Abstract
Superficially porous particles (also called Fused-Core, core shell or porous shell particles) show distinct advantages over comparable totally porous particles for separating small molecules. Columns of Fused-Core particles exhibit very high efficiency because of superior eddy dispersion properties (smaller van Deemter A term). The efficiency for columns of 2.7 μm Fused-Core particles actually rivals that for sub-2 μm totally porous particles with only about one-half the back pressure. These Fused-Core particles show special advantages with larger molecules for fast separations at high mobile phase velocities because of superior mass transfer (kinetic) properties (smaller van Deemter C term). This report describes the effect of different particle size and porous shell thicknesses on chromatographic performance for Fused-Core particles. Particle characteristics can significantly affect factors of separation importance. For example, the reduced plate height of packed columns is affected by particle diameter. Interestingly, larger Fused-Core particles show smaller reduced plate heights than smaller Fused-Core particles. Also, porous shell thickness has a strong effect on solute retention as well as separation efficiency, and particle surface area has a direct influence on sample loading characteristics. Fused-Core particles with a wide range of physical characteristics have been developed that allows the preparation of stable, efficient packed columns.
Keywords: Superficially porous particles, Fused-Core particles, Core-shell particles, Sample loading, Plates/pressure, van Deemter plots
1. Introduction
The traditional use of totally porous silica particles as support material for preparing columns for high-performance liquid chromatography (HPLC) has recently been undergoing serious changes. The reincarnation of superficially porous (often called Fused-Core®, core shell or porous shell) particles has rapidly resulted in the routine use of these materials because of superior performance for separating both small molecules [1–6] and larger compounds such as peptides [7–9] and proteins [7,10,11]. Better column performance of Fused-Core columns for small molecules is probably based on the higher efficiency of these particles as a result of improved packed bed homogeneity, largely because of very narrow particle size distributions and perhaps higher particle density [1,2]. Advantages in longitudinal diffusion caused by the fused core are also a feature of high column performance. Separations for larger compounds, such as peptides and proteins, are enhanced by the excellent mass transfer (kinetic) properties resulting from the thin shell that surrounds a solid core. Poorly diffusing larger molecules can more rapidly enter and exit the Fused-Core outer shell containing the stationary phase. For totally porous particles where diffusion paths within the porous structure are much longer, poorer mass transfer results in increased peak broadening and decreased column efficiency especially at higher mobile phase flow rates.
In this report, the term superficially porous particles (SPP) is used to designate generic particles with solid cores and porous outer shell. The term Fused-Core particles specifically relates to the materials made by Advanced Materials Technology, Inc. For separating small molecules, superficially porous particles with pores in the 80–100 Å range appear adequate for such solutes to enter the pore structure without restricted access which would denigrate column efficiency. Larger molecules require larger pores for access, and Fused-Core particles with 160 Å have been made available for separating compounds such as peptides [8]. Superficially porous particles with 200–400 Å pores have been used for separating proteins and other larger molecules [10–12]. This report describes for the first time detailed chromatographic data for a series of Fused-Core particles ranging from 2.2 to 5 μm specifically designed for separating small molecules.
The particle size of superficially porous particles can strongly affect the efficiency that can be expected from columns of these materials. Theory predicts that as particle size decreases, column efficiency improves, but the pressure increases more rapidly as a square function of reciprocal particle size. However, there appears to be a practical limit on reducing the size of all particles, both superficially porous and totally porous, since columns with particle sizes of less than about 2 μm diameter typically show lower efficiency than expected from theory [6]. This effect has been said to be a function of increased difficulty for obtaining homogeneous packed beds of these very small particles, especially for smaller diameter columns [6]. The influence of extra-column band broadening and frictional effects that cause inhomogeneity of column cross-sectional flow due to temperature changes also may be an issue.
Currently, the most popular size for superficially porous particles is in the 2.5–2.7 μm diameter range, which involves the usual compromise of chromatographic characteristics (efficiency, back pressure, sample loadability, retention). Superficially porous particles typically have about one-half to three quarters the surface area of comparable totally porous particles, resulting in somewhat smaller capacity factor k values for solutes. This feature does not appear to be a practical problem since a small decrease in organic solvent concentration in the mobile phase for reversed-phase separations restores longer retention. Columns of Fused-Core particles in this size range show efficiencies almost equivalent to those containing totally porous sub-2 μm particles, but with only about one-half the back pressure [2,13].
