Abstract
BACKGROUND
Transfusion-related acute lung injury (TRALI) mitigation strategies include the deferral of female donors from apheresis platelet (PLT) donations and the distribution of plasma for transfusion from male donors only. We studied the implications of these policies in terms of component loss at six blood centers in the United States.
STUDY DESIGN AND METHODS
We collected data from allogeneic blood donors making whole blood and blood component donations during calendar years 2006 through 2008. We analyzed the distribution of donations in terms of the sex, transfusion and pregnancy histories, and blood type.
RESULTS
A TRALI mitigation policy that would not allow plasma from female whole blood donors to be prepared into transfusable plasma components would result in nearly a 50% reduction in the units of whole blood available for plasma manufacturing and would decrease the number of type AB plasma units that could be made from whole blood donations by the same amount. Deferral of all female apheresis PLT donors, all female apheresis PLT donors with histories of prior pregnancies, or all female apheresis PLT donors with histories of prior pregnancies and positive screening test results for antibodies to human leukocyte antigens (HLAs) will result in a loss of 37.1, 22.5, and 5.4% of all apheresis PLT donations, respectively.
CONCLUSION
A TRALI mitigation policy that only defers female apheresis PLT donors with previous pregnancies and HLAs would result in an approximately 5% decrease in the inventory of apheresis PLTs, but would eliminate a large proportion of components that are associated with TRALI.
Transfusion-related acute lung injury (TRALI) is the leading cause of transfusion-related deaths in the United States. Thirty-five percent of the deaths reported to the US Food and Drug Administration in the federal fiscal year 2008 were attributed to TRALI;1 this percentage decreased to 30% in the federal fiscal years 2009.2 The AABB issued an association bulletin on TRALI mitigation in November 2006 recommending that member blood centers minimize the preparation of high-plasma-volume components from donors known to be white blood cell (WBC) alloimmunized or at increased risk for WBC alloimmunization.3
In response to these recommendations, many blood collection agencies have restricted the distribution of plasma for transfusion to plasma that is derived from male donors as much as possible, with diversion of plasma from female donors to recovered plasma for use in manufacturing derivatives. While this approach is practical for blood group A and O plasma products, it might be more difficult to collect sufficient group B and AB plasma products exclusively from male donors to support the need for transfusable plasma. The AABB also recommended mitigation steps for apheresis platelet (PLT) components. However, there is no excess of apheresis PLT products from male donors; therefore, TRALI mitigation steps must consider alternative approaches for TRALI reduction than simply the use of male-only donors.
In response to the need for data on the prevalence of WBC alloimmunization in blood donors, the National Institutes of Health–funded Retrovirus Epidemiology Donor Study-II (REDS-II) initiated an investigation of the prevalence of antibodies to human leukocyte antigens (HLA) and/or human neutrophil antigens (HNA) among blood donors from six geographically dispersed US blood collection centers. The REDS-II Leukocyte Antibody Prevalence Study-I (LAPS-I) reported that the prevalence of HLA Class I and/or HLA Class II antibodies was similar in nontransfused (1.0%) versus transfused men (1.7%) and that 24.4% of female donors with a history of a previous pregnancy had HLA antibodies.4
This study estimates the prevalence of WBC alloimmunization according to the pregnancy and transfusion history of allogeneic blood donors at each of the REDS-II blood centers. These data, together with the data from the LAPS-I study,4 were used to compare the impact of the implementation of the AABB TRALI mitigation strategies among six different blood centers.
MATERIALS AND METHODS
Data collection
Data from the National Heart, Lung, and Blood Institute’s REDS-II, a multicenter study designed to research blood safety and availability issues in the United States, was used for this study. The six US blood centers participating in REDS-II include the Blood Centers of the Pacific (San Francisco, CA), the American Red Cross Blood Services Southern Region (Atlanta, GA) and New England Region (Dedham, MA), the Hoxworth Blood Center (Cincinnati, OH), the Institute for Transfusion Medicine (Pittsburgh, PA), and the BloodCenter of Wisconsin (Milwaukee, WI). The REDS-II protocol was approved by the institutional review board at each participating blood center and the central coordinating center, Westat, Inc. (Rockville, MD).
Information on demographic characteristics (age, sex, first-time or repeat donor status, transfusion history, and pregnancy history), donation procedure (whole blood vs. apheresis component collections), and blood donation type (allogeneic vs. autologous donations) has been collected on each blood donor by the REDS-II program continuously since January 2006. Three of the REDS-II blood centers (Blood Centers of the Pacific, Institute for Transfusion Medicine, and Hoxworth Blood Center) collected the research demographic information—such as lifetime pregnancy history—by incorporating these questions among the health history questions routinely asked to all blood donors. These questions were asked during the donor health history screening process. At the two American Red Cross blood centers and at the BloodCenter of Wisconsin, the same research questions were administered to the donors via a supplemental questionnaire that was completed by the donors before or during their health histories. Transfusion history was elicited by the question “Have you ever received someone else’s blood?” The pregnancy screening question was “Have you ever been pregnant?” and if the donor had been pregnant a follow-up question was asked (“How many times?”). Donations were classified as first time if donors reported that it was the first time they donated at the blood center and there was no record of a previous donation within the REDS-II database.
Donors were provided with information stating that their demographic information could be used for research studies in an anonymous manner according to protocol of each of the REDS-II blood centers. The blood centers routinely sent their donation data with encrypted donor identification to the REDS-II’s central coordinating center (Westat, Inc.) where the data were compiled for analysis.
