Highlights
▸ Attention Bias Modification Treatment (ABMT) is emerging as an anxiety disorder treatment. ▸ Active attention training towards happy faces did not augment the clinical benefits of CBT. ▸ Unlike behavior, neural activation of threat biases was stable across time. ▸ Further research is needed to understand the clinical and neural changes of ABMT.
Keywords: Dot-probe, Attention training, Test–retest reliability, fMRI
Abstract
Attention Bias Modification Treatment (ABMT), an emerging treatment for anxiety disorders, is thought to modify underlying, stable patterns of attention. Therefore, ABMT research should take into account the impact of attention bias stability on attention training response, especially in pediatric populations. ABMT research typically relies on the dot-probe task, where individuals detect a probe following an emotional–neutral stimulus pair. The current research presents two dot-probe experiments relevant to ABMT and attention-bias stability. In Experiment 1, anxious youth receiving 8-weeks of cognitive-behavioral therapy (CBT) were randomly assigned to ABMT that trains attention towards happy faces (n = 18) or placebo (n = 18). Two additional comparison groups, anxious youth receiving only CBT (n = 17) and healthy comparison youth (n = 16), were studied. Active attention training towards happy faces did not augment clinician-rated response to CBT; however, individuals receiving training exhibited reductions on self-report measures of anxiety earlier than individuals receiving CBT only. In Experiment 2, healthy youth (n = 12) completed a dot-probe task twice while undergoing functional magnetic resonance imaging. Intra-class correlation demonstrated stability of neural activation in response to attention bias in the ventrolateral prefrontal cortex and amygdala. Together, these two studies investigate the ways in which attention-bias stability may impact future work on ABMT.
1. Introduction
Attention Bias Modification Treatment (ABMT) is emerging as a possible treatment for individuals with anxiety disorders (Bar-Haim, 2010). Individuals with anxiety disorders, both adults and youth, commonly have threat-related attention biases (Bar-Haim et al., 2007) and perturbations in neural regions involved in threat salience (e.g., amygdala) and attentional control (e.g., ventrolateral prefrontal cortex, PFC) (Monk et al., 2006, Monk et al., 2008, Britton et al., 2012). Initial evidence in adults suggests that training attention away from threat reduces anxiety symptoms (Hakamata et al., 2010) and influences PFC function (Browning et al., 2010). To have maximally beneficial therapeutic effects, ABMT might target those aspects of attention bias that are stable over time, when no intervention is administered. Two possible targets of attention bias stability include behavior and brain function. Yet, no study has examined simultaneously the stability of these measures using the dot-probe task, which assesses and trains attention biases. As the ABMT field grows, it is important to understand the impact of attention training and time on symptoms, behavior, and the brain.
Some evidence has emerged on the efficacy of ABMT as a stand-alone treatment; however, few ABMT studies have been conducted in clinical populations (Amir and Beard, 2009, Schmidt et al., 2009) or pediatric populations (Bar-Haim et al., 2011, Eldar et al., 2012, Waters et al., in press). Cognitive behavioral therapy (CBT) is an effective treatment for some youth with anxiety disorders, yet combining treatments (e.g., CBT and medication) may yield additional benefit (Walkup et al., 2008). CBT uses a didactic approach of exposure and enhances top-down cognitive processes. In contrast, ABMT attempts to train attention away from threat and is thought to alter bottom-up processing not readily accessed through typical CBT procedures. It is unclear whether CBT and ABMT would complement one another. This study aims to determine if ABMT augments the effects of CBT in youth with anxiety disorders.
Although ABMT research typically focuses on training away from threat, training towards positive stimuli may also be beneficial. First, positive biases found in healthy adults are attenuated with anxiety (Frewen et al., 2008), suggesting that positive bias may be protective. Moreover, adolescents at-risk for an anxiety disorder based on temperament (i.e., behavioral inhibition) with concurrent, high levels of social anxiety fail to exhibit a happy bias, unlike healthy adolescents or at-risk adolescents with low levels of social anxiety (Shechner et al., 2012). Finally, training adults to attend towards positive stimuli reduces stress reactivity (Dandeneau et al., 2007, Li et al., 2008), and similar training in anxious children was recently shown to have a clinical effect as a stand-alone treatment (Waters et al., in press). The current work aims to address whether training towards happy would reduce anxiety symptoms further in anxious youth also receiving CBT.
Novel treatments should target stable measures of underlying pathological processes. In other words, if attention bias is inherently unstable, it will be difficult to understand the pathological processes on which ABMT acts. For example, changes in attention bias may result from experience with a task rather than treatment effects. Some studies are beginning to include control groups to determine these possibilities (Eldar et al., 2012). Yet, no study has investigated the stability of attention biases over time in youth. Here, correlations provide measures of test–retest reliability of attention biases, independent of any training manipulation. These stability data will inform future ABMT research.
This study conducts two complementary experiments to inform ABMT research in youth. The first study examines ABMT effects in pediatric anxiety. It is hypothesized that beyond the effects of CBT, training anxious youth to attend towards positive stimuli will alter threat biases and clinical symptoms more strongly than placebo training. The second study examines stability of behavioral and neural correlates of attention bias in healthy youth. Although healthy youth typically do not exhibit threat biases, the neural processes engaged by the dot-probe task should remain stable over time. It is hypothesized that measurements of threat bias, assessed by both behavioral and neural activation, will be reliable across time in healthy youth. At first, it may seem counterintuitive to discuss changes and stability together; however, these two concepts are coupled. Understanding how behavioral and neural indices vary with training and time informs attempts to identify novel therapeutic targets for this newly emerging treatment.
2. Experiment 1: Attention training towards happy faces
2.1. Methods
2.1.1. Participants
Anxious youth (ages 8–17 years old) seeking outpatient treatment participated in this study. All participants were medication free, medically healthy, and had an IQ > 70 according to the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999). The Kiddie-Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version (K-SADS-PL) (Kaufman et al., 1997), a semi-structured diagnostic interview with child and parent, was used to assess for DSM-IV Axis I psychopathology. Anxious youth were diagnosed with current generalized anxiety disorder, social phobia, separation anxiety, and/or specific phobia. Co-morbid anxiety and major depression were allowed because these disorders often co-occur. Children with a current diagnosis of obsessive–compulsive disorder or post-traumatic stress disorder were excluded from the study, as were children with a past or current history of mania, psychosis, or severe pervasive developmental disorder.
Clinician ratings included the Clinician's Global Impression Scale (CGI) (Guy, 1976) and the Pediatric Anxiety Rating Scale (PARS) (Research Units on Pediatric Psychopharmacology Anxiety Study Group, 2002). Participants completed the following self-report measures: the Screen for Child Anxiety Related Emotional Disorders-Child Self-Report (SCARED-C) (Birmaher et al., 1997), and the Child Depression Inventory (CDI) (Kovacs, 2009). Parents also completed the SCARED (Birmaher et al., 1997).
Although all anxious youth received cognitive-behavioral therapy (CBT), anxious youth were studied as part of two cohorts. An initial cohort included anxious youth receiving CBT who then were randomized, blocking for gender, to receive either ABMT (n = 18) or placebo (n = 18) as an augmenting training method. These 36 individuals randomized to ABMT or placebo received exposure to happy–neutral face-pairs throughout CBT treatment; however, the active training group was trained towards the happy faces, while the placebo group was not. While data from this initial cohort were being analyzed to determine the direction for future ABMT research at NIMH, a second cohort was studied. This second cohort included two comparison groups not receiving computerized training. One comparison group of (n = 17) anxious youth simply received CBT. The other comparison group included healthy youth free of any current Axis I psychopathology (n = 16) to determine the stability of attention bias and symptom measures in a non-clinical sample. This second cohort underwent similar pre- and post-assessment procedures as the initial cohort studied with ABMT; however, no training procedures were completed in either of these two comparison groups.