This report presents data on Fused-Core particles in the 2.2–5.0 μm range to illustrate some of the effects on column performance largely caused by changes in particle size and thickness of the outer porous shell. Fused-Core particles with thinner porous shells show marginal improvement in efficiency for small molecules, but can exhibit appreciable efficiency improvement for larger molecules, as predicted by theory. Similar findings have previously been reported by Olah et al. [14] who compared Kinetex SPP particles to Ascentis Express SPP particles and by Omamogho et al. [15] and Gritti et al. [16] in which SPP particles with 0.15, 0.25, or 0.35 μm shells were investigated. An interesting finding of our study was that 4–5 μm Fused-Core particles can be packed into columns with significantly lower reduced plate heights than columns packed with 2.2, 2.7 μm Fused-Core particles. For a comprehensive list of minimum reduced plate heights, minimum plate heights, and maximum plate numbers that have been obtained with modern superficially porous particles, the reader is referred to the recent review of Fekete et al. [17].
2. Experimental
2.1. Chemicals
Compounds used as sample probes and in sample mixtures were from Sigma–Aldrich (St. Louis, MO). Trifluoroacetic acid was obtained from Pierce Chemicals (Rockford, IL) and acetonitrile from EMD (Gibbstown, NJ).
2.2. Columns/chromatographic conditions
Columns of Halo® Fused-Core particles were obtained from Advanced Materials Technology, Inc. (Wilmington, DE). Fig. 1 shows a Scanning electron microphotograph of 5 μm Halo particles and a graphic of the particle with appropriate size measurements. Table 1 gives the physical characteristics of the particles used in this study. Columns of 4.6 mm × 50 mm and 150 mm were used to obtain chromatographic data in this work.
Fig. 1.
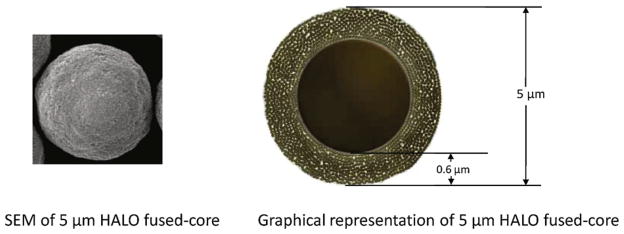
Graphics of 5 μm Fused-Core particles.
Table 1.
Characteristics of particles.
| Particle type | Porous shell thickness, μm | Solid core/particle dia. ratio | BET surface area, m2/g | Ave. pore dia., Åa | % Particle porosity | Plate numberb | Pressure, barb |
|---|---|---|---|---|---|---|---|
| 2.2 μm Fused-core | 0.3 | 0.73 | 130 | 90 | 62 | 38,700 | 530 |
| 2.7 μm Fused-core | 0.5 | 0.63 | 135 | 90 | 75 | 38,300 | 284 |
| 4 μm Fused-core | 0.6 | 0.65 | 98 | 90 | 66 | 28,900 | 97 |
| 4 μm Fused-core | 0.2 | 0.90 | 30 | 90 | 27 | 27,500 | 95 |
| 5 μm Fused-core | 0.6 | 0.71 | 90 | 90 | 56 | 28,300 | 78 |
| 3 μm Totally porous | N/A | N/A | 300 | 100 | 100 | 24,200 | 309 |
| 5 μm Totally porous A | N/A | N/A | 300 | 120 | 100 | 14,600 | 100 |
| 5 μm Totally porous B | N/A | N/A | 170 | 120 | 100 | 14,400 | 63 |
| 5 μm Totally porous C | N/A | N/A | 450 | 100 | 100 | 15,300 | 120 |
Reported by manufacturer.
Column plate number and back pressure for 4.6 mm × 150 mm columns measured with 1-CI-4-nitrobenzene at the plate height minimum with 50% acetonitrile/50% water at 30 °C using an Agilent 1100 instrument or an Agilent 1200 SL.