Information from allogeneic donations of whole blood and apheresis components collected during a 3-year period (January 2006 to December 2008) from all of the REDS-II blood centers was included in this analysis.
Procedure type classification
The type of blood component collected was classified as follows: whole blood, apheresis double red blood cells (RBCs), apheresis PLTs only, apheresis PLTs with plasma collection, apheresis PLTs with plasma and RBC collection, apheresis PLTs with RBC collection, RBC collection by automated instrument with plasma collection, plasma collection by automated instrument, leukapheresis, and others. For the majority of the analyses, the number of apheresis PLTs-only collections was combined with the number of collections of apheresis PLTs with concurrent plasma and/or RBCs. For an analysis of the blood types of the whole blood versus apheresis plasma donations, the number of apheresis plasma collections included plasma-only procedures; apheresis plasma and PLTs collections; apheresis plasma, PLTs, and RBC collections; and apheresis plasma with RBC collections. Apheresis double-RBC collections and apheresis of single RBCs with plasma and/or PLTs collections were not counted in the total number of whole blood collections.
Statistical analysis
The percentages of donations from donors with risk factors for HLA and/or HNA alloimmunization were calculated. The number of donations from female donors according to their prior pregnancy histories from this study and the proportion of female donors with positive screening test results for HLA antibodies (according to their prior pregnancy histories) from the LAPS-I study4 were used to calculate the number of donations of apheresis PLTs from female donors that could have HLA antibodies.
The percentages of donations resulting in 1, 2, or 3 units of apheresis PLTs at two REDS-II blood centers were used to calculate the numbers of apheresis PLTs products that could be lost if all female donors, all ever-pregnant female donors, or all ever-pregnant female donors with HLA antibodies are deferred from future apheresis PLT donations.
The percentages of donations from donors with AB blood type from all male donors, all female donors, all ever-pregnant female donors, and all ever-pregnant female donors with positive screening tests for HLA antibodies was calculated. It was assumed that the pregnancy history of donors of apheresis plasma was similar to the pregnancy history of the donors of apheresis PLTs and that the pregnancy history of donors of whole blood and apheresis plasma was independent of the blood type of the donors. A chi-square test was performed to assess the association between the percentage of female apheresis PLTs donors in 2006 versus 2008 (SAS 9.1.3 [2004], SAS Institute, Inc., Cary, NC).
RESULTS
During the calendar years 2006 through 2008, 3,454,375 allogeneic donations were made to the REDS-II blood centers. The donations consisted of 2,971,759 donations of whole blood, 200,316 donations of apheresis double RBCs, and 282,300 donations of apheresis PLTs (with and without concurrent RBCs and/or plasma; Table 1). Transfusion and pregnancy histories information were available for more than 90% of the donations.
TABLE 1.
Donations by sex and blood center for all blood donors*
| Sex | Blood center
|
||||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | Total | |
| Whole blood | |||||||
| Female and male | 450,790 | 293,944 | 682,117 | 211,954 | 373,468 | 959,486 | 2,971,759 |
| Female | 227,230 (50.4) | 148,146 (50.4) | 348,284 (51.1) | 112,990 (53.3) | 191,857 (51.4) | 454,271 (47.4) | 1,482,778 (49.9) |
| Male | 223,560 (49.6) | 145,798 (49.6) | 333,833 (48.9) | 98,964 (46.7) | 181,611 (48.6) | 505,215 (52.7) | 1,488,981 (50.1) |
| Double RBCs | |||||||
| Female and male | 28,051 | 34,555 | 31,578 | 29,541 | 47,939 | 28,652 | 200,316 |
| Female | 3,517 (12.5) | 2,898 (8.4) | 2,578 (8.2) | 4,365 (14.8) | 5,706 (11.9) | 1,992 (7.0) | 21,056 (10.5) |
| Male | 24,534 (87.5) | 31,657 (91.6) | 29,000 (91.8) | 25,176 (85.2) | 42,233 (88.1) | 26,660 (93.1) | 179,260 (89.5) |
| Apheresis PLTs† | |||||||
| Female and male | 43,074 | 39,286 | 50,712 | 22,796 | 22,705 | 103,727 | 282,300 |
| Female | 15,122 (35.1) | 14,799 (37.7) | 17,862 (35.2) | 9,343 (41.0) | 8,836 (38.9) | 38,771 (37.4) | 104,733 (37.1) |
| Male | 27,952 (64.9) | 24,487 (62.3) | 32,850 (64.8) | 13,453 (59.0) | 13,869 (61.1) | 64,956 (62.6) | 177,567 (62.9) |
| Total | 3,454,375 | ||||||
Data are reported as number (column %).
This number includes donations of apheresis PLTs with or without concurrent RBCs and/or plasma.
Donor sex
While the donations of whole blood were evenly distributed by sex, the majority of the apheresis double RBCs and the apheresis PLTs were made by men. Men contributed slightly more units of whole blood than women (50.1% vs. 49.9%) based on the sum of all of the donations to all REDS-II blood centers. There were more whole blood donations from women than men at five of the REDS-II blood centers (Centers A–E) and more whole blood donations from men than women at Blood Center F. Blood Center F is the REDS-II blood center with the largest number of donations enrolled in this study and the large number of donations from this blood center influenced the composite distribution of donations by sex. At all REDS-II blood centers the majority of the double-RBC donations (89.5%) as well as the majority of donations of apheresis PLTs (62.9%) were donated by men. Female donors donated 104,733 units of apheresis PLTs or 37.1% of all apheresis PLT donations (Table 1).