Of note, prior studies of ABMT focusing on threat-bias training have selected subjects who manifest a threat bias at baseline. This pre-selection criteria aims to minimize the potential adverse effects of training subjects without a pre-treatment bias to avoid threat, as a bias away from threat has been linked to stress vulnerability (Wald et al., in press). Prior studies focusing on happy bias training have not pre-selected subjects based on bias scores (Waters et al., in press), and the current study followed this practice. Moreover, the fact that all subjects receiving ABMT in the current study also received CBT further minimizes the potential for adverse effects from ABMT.
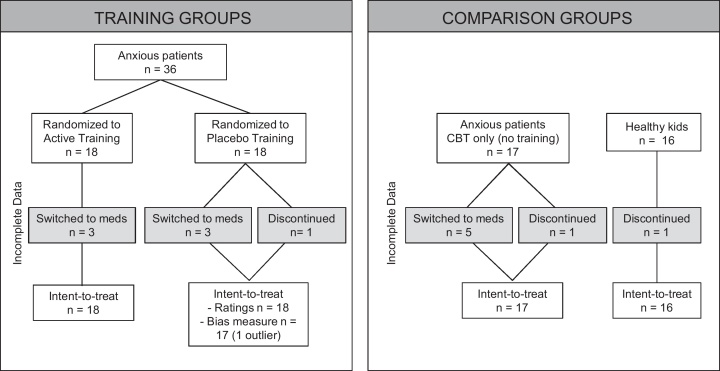
Patient groups completed a similar number of treatment sessions [active: 5.7 ± 2.1, placebo: 5.6 ± 1.9, CBT only: 4.9 ± 2.3, p > 0.5]; however, several individuals discontinued the procedures prior to 8-weeks. Four youth in the active training group, 5 youth in the placebo training group, and 8 youth in the CBT only group were transferred to a medication-treatment condition, prior to completing 8-week CBT treatment. Due to concerns about the clinical status of the child, discontinuation was deemed advisable by the families and clinicians, both blind to training condition. There was no significant difference in the number of sessions attended in individuals completing 8-week CBT treatment and individuals discontinuing the procedures across all groups [active: 5.2 ± 2.8 vs. 5.9 ± 2.0, placebo: 5.8 ± 2.3 vs. 5.5 ± 1.8, CBT-only: 5.6 ± 2.0 vs. 4.3 ± 2.4, all p > 0.3]. One healthy subject did not return to complete the follow-up procedures due to scheduling conflicts (see Fig. 1).
Fig. 1.
Attention Bias Modification Treatment (ABMT) study compliance profile (Experiment 1).
Groups were matched on gender and IQ [both p > 0.2]; however, despite the anxious groups being matched on age [p > 0.8], the healthy comparison group was older than the anxious groups [F(3,68) = 6.2, p < 0.001] (Table 1). All procedures were approved by the NIMH Institutional Review Board (IRB) and all parents and children provided informed consent and assent, respectively.
Table 1.
Demographics, clinical profile, symptom severity of individuals in Experiment 1.
| Anxious |
Healthy | |||
|---|---|---|---|---|
| Active training | Placebo training | CBT only | No training | |
| Demographics | ||||
| Number | 18 | 18 | 17 | 16 |
| Age (years) | 11.4 ± 2.5 | 10.9 ± 2.1 | 11.0 ± 2.5 | 13.9 ± 2.2* |
| Gender (males) | 6 | 6 | 10 | 8 |
| IQ | 107.7 ± 13.0 | 108.4 ± 11.3 | 116.1 ± 14.1 | 111.1 ± 9.5 |
| Diagnoses | ||||
| GAD | 9 | 14 | 11 | – |
| SoPh | 8 | 8 | 7 | – |
| SAD | 6 | 10 | 10 | – |
| Specific phobia | 4 | 8 | 4 | – |
| MDD | 1 | 1 | 0 | – |
| Baseline | Post-treatment | Baseline | Post-treatment | Baseline | Post-treatment | Baseline | Post-treatment | |
|---|---|---|---|---|---|---|---|---|
| Dot probe performance | ||||||||
| Accuracy (%) | 69 ± 24 | 77 ± 14 | 67 ± 19 | 76 ± 16 | 66 ± 17 | 72 ± 19 | 82 ± 15 | 84 ± 12 |
| Happy bias | 7.3 ± 33.5 | −10.9 ± 20.6 | −5.8 ± 32.2 | −11.6 ± 35.8 | 1.36 ± 44.3 | −8.5 ± 19.9 | −0.1 ± 32.1 | −2.8 ± 26.5 |
| Threat bias | 0.4 ± 30.6 | −4.1 ± 25.7 | −4.4 ± 44.0 | 22.5 ± 27.5 | 5.1 ± 35.1 | 5.4 ± 35.6 | −5.2 ± 20.5 | 5.1 ± 22.5 |
| Reaction time | ||||||||
| Happy congruent | 584 ± 124 | 624 ± 114 | 532 ± 101 | 575 ± 124 | 552 ± 126 | 545 ± 118 | 578 ± 83 | 572 ± 88 |
| Happy incongruent | 591 ± 117 | 613 ± 108 | 527 ± 105 | 564 ± 120 | 554 ± 115 | 536 ± 123 | 578 ± 88 | 569 ± 83 |
| Neutral (happy blocks) | 583 ± 128 | 609 ± 110 | 532 ± 89 | 580 ± 132 | 565 ± 120 | 553 ± 122 | 582 ± 90 | 577 ± 93 |
| Angry congruent | 591 ± 130 | 617 ± 111 | 525 ± 85 | 565 ± 124 | 558 ± 94 | 563 ± 108 | 586 ± 80 | 573 ± 84 |
| Angry incongruent | 592 ± 126 | 616 ± 108 | 520 ± 103 | 587 ± 127 | 563 ± 95 | 569 ± 112 | 581 ± 80 | 578 ± 94 |
| Neutral (angry blocks) | 603 ± 124 | 612 ± 113 | 524 ± 100 | 576 ± 120 | 554 ± 96 | 554 ± 134 | 572 ± 76 | 573 ± 92 |
Mean and standard deviation. CBT, cognitive-behavioral therapy; IQ, intelligence quotient; GAD, generalized anxiety disorder; SoPh, social phobia; SAD, separation anxiety disorder; MDD, major depressive disorder.
Significant group difference, p < 0.05.
2.1.2. Procedure
Trained clinical psychologists with at least five years of experience administered CBT using standard methodology (i.e., Kendall's “Coping Cat”) used in prior research (Kendall, 1994, Kendall and SouthamGerow, 1996, Walkup et al., 2008, Compton et al., 2010). The CBT sessions involved psychoeducation, practicing relaxation, cognitive restructuring and exposure. To balance the duration of CBT (e.g., 16 weekly sessions) and the duration of prior ABMT studies (e.g., 8 biweekly sessions), treatment effects were measured for 8 weeks. This approach is consistent with the prior randomized control trials that determined efficacy of CBT after 8 weeks of treatment or wait-list (Kendall, 1994, Kendall and Flannery-Schroeder, 1997). In the current study, sessions 1–2 involved psycho-education and learning relaxation techniques. Exposure therapy and cognitive restructuring usually began in session 3 or 4. Anxious youth randomized to receive active or placebo training completed the computerized training immediately prior to weekly CBT sessions. Both clinicians and anxious youth receiving attention training were blind to training group assignment.
In all anxious patients, clinicians rated CGI severity and improvement scores after each treatment session. Treatment and training continued for 8 weeks. Baseline, mid-treatment and post-treatment self-report, parent, and clinician ratings of anxiety were collected. Baseline and post-treatment attention biases towards threat and towards happy faces were assessed using the dot-probe task. In healthy youth, self-reported symptoms and attention biases were measured twice, spaced 8 weeks apart, to mimic the time schedule of assessment in the anxious groups.