Columns of 5 μm conventional totally porous particles (4.6 mm × 50 mm and 4.6 mm × 150 mm) were obtained from Mac-Mod Analytical (Chadds Ford, PA) and Supelco (Bellefonte, PA). Surface area BET measurements were conducted with a Micromeritics TriStar II instrument (Norcross, GA) using nitrogen. Particle sizes were determined with a Coulter Multisizer 3 instrument (Fullerton, CA). Shell thicknesses were determined by the difference in Coulter counter measurements for the starting solid cores and the final particles. This parameter was confirmed by cross-section microphotographs prepared by Micron, Inc. (Wilmington, DE), who also prepared electron microphotographs of Fused-Core particles.
HPLC separations with 4.6 mm i.d. columns were conducted with an Agilent Model 1100 liquid chromatograph (Palo Alto, CA) with quaternary pump and a pressure limitation of 400 bar. An Agilent Model 1200 SL instrument was used for experiments involving pressures up to 600 bar. Data acquisition and instrument control used Version B.03.02 ChemStation software for experiments conducted with the Agilent 1200SL, while Version B.01.03 ChemStation software was used for experiments with the Agilent 1100. Column stability studies and the NSAID application were performed with a Shimadzu Prominence UFLC XR instrument. Peak widths (Full Width Half Max) were used for measuring plate numbers. No corrections for extra-column band broadening effects were applied to the data obtained in this study since the authors were interested in publishing values that could be easily and conveniently duplicated by interested practitioners with the same instruments.
3. Results and discussion
3.1. Effect of particle size
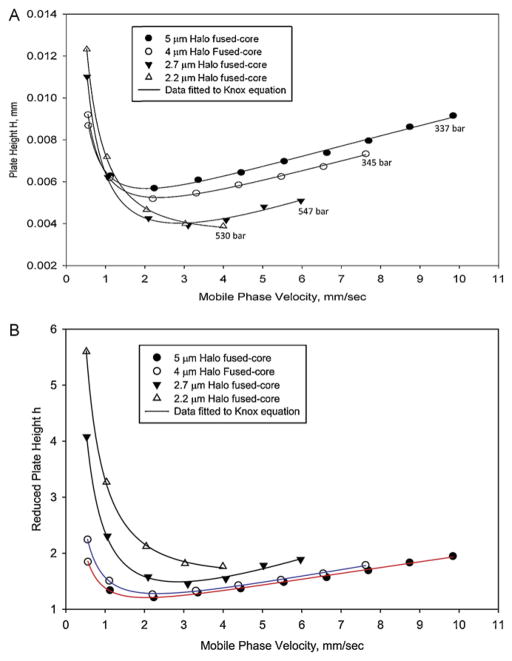
Theory predicts that as particle size is decreased, the efficiency of a packed bed improves, that is, the plate height H decreases [18]. This effect is shown in Fig. 2A for van Deemter plots of Fused-Core particles ranging in size from 2.2 to 5 μm, all having about the same porous shell thickness (Table 1). These plate height H data are in keeping with theory, with smaller plate heights resulting from smaller particles with the superficial linear mobile phase velocity used. Data for columns of the 2.2 and 2.7 μm Fused-Core particles were limited to 600 bar by the instrument utilized (Agilent 1200). Note that data for the 2.2 μm particles did not quite reach the plate height minimum with the instrument used because of pressure limitations.
Fig. 2.
Van Deemter plots for various sizes of Halo Fused-Core particles. Columns: 4.6 mm × 150 mm; mobile phase: 50% acetonitrile/50% water; temperature: 30 °C; injection volume: 1 μL; instrument: Agilent 1100 with autosampler (no correction for extra-column band broadening effects); solute: 1-Cl-4-nitrobenzene. (A) Plate height H plots. (B) Reduced plate height h plots.