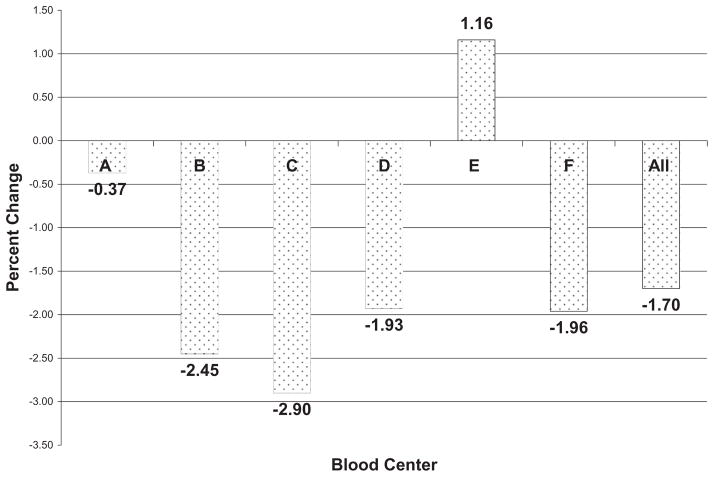
An analysis of the distribution of the sex of the apheresis PLT donors for all REDS-II blood centers of Calendar Year 2006 versus Calendar Year 2008 revealed a slight decrease in the percentage of apheresis PLTs donations from female donors (mean, −1.70%; p = 0.04 for 2006 vs. 2008) with variability in this percentage among each of the REDS-II blood centers (range, +1.16% to −2.90%; Fig. 1).
Fig. 1.
Changes in the percentage of female apheresis PLT donors from 2006 to 2008. Most REDS-II blood centers had a slight decrease in donations from female donors. Blood Center E had a policy of recruiting never-pregnant female apheresis PLT donors.
Donor pregnancy history
Table 2 shows the proportion of donations given by women of various pregnancy histories. The majority of the female whole blood and apheresis PLT donations were made by women with a history of pregnancy. The percentage of whole blood donations collected from never-pregnant female donors ranged by blood center from 33.6% to 43.7% with a mean for all REDS-II blood centers of 38.5%. The percentage of whole blood donations collected from female donors with one or more pregnancies ranged by blood center from 56.3% to 66.4% with a mean of 61.5%.
TABLE 2.
Donations by pregnancy history among female donors
| Donation type | Blood center
|
Total | |||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | ||
| Whole blood | |||||||
| Total number of pregnancies | 217,266 | 146,943 | 327,692 | 110,939 | 172,477 | 424,777 | 1,400,094 |
| None (%*) | 33.6 | 43.7 | 39.7 | 37.7 | 35.7 | 39.7 | 38.5 |
| One (%*) | 10.2 | 12.4 | 12.6 | 10.8 | 11.0 | 10.6 | 11.3 |
| Two (%*) | 23.0 | 20.0 | 22.6 | 21.5 | 22.9 | 21.6 | 22.0 |
| Three (%*) | 17.0 | 12.9 | 14.4 | 15.7 | 16.5 | 15.0 | 15.2 |
| Four or more (%*) | 16.2 | 11.1 | 10.8 | 14.3 | 13.9 | 13.1 | 13.1 |
| One or more (%*) | 66.4 | 56.3 | 60.3 | 62.3 | 64.3 | 60.3 | 61.5 |
| Apheresis PLTs† | |||||||
| Total number of pregnancies | 14,264 | 14,636 | 15,436 | 9,277 | 7,921 | 33,972 | 95,506 |
| None (%*) | 30.9 | 34.2 | 33.0 | 31.9 | 30.3 | 35.3 | 33.4 |
| One (%*) | 10.5 | 13.1 | 13.1 | 10.9 | 13.6 | 11.3 | 11.9 |
| Two (%*) | 25.1 | 20.9 | 25.0 | 24.5 | 21.7 | 21.9 | 23.0 |
| Three (%*) | 16.1 | 16.9 | 16.7 | 15.6 | 16.7 | 16.2 | 16.4 |
| Four or more (%*) | 17.5 | 14.9 | 12.1 | 17.1 | 17.7 | 15.3 | 15.4 |
| One or more (%*) | 69.1 | 65.8 | 67.0 | 68.1 | 69.7 | 64.7 | 66.6 |
Column percent.
This number includes donations of apheresis PLTs with or without concurrent RBCs and/or plasma.
The percentage of apheresis PLT donations collected from never-pregnant female donors ranged by blood center from 30.3% to 35.3% with a mean for all REDS-II blood centers of 33.4%. The percentage of apheresis PLTs donations collected from female donors with one or more pregnancies ranged by blood center from 64.7% to 69.7% with a mean of 66.6% (Table 2).