2.1.3. Dot-probe task
Attention biases were measured using the dot-probe task at baseline and approximately 8 weeks later (Mogg et al., 2004). In this task, an emotional face (angry or happy) and a neutral face of the same individual were presented simultaneously. In addition to emotional–neutral face-pairs, trials with pairs of neutral faces were used as a control condition. The face stimuli were presented in black and white and consisted of twelve identities, balanced in gender, selected from the NimStim Face Stimulus set (Tottenham, 2009). Immediately following the face-pair, a probe requiring a response replaced either the emotional face (congruent trial) or the neutral face (incongruent trial).
Using Eprime 1.1, the task was administered in four blocks of 72 trials (24 congruent, 24 incongruent, and 24 neutral). Blocks of angry and happy trials alternated and block order was counterbalanced across subjects. Participants took a short break between each block. Each trial began with a central fixation cross (+) for 500 ms, then a face-pair (angry–neutral, happy–neutral, or neutral–neutral) appeared side-by-side against a black background for 500 ms. A 400 ms probe (two horizontal [..] or vertical [:] dots) replaced the emotional face with equal probability and was followed by an 1100 ms blank screen before the next trial began. Participants were asked to identify the probe via button press. Response mappings for the probe orientations were counterbalanced across participants.
2.1.4. Attention training task
The ABMT task (Bar-Haim, 2010) was designed to train participants to attend towards happy stimuli. In this task, only happy–neutral and neutral–neutral face-pairs were presented. In the active version, the probe always replaced the happy face. In the placebo version, the probe replaced the happy and neutral faces with equal probability. The task consisted of one block of 160 trials. The active version consisted of 128 congruent and 32 neutral trials. The placebo version had 64 congruent, 64 incongruent, and 32 neutral trials.
The face stimuli, stimulus orientation, and probe in the ABMT task were different from the dot-probe task used for bias measurement to demonstrate generalization of training effects. Four faces were selected from a set of emotional expressions used in previous research (Matsumoto and Ekman, 1989). A central fixation cross appeared for 500 ms, then a face-pair (happy–neutral or neutral–neutral), aligned vertically, appeared against a white background for 500 ms. A probe (E or F) replaced one face until the participant identified the probe. The next trial began upon response. Participants were told to respond as quickly and accurately as possible.
2.1.5. Behavioral analyses
Overall accuracy rates were calculated. Attention bias was calculated by subtracting the reaction time to the congruent trials from the reaction time to the incongruent trials. A positive score suggested a bias towards the emotion, while a negative score suggested a bias away from the emotion. Threat bias and happy bias were calculated separately. Dot-probe trials with incorrect responses and trials with reaction times less than 200 ms or greater than 1000 ms were excluded from attention bias calculations.
2.1.6. Baseline measures
Group differences in demographics, pre-treatment clinical presentation and dot-probe performance (i.e., accuracy rates, reaction times, attention biases) were tested using ANOVAs. Bonferroni correction was applied, where applicable. Within each group, one-sample, two-tailed t-tests were used to assess whether threat and happy biases were significantly different from zero. Statistical significance was determined using α = 0.05.
2.1.7. Treatment effects
Due to scheduling and/or treatment difficulties (i.e., transferred to medication treatment), participants completed a variable number of CBT sessions within the 8-week treatment period. All available data were used in linear mixed-effects (LME) analysis, which assumes missing data were missing at random.
To assess the effect of ABMT on the clinical outcome, symptom scores were analyzed using an LME model with assessment period (baseline, mid-treatment, and post-treatment) as a within-subject factor and group (active training, placebo training, and CBT only) as a between-subjects factor. To assess the effect of ABMT on attention biases, an LME model was used with emotion (angry, happy) and assessment period (baseline and post-treatment) as within-subjects factors, group (active, placebo, CBT only, and healthy) as a between-subjects factor, and age as a covariate of non-interest. Significant main effects and interaction effects were determined. Post hoc analyses with Bonferroni correction were applied where needed. Within each group, one-sample, two-tailed t-tests were used to assess whether threat and happy biases were significantly different from zero. Statistical significance was determined using α = 0.05.
2.2. Results
2.2.1. Baseline measures
Anxious groups had similar diagnoses [Table 1, all p > 0.2] and baseline symptom severity [Fig. 2, all p > 0.3]; as expected, anxiety was markedly higher in the patient groups compared to the healthy comparison group [data not shown, all p < 0.01].
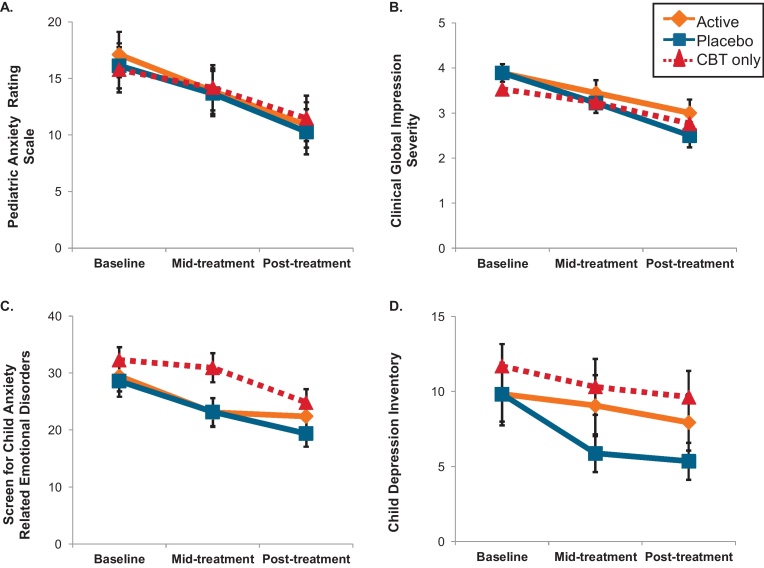
Fig. 2.
Symptom changes in anxious youth receiving cognitive-behavioral therapy (CBT) with and without Attention Bias Modification Treatment (ABMT). Legend: Independent of training status, anxious youth receiving CBT improved over time [panels A–D, all p > 0.001]. However, based on the combined parent and child Screen for Child Anxiety-Related Emotional Disorders (panel C, SCARED), families of youth receiving active ABMT in addition to CBT reported reductions earlier than families receiving CBT only [p < 0.05]. Of note, across all anxious individuals, the parent and child SCARED scores positively correlated [r = 0.38, p < 0.008]. Mean and standard error bars of intent-to-treat variables displayed.
Baseline accuracy rates and attention bias scores were similar across anxious groups [Table 1, all p > 0.9] and compared to the healthy comparison group [all p > 0.13]. Before treatment, no significant happy bias or threat biases were detected in any group [all p > 0.3]. In other words, attention biases were not significantly different than zero. Of note, these attention biases were normally distributed in all groups.
2.2.2. Training
Both groups completed a similar number of training sessions across the 8-week treatment period [active: 5.7 ± 2.1, placebo: 5.6 ± 1.9, t(34) = 0.2, p > 0.9] and had similar accuracy rates [active: 86.9% ±6.4, placebo: 87.6% ±4.1, t(34) = −0.3, p > 0.8] and reaction times [active: 574.4 ms ±89.8, placebo: 584.2 ms ±93.4, t(34) = −0.3, p > 0.8] across training sessions.
2.2.3. Treatment effects
As shown in Fig. 2, clinician ratings of anxiety symptoms (i.e., PARS and CGI severity) continually decreased throughout treatment in all groups [all p < 0.001]; however, no group differences were detected [all p > 0.6]. Using the definition of “responder” from the RUPP Anxiety Study (Research Units on Pediatric Psychopharmacology Anxiety Study Group, 2002), 51% of patients responded to treatment (i.e., had a CGI improvement score less than 4). Based on this criterion, the number of treatment responders did not vary across any patient group [χ2(2) = 2.7, p > 0.2].
