Abstract
Mutations in COL4A1 have been identified in families with hereditary small vessel disease of the brain presumably due to a dominant-negative mechanism. Here, we report on two novel mutations in COL4A1 in two families with porencephaly, intracerebral hemorrhage and severe white matter disease caused by haploinsufficiency. Two families with various clinical presentations of cerebral microangiopathy and autosomal dominant inheritance were examined. Clinical, neuroradiological and genetic investigations were performed. Electron microscopy of the skin was also performed. In one of the families, sequence analysis revealed a one base deletion, c.2085del, leading to a frameshift and a premature stopcodon, p.(Gly696fs). In the other family, a splice site mutation was identified, c.2194-1G>A, which most likely leads to skipping of an exon with a frameshift and premature termination as a result. In fibroblasts of affected individuals from both the families, nonsense-mediated decay (NMD) of the mutant COL4A1 messenger RNAs (mRNAs) and a clear reduction of COL4A1 protein expression were demonstrated, indicating haploinsufficiency of COL4A1. Moreover, thickening of the capillary basement membrane in the skin was documented, similar to reports in patients with COL4A1 missense mutations. These findings suggest haploinsufficiency, a different mechanism from the commonly assumed dominant-negative effect, for COL4A1 mutations as a cause of (antenatal) intracerebral hemorrhage and white matter disease.
INTRODUCTION
Mutations in the collagen 4 A1 gene encoding procollagen type IV α1, COL4A1, have initially been identified in mice and humans with porencephaly (1). Later mutations in the same gene were reported in patients with (recurrent) hemorrhagic stroke perinatally and in adult life as well as in patients with symptomatic small vessel disease (2–10). Recently, a role for COL4A1 mutations in the etiology of sporadic late-onset intracranial hemorrhage has been identified by sequencing COL4A1 in 96 sporadic patients (11). Hereditary angiopathy, nephropathy, aneurysms and cramps, possibly a disease within the same spectrum, was described as an allelic disorder (12–14). Moreover, novel mutations were described in muscle–eye–brain disease and Walker–Warburg syndrome (15). Because COL4A1 and COL4A2 have similar structural and functional properties, the COL4A2 gene has been analyzed in familial and sporadic patients with similar phenotypes and mutations have indeed been identified (16–18).
Various phenotypes have been documented within families carrying the same COL4A1 mutation, suggesting a role for environmental factors, as for instance cranial trauma, use of oral anticoagulants and genetic modifiers in the phenotypic expression (1,6,8). COL4A1 is ubiquitously expressed in the basement membrane and is of importance for its stability (19). Mutations in COL4A1 have been shown to cause structural disruptions in the basement membrane potentially resulting in vascular defects (1,3). Almost all mutations reported are missense mutations in highly conserved regions in a triple helix domain of the gene. Based on the autosomal dominant inheritance pattern and lack of a phenotype in mice heterozygous for the null alleles of Col4a1 and Col4a2, a dominant-negative mechanism has been suggested as opposed to haploinsufficiency (1,19).
Here, we report on two families with porencephaly, hemorrhagic stroke and small vessel disease, due to novel mutations in COL4A1, suggesting haploinsufficiency as the pathogenic mechanism.