However, an interesting result is that columns of larger Fused-Core particles actually show smaller reduced plate heights and higher performance relative to comparable columns of smaller Fused-Core particles, as shown in Fig. 2B. Smaller reduced plate heights for larger totally porous particles have previously been reported [19], but as far as the authors could determine, this effect has not yet been verified for superficially porous particles other than the comparison between 2.6 and 1.7 μm superficially porous particles [20]. The 150 mm column of 5 μm Fused-Core particles was found to have a reduced plate height minimum of 1.2 under these operating conditions even when no correction for instrumental extra-column band broadening effects was used. Results for 2.7 μm and especially for 2.2 μm particles show larger reduced plate heights than 4 and 5 μm Fused-Core particles. These data suggest that larger Fused-Core particles can be packed even more homogeneously into column beds than the smaller Fused-Core particles which already exhibit excellent bed homogeneity [1,2].
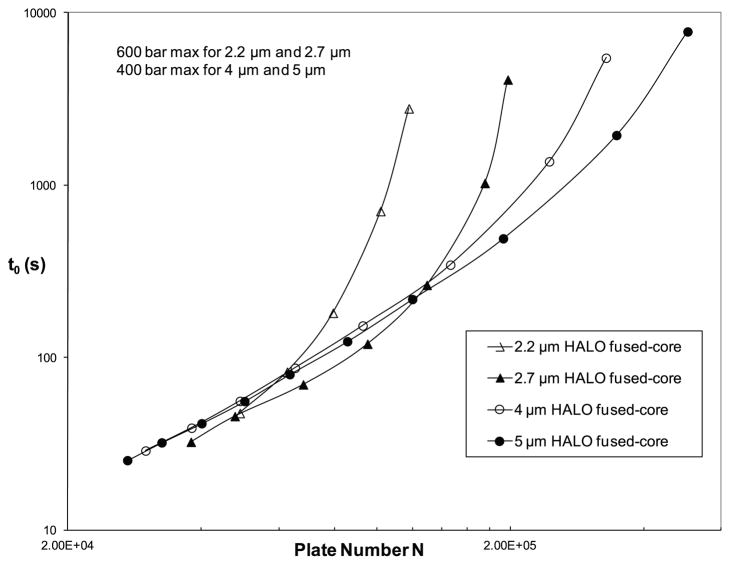
The particle size effect is further illustrated by the kinetic plots of Fig. 3 using the method described by Desmet et al. [21]. The velocity and plate height data acquired were used for the calculation of kinetic plots using the free Excel template available online from Desmet’s group [21]. The permeability (Kv) of each particle type was obtained from the slope of a plot of pressure drop/column length (ΔP/L) versus linear velocity (u0). For very fast separations, smaller particles generate more plates in shorter times. As particle size increases, available plate numbers also can increase, but at the cost of longer analysis times. These results suggest that for very fast separations, smaller particles should be used, as previously noted [22]. On the other hand, columns of larger Fused-Core particles should be used for separating complex mixtures since columns of these larger particles have lower back pressure, allowing the use of longer columns for higher total plate numbers (but at the cost of longer separation times) [23].
Fig. 3.
Kinetic plot: Effect of particle size on Halo Fused-Core particles. Conditions as in Fig. 2.
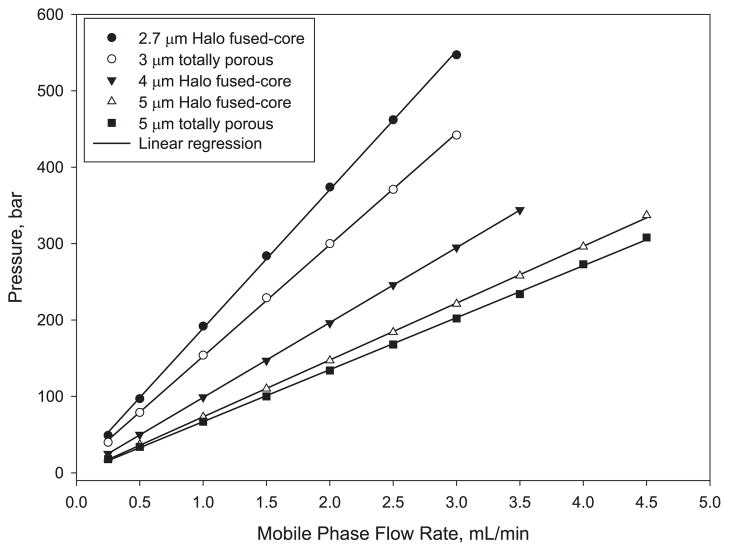
The pressure versus flow rate plots of Fig. 4 show the effect of particle size and particle type. As predicted by theory, the smaller particles show the highest back pressure. The pressure P in p.s.i. for a packed column can be estimated by [18]
Fig. 4.