Using combined parent and child SCARED, symptoms reduced over time [F(2,80) = 23.0, p < 0.001]; however, anxious groups reported different patterns of anxiety symptom reductions across treatment [group × time: F(4,80) = 3.1, p < 0.02]. As shown in Fig. 2C, both groups receiving training (i.e., active and placebo) reported reduced symptoms from baseline to mid-treatment [both p < 0.003]. The placebo group showed additional reduction at post-treatment [p < 0.03]. However, the CBT-only group showed delayed reductions from mid-treatment to post-treatment [p < 0.001], contributing to a significant difference between active training group and CBT only group at mid-treatment [p < 0.05]. Of note, across all anxious individuals, the parent and child SCARED scores positively correlated [r = 0.38, p < 0.008].
Based on CDI self-report, all groups reported similar depression symptom reduction from baseline to mid-treatment without further reduction at post-treatment [time: F(2,79) = 5.8, p < 0.004]; no group differences were noted [both p > 0.3].
2.2.4. Attention bias
Across both training groups, the group × time × emotion interaction was non-significant [p > 0.4]; however, a time × emotion interaction in attention bias was detected [F(1,117) = 4.0, p < 0.05]. Although attention biases for happy and threat were not significantly different at baseline [both p > 0.7], a significant bias away from happy was detected post-treatment [t(34) = 2.3, p < 0.03]. Of note, the attention biases for either happy or threat bias were not significantly different across visits [both p > 0.1]. Similar effects were detected when examining all patient groups together.
Unlike in the anxious patients receiving ABMT or placebo training, stability across time, independent of treatment manipulation, could be assessed in the healthy comparison group. In healthy youth, happy bias correlated positively across time [r = 0.52, p < 0.05]; however, threat bias was not stable in this group [p > 0.3].
2.3. Discussion
This study is the first to examine the effects of augmenting CBT with attention training towards happy faces in pediatric anxiety. Active attention training towards happy faces did not augment the clinical benefits of CBT; however, combined self- and parental-reports of symptom ratings among individuals receiving active training did decrease more quickly than among individuals receiving CBT only. Behaviorally, attention biases in the anxious groups were not correlated across time; however, the biases towards happy faces across visits were correlated positively in the healthy comparison group.
Anxiety symptom reduction was demonstrated in groups of youth receiving CBT; however, individuals receiving computerized attention training towards happy faces did not demonstrate additional clinical benefits from training. Both CBT and ABMT designed to train attention away from threat have been effective as stand-alone treatments of anxiety, though clearly CBT is a far more established treatment than ABMT (Kendall, 1998, Walkup et al., 2008, Amir and Beard, 2009, Schmidt et al., 2009, Eldar et al., 2012). Here, according to clinician report, no additional benefit was gained from combining CBT and ABMT designed to train towards happy faces. It would be difficult to detect augmentation effects of ABMT if CBT treatment produced ceiling effects. In the current study, the modest treatment response rate, measured by CGI improvement, suggests that the ability to detect augmentative effects of combining treatments with CBT is possible; however, the augmentative procedures of ABMT employed here did not yield additional benefit. The lack of group difference may be explained by the training type, population studied, or time interval between measurements. Moreover, because few studies have examined the effects of ABMT in anxious children, the possibility remains that it could have adverse effects in some children, even interfering with CBT. Clearly, no evidence of this emerged in the current study, though the tendency for training to induce happy-face avoidance in the current study serves as a reminder of the early state of ABMT research. The field should remain vigilant for unintended consequences, given the early stage of this research.
Stand-alone ABMT treatments typically train individuals to attend away from threat (Hakamata et al., 2010), and few studies examine training towards happy. One recent trial using different procedures than in the current study did show happy-bias training to reduce anxiety in children (Waters et al., in press); thus, training towards happy may be ineffective at reducing anxiety symptoms beyond the effects of CBT. Thus, it is clear that more research is needed. For example, it is unclear whether different types of training, such as ABMT to train bias away from threat as an augmentation, would yield similar results. Some studies only treated individuals with a threat bias at baseline (Eldar et al., 2012). However, including baseline attention biases (happy or threat bias) as covariates in the LME models did not alter the current results. Future studies should examine the effects of ABMT in individuals demonstrating attention biases at baseline. Group differences based on ABMT assignment were not detected after 8 weeks of treatment; however, follow-up assessments were not completed in this sample. In a recent study of euthymic individuals with past depression, anxiety symptom reduction was detected one month following ABMT treatment towards positive stimuli, suggesting that attention training can have delayed effects (Browning et al., 2012). Future work should assess the long-term treatment outcomes of ABMT involving training towards positive and away from negative stimuli as these types of treatments may operate on different time scales.
Patterns of anxiety symptom changes differed between clinician report and parent and child reports. Based on the clinician-rated measures, there were no group differences in anxiety symptom reduction; however, based on parent and child reports, the active training group improved more quickly than the CBT only group. The addition of computerized training may facilitate the treatment process by allowing patients to engage in more difficult exposure steps of CBT. In fact, both active and placebo training groups showed anxiety symptom reductions from baseline at mid-treatment; whereas, the CBT only group did not. These results may reflect self-assessed improvement in the training groups due to exposure to happy faces. In addition, parents may be reporting improvements seen outside of the clinical setting, possibly reflecting generalizability of the training to “real-world” experiences not observed by the clinician. Alternatively, treatment outcome expectations may be greater in the training groups than the CBT only group. Parents and children were told that the child would be randomly assigned to one of the two versions of a training task that may or may not help them. Thus, these self-report measures may be particularly vulnerable to expectancy, more so than clinician ratings.
Attention biases changed with treatment in unusual ways. First, across all groups undergoing training, no significant biases towards or away from happy faces were found at baseline, yet although not significantly different from baseline, a significant bias away from happy faces developed post-treatment. This pattern is opposite from what one might expect in individuals receiving active attention training, designed to induce a bias towards happy faces. As noted above, this finding should alert the field to remain vigilant for unintended clinical consequences of ABMT. However, the bias did not significantly change from baseline and is consistent with other studies that also fail to find increased happy bias following training towards positive stimuli (Browning et al., 2012). Second, threat biases did not change with CBT + ABMT towards happy or CBT treatment, which is consistent with prior work examining attention biases following CBT treatment (Waters et al., 2008). Contrary to other research (Wadlinger and Isaacowitz, 2008), the results of the current study may suggest that threat biases are unaffected by attention training towards happy or alternatively, it is more difficult to alter threat biases by training towards happy faces than by training away from threat faces.
Yielding more questions than answers, this study highlights the need for further ABMT research. In particular, it remains unclear when attention training procedures are effective and translate into therapeutic benefit detected by clinicians. Here, healthy comparison youth exhibited relatively stable happy bias (Frenkel et al., 2009), evidenced by correlations across visits; however, threat biases were uncorrelated. This result raises questions about stability of the threat bias. In fact, stressful situations often alter threat biases (Constans et al., 2004, Bar-Haim et al., 2010), suggesting that behavioral indices of attention towards threat are adaptable. If threat bias is not stable, it will be difficult to study treatments designed to alter it in significant ways. Neural correlates of attention bias may be more robust than behavioral indices, providing a more suitable target to manipulate. Before understanding stability in anxious youth, it is important to understand stability in healthy youth. Experiment 2 begins to examine the stability of neural activation in healthy youth performing the dot-probe task to set the stage for future neuroimaging work examining the neural changes associated with ABMT.
3. Experiment 2: Stability of attention biases in healthy youth
3.1. Methods
3.1.1. Participants
Twenty-one individuals were recruited from local advertisements to participate in this functional magnetic resonance imaging (fMRI) experiment as paid volunteers. All participants were between 8 and 17 years old, English-speaking, and had normal or corrected-to-normal vision. All participants were free from any past or current Axis I psychiatric disorder. To assess psychopathology, experienced clinicians administered the K-SADS-PL (Kaufman et al., 1997) to each participant and one parent. All participants were free of any medical illness, based on history and physical exam. Participants denied current or past history of head injury, learning disability, or substance abuse/dependence (>6 months). All subjects were free of any psychotropic medication and any fMRI contraindications. Each participant had an IQ > 70. For all participants, indices of anxiety and depressive symptom severity were measured using the SCARED and CDI.