RESULTS
Case reports
Family A, II:1
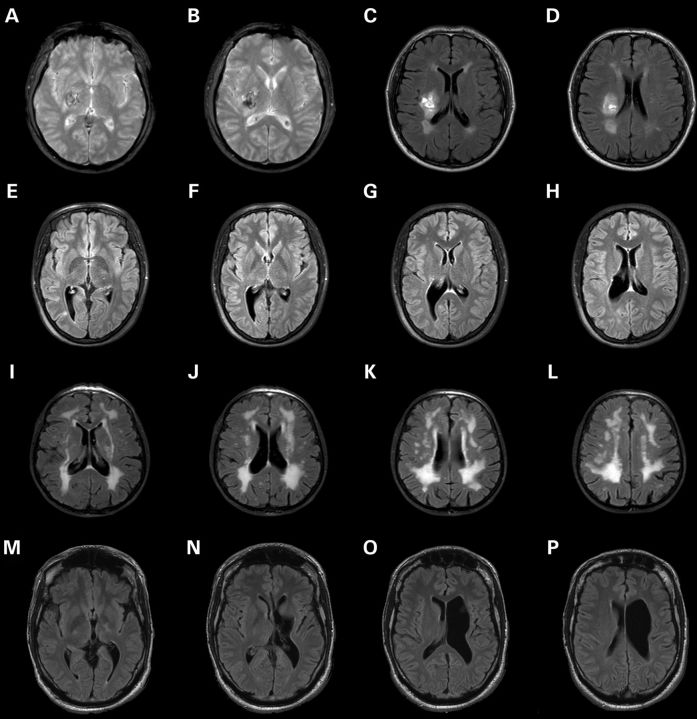
The proband, a 48-year-old male, with a history of retinal detachment, was hospitalized with an acute left-sided hemiparesis with sensory involvement and an epileptic seizure. A computed tomography scan demonstrated a right thalamic hemorrhage. Magnetic resonance imaging (MRI) of the brain showed white matter changes as presented in Figure 2A–D. Initially, hypertension was suggested as a risk factor for both pathological features; however, this could not be documented on several clinical examinations or by a 24 h blood pressure measurement. The patient had no other cardiovascular risk factors (see Fig. 1A for pedigrees).
Figure 2.
MRI of the brain was obtained in affected individuals of families A and B and gradient echo (GE) and fluid attenuated inversion recovery (FLAIR) imaging is shown in the different panels. In the proband, the intracranial hemorrhage is demonstrated (GE: A and B and FLAIR: C and D), while imaging of his daughter revealed porencephaly (FLAIR: E–H) and severe white matter disease was documented in the mother (FLAIR: I–L). Brain MRI in the proband of family B showed porencephaly in the left hemisphere with partial destruction of the basal ganglia and left pyramidal tract (FLAIR: M–P).
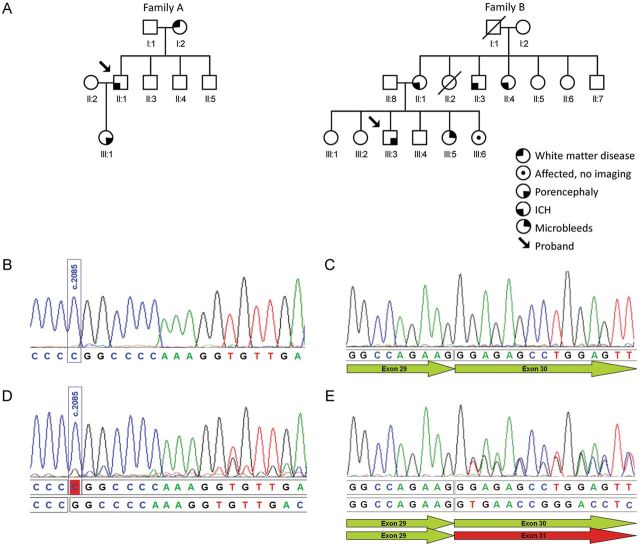
Figure 1.
Pedigree of Belgian (family A) and Dutch (family B) families show affected individuals with various clinical presentation (A). Analysis of the effect at RNA level of the c.2085del mutation was performed in patient III:1 of family A (B and D) and the splicing mutation c.2194-1G>A in patient III:3 of family B (C and E). Because of mRNA instability, due to nonsense-mediated mRNA decay, the transcript of the mutant alleles is not detectable under standard conditions as shown in the sequence of COL4A1 cDNA in patient III:1 of family A (B) and in patient III:3 of family B (C). Additionally, the sequence of the patients' cDNA, obtained after incubation of fibroblast cells in a medium containing cycloheximide, was determined (D and E). Cycloheximide prevents, although only partially in fibroblasts of patient III:1 of family A (D), nonsense-mediated decay of the mutant mRNAs. Sequence analysis shows transcripts from both the normal (upper lane) and the mutant allele (lower lane).