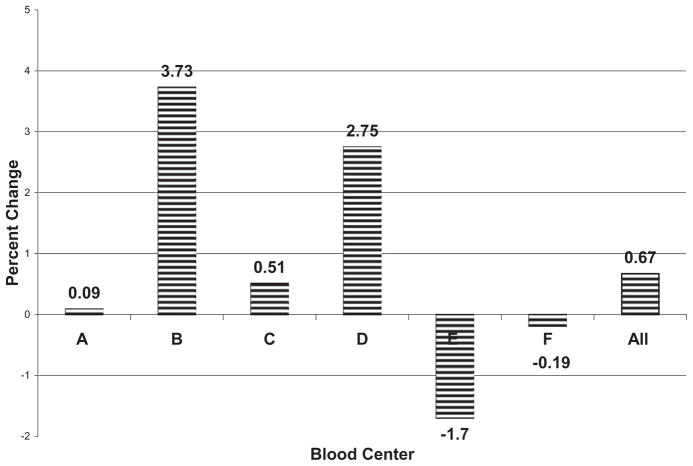
An analysis of the distribution of the pregnancy history of the apheresis PLTs donors for all REDS-II blood centers of Calendar Year 2006 versus Calendar Year 2008 revealed an increase in the percentage of donations of apheresis PLTs from female donors with one or more pregnancies (mean, +0.67%) with variability in this percentage among the REDS-II blood centers (range, +3.73% to −1.70%; Fig. 2). These data show that there have been minimal changes in the number of donations of apheresis PLTs by sex and pregnancy history during the study period at the REDS-II blood centers.
Fig. 2.
Changes in the percentage of ever-pregnant apheresis PLT donors from 2006 to 2008. Most REDS-II blood centers had small increases in the number of donations from ever-pregnant female donors. Blood Center E had a policy of reducing the number of ever-pregnant apheresis PLT donors.
Combined effect of donor sex and pregnancy history
Previously pregnant female blood donors donated 29.0% of the whole blood donations and 22.5% of all apheresis PLTs donations. The pregnancy history of the female donors in this study and the prevalence of positive screening tests for HLA antibodies according to gravidity from the LAPS-I study4 were used to estimate the number of HLA antibody–positive donations from female donors. Assuming that only previously pregnant female donors will be tested for HLA antibodies and that 11% to 32% of these women will have positive screening tests for HLA antibodies (depending on their number of prior pregnancies), then 6.9% of the whole blood donations and 5.4% of the apheresis PLT donations would be lost if previously pregnant female donors with positive screening tests for HLA antibodies are deferred from whole blood and apheresis PLT donations (Tables 3A and 3B).
TABLE 3A.
Estimated number of whole blood donations lost based on the deferral of ever-pregnant female donors with positive screening tests for HLA antibodies
| Donation characteristic | Number of whole blood donations from female donors | Percentage of total donations from donors of both sexes* | Proportion of female donors who could potentially have HLA antibodies† | Number of whole blood donations from female donors with HLA antibodies | Percentage of total donations from donors of both sexes* |
|---|---|---|---|---|---|
| Number of pregnancies | |||||
| None | 539,010 | 18.1 | NA | NA | |
| One | 157,503 | 5.3 | 0.112 | 17,640 | 0.6 |
| Two | 308,407 | 10.4 | 0.223 | 68,775 | 2.3 |
| Three | 212,521 | 7.2 | 0.275 | 58,443 | 2.0 |
| Four or more | 182,653 | 6.1 | 0.322 | 58,814 | 2.0 |
| Pregnancy history information | |||||
| Donations from female donors without information on pregnancy history | 82,684 | 2.8 | |||
| Donations from never-pregnant donors | 539,010 | 18.1 | |||
| Donations from ever-pregnant donors | 861,084 | 29.0 | 203,673 | 6.9 | |
| Total number of donations | |||||
| Donations from female donors | 1,482,778 | 49.9 | |||
| Donations from male donors | 1,488,981 | 50.1 | |||
| Donations from female and male donors‡ | 2,971,759 | 100.0 | 2,971,759 | 100.0 | |
Column percentages.
Based on the REDS-II LAPS-I study and assuming that never-pregnant donors will not have any HLA antibodies.
From Table 1.
TABLE 3B.
Estimated number of apheresis PLT donations lost based on the deferral of ever-pregnant female donors with positive screening tests for HLA antibodies
| Donation characteristic | Number of apheresis PLTs donations from female donors* | Percentage of total donations from donors of both sexes† | Proportion of female donors who could potentially have HLA antibodies‡ | Number of apheresis PLT donations from female donors with HLA antibodies | Percentage of total donations from donors of both sexes† |
|---|---|---|---|---|---|
| Number of pregnancies | |||||
| None | 31,855 | 11.3 | NA | NA | |
| One | 11,373 | 4.0 | 0.112 | 1,274 | 0.5 |
| Two | 21,943 | 7.8 | 0.223 | 4,893 | 1.7 |
| Three | 15,616 | 5.5 | 0.275 | 4,294 | 1.5 |
| Four or more | 14,719 | 5.2 | 0.322 | 4,740 | 1.7 |
| Pregnancy history information | |||||
| Donations from female donors without information on pregnancy history | 9,227 | 3.3 | |||
| Donations from never-pregnant donors | 31,855 | 11.3 | |||
| Donations from ever-pregnant donors | 63,651 | 22.5 | 15,201 | 5.4 | |
| Total number of donations | |||||
| Donations from female donors | 104,733 | 37.1 | |||
| Donations from male donors | 177,567 | 62.9 | |||
| Donations from female and male donors§ | 282,300 | 100.0 | 282,300 | 100.0 | |
This number includes donations of apheresis PLTs with or without concurrent RBC and/or plasma.
Column percentages.
Based on the REDS-II LAPS-I study and assuming that never-pregnant donors will not have any HLA antibodies.
From Table 1.