Effect of flow rate on column pressure. Conditions as for Fig. 2.
| (1) |
where L is column length (mm), η is mobile phase viscosity (cP), F is the flow rate (mL/min), dp is the particle diameter (μm) and dc is the column internal diameter (mm). The larger 4 μm Fused-Core particles show a back pressure smaller than for 3 μm particles and larger than for 5 μm particles, as expected. The small difference in the back pressure of columns for 5 μm Halo Fused-Core and totally porous particles is believed to be a result of a larger particle size distribution for the totally porous particles.
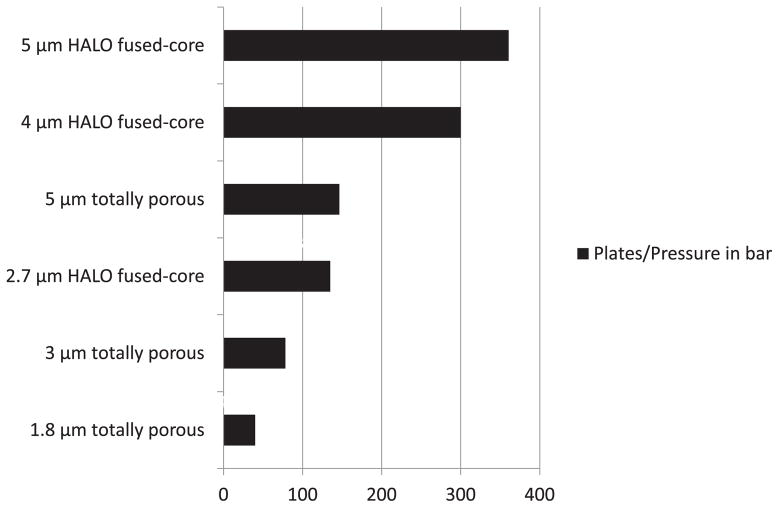
The effect of pressure for Fused-Core and totally porous particles of different sizes on column efficiency is shown in Fig. 5. Here, plate number/pressure for 4.6 mm × 150 mm columns is calculated for some of the particles studied. The 5 μm Halo Fused-Core particle shows more than double the plates/pressure than 5 μm totally porous particles and four times the number of plates/pressure of the 3 μm totally porous particles. This advantage is due to the lower column pressure required for the larger Fused-Core particle and the enhanced efficiency of the homogeneously packed column bed (Fig. 2).
Fig. 5.
Plates/pressure for various particle sizes. Columns: 4.6 mm × 150 mm. Conditions as in Fig. 2.
3.2. Effect of porous shell thickness
The effect of the thickness of the outer porous shell and the solute molecular weight on column performance for Fused-Core particles is shown by the van Deemter plots of Fig. 6 for a small molecule, 1-Cl-4-nitrobenzene (MW = 158) and a larger solute, the drug verapamil (MW = 491). The van Deemter relationship provides information regarding the band broadening processes within a column and is expressed as [18,24]
Fig. 6.