Family A, III:1
This 21-year-old female is the daughter of the proband. Intrauterine growth retardation (IUGR) was diagnosed during pregnancy at 32 weeks gestation. This IUGR resolved spontaneously and no placental abnormalities were documented at birth. She was delivered by caesarian section at full-term pregnancy. At 1 year, a left-sided motor deficit was discovered which required rehabilitation. Brain imaging showed porencephaly at the right lateral ventricle potentially caused by stroke in utero. MRI of the brain was repeated at the current age which confirmed the known abnormalities and identified some supratentorial white matter signal abnormalities (Fig. 2E–H).
Family A, I:2
The 74-year-old mother of the proband reported no neurological symptoms. Clinical neurological examination and cognitive examination did not reveal any abnormalities. MRI of the brain identified severe white matter disease comparable with what has been reported in families with mutations in COL4A1 (Fig. 2I–L).
Family B, III:3
The proband, a 39-year-old male, visited our outpatient clinic after genetic counseling of his clinically unaffected sister (III:4). The patient was born after an uneventful pregnancy and the delivery was uncomplicated. During early childhood, spasticity and a delayed motor development were diagnosed, which were considered to be caused by prolonged neonatal jaundice. Brain imaging was initially not performed. Currently, he walks with crutches and his cognition is normal. A neurological examination revealed a right-sided pyramidal syndrome. Brain imaging showed left paraventricular porencephalic cyst and partial destruction of the basal ganglia and left pyramidal tract (Fig. 2M–P).
Family B, III:4
The 37-year-old healthy sister of the proband was not evaluated clinically. However, analysis of the familial mutation in COL4A1 was performed.
Family B, III:5
The 35-year-old sister of the proband has no complaints. At the age of 33 years, brain MRI has been performed because of headache. A review of the brain MRI revealed cerebral microhemorrhages. Analysis of the familial mutation in COL4A1 has been performed.
Family B, III:6
Infantile hemiplegia and intellectual disability were diagnosed during infancy in the sister of the proband, a 33-year-old women. She was unwilling to visit the hospital for clinical or radiological studies, but agreed on DNA analysis of the familial mutation.
Family B, patient II:1
The 66-year-old mother of patients III: 3, 5 and 6. At the age of 51 years, she suffered from an intracerebral hemorrhage. Brain imaging showed a previous intracerebral hemorrhage, and no other abnormalities. DNA analysis of the familial mutation was performed. In two siblings of this patient II: 1, (II:3 and II:4) intracerebral hemorrhage or ischemic stroke was diagnosed at the age of 43 and 47 years.
Genetic study
DNA sequencing of patient II:1 of family A showed a heterozygous deletion at position 2085 (c.2085del) resulting in a frameshift and a premature stopcodon (p.Gly696fs) in the COL4A1 gene. The mutation cosegregated with disease as the sequencing of patient III:1 confirmed the presence of the same deletion. The presence of this mutation was studied in the parents of patient II:1 and was confirmed in the mother (I:2) with severe white matter disease and was absent in healthy subject I:1. The mutation identified in this family was not present in 744 controls from the same population as this family and not present in the public databases 1000 Genomes (May 2012) and dbSNP135, nor in up to 5379 exomes from individuals of different ancestry [ESP5400; Exome Variant Server, NHLBI Exome Sequencing Project (ESP), Seattle, WA (http://evs.gs.washington.edu/EVS/) (May 2012 accessed)]. In family B, sequence analysis of the COL4A1 gene in patient III:1 showed a c.2194-1G>A mutation affecting the consensus sequence of an acceptor splice site. Mutation c.2194-1G>A was also identified in patients III:2, III:3 and II:1. In (healthy) subject III:4, the familial COL4A1 mutation was not present. As such, the mutation cosegregated with the phenotype in this family. Also this mutation was not present in 1000 G, dbSNP135 or ESP5400 databases.
Analysis of RNA in fibroblasts
In family A, sequence analysis of complementary DNA (cDNA) obtained from fibroblasts cultured without cycloheximide showed no expression of mutant COL4A1 allele (Fig. 1B), suggesting nonsense-mediated decay (NMD) of mutant messenger RNA (mRNA) and haploinsufficiency. Culturing of fibroblasts with cycloheximide led to a partial inhibition of NMD, allowing sequencing of the mutant allele (Fig. 1C).