If all female donors were deferred from making apheresis PLT donations and assuming that 43.7, 50.4, and 6.0% of the apheresis PLTs donations from female donors result in 1, 2, or 3 units of apheresis PLTs (the single-, double-, and triple-split rates for female apheresis PLT donations at the REDS-II Blood Center F—the blood center with the largest number of apheresis PLT donations), then the number of units of apheresis PLT components that would be lost at the six REDS-II blood centers in 3 years as a consequence of the deferral of all female donors could be as high as 169,971 units (or 34.9% of the total inventory of apheresis PLTs after accounting for donations resulting in one or more units of apheresis PLTs; Table 4A).
TABLE 4A.
Estimated number of apheresis PLT donations after splits from all male donors versus all female donors
| Donation outcome | Number of apheresis PLTs donations from all female donors* (n = 104,733)
|
Number of apheresis PLTs donations from all male donors* (n = 177,567)
|
||||
|---|---|---|---|---|---|---|
| Split %† | Donations × split rates | Number of products with splits | Split %† | Donations × split rates | Number of products with splits | |
| Estimated number of total products based on split rates of REDS Blood Center C | ||||||
| One product | 60.29 | 63,144 | 63,144 | 47.59 | 84,504 | 84,504 |
| Two products | 37.01 | 38,762 | 77,523 | 47.92 | 85,090 | 170,180 |
| Three products | 2.70 | 2,828 | 8,483 | 4.49 | 7,973 | 23,918 |
| Total number of products | 149,150 | 278,603 | ||||
| Estimated number of total products based on split rates of REDS Blood Center F | ||||||
| One product | 43.66 | 45,726 | 45,726 | 30.15 | 53,536 | 53,536 |
| Two products | 50.39 | 52,775 | 105,550 | 60.92 | 108,174 | 216,348 |
| Three products | 5.95 | 6,232 | 18,695 | 8.93 | 15,857 | 47,570 |
| Total number of products | 169,971 | 317,454 | ||||
| Percentage of total donations by donor category | 34.87 | 65.13 | ||||
| Total number of apheresis products from all male and all female donors after splits for Blood Center F | 487,425 | |||||
From Table 1.
2006 through 2008 percentage of apheresis donations resulting in 1, 2, or 3 units of apheresis PLTs from male and female donors.
With a less restrictive policy of only deferring previously pregnant female donors, the number of units of apheresis PLTs lost in 3 years will be reduced to 103,299 units (or 21.2% of the total inventory of apheresis PLTs after accounting for donations resulting in 1 or more units of apheresis PLTs). With a very selective policy of only testing previously pregnant female apheresis donors for HLA antibodies and only deferring donors with positive screening tests for HLA antibodies, the number of units of apheresis PLTs lost in 3 years would be reduced to 24,670 (or 5.1% of the total inventory of apheresis PLTs after accounting for donations resulting in 1 or more units of apheresis PLTs; Table 4B).
TABLE 4B.
Estimated number of apheresis PLT donations after splits from ever-pregnant donors versus ever-pregnant donors with positive screening tests for HLA antibodies
| Donation outcome | Number of apheresis PLTs donations from ever-pregnant female donors*† (n = 63,651)
|
Number of apheresis PLTs donations from ever-pregnant female donors with positive screening tests for HLA antibodies*† (n = 15,201)
|
||||
|---|---|---|---|---|---|---|
| Split %‡ | Donations × split rates | Number of products with splits | Split %‡ | Donations × split rates | Number of products with splits | |
| Estimated number of total products based on split rates of REDS Blood Center C | ||||||
| One | 60.29 | 38,375 | 38,375 | 60.29 | 9,165 | 9,165 |
| Two | 37.01 | 23,557 | 47,114 | 37.01 | 5,626 | 11,252 |
| Three | 2.70 | 1,719 | 5,156 | 2.70 | 410 | 1,231 |
| Total number of products | 90,645 | 21,648 | ||||
| Estimated number of total products based on split rates of REDS Blood Center F | ||||||
| One product | 43.66 | 27,790 | 27,790 | 43.66 | 6,637 | 6,637 |
| Two products | 50.39 | 32,074 | 64,147 | 50.39 | 7,660 | 15,320 |
| Three products | 5.95 | 3,787 | 11,362 | 5.95 | 904 | 2,713 |
| Total number of products | 103,299 | 24,670 | ||||
| Percentage of total donations by donor category | 21.19 | 5.06 | ||||
| Total number of apheresis products from all male and all female donors after splits for Blood Center F§ | 487,425 | |||||
Transfusion history
During the 3-year study period, an evaluation of transfusion history data revealed that 4.2% of whole blood, 3.4% of double RBCs, and 4.2% of apheresis PLT donations were collected from donors with histories of prior transfusion(s) (Table 5).
TABLE 5.
