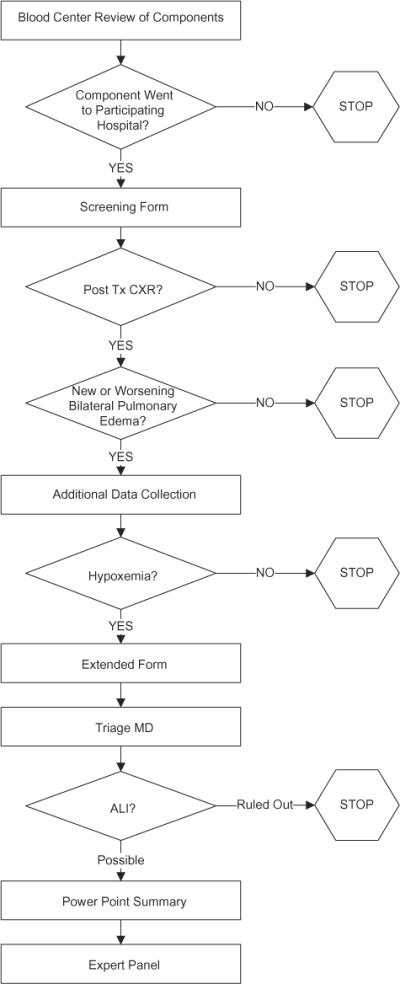
Flow chart of data acquisition at various stages of retrospective medical record review. CXR = chest X-ray; Tx = transfusion.
The expert panel consisted of three pulmonary or critical care specialists (OG, RH, and ML) who each had previous involvement with TRALI research studies. Each case was reviewed by two of the three experts. If both experts independently arrived at the same diagnosis, the case was assigned this diagnosis. However, if the two experts made different diagnoses, the case was initially classified as nonconcordant and sent to the third panel member for review. All nonconcordant cases were discussed by all three experts on regularly scheduled conference calls where a consensus diagnosis was assigned.
All recipients whose cases were reviewed by the expert panel were assigned one of the following final diagnoses: TRALI, possible TRALI, cannot distinguish between TRALI and transfusion-associated circulatory overload (TACO; designated as TRALI/TACO), TACO, and “other.” Criteria for each diagnosis are specified in Table 1.
TABLE 1.
Expert panel diagnostic classification
| Diagnosis | Criteria |
|---|---|
| TRALI | Conforms to the Canadian Consensus Conference definition |
| No ALI risk factors and no existing ALI | |
| Cases with a possible finding of fluid overload could be classified as TRALI if the panel judged that ALI was present | |
| Possible TRALI | Posttransfusion ALI occurring in the presence of an ALI risk factor listed in the Canadian Consensus Conference publication |
| TRALI/TACO | Bilateral pulmonary edema and hypoxemia with insufficient information on the PowerPoint file to differentiate between a diagnosis of TACO and TRALI (usually insufficient clinical evidence to rule out TACO) |
| TACO | Posttransfusion hypoxemia due to hydrostatic pulmonary edema as judged by the expert panel's evaluation of fluid balance, vascular pressures, cardiac and renal function, and other comorbidities |
| Other | Pulmonary edema before transfusion of the LAPS-II component due to ALI or other causes |
| Pulmonary edema at an interval of greater than 8 hours posttransfusion* of the LAPS-II component due to ALI or other causes | |
| Diagnosis other than ALI or TACO |
An 8-hour rather than a 6-hour interval was chosen based on the premise that the objective measurements (blood gas, oxygen saturation, or chest X-ray) used to define the time from transfusion to the onset of TRALI could have been somewhat delayed (due to the time taken to order and perform such tests) relative to the time of onset of TRALI symptoms.
Additional data were obtained for cases classified as TRALI, possible TRALI, and TRALI/TACO. Data concerning recipient admitting diagnosis, outcome, clinical course, and number of components transfused within 6 hours of the study unit were abstracted from the Power-Point files by two REDS-II investigators (RK and SK). Hospital records (recipient charts and HLA testing laboratory records) were searched to determine if any of these recipients had HLA antigen typing results on file. The HNA antibody status of the LAPS-II donors whose components were transfused to these recipients was determined by testing LAPS-I frozen repository samples using flow cytometry methods previously described.22
Human subjects
The LAPS-II study protocol was approved by each participating blood center and hospital institutional review board as well as the Westat institutional review board. No patient identifiers were forwarded from the hospitals to either the blood centers or the coordinating center and no patients were contacted.
Statistical analysis
Sample size calculation
The sample size calculation was based on estimating the number of cases of TRALI and possible TRALI that would be likely to occur in the HLA antibody–positive (designated as the study arm) and HLA antibody–negative (designated as the control arm) arms. The starting point for this calculation was a study by Finlay and colleagues,26 which identified three TRALI and four possible TRALI cases in 820 recipients of 6888 components (including plasma, PLTs, and red blood cells [RBCs]) of unknown HLA antibody status, yielding a TRALI/possible TRALI rate of 0.85% (7/820). These data were combined with several assumptions (e.g., 80% of TRALI is antibody-mediated, high-volume-plasma components carry a sixfold higher risk of TRALI than low-plasma components, the average transfusion episode consists of six components) to determine that a sample size of 1175 recipients in each arm would have 90% power to detect a difference in rates of TRALI/possible TRALI, which were projected to be 2.3% among the study arm recipients (30 cases) and 0.8% (10 cases) among the control arm recipients. Based on a 10% buffer for unavailable records or nontransfused components, a final sample size of 2596 components with 1298 in each arm was used.
Other statistics
Diagnostic outcomes (e.g., TRALI) were compared by arm (study arm vs. control arm) using exact p values based on the likelihood ratio statistic (StatXact 9.0, Cytel, Cambridge, MA). Additional covariates (e.g., sex/parity) were similarly individually tested. Due to the small number of TRALI cases (and small number of other diagnostic outcomes) no models involving multiple covariates were developed. The interrate agreement among the three members of the expert panel was assessed using Cohen's kappa. Ordinal outcomes (e.g., number of components in the transfusion episode) were compared across groups using the Kruskal-Wallis test.