The final sample used for test–retest reliability was 12, obtained after 1 participant in visit 1 was excluded for technical problems, 2 participants in visit 2 were excluded for low accuracy rates and 6 participants in visit 1 and 1 participant in visit 2 were excluded for excessive head movement (>75% exceeding 3 mm). Group characteristics are outlined in Table 2. All procedures were approved by the NIMH IRB and all parents and children provided informed consent and assent, respectively.
Table 2.
Group characteristics and behavioral results from individuals in Experiment 2.
| Visit 1 | Visit 2 | |
|---|---|---|
| Demographics | ||
| Number of subjects (males) | 12 (6) | 12 (6) |
| Age (years) | 15.6 ± 1.9 | 15.9 ± 2.0 |
| IQ | 108.0 ± 12.2 | |
| SCARED | 10.6 ± 6.2 | 9.3 ± 6.9 |
| CDI | 0.6 ± 1.3 | 0.4 ± 0.9 |
| Duration between visits (days) | – | 126.1 ± 50.8 |
| Behavioral data | ||
| % accuracy | 96.0 ± 4.0 | 95.8 ± 4.4 |
| Attention bias | ||
| Masked fear bias | 5.4 ± 28.6 | −12.9 ± 37.5 |
| Masked angry bias | −7.9 ± 25.8 | −11.0 ± 26.7 |
| Unmasked fear bias | −2.5 ± 19.8 | 3.3 ± 23.5 |
| Unmasked angry bias | 4.8 ± 26.5 | 6.0 ± 27.3 |
| Reaction time | ||
| Masked fear congruent | 659.4 ± 115.7 | 639.5 ± 119.5 |
| Mask fear incongruent | 664.8 ± 128.0* | 626.7 ± 121.3 |
| Masked angry congruent | 665.0 ± 131.6 | 644.8 ± 112.5 |
| Masked angry incongruent | 657.1 ± 110.2 | 634.3 ± 106.3 |
| Masked neutral | 650.4 ± 106.1 | 634.4 ± 112.2 |
| Unmasked fear congruent | 627.2 ± 126.4 | 606.5 ± 102.0 |
| Unmasked fear incongruent | 624.6 ± 116.5 | 609.9 ± 109.3 |
| Unmasked angry congruent | 625.2 ± 112.3 | 616.1 ± 111.5 |
| Unmasked angry incongruent | 630.0 ± 121.3 | 622.1 ± 94.0 |
| Unmasked neutral | 625.4 ± 121.3 | 620.3 ± 114.7 |
Mean and standard deviation.
Significant visit difference (p < 0.05).
3.1.2. Dot-probe task
In two separate visits, participants completed a dot-probe task in the MRI scanner (Mogg et al., 2004). In this dot-probe task, an emotional face (angry or fearful) and a neutral face of the same individual were presented simultaneously. Participants were asked to identify the location of probe (*) that followed one of the faces via button press, yielding reaction time differentials between incongruent and congruent trials to assess attention bias.
Four runs of this task were completed. In each event-related fMRI run, 32 angry–neutral, 32 fearful–neutral and 16 neutral–neutral trials were presented. The face stimuli were presented in black and white and consisted of 24 identities selected from the NimStim Face Stimulus set (Tottenham, 2009). Four females and four males were selected to present each emotional category (angry, fearful, and neutral). In this version, participants were instructed to identify the location of the probe (an asterisk) via button-press. The emotional face and probe location were counterbalanced across the entire experiment. In addition, 16 null trials per run were presented to provide reliable estimates for the hemodynamic response magnitude.
During half of the trials, faces were presented overtly (i.e., unmasked) and during half of the trials, faces were presented subliminally (i.e., believed to be masked below conscious awareness). Both trials were presented for 3100 ms, including an average intertrial interval (ITI) of 1000 ms. Each unmasked trial consisted of a 500 ms fixation (i.e., a white fixation cross centered on a black background), a 500 ms face-pair, and a 1100 ms probe. Each masked trial consisted of a 500 ms fixation, a 17 ms face-pair, a 68 ms scrambled mask image, an 1100 ms probe and 415 ms blank screen. The trials were separated by a variable length ITI, which ranged from 750 to 1250 ms. This ITI was introduced to reduce anticipation effects in the behavioral response.
3.1.3. Apparatus and acquisition
Stimuli were presented on a black background via standardized software (E-Prime, 1.1) running on a PC. These images were front-projected onto a screen and viewed by participants through a mirror mounted on the head coil.
For each participant, MRI data was collected with a 3.0 T whole-body high-speed imaging device (GE Signa, Milwaukee, WI) and an 8-channel gradient head coil. After automated shimming, a T2*-sensitive gradient echo pulse sequences was used for all functional imaging (TR = 2100 ms, TE = 30 ms, flip angle = 78°, FOV = 22 cm, 64 × 64, 29 axial slices with 4 mm slice thickness and no skip). A high resolution 3D MPRAGE MRI spin echo sequence (TR = 700 ms, TE = min, FOV = 22 cm, 256 × 256 matrix. 1.2 mm slice thickness) was collected for spatial co-registration and normalization.
3.1.4. Behavioral analysis
For each visit, overall accuracy rates were calculated. In addition, attention bias scores were calculated for each condition (masked anger, unmasked anger, masked fear, unmasked fear). Dot-probe trials with incorrect responses and trials with reaction times less than 200 ms or greater than 1000 ms were excluded from attention bias calculations.
For each visit, one-sample, two-tailed t-tests were used to detect significant attention biases in each condition. Paired t-tests were used to test for significant differences in bias scores between visits. In addition, bias scores between visits were tested for significant correlations. In all statistical tests, a significance level of α = 0.05 was used.
3.1.5. fMRI analysis
fMRI data were preprocessed using AFNI (http://afni.nimh.nih.gov/afni). Preprocessing included slice-timing correction and the series of functional images was realigned to a functional image collected closest to when the anatomical was collected (i.e., third image or last image of the series). To co-register the images between visits in an unbiased manner, the two anatomical images from each visit were skull stripped, co-registered to the intermediate image and then these images were averaged together (Glen, 2012). All functional images were co-registered to this resulting image (Saad et al., 2009) and normalized to Talaraich space (Talairach and Tournoux, 1988). The data were smoothed using a 6 mm full width at half maximum, isotropic Gaussian filter and scaled to the mean of the voxel-wise time series. After all preprocessing steps, the resulting images contained 3 mm isotropic voxels.
A general linear model was created for each individual. For both unmasked and masked conditions, regressors were included for angry-congruent, angry-incongruent, fearful-congruent, fearful-incongruent, and neutral conditions. Trials with errors were included as a separate regressor of no interest. Finally, six motion regressors corresponding to translation and rotation in each xyz direction were included. Contrasts among the effect estimates were generated by comparing the beta weights associated with BOLD activation. Our main interest was on contrasts of incongruent relative to congruent conditions to assess bias for angry and fearful conditions in both masked and unmasked separately. In addition, contrasts of all angry conditions and all fearful conditions vs. fixation were investigated based on unmasked and masked conditions.
For each condition, intra-class correlation (ICC) analysis was conducted in AFNI on the whole-brain level using subject and visit as random variables in an LME model (Chen, 2010). To measure agreement across visits, ICC (2,1) values were calculated (Shrout and Fleiss, 1979). Significant regions were identified by peak threshold and, to control for multiple comparisons at a α < 0.05 corrected-level, a cluster threshold. The initial ICC threshold was set to correspond to an ICC = 0.56, based on an uncorrected p < 0.05 with 11 degrees of freedom (Bartko, 1966). Additional corrections were implemented to account for the multi-voxel nature of the fMRI data. Based on AlphaSim calculations with an estimated spatial smoothness of 11 mm, minimum cluster sizes of 17 voxels (459 mm3) for the amygdala, 52 voxels (1404 mm3) for the ventrolateral prefrontal cortex as a priori-defined regions, and 468 voxels (12,636 mm3) were needed to correct for multiple comparisons (i.e., Family-wise Error, FWE) at the whole-brain level.