Donations by transfusion history, sex, and blood center among all blood donors
| Sex | Blood center
|
||||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | Total | |
| Whole blood | |||||||
| Female and male (n) | 424,007 | 293,629 | 629,011 | 206,873 | 340,916 | 900,036 | 2,794,472 |
| Female (%*) | 51.0 | 50.4 | 51.9 | 53.5 | 52.1 | 48.0 | 50.5 |
| Male (%*) | 49.0 | 49.6 | 48.1 | 46.5 | 47.9 | 52.0 | 49.5 |
| Transfused donors | |||||||
| Female and male (%*) | 5.1 | 4.3 | 4.0 | 3.5 | 4.7 | 3.9 | 4.2 |
| Female (%*) | 3.2 | 2.5 | 2.4 | 2.5 | 2.8 | 2.2 | 2.5 |
| Male (%*) | 1.9 | 1.8 | 1.6 | 1.1 | 1.9 | 1.7 | 1.7 |
| Double RBCs | |||||||
| Female and male (n) | 26,445 | 34,528 | 28,852 | 28,909 | 44,182 | 26,543 | 189,459 |
| Female (%*) | 12.7 | 8.4 | 8.4 | 14.9 | 12.2 | 7.1 | 10.7 |
| Male (%*) | 87.3 | 91.6 | 91.6 | 85.1 | 87.8 | 92.9 | 89.3 |
| Transfused donors | |||||||
| Female and male (%*) | 4.0 | 3.0 | 3.0 | 2.4 | 3.9 | 3.7 | 3.4 |
| Female (%*) | 0.9 | 0.4 | 0.4 | 0.6 | 0.7 | 0.4 | 0.5 |
| Male (%*) | 3.2 | 2.6 | 2.6 | 1.8 | 3.3 | 3.3 | 2.8 |
| Apheresis PLTs† | |||||||
| Female and male (n) | 39,882 | 39,263 | 42,680 | 22,475 | 20,644 | 90,489 | 255,433 |
| Female (%*) | 35.6 | 37.7 | 36.3 | 41.1 | 39.6 | 38.9 | 38.0 |
| Male (%*) | 64.4 | 62.3 | 63.7 | 58.9 | 60.4 | 61.1 | 62.0 |
| Transfused donors | |||||||
| Female and male (%*) | 5.2 | 4.7 | 4.3 | 2.8 | 3.7 | 3.9 | 4.2 |
| Female (%*) | 2.7 | 2.7 | 1.8 | 1.5 | 2.0 | 2.2 | 2.2 |
| Male (%*) | 2.5 | 2.0 | 2.6 | 1.3 | 1.7 | 1.7 | 2.0 |
Column percent.
This number includes donations of apheresis PLTs with or without concurrent RBCs and/or plasma.
Blood types
A valid blood type was available for 99% of the whole blood (2,971,759) and apheresis plasma (55,093) donations. The percentage of whole blood donations from female donors with the AB blood type ranged from 1.6% to 2.4% with a mean of 1.9% for all six REDS-II blood centers. The percentage of whole blood donations from male donors with the AB blood type was similar and ranged from 1.5% to 2.5% with a mean of 2.1% for all six REDS-II blood centers.
The percentage of apheresis plasma donations from female donors with the AB blood type ranged from 1.6% to 21.6% by blood center with a mean of 9.9% for all six REDS-II blood centers. The percentage of apheresis plasma donations from male donors with the AB blood type was substantially higher than for women and ranged from 4.8% to 41.3% by blood center with a mean of 17.6% for all six REDS-II blood centers.
The deferral of all female donors, all ever-pregnant female donors, and all ever-pregnant female donors with positive screening tests for HLA antibodies from whole blood donations would decrease the inventory of type AB plasma units by 49.9, 29.0, and 6.9%, respectively. The deferral of all female donors, all ever-pregnant female donors, and all ever-pregnant female donors with positive screening tests for HLA antibodies from apheresis plasma donations would decrease the inventory of type AB plasma by 33.6, 20.4, and 4.9%, respectively (Table 6).
TABLE 6.
Percentage of AB blood type donations by sex, pregnancy history, and positive screening tests for HLA antibodies
| Donor category | Donation type
|
|
|---|---|---|
| Whole blood–derived AB plasma (%)* | Apheresis-derived AB plasma† | |
| Male donors | 50.1 | 66.4 |
| Female donors | 49.9 | 33.6 |
| Female donors without pregnancy history information | 2.8 | 3.0 |
| Never-pregnant female donors | 18.1 | 10.2 |
| Ever-pregnant female donors | 29.0 | 20.4 |
| Ever-pregnant female donors with positive screening tests for HLA antibodies | 6.9 | 4.9 |
Assuming that the distribution of donations by pregnancy history of AB whole blood donations is the same as the distribution of donations by pregnancy history of whole blood donations of all blood types.
Assuming that distribution of donations by pregnancy history of AB apheresis plasma donations is the same as the distribution of donations by pregnancy history of apheresis PLT donations of all blood types.
DISCUSSION
The AABB recommended to its constituency to take actions to mitigate TRALI by minimizing the distribution of high-plasma-volume blood components from donors who are potentially alloimmunized to WBCs by November 2008.5 Data on the prevalence of risk factors for WBC alloimmunization are needed to assess the extent of blood component losses associated with this policy. This study provides large-scale data on the frequency of risk factors for alloimmunization to WBC antigens among US blood donors from six geographically dispersed blood centers that are part of the REDS-II studies. It also allowed us to estimate the potential loss of blood products under different TRALI mitigation strategies.
Women accounted for 49.9% of whole blood donations and 37.1% of apheresis PLT donations at the six REDS-II centers. Whole blood donations are manufactured into plasma-rich components such as fresh-frozen plasma (FFP) and cryopoor plasma. Based on our data, we estimate that a policy for TRALI mitigation that would not allow plasma from whole blood donations from women to be prepared into transfusable plasma components would result in a 50% reduction in the number of the units of whole blood available for plasma manufacturing and would decrease the number of AB blood type plasma units made from whole blood by the same amount. While it may be possible to compensate for the loss of all female-derived transfusable plasma units, the loss of all apheresis PLTs from female donors would impact clinical care. Thus, alternative ways to eliminate PLTs units most likely to cause TRALI must be found.