RESULTS
The 2596 components selected for study were donated by 853 donors at the five participating REDS-II centers for a mean of three components per donor. Transfusable plasma products accounted for 55% of components and plateletpheresis for 45%. For the 682 donors who made at least one plasma donation, the mean number of components included in LAPS-II was 1.9 whereas for the 220 donors with at least one plateletpheresis donation the mean number was 5.5. With regard to timing, 15% of components were index donations, 56% were subsequent donations, and 30% were previous donations (Fig. 1).
The baseline characteristics of recipients of study and control arm components showed no statistical differences for any variables including sex, type of component, ABO/Rh type, percentage of recipients with pre- and posttransfusion chest X-rays, and ICD-9 diagnostic codes. The 10 most prevalent ICD-9 codes in study and control arm recipients are listed in Table 2.
TABLE 2.
Ten most prevalent ICD-9 codes in LAPS-II recipients
| Diagnosis | Percentage in total study | Percentage in study arm | Percentage in control arm |
|---|---|---|---|
| 518: Other diseases of lung | 4.1 | 4.1 | 4.0 |
| 276: Disorders of fluid, electrolyte, and acid-base balance | 3.0 | 3.1 | 3.0 |
| 584: Acute kidney failure | 2.9 | 3.2 | 2.7 |
| 427: Cardiac dysrhythmias | 2.2 | 2.0 | 2.5 |
| 286: Coagulation defects | 1.8 | 1.9 | 1.8 |
| 038: Septicemia | 1.8 | 1.7 | 1.8 |
| 285: Other and unspecified anemias | 1.8 | 1.8 | 1.7 |
| 287: Purpura and other hemorrhagic conditions | 1.8 | 1.7 | 1.8 |
| 996: Complications peculiar to certain specified procedures | 1.7 | 2.0 | 1.4 |
| 998: Other complications of procedures, not elsewhere classified | 1.6 | 1.6 | 1.6 |
Figure 3 describes the results of the stepwise retrospective recipient investigations. Initial adjustments in each arm resulted in 2428 eligible recipients who received only one LAPS-II component: 1195 were in the study arm and 1233 were in the control arm. A chest X-ray within 24 hours of transfusion was on record for 1188 recipients (49%), resulting in their progressing to the next stage of evaluation where 246 (21% of those with X-rays) had a finding of possible bilateral pulmonary edema. Of these, 173 (70% of those with possible pulmonary edema) had documented hypoxemia and 112 (65% of those with hypoxemia) were judged by the triage physician to have chest X-ray findings that could be consistent with TRALI. These 112 recipient cases, representing 4.6% of the 2428 eligible recipients, were sent to the expert panel for final diagnostic review. The percentage of recipient cases that completed the protocol at each stage did not differ between study and control arms.
Fig. 3.
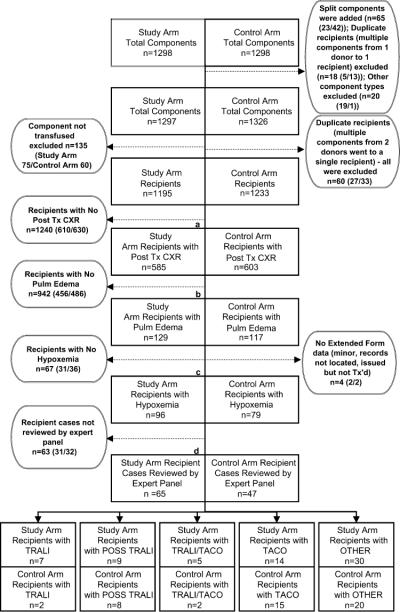
Results for LAPS-II component tracing and recipient medical record review in study and control arms. Boxes indicate number of recipients progressing through each stage of data collection. Circles indicate number of recipients completing data review at each stage and provide further details about recipients not progressing to the next stage of data collection. Due to space limitations, circles are displayed on either side of the primary flow chart. Within each circle, numbers in parentheses indicate study and control arm recipients. Split components are donations that were divided into two or more components that were transfused to different LAPS-II recipients, duplicate recipients are recipients who received more than one LAPS-II component and were excluded from the study for that reason, and other component types are components that were initially classified as high-plasma-volume components but which were volume reduced before transfusion. The percentage of study arm and control arm recipients who completed data collection at various junctures of the protocol was equivalent by exact p value of likelihood ratio statistic: (a) p = 0.98 for study or control arm by posttransfusion chest X-ray or no posttransfusion chest X-ray; (b) p = 0.26 for study or control arm by pulmonary edema or no pulmonary edema; (c) p = 0.23 for study or control arm by full or partial extended review; (d) p = 0.32 for study or control arm by expert panel review or no expert panel review. CXR = chest X-ray; Tx = transfusion.
Final diagnoses for the 112 recipients are given in Table 3. The most frequent diagnosis was “other” (pulmonary syndromes probably unrelated to the transfusion of the LAPS-II component; 45%), followed by TACO (26%), possible TRALI (15%), TRALI (8%), and TRALI/TACO (6%). There were nine TRALI cases (seven in the study arm, two in the control arm) and 17 possible TRALI cases (nine in the study arm, eight in the control arm). TRALI incidence in the study arm was 0.59% versus 0.16% in the control arm; this gave an odds ratio (OR) of 3.6 (95% confidence interval [CI], 0.7–17.4) and a p value that was not significant (p = 0.10). The OR for possible TRALI cases was 1.2 (95% CI, 0.4–3.0; p = 0.81), and for TRALI and possible TRALI aggregated together, it was 1.7 (95% CI, 0.7–3.7; p = 0.24). The rate of TACO was almost identical in the two arms (1.17 and 1.22%, respectively; OR, 1.0; p = 1.0) while the rate of the diagnostic category of other was somewhat higher in the study arm (2.51 and 1.62%, respectively; OR, 1.6; p = 0.15). Concordant diagnosis from the initial independent review by two expert panel members was 49.1%; this computes to a kappa statistic of 0.31 (95% CI, 0.18–0.44). After teleconference review and discussion, the three-member-panel reached agreement on all cases.