A whole-brain voxel-wise analysis also was performed at the group level, with contrast images from individual analyses. Unlike the initial ICC analysis, which examined stability of response, this second analysis examined task-related activation in the group as a whole. This analysis was performed for each visit, to identify regions showing condition effects (e.g., unmasked angry bias). Significant regions were identified using FWE correction as described above. With a voxel-wise p < 0.005 uncorrected threshold, 2 voxels (54 mm3) for the amygdala, 9 voxels (243 mm3) for ventrolateral prefrontal cortex, and 71 voxels (1917 mm3) were needed to correct for multiple comparisons at the corrected significance α < 0.05 whole-brain level. Coordinates at the peak are presented using LPI coordinate system.
3.2. Results
3.2.1. Behavioral results
The accuracy rates were 96% ±4 for both visits. No significant attention biases were detected during either visit (all p > 0.20) and no significant differences were detected in bias scores between visits (all p > 0.20). The unmasked anger biases on the two visits were negatively correlated (r = −0.75, p < 0.005); however, no other bias condition showed a significant association across visits (all p > 0.20). The reaction times for all individual conditions (e.g., unmasked angry congruent, unmasked angry incongruent) were strongly, positively correlated across visits (all r > 0.85, p < 0.001).
3.2.2. Intra-class coefficient (ICC) fMRI results
3.2.2.1. Ventrolateral prefrontal cortex
The activation within the inferior frontal gyrus/BA47 was consistent across visits for unmasked angry bias (i.e., unmasked angry incongruent vs. unmasked angry congruent) [Fig. 3A, (−32, 29, 3), 64 voxels (1728 mm3), ICC = 0.73] and unmasked fearful bias (i.e., unmasked fearful incongruent vs. unmasked fearful congruent) [Fig. 3B, (41, 20, −4), 77 voxels (2079 mm3), ICC = 0.75]. Stability within this region was not detected within the individual congruent and incongruent conditions that comprise these bias contrasts (i.e., incongruent > congruent).
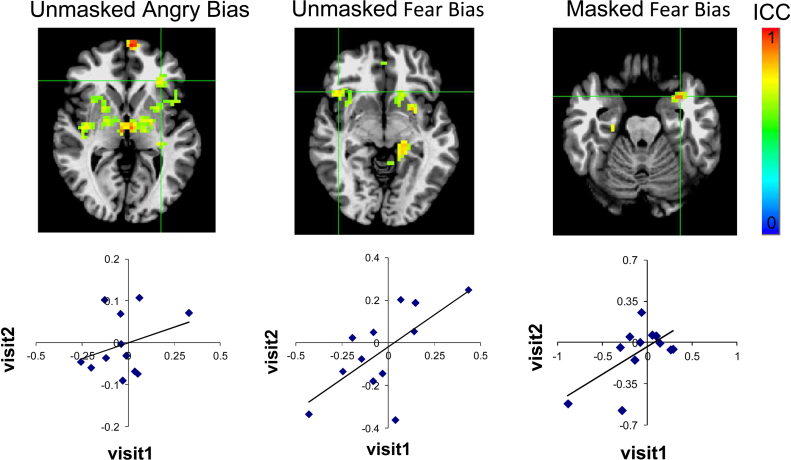
Fig. 3.
Test–retest reliability of neural activation in healthy youth across visits. Legend: Activation in regions showing significant intra-class correlation (ICC) values include the ventrolateral prefrontal cortex and amygdala for unmasked threat (both angry [(−32, 29, 3), 64 voxels (1728 mm3), ICC = 0.73] and fear bias [(41, 20, −4), 77 voxels (2079 mm3), ICC = 0.75]) and masked fear bias [(−31, 5, −19), 24 voxels (648 mm3), ICC = 0.83], respectively. Images display ICC surviving p < 0.05 corrected threshold for these regions. Images are displayed in radiological convention (left = right). Percent signal change values from each functionally defined region are shown in the plots below each image. Grid lines indicate region of extracted data.
3.2.2.2. Amygdala
Significant reliability was detected in the left amygdala/parahippocampal gyrus for the masked fearful bias contrast (i.e., masked fearful incongruent vs. masked fearful congruent) [Fig. 3C, (−31, 5, −19), 24 voxels (648 mm3), ICC = 0.83]. Significant reliability in the amygdala/parahippocampus was also detected in the masked fearful incongruent relative to fixation [left: (−29, 5, −19), 44 voxels (1188 mm3), ICC = 0.81, right: (17, −8, −19), 24 voxels, (648 mm3), ICC = 0.73], but was not detected in the masked congruent fearful condition.
3.2.3. Activation patterns
Only one bias contrast showed activation in a priori selected regions, the amygdala and ventrolateral prefrontal cortex. During visit 2, amygdala activation was detected in response to unmasked fear bias contrast [(26, 2, −13), 2 voxels (54 mm3), t-value 3.69]. Amygdala activation was detected in response to the unmasked fearful incongruent condition relative to fixation [(26, 2, −13), 46 voxels (1242 mm3), t-value 3.94; (−16, −4, −10), 39 voxels (1053 mm3), t-value 4.25], but not in the unmasked fearful congruent condition. Activation surviving whole-brain correction was detected in a non-a priori region, the cerebellum, in response to unmasked angry bias [(−47, −65, −22), 120 voxels (3240 mm3), t-value 4.45]. Cerebellar activation was detected in the unmasked angry incongruent condition relative to fixation [(32, −82, −19), 2003 voxels (54,081 mm3), t-score 5.43].
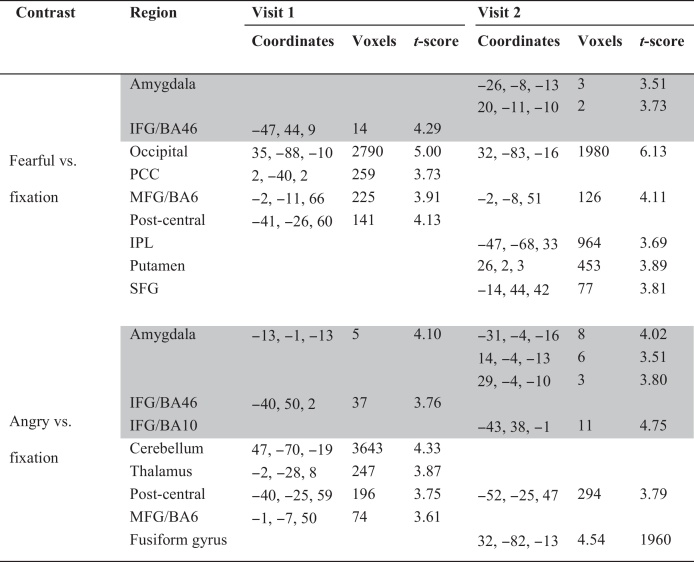
Additional activations for fearful faces and angry faces relative to fixation are listed in Table 3.
Table 3.
Activation patterns in healthy youth for fearful and angry faces relative to fixation.
p < 0.05 corrected for a priori regions (highlighted in gray) and whole-brain (in white). IFG, inferior frontal gyrus; PCC, posterior cingulate cortex; MFG, medial frontal gyrus; IPL, intra parietal lobule; SFG, superior frontal gyrus; BA, Brodmann's area.
3.3. Discussion
In summary, initial evidence suggests stability in response to attention bias contrasts was demonstrated in the ventrolateral prefrontal cortex and amygdala. Stability in ventrolateral prefrontal cortex activation was demonstrated in both unmasked bias conditions; whereas, stability of amygdala activation was limited to masked fearful bias. Despite finding stability with neural activation, behavioral attention biases were inconsistent across visits.