Previous transfusions are a possible cause of WBC alloimmunization in blood donors. Our study revealed that between the 2006 through 2008 calendar years 3% to 4% of donors reported a history of a prior transfusion. In a previous study from the REDS-I that used data collected from 1991 to 2000, 4.9% of donations were made by donors with a history of a transfusion.6 However, the deferral of transfused blood donors is not considered to be an effective TRALI mitigation strategy since the prevalence of HLA antibodies among blood donors seems to be independent of the history of prior transfusions. According to another publication from the LAPS-I study, the prevalence of positive screening tests for HLA antibodies among blood donors is not significantly different between transfused men and nontransfused men and between transfused nulliparous women and nontransfused nulliparous women.7 Blood transfusions, when received many years ago as was the case in our donor population, do not seem to produce HLA antibodies that are detectable years later, and screening donors for a history of a prior transfusion does not appear to be an effective TRALI mitigation strategy based on LAPS-I data.
Pregnancy is another risk factor for HLA and/or HNA antibody formation. Before the primary LAPS-I publication,3 published data on the history of pregnancy in blood donors were limited. In a small study from the United States, a history of one or more pregnancies was obtained in 38.2% of 322 female apheresis PLTs donors.8 A more recent study of the pregnancy history of blood donors at a US hospital-based donation program revealed that 52.6% of 1009 female whole blood or apheresis PLTs donors had been pregnant.9 Using data from donations made to the REDS-II blood centers during calendar years 2006 through 2008, we report that 66.7% of 95,000 donations of apheresis PLTs from female donors were donated by women reporting a history of one or more pregnancies.
We used data from our study to estimate the impact of three different TRALI mitigation strategies for female apheresis PLT donors. REDS-II centers collect approximately 7.0% of the whole blood donations and 8.1% of the apheresis PLT donations of the United States and donors at the REDS-II centers were assumed to be representative of donors giving blood at other centers across the United States. One potential strategy would be the deferral of all female donors from future apheresis PLT donations. The most significant benefit from this policy would be a potential substantial reduction in the cases of TRALI. In the United Kingdom, where plasma for transfusion is now predominately manufactured from male donors, the risk for highly likely or probable TRALI cases has been reduced from 15.5 cases per 1 million units of FFP issued in 1999 through 2004 (when both male and female plasma was transfused) to 3.2 cases per 1 million units of FFP issued per year in 2005 through 2006,10 and the number of TRALI cases by year of transfusion has decreased from 32 cases in 2002 (before the introduction of preferential male plasma in late 2003) to 14 cases in 2009.11 Deferring all women from apheresis PLT donations could potentially have a similar effect on the incidence of TRALI. However, this approach may also result in an unacceptably high loss of more than one-third of apheresis PLT donations (37%).
An alternative policy that would only defer female donors who had at least one prior pregnancy could result in the loss of approximately one-fourth of the apheresis PLT donations (23%). In contrast, a deferral of previously pregnant apheresis PLT donors with HLA antibodies could limit this loss to an estimated 5% of all apheresis PLT donations. While these last two strategies would less severely impact the supply of apheresis PLTs than the deferral of all female donors, blood collection agencies would nonetheless still have to replace the 5% shortfall in PLT products either by increasing the production of PLT concentrates from whole blood or by replacing the deferred female apheresis donors with male donors, with never-pregnant female donors, or with ever-pregnant female donors without HLA antibodies.
We do not believe that the results of this study are affected by selection bias since the donors provided the demographic information on pregnancy and transfusion histories without any knowledge of the subject matter being studied. We had complete information on required demographic factors needed to accept a person for a blood donation (such as age, sex, donation type, and procedure type) from every donor and nearly complete information of the pregnancy and transfusion histories of the donors who agreed to provide that information. Therefore, the degree of information bias in the study should be small. We did not have data on the pregnancy and transfusion history of donors who were recently pregnant or transfused since donors who were transfused during a 12-month period before a blood donation attempt or were pregnant at the time of a donation attempt were deferred from donating blood. We did not include the potential donation losses from donors with HNA antibodies since the prevalence of HNA antibodies among blood donors is small in comparison to the number of donors with HLA antibodies.12
We found similar distributions of donations in terms of sex, transfusion histories, pregnancy histories, and blood type among donors of whole blood and apheresis PLTs among the six REDS-II blood centers and more variability in the blood types of the donors of apheresis plasma. It could be the case that other blood collection agencies will have a different distribution of donations in terms of sex, transfusion histories, pregnancy histories, and blood type and that percentage of components lost by other blood centers from donors deferred for being potentially alloimmunized to WBC antigens is different from the percentage of components lost by our centers. Therefore, each blood collection agency needs to study its donor base to determine which TRALI mitigation strategy is best suited to provide blood components for its customers.
In summary, a TRALI mitigation strategy that only defers previously pregnant female donors with HLA and/or HNA antibodies from making apheresis PLT donations would result in an approximately 5% decrease in the apheresis PLT inventory. Since 65% cases of TRALI (according to the UK SHOT Study10) are estimated to be related to the presence of HLA antibodies in transfused components, such a strategy would be expected to reduce the risk of TRALI.
Acknowledgments
This work was supported by NHLBI Contracts N01-HB-47168, -47169, -47170, -47171, -47172, -47174, -47175, and -57181.
The authors thank the staff at each REDS-II blood center. Without their help, this study would not have been possible.