TABLE 3.
Recipient diagnoses by HLA antibody status of transfused LAPS-II component
| Diagnosis | Number of cases in both arms | Study (HLA antibody positive) arm (n = 1195)* | Control (HLA antibody negative) arm (n = 1233)* | OR (95% CI) | p value (exact test) |
|---|---|---|---|---|---|
| TRALI | 9 | 7 (0.59) | 2 (0.16) | 3.6 (0.7–17.4) | 0.10 |
| Possible TRALI | 17 | 9 (0.75) | 8 (0.65) | 1.2 (0.4–3.0) | 0.81 |
| TRALI or possible TRALI | 26 | 16 (1.34) | 10 (0.81) | 1.7 (0.7–3.7) | 0.24 |
| TRALI/TACO | 7 | 5 (0.42) | 2 (0.16) | 2.6 (0.5–13.4) | 0.28 |
| TACO | 29 | 14 (1.17) | 15 (1.22) | 1.0 (0.4–2.0) | 1.00 |
| Other | 50 | 30 (2.51) | 20 (1.62) | 1.6 (0.8–2.7) | 0.15 |
Data are reported as number (%).
Details of each TRALI case are given in Table 4. Four of the nine cases had been clinically diagnosed; however, two of these were part of the prospective NHLBI SCCOR conducted at UCSF and it is unclear if their diagnosis was made because of participation in the SCCOR study. All four of these cases were reported to the transfusion service as having transfusion reactions. One additional case was clinically diagnosed as ARDS but not as TRALI. Recipient HLA antigen typing was available for two of the seven TRALI study arm cases; in both cases there was a cognate antigen-antibody match based on the cross-reactive nature of the DR3 antibody specificity with other DR antigens. Two deaths occurred in the study arm TRALI cases but the extent to which TRALI contributed to recipient mortality in these cases is unclear.
TABLE 4.
Details of TRALI cases
| Case | Arm | Age/sex | Admitting diagnosis | LAPS-II component* | Donor HLA antibody specificity | Recipient HLA type | Clinical TRALI noted† | Reaction reported to transfusion service | Other components transfused
within 6 hr |
Posttransfusion fluid balance | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasma | All | ||||||||||
| 1 | Study | 69/F | Renal Ca surgery | Plasma | DQ7 | NA | No | No | 5 | 28 | Pos |
| 2 | Study‡ | 58/M | Thoracic aorta dissection | Plasma | DR10,DR53§ | NA | Yes | Yes | 5 | 20 | NA |
| 3 | Study‡ | 21/M | Spinal surgery | PLT | A68 | NA | Yes | Yes | 22 | 30 | Pos |
| 4 | Study | 39/M | Abdominal surgery | Plasma | Multiple∥ | NA | No; ARDS |
No | 1 | 1 | Pos |
| 5 | Study | 78/F | Subdural hematoma | Plasma | DR3¶ | A2,A74 B53,B72 DR13,DR52 DQ6, DQ7 |
Yes | Yes | 1 | 1 | Neg |
| 6 | Study | 79/M | Subdural hematoma | Plasma | DR3,DR7,DR8,DR11,DR12,DR13 | NA | No | No | 1 | 2 | Neg |
| 7 | Study | 70/F | Liver transplant | Plasma | B8, B15, B37, B57, DR3¶ | A1, B8, DR17, DQ2 | No | No | 1 | 15 | Pos |
| 8 | Control | 58/M | Alcohol enceph and acute renal failure | Plasma | Negative | NA | No | No | 0 | 0 | Neg |
| 9 | Control | 82/F | GI bleed | Plasma | Negative | NA | Yes | Yes | 1 | 1 | Neg |
Eight LAPS-II components were from females with three or more pregnancies and one was from a donor with two pregnancies. Three cases received index LAPS-II components, three received subsequently donated components, and three received previously donated components.
All cases except Case 8 required mechanical ventilation. In-hospital death occurred in Cases 1 and 5. Frothy endotracheal tube secretions were observed in Cases 3 and 6.
These two recipients were also part of the NHLBI SCCOR prospective TRALI study.
The HLA antibody screening assay was strongly positive for Class I antibody but the SAB assay failed to define a Class I specificity.
The multiple antibody specificities were B13, B15, B27, B41, B44, B45, B46, B40, B47, B49, B50, B53, Cw1, Cw3, Cw4, Cw14, Cw18, DR3, DR8, DR11, DR12, DR13, DR14, DQ6, DQ8, DQ9; this donor also had a nonspecific HNA antibody.
DR3 antibody crossreacts with DR13, DR52 (Case 5), and DR17 antigens (Case 7).
NA = not available.
Four of the LAPS-II donors linked to the recipients with TRALI had high-plasma-volume components from other donations included in LAPS-II. There were no cases of TRALI, possible TRALI, or TRALI/TACO identified in 34 additional recipients transfused with other high-plasma-volume components donated by these four donors.
TRALI incidence was not statistically different in recipients of HLA Class I only, HLA Class II only, and dual HLA Class I and II antibody–positive components; incidence was 0.33, 0.53, and 0.91%, respectively (p = 0.37). In the study arm, TRALI cases did not differ from non-TRALI cases in the mean NBG value of the HLA antibody screening assay or in the mean number of HLA specificities identified (Fig. 4).
Fig. 4.