To assess the effect of the c.2194-1G>A mutation on splicing, cDNA analysis was performed in the proband of family B. Sequence analysis of cDNA obtained from fibroblasts cultured without cycloheximide showed no expression of mutant COL4A1 allele (Fig. 1D), suggesting NMD of mutant mRNA and haploinsufficiency also in this family. Culturing of fibroblasts with cycloheximide led to stabilization of the mutant allele and revealed a deletion (r.2194_2344del) due to skipping of exon 30, leading to a frameshift and a premature termination: p.(Gly732fs) (Fig. 1E).
Reduction in protein expression in human fibroblasts
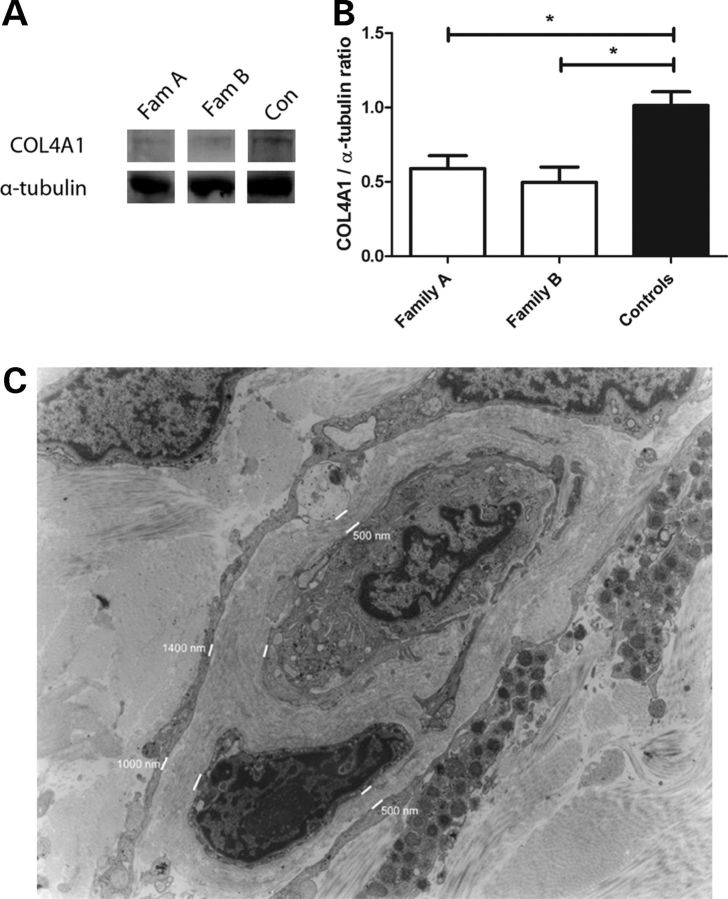
Additionally, COL4A1 RNA expression was examined in cultured fibroblasts and compared with expression in healthy controls. Western blotting revealed a clear reduction in COL4A1 protein in both families which was confirmed by semi-quantitative analysis: 41% reduction for family A (P = 0.009) and 50% reduction for family B (P = 0.01), suggesting haploinsufficiency to be the cause of the disease in these particular families (Fig. 3A and B).
Figure 3.
COL4A1 expression was analyzed in fibroblasts of patients (patient II:1 and III:1 of family A; patient III:3 of family B) and four controls and normalized to α-tubulin. A marked reduction was observed in both the families (A) which was confirmed by quantification (B) (data are expressed as mean ± SEM, *P < 0.05). Electron micrograph of a skin capillary of patient II:1 (magnification ×1200) showed striking thickening of the basement membrane (C). The thickness of the basement membrane is indicated in nanometer.
Skin basement membrane abnormalities
In patient II:1 of family A, capillaries displayed striking ultrastructural abnormalities. In these capillaries, the basement membrane of the endothelial cells showed an increase in the thickness of up to 4030 nm (normal is approximately 40 nm as we have previously shown (3)), with focal interruptions and formation of pools of fragmented basement membranes (Fig. 3C). A total of seven sections with thickened basement membranes were evaluated for thickness of the basement membrane. The mean width of the basement membrane in these sections was 1150 ± 136 nm (minimum 280 nm, maximum 4030 nm).