The Retrovirus Epidemiology Donor Study (REDS)-II is presently the responsibility of the following persons:
Blood centers
American Red Cross Blood Services, New England Region: R. Cable, J.A. Rios, R.J. Benjamin
American Red Cross Blood Services, Southern Region/Emory University: J.D. Roback, C. Shepard
BloodCenter of Wisconsin: J.L. Gottschall, A.E. Mast
Hoxworth Blood Center, University of Cincinnati Academic Health Center: R.A. Sacher, S.L. Wilkinson, P.M. Carey
Regents of the University of California/Blood Centers of the Pacific/BSRI: E.L. Murphy, M.P. Busch, B.S. Custer
The Institute for Transfusion Medicine (ITxM)/LifeSource Blood Services: D.J. Triulzi, R.M. Kakaiya, J. Kiss
Central laboratory:
Blood Systems Research Institute: M.P. Busch, P. J. Norris
Coordinating center:
Westat, Inc.: J Schulman, M.R. King, D.J. Wright, T.L. Simon, S.H. Kleinman, P.M. Ness
National Heart, Lung, and Blood Institute, NIH
G.J. Nemo
Steering committee chairman
R.Y. Dodd
ABBREVIATIONS
- HLA
human leukocyte antigen
- HNA
human neutrophil antigen
- LAPS
Leukocyte Antibody Prevalence Antibody Study
- REDS
Retrovirus Epidemiology Donor Study
Footnotes
CONFLICT OF INTEREST
None.
References
- 1.US Food and Drug Administration. Fatalities reported to FDA following collection and transfusion, annual summary for fiscal year 2008. Center for Biologics Evaluation and Research, US Food and Drug Administration; Rockville, MD: [cited 2010 Jan 10]. Available from: URL: http://www.fda.gov. [Google Scholar]
- 2.US Food and Drug Administration. Fatalities reported to FDA following collection and transfusion, annual summary for fiscal year 2009. Center for Biologics Evaluation and Research, US Food and Drug Administration; Rockville, MD: [cited 2010 Aug 11]. Available from: URL: http://www.fda.gov. [Google Scholar]
- 3.Strong D, Lipton K. AABB Association Bulletin #06-07. Bethesda (MD): AABB; Nov 3, 2006. Transfusion-related acute lung injury; pp. 1–11. [Google Scholar]
- 4.Triulzi DJ, Kleinman S, Kakaiya RM, Busch MP, Norris PJ, Steele WR, Glynn SA, Hillyer CD, Carey P, Gottschall JL, Murphy EL, Rios JA, Ness PM, Wright DJ, Carrick D, Schreiber GB. The effect of previous pregnancy and transfusion of HLA alloimmunization in blood donors: implications for a transfusion-related acute lung injury reduction strategy. Transfusion. 2009;49:1825–35. doi: 10.1111/j.1537-2995.2009.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connor JD, Lipton KS. Association bulletin #07-03: clarifications to recommendations to reduce the risk of TRALI. Bethesda (MD): AABB; 2007. [Google Scholar]
- 6.Wang B, Higgins MJ, Kleinman S, Schreiber GB, Murphy EL, Glynn SA, Wright DJ, Nass CC, Chang D, Busch MP Retrovirus Epidemiology Donor Study. Comparison of demographic and donation profiles and transfusion-transmissible disease markers and risk rates in previously transfused and nontransfused blood donors. Transfusion. 2004;44:1243–51. doi: 10.1111/j.1537-2995.2004.04034.x. [DOI] [PubMed] [Google Scholar]
- 7.Kakaiya RM, Triulzi DJ, Wright DJ, Steele WR, Kleinman SH, Busch MP, Norris PJ, Hillyer CD, Gottschall JL, Rios JA, Carey P, Glynn SA National Heart, Lung, and Blood Institute (NHLBI) Retrovirus Epidemiology Donor Study-II. Prevalence of HLA antibodies in remotely transfused or alloexposed volunteer blood donors. Transfusion. 2010;50:1328–34. doi: 10.1111/j.1537-2995.2009.02556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Densmore TL, Goodnough LT, Ali S, Dynis M, Chaplin H. Prevalence of HLA sensitization in female apheresis donors. Transfusion. 1999;39:103–6. doi: 10.1046/j.1537-2995.1999.39199116901.x. [DOI] [PubMed] [Google Scholar]
- 9.Powers A, Stowell CP, Dzik WH, Saiman S, Lee H, Makar RS. Testing only donors with a prior history of pregnancy or transfusion is a logical and cost-effective transfusion-related acute lung injury prevention strategy. Transfusion. 2008;48:2549–58. doi: 10.1111/j.1537-2995.2008.01902.x. [DOI] [PubMed] [Google Scholar]
- 10.Chapman CE, Stainsby D, Jones H, Love E, Massey E, Win N, Navarrete C, Lucas G, Soni N, Morgan C, Choo L, Cohen H, Williamson LM Serious Hazards of Transfusion Steering Group. Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49:440–52. doi: 10.1111/j.1537-2995.2008.01948.x. [DOI] [PubMed] [Google Scholar]
- 11.Serious Hazards of Transfusion Steering Committee. Serious hazards of transfusion: annual report 2009 [monograph on the Internet] Manchester (UK): SHOT Office; 2010. [cited 2010 Sep 13]. Available from: URL: http://www.shotuk.org/wp-content/uploads/2010/07/SHOT2009.pdf. [Google Scholar]
- 12.Triulzi D, Kakaiya R, Kleinman S, Norris P, Steele W, Busch MP, Curtis B, Carey P, Carrick D, Hillyer C, Gottschall JL, Schreiber G, deCastro BR. Retrovirus Epidemiology Donor Study II. Human neutrophil antibody prevalence in US blood donors. Transfusion. 2008;48(Supplement):1A-P1-020A. (abstract) [Google Scholar]