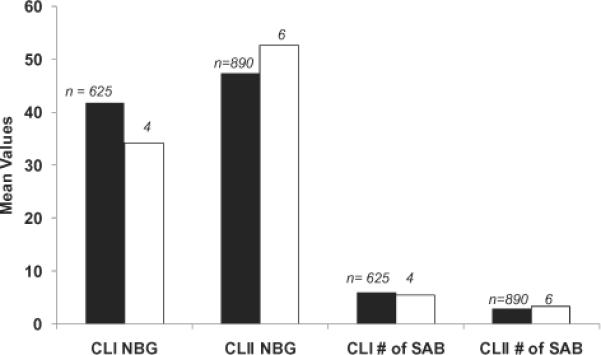
HLA antibody NBG screening value and number of single-antigen bead (SAB) reactivities in LAPS-II donations that were transfused into recipients who developed TRALI or did not develop TRALI. (∎) No TRALI; (◻) TRALI. There was no significant difference (using Kruskal-Wallis test) between TRALI and non-TRALI cases for NBG signal strength or number of SAB reactivities. Of the seven study arm recipients with TRALI, one received a LAPS-II component with Class I (CLI) antibody only, three received LAPS-II components with Class II (CLII) antibody only, and three received LAPS-II components with Class I and Class II antibody.
ALI risk factors in the 17 possible TRALI cases were sepsis (seven cases), aspiration (four cases), trauma (two cases), pancreatitis (one case), burn (one case), trauma and pneumonia (one case), and extended cardiopulmonary bypass (one case). One study arm case had been clinically diagnosed as suspected TRALI and had been reported to the transfusion service and blood center. This clinical reporting resulted in an HLA antigen typing being obtained on the recipient. The donor of the LAPS-II component (a plateletpheresis unit) was demonstrated to have HLA antibodies that were cognate to the HLA antigen profile of the recipient. None of the other cases of possible TRALI were identified as suspected cases at the time of their occurrence.
The median number of additional plasma and total components transfused within 6 hours of the first pulmonary finding (or during the surgical episode if precise times of transfusion could not be determined) in the 33 cases of TRALI, possible TRALI, and TRALI/TACO are shown in Fig. 5. The median number was highest in the TRALI/TACO cases and lowest in the TRALI cases; however, these trends did not reach significance (p = 0.11 for plasma and p = 0.05 for all components). As shown in Table 3, there was substantial variability in the total number of components transfused to the nine TRALI cases. Of note is that some recipients received limited number of components; e.g., one control arm case received only the LAPS-II component, one other control arm case received a total of two components, and three study arm cases received two or three components. In four study arm cases, the number of transfused components ranged from 16 to 31. Two study arm and both control recipients were transfused only with plasma whereas the component mix for the other five study arm recipients included plasma, PLTs, and RBCs.
Fig. 5.
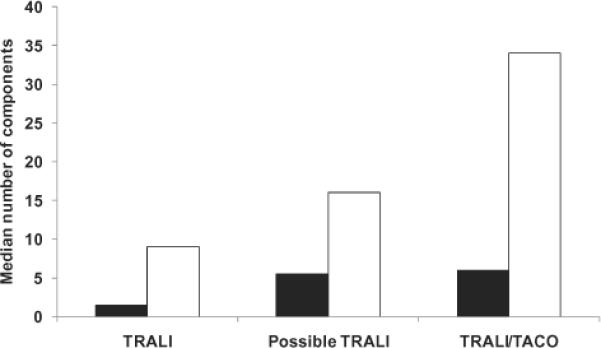
Median number of other components transfused within 6 hours of the first pulmonary finding. (∎) Plasma; (◻) all components. There was no significant difference among the three groupings (TRALI, possible TRALI, TRALI/TACO) in terms of the median number of plasma components (p = 0.11), nor in terms of the median number of all types of components (p = 0.05).
HNA antibody was negative in 32 of the 33 LAPS-II donors whose components were transfused to TRALI, possible TRALI, or TRALI/TACO recipients. The only HNA antibody–positive donor had an HNA immunoglobulin G antibody that was nonspecific, but this donor also had multiple HLA Class I and Class II antibodies.
We performed two additional exploratory analyses for TRALI and possible TRALI cases. First, we determined the incidence of these conditions separately and in aggregate by the sex and parity of the LAPS-II donor (Table 5). For TRALI cases, incidence in the study arm, the control arm, and the total data set was highest when the LAPS-II donor had three or more pregnancies; however, this observation did not achieve significance (p = 0.13). For possible TRALI cases, no such difference was observed. Second, we determined incidence by the LAPS-II component type (plasma vs. plateletpheresis; Table 6). TRALI incidence in LAPS-II plasma recipients was 0.62% compared to 0.09% in plateletpheresis recipients, giving an OR of 7.1 (95% CI, 0.9–314.6) and a p value of borderline significance at a p value of 0.06. In contrast, the rate of possible TRALI was approximately equal in recipients of both types of products with an OR of 0.8 (95% CI, 0.3–2.3).
TABLE 5.
TRALI and possible TRALI diagnoses by sex, parity, and HLA antibody of the LAPS-II donor whose component was transfused*
| Study |
Control |
Total |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Females with
pregnancies |
Females with
pregnancies |
Females with
pregnancies |
|||||||||||
| Diagnosis | Male and never-pregnant females (n = 99) | One pregnancy (n = 99) | Two pregnancies (n = 328) | Three or more pregnancies (n = 669) | Male and never-pregnant females (n = 103) | One pregnancy (n = 103) | Two pregnancies (n = 349) | Three or more pregnancies) (n = 678) | Male and never-pregnant females (n = 202) | One pregnancy (n = 202) | Two pregnancies (n = 677) | Three or more pregnancies (n = 1347) | p value† (exact test) |
| TRALI | 0 | 0 | 1 (0.30) | 6 (0.90) | 0 | 0 | 0 | 2 (0.29) | 0 | 0 | 1 (0.15) | 8 (0.59) | 0.13 |
| Possible TRALI | 1 (1.01) | 2 (2.02) | 2 (0.61) | 4 (0.60) | 0 | 0 | 3 (0.86) | 5 (0.74) | 1 (0.50) | 2 (0.99) | 5 (0.74) | 9 (0.67) | 0.97 |
| TRALI or possible TRALI | 1 (1.01) | 2 (2.02) | 3 (0.91) | 10 (1.49) | 0 | 0 | 3 (0.86) | 7 (1.03) | 1 (0.50) | 2 (0.99) | 6 (0.89) | 17 (1.26) | 0.71 |
Data are reported as number (%).
p value is computed only among the total columns and compares rates among the four sex/parity classifications.