DISCUSSION
We report on a Belgian and Dutch family with porencephaly, intracranial hemorrhage and white matter disease caused by COL4A1 haploinsufficiency. In family A, these clinical symptoms and concomitant neuroradiological findings were associated with a novel c.2085del mutation in the COL4A1 gene, occurring in three generations. However, striking clinical features were lacking in the mother of the proband, although neuroimaging showed severe white matter disease. Potentially thorough cognitive testing could have revealed abnormalities, but this was not performed. In family B, a splice site mutation leading to a deletion and a frameshift due to skipping of exon 30 was identified in four patients with clinical or neuroimaging features of COL4A1-related disorders and was not present in one family member without complaints. In contrast to almost all mutations reported, which are mainly missense mutations in highly conserved regions in a triple helix domain of the gene, both these novel mutations result in premature termination. Functional studies indicate instability of mutant mRNAs and deficiency of COL4A1 protein in both the families.
A dominant-negative effect of the mutations in COL4A1 was suggested as a pathological mechanism based on the findings thus far. This was underscored by animal models of this disease. Mice heterozygous for the null alleles of Col4a1 and Col4a2 are reported to be normal (19). A semi-dominant mutation in Col4a1 in mice results in cerebral hemorrhage (1), a phenotype that is lethal after mid-embryogenesis in homozygous mutant mice and less severe in heterozygotes, but uniformly present in naturally born pups (1). It was proposed that the presence of a mutant allele is required to induce defects in the basal membrane resulting in weakening of the vessels due to increased intracellular accumulation of COL4A1 and/or decreased extracellular COL4A1 (11). However, functional studies on both mutations identified in the families in this report suggest haploinsufficiency to be the cause of the disease. It is known that the presentation of disease in COL4A1-related disorders can vary depending on the mutation but, our insights on genotype–phenotype relationships are highly limited. Moreover, also within subjects in families with the same mutation, striking variability has been reported. Potentially the clinical presentation may be more severe in patients with missense mutations resulting in a dominant-negative effect than in patients with mutations leading to haploinsufficiency. However, the clinical features and MRI findings in the families described here are comparable with those previously described families with missense mutations. Additionally, characteristic histology that has been described in other families with mutations in COL4A1 was documented in our family, since skin basement membrane abnormalities were clearly identified. The extent of the thickening of the basement membrane was indeed similar as in a reported patient with a missense mutation (3). Therefore, the potential mechanism might more likely involve a reduction of COL4A1 in the extracellular matrix, rather than a toxic intracellular accumulation, reducing the stability of the basement membrane resulting in cerebral small vessel disease (19,20). It remains speculative by which mechanism thickening of the basement membrane might result in both intracranial hemorrhage as well as ischemic infarction and white matter disease. The thickening of the wall with focal disruptions can contribute to the development of small aneurysms which can induce cerebral hemorrhage, but may additionally lead to the obliteration of small penetrating vessels which can present as lacunar infarctions and white matter disease.
The numbers of known families with missense mutations or haploinsufficiency are too small to calculate or compare penetrance. The phenotype caused by haploinsufficiency might be less penetrant, and reduced penetrance in families carrying null mutations might lead to misdiagnosis of patients as sporadic, while actually the clinical presentation is due to an inherited disorder.
These and other recent findings in sporadic patients (11) point to a broader role of mutations in COL4A1 in the clinical presentation of intracranial hemorrhage and small vessel disease than has been commonly assumed. Sequencing and copy number quantification of COL4A1 aimed to identify mutations, deletions or rare variants in large cohorts of sporadic patients presenting with these phenotypes might provide further evidence for this hypothesis. These studies might elucidate whether reduced expression of COL4A1 might be associated with an increased susceptibility for cerebral microangiopathy.
MATERIALS AND METHODS
Human data
Family A: Two patients and one asymptomatic individual within the same family were identified and examined. They underwent a clinical neurological (and the two patients underwent an ophthalmological) examination and an imaging protocol including sagittal T1-weighted, axial T2-weighted, fluid-attenuated inversion recovery (FLAIR) imaging plus diffusion-weighted imaging and gradient echo (GE) imaging. The control individuals have been previously described (21).