Although stability of activation was not compared directly, stability of activation within vlPFC and amygdala appeared to depend on the type of face presentation. Unmasked face presentations of threat recruited vlPFC reliably; whereas, masked fearful face presentations recruited amygdala reliably. The regional patterns of stable engagement for unmasked and masked face presentations may reflect the recruitment of bottom-up control when faces are presented outside of awareness, and top-down control when the faces are presented for longer durations (e.g., unmasked presentations). In fact, this is consistent with prior work showing group differences between pediatric anxiety and healthy youth have been detected in the vlPFC for unmasked faces (Monk et al., 2006) and in the amygdala for masked faces (Monk et al., 2008).
More stability of the attention bias contrast was demonstrated in the neural activation patterns than the behavioral patterns of dot-probe performance. While the overall reaction times are highly reliable, attention bias scores were not. Interestingly, attention bias to unmasked angry faces was correlated negatively across visits, indicative of opposite behavioral patterns of threat bias. It is difficult to interpret this effect as the attention biases were not significant at either visit. Yet, ICC values detected in vlPFC and amygdala regions for attention bias contrasts were moderately reliable (i.e., >0.7). The inherent differences in the types of measurements may dictate the level of stability. First, the dot-probe trial involves the presentation of a face-pair that contains an emotional stimulus followed by a target requiring a response. Therefore, the behavioral response captures the end result of multiple cognitive processes, i.e., perceiving both emotional/neutral faces and probe, inhibiting task-irrelevant faces, determining the location of the probe, and pressing the correctly mapped button. Second, behavioral bias scores have decreased reliability due to measurement error (Eide et al., 2002, Strauss et al., 2005). On the other hand, the neural signal is measured throughout the dot-probe trial and this continuous physiological measure may be more reliable. In addition, the measurement error for fMRI studies is less because this neuroimaging technique relies on a relative measure (i.e., contrast of two conditions) rather than being an absolute measure like reaction time.
4. General discussion and conclusions
In summary, two experiments were conducted in youth to inform ABMT research. In the first study, active training towards happy did not provide any additional therapeutic benefit beyond CBT; however, families of patients receiving active training reported symptom reduction faster than those that did not receive computer-based behavioral treatment. In the second study in healthy youth, stability of threat bias was detected in neural activation but not behaviorally.
To be meaningful, treatment studies must establish outcome measures that endure over time. In both experiments, reaction time measures of threat biases in healthy youth were found to be unstable (i.e., not correlated) over time. However, finding initial evidence of stable neural activation patterns in healthy youth is important and demonstrates the need for larger studies focused on understanding the stability of attention biases using physiological and behavioral measures. Future studies using ABMT should examine group differences in neural changes associated with training and determine whether neural changes mediate changes in treatment outcome (Eldar et al., 2010).
The results of these studies should be viewed with consideration of some limitations. First, unlike more-widely used ABMT procedures which train attention away from threat, the current study examined the effects of training attention towards positive. Future studies will examine benefit of combining CBT and attention training away from threat. Second, the small sample size limits generalizability and the ability to investigate potential mediating or moderating factors. Few studies have examined the test–retest reliability of neural activation in cognitive/affective tasks (Clement and Belleville, 2009, Plichta et al., 2012); however, in prior work, the sample sizes vary between 10 and 25 individuals, yet this range encompasses the current sample size (n = 12). In addition, a small sample size typically yields Type II errors; however, with this sample size, a significant intra-class correlation was detected. Contrary to this argument, the small samples may lead to an over estimation of effect sizes. Replication is needed. The findings were also complicated by participation compliance, as not all participants completed all eight CBT and training sessions due to changing treatment course or to normal scheduling conflicts. To increase the number of training sessions, future studies should examine the effects of ABMT using a longer treatment period (e.g., 16-week) as is done in standard “Coping Cat” procedures (Kendall, 1994, Walkup et al., 2008). Although this mimics typical treatment compliance, the variability may reduce the effects of ABMT. Compliance was also an issue in the second study examining reliability across two visits. It was difficult to obtain usable data in pediatric participants for both visits because the likelihood of behavioral incompliance and excessive motion increases with repetition. Large scale studies of training and reliability of attention biases to both positive and negative stimuli using standardized procedures are needed to understand the potential impact of ABMT. Another limitation concerns participants low accuracy rates during assessment of attention bias in the ABMT study. Although only accurate trials in the dot probe task were analyzed, future studies should consider simplifying the dot probe task to a developmentally appropriate level for a pediatric sample. For example, higher accuracy rates were achieved during training when the probe discrimination was simpler (i.e., E/F) and the probe remained on the screen until response. Finally, the pediatric anxiety population included many anxiety disorders. Future studies should examine disorder specificity of these results.
Despite these limitations, these results add to the growing ABMT literature, providing some direction for future research. Understanding when ABMT procedures are effective is essential to provide therapeutic benefit for pediatric anxiety disorders. Together, these two studies suggest that the training (i.e., ABMT towards positive stimuli), combining treatments (i.e., ABMT + CBT), and the treatment target (i.e., behavioral vs. neural) are important factors to consider.
Conflicts of interest
The authors declare no conflicts of interest or financial interests related to this manuscript.
Acknowledgments
This research was supported in part by theIntramural Research Program of the National Institute of Mental Health (NIMH), National Institutes of Health and the National Alliance of Research on Schizophrenia and Depression (NARSAD) Young Investigator Award (JCB). A version of these findings was presented at the Anxiety Disorders Association of America annual conference in April 2012. The imaging data partially overlap with data presented in Thomason et al. (2010). We thank the clinicians in the Section on Development and Affective Neuroscience Program at the NIMH for their assistance.
Contributor Information
Jennifer C. Britton, Email: j.britton@miami.edu.
Yair Bar-Haim, Email: yair1@post.tau.ac.il.
Michelle A. Clementi, Email: mclementi@uh.edu.
Lindsey S. Sankin, Email: LindseySankin2016@u.northwestern.edu.
Gang Chen, Email: gangchen@mail.nih.gov.
Tomer Shechner, Email: shechnert@mail.nih.gov.
Maxine A. Norcross, Email: norcrossm@gmail.com.
Carolyn N. Spiro, Email: spirocn@mail.nih.gov.
Kara M. Lindstrom, Email: karalindstrom@gmail.com.
Daniel S. Pine, Email: pined@mail.nih.gov.