TABLE 6.
Recipient diagnoses by LAPS-II component type
| Diagnosis | Plasma (n = 1291)* | Plateletpheresis (n = 1137)* | OR (95% CI) | p value (exact test) |
|---|---|---|---|---|
| TRALI | 8 (0.62)† | 1 (0.09) | 7.1 (0.9–314.6) | 0.06 |
| Possible TRALI | 8 (0.62) | 9 (0.79) | 0.8 (0.3–2.3) | 0.63 |
| TRALI or possible TRALI | 16 (1.24) | 10 (0.88) | 1.4 (0.6–3.5) | 0.43 |
Data are reported as number (%).
Three were from plasma collected by apheresis and five from plasma manufactured from whole blood.
In seven cases (five study arm and two control arm), the expert panel could not distinguish between a diagnosis of TRALI or TACO due primarily to incomplete clinical data especially regarding fluid balance. As shown in Fig. 5, the median number of components transfused to these recipients was higher than in the TRALI and possible TRALI cases. None of these cases had other ALI risk factors. Due to the lack of a firm diagnosis and the small number of such cases, these were not included in a sensitivity analysis of TRALI incidence.
DISCUSSION
To our knowledge, this is the largest retrospective study measuring incidence of TRALI in recipients of HLA antibody–positive components. It differs in whole or in part from previous lookback studies in several ways. Most LAPS-II study arm recipients (70%) were transfused with known HLA antibody–positive components or with components donated subsequent to HLA antibody detection. To maximize TRALI incidence, LAPS-II was confined to recipients of high-plasma-volume components. A carefully matched set of control donors was selected in order to be able to account for TRALI cases due to nonantibody mechanisms or to transfusions of non–LAPS-II components as well as to evaluate study robustness by comparing medical diagnoses that would be expected to occur equally in the study and control arms. Recipients in the study and control arms were documented to have similar baseline characteristics. All study personnel retrieving and evaluating recipient data were blinded to donor HLA antibody results. An expert panel of three experienced pulmonary and critical care physicians applied the Canadian Consensus Conference criteria for TRALI and possible TRALI to arrive at consensus diagnoses.
One major finding of this study was that TRALI incidence in recipients of HLA antibody positive high-plasma-volume components was 0.59% (1 in 170). This is somewhat higher than the aggregate result of 0.26% in two previously published studies of 378 recipients of components from HLA antibody–positive donors but lower than the aggregate results of 2.9% from four published studies of donors implicated in TRALI cases.13–18 LAPS-II data corroborate the consensus finding that TRALI occurs only infrequently after the infusion of components containing HLA antibodies.
As stated in the original Canadian Consensus Conference publication, the category of possible TRALI was established in order to differentiate definite TRALI cases from those that occur after transfusion but which have a substantial likelihood of being caused by another etiologic factor for ALI.25 In LAPS-II, TRALI and possible TRALI cases segregated differently in the study and control arms: the OR for TRALI between study and control arms was 3.6 whereas it was 1.2 for possible TRALI. Almost all of the possible TRALI cases in each arm had medical conditions associated with a high rate of ALI development. Thus, one plausible interpretation of LAPS-II data is that the reason possible TRALI was unrelated to the HLA antibody status of the transfused LAPS-II component was because the ALI was not a result of transfusion.
These considerations are important for interpreting the observation that while TRALI was more common in recipients of a known HLA antibody–positive component than in recipients of a known HLA antibody–negative component, this finding did not reach significance. It is plausible that this trend may reflect a true difference, but that the lack of significance was because LAPS-II was not appropriately powered for TRALI as an endpoint. The original sample size calculations were based on the projected aggregate incidence of TRALI and possible TRALI in the two arms, which in retrospect may have been inappropriate. Thus it is possible that a larger study would have found a significant association and based on results from other studies, we favor this explanation.2,9 The second possible explanation is that there is no strong association between donor HLA antibodies and TRALI. Third, it is possible that the association of HLA antibodies with TRALI was confounded (masked) by the fact that recipients were transfused with multiple non–LAPS-II components of unknown HLA antibody status in the relevant time interval.
While the presence of donor HLA antibody does not establish that TRALI resulted through this mechanism, this interpretation is strengthened when the recipient is demonstrated to have cognate HLA antigen. This cognate relationship was present in the two cases where recipient HLA antigen test results were recorded but could not be evaluated in the remaining five cases that lacked recipient HLA antigen data.
It has been speculated that TRALI occurrence may be dependent on HLA antibody class and/or antibody strength and the latter association has been reported in a recent study from Japan.27 However, in LAPS-II there was no significant association of TRALI incidence with donor HLA antibody class, the NBG value of the HLA antibody screening assay, or the number of donor HLA antibody specificities identified. These LAPS-II findings are limited by the small number of identified TRALI cases and additional studies are needed for a more definitive evaluation of these associations.
No LAPS-II TRALI case was definitively associated with donor HNA antibodies and only one TRALI case arose from a LAPS-II component given by a donor with a nonspecific HNA antibody who also had multiple HLA antibodies. Our assessment is that the HNA antibody was unlikely to be causative in this case. Although TRALI cases have previously been associated with transfused nonspecific neutrophil antibodies,2 proof that such nonspecific HNA antibodies are causative of TRALI is currently lacking. Furthermore, additional lookback studies, to be reported separately, on five LAPS-II donors with HNA antibodies (three of whom lacked HLA antibody) did not demonstrate any additional cases of TRALI.