Family B: Within the family, five patients with intracerebral hemorrhages or spasticity were identified. In the proband, clinical neurological examination, DNA analysis and brain MRI were performed. In four other family members, DNA analysis has been performed and in two family members, brain imaging has been performed.
All consent was obtained according to the Declaration of Helsinki from all subjects in this study and approved by the Ethical Committee of the Institution in which the work was performed.
Sequencing
DNA was extracted from venous blood samples using standard methods. PCR amplification of the 52 coding exons and flanking regions of the COL4A1 gene was performed in the proband of both families using a PE 9700 thermocycler (Applied Biosystems). Primers and conditions are available on request. Sequencing reactions were performed using an ABI 3130 and an ABI 3730 DNA analyzer from Applied Biosystems. In family B, segregation of the mutation was studied using the same primers and conditions. In family A, the identified mutation was specifically investigated in the second patient of the same family using specific primers. Controls were analyzed for the identified mutation by using genotyping using allele-specific PCR (KASPar) by KBiosciences (http://www.kbioscience.co.uk) run on a 7300 real-time PCR system. On every plate, the two patients were run as positive controls.
Fibroblasts
To assess the effect of the mutations and the stability of the mutant COL4A1 mRNAs, fibroblast cell lines were established from a skin biopsy of affected individuals from both the families (II:1 and III:1 of family A and III:3 of family B, respectively). Before RNA extraction, half of the cultured cells were incubated for 4.5 h in a medium containing cycloheximide. In cells grown with cycloheximide, a protein synthesis inhibitor, the nonsense-mediated mRNA decay process is prevented (22). After RNA extraction and reverse transcription-PCR (RT-PCR), a fragment of the complementary DNA (cDNA) encompassing the mutations was amplified. cDNA obtained from cells grown with or without cycloheximide from patient III:1 of family A was amplified using the set of primers 5′′-CAGGTCCAAAGGGTGAAC-3′ and 5′-GTAGACCAACTCCAGGCT-3′. For family B, the set of primers 5′-AAGGAGACCGAGGCTTTC-3′ and 5′-TCCAGTCCAGGGAATCCG-3′ was used.
Fibroblasts of II:1 and III:1 from family A, III:3 from family B and four controls were cultured under standard medium conditions for protein expression studies. Protein extractions were analyzed for COL4A1 expression by western blotting. Antibodies used were rat anti-human COL4A1 (H22), a kind gift from Dr Yoshikazu Sado, mouse anti-α-tubulin and goat-anti-rabbit-HRP. Bands were visualized using enhanced chemiluminescence and scanned with the Image Quant LAS 4000. Semi-quantitative analysis of COL4A1 and α-tubulin was carried out by densitometry using ImageQuantTL software. Each mutation was analyzed in at least three different samples and compared with at least three controls per blot. Expression was statistically analyzed by the Student's t-test and all P-values reported are two tail-tailed and the significance level was set at 0.05.
Skin biopsy
Skin biopsies were fixed in 2% (vol/vol) glutaraldehyde for 30 min and 1.5% (wt/vol) osmium tetroxide, dehydrated with acetone and embedded in Epon 812 (Sigma, Zwijndrecht, the Netherlands). Ultrathin sections were collected on 300-mesh Formavar-coated nickel grids (Merck, Darmstadt, Germany). The sections were contrasted with uranyl acetate and lead citrate; they were examined using a Jeol 1200 EX electron microscope (Jeol Ltd, Tokyo, Japan). Sections with thickened basement membranes were measured for thickness of the basement membrane at 5–7 positions per section. The thickness of the basement membrane is given in mean ± SEM (nm).
FUNDING
R.L. is supported by Research Fund KU Leuven and is a senior clinical investigator of FWO Flanders. V.N.T. is also a senior clinical investigator of FWO Flanders. W.R. is supported by the E von Behring Chair for Neuromuscular and Neurodegenerative Disorders at the University of Leuven. AG is supported by Onderzoeksfonds KU Leuven/ Research Fund KU Leuven (OT/11/087), the Belgian Neurological Society and the Belgian Charcot Foundation.