References
- Amir N., Beard C. Attention training in individuals with generalized social phobia: a randomized controlled trial. Journal of Consulting and Clinical Psychology. 2009;77(5):961–973. doi: 10.1037/a0016685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y. Research review: attention bias modification (ABM): a novel treatment for anxiety disorders. Journal of Child Psychology and Psychiatry. 2010;51(8):859–870. doi: 10.1111/j.1469-7610.2010.02251.x. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y., Holoshitz Y. Life-threatening danger and suppression of attention bias to threat. American Journal of Psychiatry. 2010;167(6):694–698. doi: 10.1176/appi.ajp.2009.09070956. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y., Lamy D. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological Bulletin. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y., Morag I. Training anxious children to disengage attention from threat: a randomized controlled trial. Journal of Child Psychology and Psychiatry. 2011;52(8):861–869. doi: 10.1111/j.1469-7610.2011.02368.x. [DOI] [PubMed] [Google Scholar]
- Bartko J. The intraclass correlation coefficient as a measure of reliability. Psychological Reports. 1966;19:3–11. doi: 10.2466/pr0.1966.19.1.3. [DOI] [PubMed] [Google Scholar]
- Birmaher B., Khetarpal S. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(4):545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Britton J.C., Bar-Haim Y. Isolating neural components of threat bias in pediatric anxiety. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2012;53(6):678–686. doi: 10.1111/j.1469-7610.2011.02503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning M., Holmes E.A. Using attentional bias modification as a cognitive vaccine against depression. Biological Psychiatry. 2012;72(7):572–579. doi: 10.1016/j.biopsych.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning M., Holmes E.A. Lateral prefrontal cortex mediates the cognitive modification of attentional bias. Biological Psychiatry. 2010;67(10):919–925. doi: 10.1016/j.biopsych.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. 2010. Intra-Class Correlation with ANOVA Method.http://afni.nimh.nih.gov/sscc/gangc/ICC [retrieved 2010] [Google Scholar]
- Clement F., Belleville S. Test–retest reliability of fMRI verbal episodic memory paradigms in healthy older adults and in persons with mild cognitive impairment. Human Brain Mapping. 2009;30(12):4033–4047. doi: 10.1002/hbm.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton S.N., Walkup J.T. Child/Adolescent Anxiety Multimodal Study (CAMS): rationale, design, and methods. Child and Adolescent Psychiatry and Mental Health. 2010;4:1. doi: 10.1186/1753-2000-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constans J.I., McCloskey M.S. Suppression of attentional bias in PTSD. Journal of Abnormal Psychology. 2004;113(2):315–323. doi: 10.1037/0021-843X.113.2.315. [DOI] [PubMed] [Google Scholar]
- Dandeneau S.D., Baldwin M.W. Cutting stress off at the pass: reducing vigilance and responsiveness to social threat by manipulating attention. Journal of Personality and Social Psychology. 2007;93(4):651–666. doi: 10.1037/0022-3514.93.4.651. [DOI] [PubMed] [Google Scholar]
- Eide P., Kemp A. Test–retest reliability of the emotional stroop task: examining the paradox of measurement change. Journal of Psychology. 2002;136(5):514–520. doi: 10.1080/00223980209605547. [DOI] [PubMed] [Google Scholar]
- Eldar S., Apter A. Attention bias modification treatment for pediatric anxiety disorders: a randomized controlled trial. American Journal of Psychiatry. 2012;169(2):213–220. doi: 10.1176/appi.ajp.2011.11060886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar S., Bar-Haim Y. Neural plasticity in response to attention training in anxiety. Psychological Medicine. 2010;40(4):667–677. doi: 10.1017/S0033291709990766. [DOI] [PubMed] [Google Scholar]
- Frenkel T.I., Lamy D. Individual differences in perceptual sensitivity and response bias in anxiety: evidence from emotional faces. Cognition and Emotion. 2009;23(4):688–700. [Google Scholar]
- Frewen P.A., Dozois D.J. Selective attention to threat versus reward: meta-analysis and neural-network modeling of the dot-probe task. Clinical Psychology Review. 2008;28(2):307–337. doi: 10.1016/j.cpr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Glen D.R. 2012. Alignment of EPI Data Across two Sessions.http://afni.nimh.nih.gov/sscc/dglen/alignmentacross2sessions (retrieved 01.06.12) [Google Scholar]
- Guy W. Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs; Rockville, MD, U.S.: 1976. ECDEU Assessment Manual for Psychopharmacology – Revised (DHEW Publ No ADM 76-338) [Google Scholar]
- Hakamata Y., Lissek S. Attention bias modification treatment: a meta-analysis toward the establishment of novel treatment for anxiety. Biological Psychiatry. 2010;68(11):982–990. doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of American Academy of Child and Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kendall P.C. Treating anxiety disorders in children – results of a randomized clinical-trial. Journal of Consulting and Clinical Psychology. 1994;62(1):100–110. doi: 10.1037//0022-006x.62.1.100. [DOI] [PubMed] [Google Scholar]
- Kendall P.C., Flannery-Schroeder E. Therapy for Youths with Anxiety Disorders: A Second Randomized Clinical Trial. Journal of Consulting and Clinical Psychology. 1997;65(3):366–380. doi: 10.1037//0022-006x.65.3.366. [DOI] [PubMed] [Google Scholar]
- Kendall P.C. Empirically supported psychological therapies. Journal of Consulting and Clinical Psychology. 1998;66(1):3–6. doi: 10.1037//0022-006x.66.1.3. [DOI] [PubMed] [Google Scholar]
- Kendall P.C., SouthamGerow M.A. Long-term follow-up of a cognitive-behavioral therapy for anxiety-disordered youth. Journal of Consulting and Clinical Psychology. 1996;64(4):724–730. doi: 10.1037//0022-006x.64.4.724. [DOI] [PubMed] [Google Scholar]
- Kovacs M. 2009. Children's Depression Inventory.http://www.pearsonassessments.com/depressioninvent.aspx [retrieved 2009] [Google Scholar]
- Li S., Tan J. Continual training of attentional bias in social anxiety. Behaviour Research and Therapy. 2008;46(8):905–912. doi: 10.1016/j.brat.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Matsumoto D., Ekman P. American–Japanese cultural-differences in intensity ratings of facial expressions of emotion. Motivation and Emotion. 1989;13(2):143–157. [Google Scholar]
- Mogg K., Philippot P. Selective attention to angry faces in clinical social phobia. Journal of Abnormal Psychology. 2004;113(1):160–165. doi: 10.1037/0021-843X.113.1.160. [DOI] [PubMed] [Google Scholar]
- Monk C.S., Nelson E.E. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. American Journal of Psychiatry. 2006;163(6):1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- Monk C.S., Telzer E.H. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry. 2008;65(5):568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta M.M., Schwarz A.J. Test–retest reliability of evoked BOLD signals from a cognitive-emotive fMRI test battery. Neuroimage. 2012;60(3):1746–1758. doi: 10.1016/j.neuroimage.2012.01.129. [DOI] [PubMed] [Google Scholar]
- Research Units on Pediatric Psychopharmacology Anxiety Study Group The Pediatric Anxiety Rating Scale (PARS): development and psychometric properties. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(9):1061–1069. doi: 10.1097/00004583-200209000-00006. [DOI] [PubMed] [Google Scholar]
- Saad Z.S., Glen D.R. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. Neuroimage. 2009;44(3):839–848. doi: 10.1016/j.neuroimage.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N.B., Richey J.A. Attention training for generalized social anxiety disorder. Journal of Abnormal Psychology. 2009;118(1):5–14. doi: 10.1037/a0013643. [DOI] [PubMed] [Google Scholar]
- Shechner T., Britton J.C. Attention biases, anxiety, and development: toward or away from threats or rewards? Depression and Anxiety. 2012;29(4):282–294. doi: 10.1002/da.20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout P.E., Fleiss J.L. Intraclass correlations: uses in assessing rater reliability. Psychological Bulletin. 1979;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Strauss G.P., Allen D.N. Test–retest reliability of standard and emotional stroop tasks – an investigation of color–word and picture–word versions. Assessment. 2005;12(3):330–337. doi: 10.1177/1073191105276375. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Stuttgart; Thieme: 1988. Co-planar Stereotactic Atlas of the Human Brain. [Google Scholar]
- Thomason M.E., Henry M.L. Neural and behavioral responses to threatening emotion faces in children as a function of the short allele of the serotonin transporter gene. Biological Psychology. 2010;85(1):38–44. doi: 10.1016/j.biopsycho.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N. 2009. NimStim Face Stimulus Set.http://www.macbrain.org/resources.htm (retrieved January 2009) [Google Scholar]
- Wadlinger H.A., Isaacowitz D.M. Looking happy: the experimental manipulation of a positive visual attention bias. Emotion. 2008;8(1):121–126. doi: 10.1037/1528-3542.8.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald, I., Degnan, K.A., et al. Attention to threats and combat-related post-traumatic stress symptoms: prospective associations and moderation by the serotonin transporter gene. Achives of General Psychiatry, in press. [DOI] [PMC free article] [PubMed]
- Walkup J.T., Albano A.M. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. New England Journal of Medicine. 2008;359(26):2753–2766. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters, A.M., Pittaway, M., et al. Attention training towards positive stimuli in clinically anxious children. Developmental Cognitive Neuroscience, in press. [DOI] [PMC free article] [PubMed]
- Waters A.M., Wharton T.A. Threat-based cognitive biases in anxious children: comparison with non-anxious children before and after cognitive behavioural treatment. Behaviour Research and Therapy. 2008;46(3):358–374. doi: 10.1016/j.brat.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Harcourt Assessment, Inc.; San Antonio, TX: 1999. Wechsler Abbreviated Scale of Intelligence (WASI) [Google Scholar]