Although LAPS-II was not designed to evaluate recipient risk factors that could predispose to TRALI, data concerning the lack of TRALI, possible TRALI, or TRALI/TACO occurrence in 34 other recipients of high-plasma-volume components from four LAPS-II donors associated with TRALI cases is consistent with the hypothesis that recipient factors influence TRALI development. The occurrence of TRALI in two recipients in the control arm likely was due to transfusion of the LAPS-II component as this was the only component transfused in one case and one of only two components transfused in the second case. These findings point to a non–HLA/HNA antibody–mediated mechanism in these two cases: possibilities include infusion of nonimmune biologic response modifiers or of undetected antibodies (in systems other than HLA or HNA) in the LAPS-II female donors, each of whom had three or more pregnancies. However, LAPS-II data are too limited to be highly informative on the issues of the relative importance and incidence of HLA antibody–mediated versus non–HLA antibody–mediated TRALI and the contribution of recipient risk factors to TRALI pathogenesis.
Surveillance systems have been fairly robust in determining TRALI incidence from plasma transfusions but have produced less definitive data regarding plateletpheresis, probably due to the lower number of plateletpheresis components transfused in some jurisdictions.2,9,28 Based on the amount of plasma contained in a plateletpheresis, it has been assumed on theoretical grounds that the TRALI risk from plasma and plateletpheresis are equivalent.29,30 While the LAPS-II finding of a borderline significantly higher TRALI incidence in recipients of plasma versus plateletpheresis is intriguing, it does not definitively answer this question because the number of TRALI cases was small, the medical diagnoses in recipients of these two types of components were significantly different (data not shown), and other possible confounders also may have been present in the retrospectively collected LAPS-II data set. In our opinion, further study of this issue is warranted.
The LAPS-II incidence of TACO (1.19% per transfused recipient) was higher than the incidence of TRALI, as has also been demonstrated in other studies.28,31 True TACO incidence may have been underestimated in LAPS-II, which only captured those TACO cases severe enough to result in bilateral pulmonary edema and hypoxemia. It is important to note that studying TACO was not a goal of LAPS-II and thus there were no uniform formal criteria used to make this diagnosis. Our finding of a trend toward increasing numbers of units transfused in cases diagnosed as TACO (compared to TRALI) may be somewhat confounded in that it may have been influenced by infused fluid volume being one of the factors used by the expert panel in assigning a diagnosis of TACO.
Despite the strengths of this study, there are some limitations. It is difficult to make the diagnosis of TRALI in critically ill patients and this difficulty is likely to be greater in retrospective studies. However, the LAPS-II methodology of using an expert physician review panel to arrive at a consensus diagnosis has also been used in other published TRALI studies.24,26,31,32 We interpret our data showing that independent expert reviewers agreed on the case diagnosis slightly less than 50% of the time as supporting the need for multiple expert reviewers to adjudicate diagnoses. Despite this procedure, the possibility of case misclassification exists in LAPS-II. The effects of such misclassification should be somewhat mitigated due to the inclusion of a control arm. Consistency of diagnosis in each arm is supported by the ORs for TACO (OR, 1.0) and other (OR, 1.6), which indicate that these cases distributed relatively equally between study and control arms; this is expected given that there is no known biologic reason for the HLA antibody status of a single transfused component to influence these outcomes. Nevertheless, given the small number of TRALI cases detected, misclassification of only a few cases relative to this diagnosis could affect the overall study interpretation as could the inability to reach a diagnosis in the seven cases designated as TRALI/TACO.
The sequential stopping points for LAPS-II data collection assume no loss of TRALI outcomes for cases that did not go through full data extraction procedures. We chose this method of data collection to make this large-scale study feasible with regard to personnel time and costs. We used conservative criteria at each evaluation step (i.e., when in doubt the case was sent to the next step) and therefore believe that all cases with bilateral pulmonary edema and hypoxemia reached the expert review panel. However, one significant limitation that resulted from this sequential review process was that we did not retrieve detailed transfusion histories on the large majority of recipients. We were thus unable to compute TRALI incidence per transfused component as this denominator was unavailable for the total cohort. Finally, due to the small number of TRALI cases and the very large number of ICD-9 codes, we determined that any formal statistical analysis of the relationship of these two variables would be noninformative; instead, we provided the admitting diagnosis of all TRALI cases in Table 4.
Finally, we can ask if LAPS-II results provide guidance as to the appropriateness of current TRALI risk reduction policies. Based on the trends of increased TRALI cases in the HLA antibody–positive study arm and from females with three or more pregnancies, the data are consistent with the likelihood that TRALI risk is decreased by selecting high-volume-plasma components for transfusion from donors at low risk of having HLA antibodies. Previous studies indicate that while HLA antibody prevalence increases with the number of pregnancies, even those female donors who have been pregnant only one or two times have significantly higher HLA antibody prevalence than never-pregnant female donors.21 Thus the donor populations with the lowest risk for HLA antibodies are male or never-pregnant female donors or previously pregnant female donors tested and found negative for HLA antibodies.
ACKNOWLEDGMENTS
We acknowledge the excellent work performed by the study coordinators at each site. These individuals performed reviews of recipient data, completed data forms, and prepared the Power-Point presentations. We thank Pam D'Andrea (ITXM, Pittsburgh, PA), Linda Banks (Hoxworth, Cincinnati, OH), Debbie Devita (UCSF, San Francisco, CA), Elizabeth Hartman (Hoxworth, Cincinnati, OH), Daniel Hindes (UCSF, San Francisco, CA), Dr Sulaiman Karatela (Emory, Atlanta, GA), Debora Nischik (BloodCenter of Wisconsin, Milwaukee, WI), Jim Newman (Emory, Atlanta, GA), and Eric Scott (BloodCenter of Wisconsin, Milwaukee, WI). We also thank Dr Whitney Steele (Westat, Rockville, MD) for developing the component selection algorithm, Monique Koenigsberg (UCSF, San Francisco, CA) for conducting a training session on data collection and extraction, Dr Michael Donahoe (University of Pittsburgh, Pittsburgh, PA) for serving as the physician who reviewed all cases with hypoxemia and triaged them before submission to the expert panel, and Dr Pearl Toy (UCSF, San Francisco, CA) for expert planning and advice.