ACKNOWLEDGEMENTS
The authors would like to thank the NHLBI GO Exome Sequencing Project and its ongoing studies which produced and provided exome variant calls for comparison: the Lung GO Sequencing Project (HL-102923), the WHI Sequencing Project (HL-102924), the Broad GO Sequencing Project (HL-102925), the Seattle GO Sequencing Project (HL-102926) and the Heart GO Sequencing Project (HL-103010).
Conflict of Interest statement. None declared.
References
- 1.Gould D.B., Phalan F.C., Breedveld G.J., van Mil S.E., Smith R.S., Schimenti J.C., Aguglia U., van der Knaap M.S., Heutink P., John S.W. Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science. 2005;308:1167–1171. doi: 10.1126/science.1109418. [DOI] [PubMed] [Google Scholar]
- 2.Breedveld G., de Coo I.F., Lequin M.H., Arts W.F., Heutink P., Gould D.B., John S.W., Oostra B., Mancini G.M. Novel mutations in three families confirm a major role of COL4A1 in hereditary porencephaly. J. Med. Genet. 2006;43:490–495. doi: 10.1136/jmg.2005.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Knaap M.S., Smit L.M., Barkhof F., Pijnenburg Y.A., Zweegman S., Niessen H.W., Imhof S., Heutink P. Neonatal porencephaly and adult stroke related to mutations in collagen IV A1. Ann. Neurol. 2006;59:504–511. doi: 10.1002/ana.20715. [DOI] [PubMed] [Google Scholar]
- 4.Vahedi K., Kubis N., Boukobza M., Arnoult M., Massin P., Tournier-Lasserve E., Bousser M.G. COL4A1 mutation in a patient with sporadic, recurrent intracerebral hemorrhage. Stroke. 2007;38:1461–1464. doi: 10.1161/STROKEAHA.106.475194. [DOI] [PubMed] [Google Scholar]
- 5.Sibon I., Coupry I., Menegon P., Bouchet J.P., Gorry P., Burgelin I., Calvas P., Orignac I., Dousset V., Lacombe D., et al. COL4A1 mutation in Axenfeld-Rieger anomaly with leukoencephalopathy and stroke. Ann. Neurol. 2007;62:177–184. doi: 10.1002/ana.21191. [DOI] [PubMed] [Google Scholar]
- 6.de Vries L.S., Koopman C., Groenendaal F., Van Schooneveld M., Verheijen F.W., Verbeek E., Witkamp T.D., van der Worp H.B., Mancini G. COL4A1 mutation in two preterm siblings with antenatal onset of parenchymal hemorrhage. Ann. Neurol. 2009;65:12–18. doi: 10.1002/ana.21525. [DOI] [PubMed] [Google Scholar]
- 7.Bilguvar K., DiLuna M.L., Bizzarro M.J., Bayri Y., Schneider K.C., Lifton R.P., Gunel M., Ment L.R. COL4A1 mutation in preterm intraventricular hemorrhage. J. Pediatr. 2009;155:743–745. doi: 10.1016/j.jpeds.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah S., Kumar Y., McLean B., Churchill A., Stoodley N., Rankin J., Rizzu P., van der Knaap M., Jardine P. A dominantly inherited mutation in collagen IV A1 (COL4A1) causing childhood onset stroke without porencephaly. Eur. J. Paediatr. Neurol. 2010;14:182–187. doi: 10.1016/j.ejpn.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Meuwissen M.E., de Vries L.S., Verbeek H.A., Lequin M.H., Govaert P.P., Schot R., Cowan F.M., Hennekam R., Rizzu P., Verheijen F.W., et al. Sporadic COL4A1 mutations with extensive prenatal porencephaly resembling hydranencephaly. Neurology. 2011;76:844–846. doi: 10.1212/WNL.0b013e31820e7751. [DOI] [PubMed] [Google Scholar]
- 10.Gould D.B., Phalan F.C., van Mil S.E., Sundberg J.P., Vahedi K., Massin P., Bousser M.G., Heutink P., Miner J.H., Tournier-Lasserve E., et al. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N. Engl. J. Med. 2006;354:1489–1496. doi: 10.1056/NEJMoa053727. [DOI] [PubMed] [Google Scholar]