The Retrovirus Epidemiology Donor Study-II (REDS-II Study Group) is the responsibility of the following persons:
Blood Centers:
American Red Cross Blood Services, New England Region: R. Cable, J. Rios, and R. Benjamin
American Red Cross Blood Services, Southern Region/Department of Pathology and Laboratory Medicine, Emory University School of Medicine: J.D. Roback
Hoxworth Blood Center, University of Cincinnati Academic Health Center: R.A. Sacher, S.L. Wilkinson, and P.M. Carey
Blood Centers of the Pacific, University of California San Francisco, Blood Systems Research Institute: E.L. Murphy, B. Custer, and N. Hirschler
The Institute for Transfusion Medicine: D. Triulzi, R. Kakaiya, and J. Kiss
BloodCenter of Wisconsin: J. Gottschall and A. Mast
Coordinating Center:
Westat, Inc.: J. Schulman and M. King
National Heart, Lung, and Blood Institute, NIH:
G.J. Nemo and S.A. Glynn
Central Laboratory:
Blood Systems Research Institute: M.P. Busch and P. Norris
This work was supported by NHLBI Contracts N01-HB-47168, -47169, -47170, -47171, -47172, -47174, -47175, and -57181.
ABBREVIATIONS
- ALI
acute lung injury
- ARDS
adult respiratory distress syndrome
- LAPS-II
Leukocyte Antibody Prevalence Study-II
- NBG
normalized background ratio
- NHLBI
National Heart, Lung, and Blood Institute
- REDS-II
Retrovirus Epidemiology Donor Study-II
- SCCOR
Specialized Centers of Clinically Oriented Research
- TACO
transfusion-associated circulatory overload
- UCSF
University of California San Francisco
Footnotes
CONFLICT OF INTEREST
The authors report no conflicts of interest or other financial involvement to declare for this work.
REFERENCES
- 1.Kleinman S, Grossman B, Kopko P. A national survey of transfusion-related acute lung injury risk reduction policies for platelets and plasma in the United States. Transfusion. 2010;50:1312–21. doi: 10.1111/j.1537-2995.2010.02659.x. [DOI] [PubMed] [Google Scholar]
- 2.Chapman C, Stainsby D, Jones H, Love E, Massey E, Win N, Navarrete C, Lucas G, Soni N, Morgan C, Choo L, Cohen H, Williamson L. Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49:440–52. doi: 10.1111/j.1537-2995.2008.01948.x. [DOI] [PubMed] [Google Scholar]
- 3.Engelfriet CP, Reesnik HW. International forum: measures to prevent TRALI. Vox Sang. 2007;92:258–77. doi: 10.1111/j.1423-0410.2006.00870.x. [DOI] [PubMed] [Google Scholar]
- 4.Reil A, Keller-Stanislawski B, Günay S, Bux J. Specificities of leucocyte alloantibodies in transfusion-related acute lung injury and results of leucocyte antibody screening of blood donors. Vox Sang. 2008;95:313–7. doi: 10.1111/j.1423-0410.2008.01092.x. [DOI] [PubMed] [Google Scholar]
- 5.Sachs U, Link E, Hofmann C, Wasel W, Bein G. Screening of multiparous women to avoid transfusion-related acute lung injury: a single centre experience. Transfus Med. 2008;18:348–54. doi: 10.1111/j.1365-3148.2008.00889.x. [DOI] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration. US Department of Health and Human Services Fatalities reported to FDA following blood collection and transfusion: annual summary for fiscal year. 2009 [cited 2010 Nov 10]. Available from: URL: http://www.fda.gov/BiologicsBloodVaccines/ SafetyAvailability/ReportaProblem/ TransfusionDonationFatalities/ucm204763.htm.
- 7.Middelburg RA, van Stein D, Briet E, van der Bom JG. The role of donor antibodies in the pathogenesis of transfusion-related acute lung injury: a systematic review. Transfusion. 2008;48:2167–76. doi: 10.1111/j.1537-2995.2008.01810.x. [DOI] [PubMed] [Google Scholar]
- 8.Eder AF, Herron R, Strupp A, Dy B, Notari EP, Chambers LA, Dodd RY, Benjamin RJ. Transfusion-related acute lung injury surveillance (2003–2005) and the potential impact of the selective use of plasma from male donors in the American Red Cross. Transfusion. 2007;47:599–607. doi: 10.1111/j.1537-2995.2007.01102.x. [DOI] [PubMed] [Google Scholar]
- 9.Eder AF, Herron RM, Strupp A, Dy B, White J, Notari EP, Dodd RY, Benjamin RJ. Effective reduction of transfusion-related acute lung injury risk with male-predominant plasma strategy in the American Red Cross (2006–2008) Transfusion. 2010;50:1732–42. doi: 10.1111/j.1537-2995.2010.02652.x. [DOI] [PubMed] [Google Scholar]
- 10.Bux J, Sachs UJ. The pathogenesis of transfusion-related acute lung injury (TRALI) Br J Haematol. 2007;136:788–99. doi: 10.1111/j.1365-2141.2007.06492.x. [DOI] [PubMed] [Google Scholar]
- 11.Silliman CC, Ambruso DR, Boshkov LK. Transfusion-related acute lung injury. Blood. 2005;105:2266–73. doi: 10.1182/blood-2004-07-2929. [DOI] [PubMed] [Google Scholar]
- 12.Fung Y, Silliman C. The role of neutrophils in the pathogenesis of transfusion-related acute lung injury. Transfus Med Rev. 2009;23:266–83. doi: 10.1016/j.tmrv.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooling L. Transfusion-related acute lung injury [letters] JAMA. 2002;288:315–6. doi: 10.1001/jama.288.3.315. [DOI] [PubMed] [Google Scholar]
- 14.Toy P, Hollis-Perry KM, Jun J, Nakagawa M. Recipients of blood from a donor with multiple HLA antibodies: a lookback study of transfusion-related acute lung injury. Transfusion. 2004;44:1683–88. doi: 10.1111/j.0041-1132.2004.04193.x. [DOI] [PubMed] [Google Scholar]
- 15.Win N, Ranasinghe E, Lucas G. Transfusion-related acute lung injury: a 5-year look-back study. Transfus Med. 2002;12:387–9. doi: 10.1046/j.1365-3148.2002.00401_1.x. [DOI] [PubMed] [Google Scholar]
- 16.Nicolle AL, Chapman CE, Carter V, Wallis JP. Transfusion-related acute lung injury caused by two donors with anti-human leukocyte antigen class II antibodies: a look-back investigation. Transfus Med. 2004;64:225–30. doi: 10.1111/j.0958-7578.2004.00504.x. [DOI] [PubMed] [Google Scholar]
- 17.Maslanka K, Michur H, Zupanska B, Ulhrynowska M, Nowak J. Leucocyte antibodies in blood donors and a look back on recipients of their blood components. Vox Sang. 2007;92:247–9. doi: 10.1111/j.1423-0410.2007.00890.x. [DOI] [PubMed] [Google Scholar]
- 18.Fadeyi EA, Adams S, Sheldon S, Leitman SF, Wesley R, Klein HG, Stroncek DF. A preliminary comparison of the prevalence of transfusion reactions in recipients of platelet components from donors with or without leucocyte antigen antibodies. Vox Sang. 2008;94:324–8. doi: 10.1111/j.1423-0410.2008.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quillen K, Medrano C, Adams S, Peterson B, Hackett J, Leitman SF, Klein HG, Stoncek DF. Screening plateletpheresis donors for HLA antibodies on two high-throughput platforms and correlation with recipient outcome. Transfusion. 2011;51:504–10. doi: 10.1111/j.1537-2995.2010.02874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kopko PM, Marshall CS, MacKenzie MR, Holland PV, Popovsky MA. Transfusion-related acute lung injury. Report of a clinical look-back investigation. JAMA. 2002;287:1968–71. doi: 10.1001/jama.287.15.1968. [DOI] [PubMed] [Google Scholar]
- 21.Triulzi DJ, Kleinman S, Kakaiya RM, Busch MP, Norris PJ, Steele WR, Glynn SA, Hillyer CD, Carey P, Gottschall JL, Murphy EL, Rios JA, Ness PM, Wright DJ, Carrick D, Schreiber GB. The Retrovirus Epidemiology Donor Study-II. The effect of previous pregnancy and transfusion on HLA alloimmunization in blood donors: implications for a transfusion-related acute lung injury risk reduction strategy. Transfusion. 2009;49:1825–35. doi: 10.1111/j.1537-2995.2009.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottschall JL, Triulzi DJ, Curtis B, Kakaiya RM, Busch MP, Norris P, Glynn SA, Carrick D, Wright DJ, Kleinman S. The National Heart, Lung, and Blood Institute Retrovirus Epidemiology Donor Study-II (REDS-II). The frequency and specificity of human neutrophil antigen antibodies in a blood donor population. Transfusion. 2011;51:820–7. doi: 10.1111/j.1537-2995.2010.02913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Endres RO, Kleinman SH, Carrick DM, Steele WR, Wright DJ, Norris PJ, Triulzi D, Kakaiya R, Busch MP. Identification of specificities of antibodies against human leukocyte antigens in blood donors. Transfusion. 2010;50:1749–60. doi: 10.1111/j.1537-2995.2010.02589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toy P, Gajic O, Gropper M, Hubmayr R, Looney M, Matthay M. Prospective assessment of the incidence of TRALI [abstract] Transfusion. 2007;47(Suppl):S10. [Google Scholar]
- 25.Kleinman S, Caufield T, Chan P, Davenport R, McFarland J, McPhedran S, Meade M, Morrison D, Pinsent T, Robillard P, Slinger P. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion. 2004;44:1774–89. doi: 10.1111/j.0041-1132.2004.04347.x. [DOI] [PubMed] [Google Scholar]
- 26.Finlay HE, Cassorla L, Feiner J, Toy P. Designing and testing a computer-based screening system for transfusion-related acute lung injury. Am J Clin Pathol. 2005;124:601–9. doi: 10.1309/1XKQKFF83CBU4D6H. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto S, Nakajima F, Kamada H, Kawamura K, Satake M, Tadokoro K, Okazaki H. Relationship of donor HLA antibody strength to the development of transfusion-related acute lung injury. Transfusion. 2010;50:2582–91. doi: 10.1111/j.1537-2995.2010.02779.x. [DOI] [PubMed] [Google Scholar]
- 28.Robillard P, Itaj NK, Chapdelaine A. Nine-year trends in incidence of adverse transfusion reactions with respiratory complications in the Quebec Hemovigilance System [abstract] Transfusion. 2010;50(Suppl):11A. [Google Scholar]
- 29.Strong DM, Lipton KS. Transfusion related acute lung injury. American Association of Blood Banks; Bethesda (MD): 2006. AABB Association bulletin #06-07. [Google Scholar]
- 30.Vamvakas EC. Relative safety of pooled whole blood–derived versus single-donor (apheresis) platelets in the United States: a systematic review of disparate risks. Transfusion. 2009;49:2743–58. doi: 10.1111/j.1537-2995.2009.02338.x. [DOI] [PubMed] [Google Scholar]
- 31.Gajic O, Rana R, Winters JL, Yilmaz M, Mendez JL, Rickman OB, O'Byrne MM, Evenson LK, Malinchoc M, DeGoey SR, Afessa B, Hubmayr RD, Moore SB. Transfusion-related acute lung injury in the critically ill: prospective nested case control study. Am J Respir Crit Care Med. 2007;176:886–91. doi: 10.1164/rccm.200702-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rana R, Fernandez-Perez ER, Khan SA, Rana S, Winters JL, Lesnick TG, Moore SB, Gajic O. Transfusion-related acute lung injury and pulmonary edema in critically ill patients: a retrospective study. Transfusion. 2006;46:1478–83. doi: 10.1111/j.1537-2995.2006.00930.x. [DOI] [PubMed] [Google Scholar]