- 11.Weng Y.C., Sonni A., Labelle-Dumais C., de Leau M., Kauffman W.B., Jeanne M., Biffi A., Greenberg S.M., Rosand J., Gould D.B. COL4A1 mutations in patients with sporadic late-onset intracerebral hemorrhage. Ann. Neurol. 2012;71:470–477. doi: 10.1002/ana.22682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plaisier E., Gribouval O., Alamowitch S., Mougenot B., Prost C., Verpont M.C., Marro B., Desmettre T., Cohen S.Y., Roullet E., et al. COL4A1 mutations and hereditary angiopathy, nephropathy, aneurysms, and muscle cramps. N. Engl. J. Med. 2007;357:2687–2695. doi: 10.1056/NEJMoa071906. [DOI] [PubMed] [Google Scholar]
- 13.Alamowitch S., Plaisier E., Favrole P., Prost C., Chen Z., Van Agtmael T., Marro B., Ronco P. Cerebrovascular disease related to COL4A1 mutations in HANAC syndrome. Neurology. 2009;73:1873–1882. doi: 10.1212/WNL.0b013e3181c3fd12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plaisier E., Chen Z., Gekeler F., Benhassine S., Dahan K., Marro B., Alamowitch S., Paques M., Ronco P. Novel COL4A1 mutations associated with HANAC syndrome: a role for the triple helical CB3[IV] domain. Am. J. Med. Genet. A. 2010;152A:2550–2555. doi: 10.1002/ajmg.a.33659. [DOI] [PubMed] [Google Scholar]
- 15.Labelle-Dumais C., Dilworth D.J., Harrington E.P., de Leau M., Lyons D., Kabaeva Z., Manzini M.C., Dobyns W.B., Walsh C.A., Michele D.E., et al. COL4A1 mutations cause ocular dysgenesis, neuronal localization defects, and myopathy in mice and Walker–Warburg syndrome in humans. PLoS Genet. 2011;7:e1002062. doi: 10.1371/journal.pgen.1002062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeanne M., Labelle-Dumais C., Jorgensen J., Kauffman W.B., Mancini G.M., Favor J., Valant V., Greenberg S.M., Rosand J., Gould D.B. COL4A2 mutations impair COL4A1 and COL4A2 secretion and cause hemorrhagic stroke. Am. J. Hum. Genet. 2012;90:91–101. doi: 10.1016/j.ajhg.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verbeek E., Meuwissen M.E., Verheijen F.W., Govaert P.P., Licht D.J., Kuo D.S., Poulton C.J., Schot R., Lequin M.H., Dudink J., et al. COL4A2 mutation associated with familial porencephaly and small-vessel disease. Eur. J. Hum. Genet. 2012;8:844–851. doi: 10.1038/ejhg.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoneda Y., Haginoya K., Arai H., Yamaoka S., Tsurusaki Y., Doi H., Miyake N., Yokochi K., Osaka H., Kato M., et al. De novo and inherited mutations in COL4A2, encoding the type IV collagen alpha2 chain cause porencephaly. Am. J. Hum. Genet. 2012;90:86–90. doi: 10.1016/j.ajhg.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poschl E., Schlotzer-Schrehardt U., Brachvogel B., Saito K., Ninomiya Y., Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131:1619–1628. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- 20.Wang X., Harris R.E., Bayston L.J., Ashe H.L. Type IV collagens regulate BMP signalling in Drosophila. Nature. 2008;455:72–77. doi: 10.1038/nature07214. [DOI] [PubMed] [Google Scholar]
- 21.Lemmens R., Abboud S., Vanhees L., Goris A., Thijs V. Lack of association between variants in the VKORC1 gene and cerebrovascular or coronary heart disease. J. Thromb. Haemost. 2008;6:2220–2223. doi: 10.1111/j.1538-7836.2008.03164.x. [DOI] [PubMed] [Google Scholar]
- 22.Bateman J.F., Freddi S., Lamande S.R., Byers P., Nasioulas S., Douglas J., Otway R., Kohonen-Corish M., Edkins E., Forrest S. Reliable and sensitive detection of premature termination mutations using a protein truncation test designed to overcome problems of nonsense-mediated mRNA instability. Hum. Mutat. 1999;13:311–317. doi: 10.1002/(SICI)1098-1004(1999)13:4<311::AID-HUMU8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]