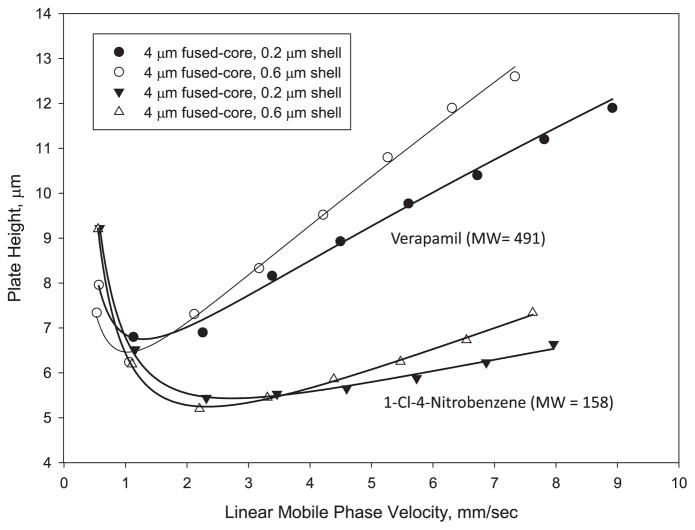
Effect of porous shell thickness and solute molecular weight on column efficiency. Columns: 4.6 mm × 150 mm; particles: 4 μm; instrument: Agilent 1100 with autosampler. 1-Cl-4-nitrobenzene (MW = 158) – Conditions as in Fig. 2; capacity factors k of 1-chloro-4-nitrobenzene = 2.7, 1.2 for 0.6 and 0.2 μm shell thickness, respectively. Verapamil (MW = 491) – Mobile phase: 30% acetonitrile/70% 0.1% aqueous trifluoroacetic acid; temperature: 40 °C; injection: 0.5 μL; capacity factors k of verapamil = 7.3, 3.6 for 0.6 and 0.2 μm shell thickness, respectively.
| (2) |
where H is the plate height, A represents the band broadening contribution by eddy diffusion, B is the effect of longitudinal mass transfer, C is the contribution by mobile phase and stationary phase mass transfer and u is the mobile phase velocity. The van Deemter relationship can also be restated as the Knox equation [18,25]:
| (3) |
where h is the reduced plate height H/dp, dp is the particle diameter and A, B, C are another set of constants representing the same band broadening effects as in the van Deemter equation, and v = uedp/Dm is the reduced velocity of the mobile phase where ue is the interstitial mobile phase velocity and Dm is the solute diffusion coefficient. In this report, the observed average mobile phase velocity v based on the column dead time t0 is used in the Knox equation as many practitioners for convenience use this observed velocity term for measurements. The reduced plate height value h so determined conveniently describes Fused-Core column performance for changes in particle size, shell thickness, and other such variables that are studied. The reduced plate height and reduced mobile phase velocity are of special interest for column performance when solutes of different diffusion characteristics are compared.
The 4 μm Fused-Core particles with a thinner (0.2 μm) shell in Fig. 6 resulted in a column with similar minimum plate height reduced plate height (van Deemter A term) for the 4 μm Fused-Core particles with a 0.6 μm shell, as anticipated. However, at higher mobile phase velocities, the particle with a 0.2 μm shell showed a somewhat smaller increase in reduced plate height (van Deemter C term) compared to the particles with a thicker porous shell. This effect is in keeping with the expected improved mass transfer (smaller van Deemter C term) for a thinner porous shell. However, mass transfer differences for this small molecule are quite small as shown because of the relatively large diffusion coefficient for the small solute in this mobile phase.
The effect of solute molecular weight on mass transfer and column efficiency also is shown in Fig. 6, using a higher molecular weight solute, the drug verapamil. Here, data from columns of 4 μm Fused-Core particles with 0.6 and 0.2 μm porous shell thicknesses are both shown for verapamil (MW = 491)and 1-Cl-4-nitrobenzene (MW = 158). As expected, mass transfer for the higher molecular weight solute is poorer and reduced plate heights somewhat larger than for the lower molecular weight compound. The plate height minimum for the larger solute also occurs at a slower mobile phase velocity than for the smaller solute. However, the difference in column efficiencies at the plate height minimum is quite small. Note that even with the larger solute, the increase in plate height (decrease in column efficiency) is modest with increased mobile phase velocity. This effect means that the mobile phase flow rate can be substantially increased and separation time decreased with only a small decrease in column efficiency and separation resolution. For example, the mobile phase velocity for verapamil in Fig. 6 can be increased four-fold from the velocity at the plate height minimum (resulting in one-fourth the separation time) with only about a 35% decrease in column efficiency and 18% loss in separation resolution.
It should be noted that a thinner outer porous shell on particles represents a chromatographic compromise that is not optimum for some applications. A thinner shell means that the particles in the column have a smaller surface area. This factor causes smaller retention (k values) and reduced sample loading, as evidenced in data below.
3.3. Effect of particle type on sample loading
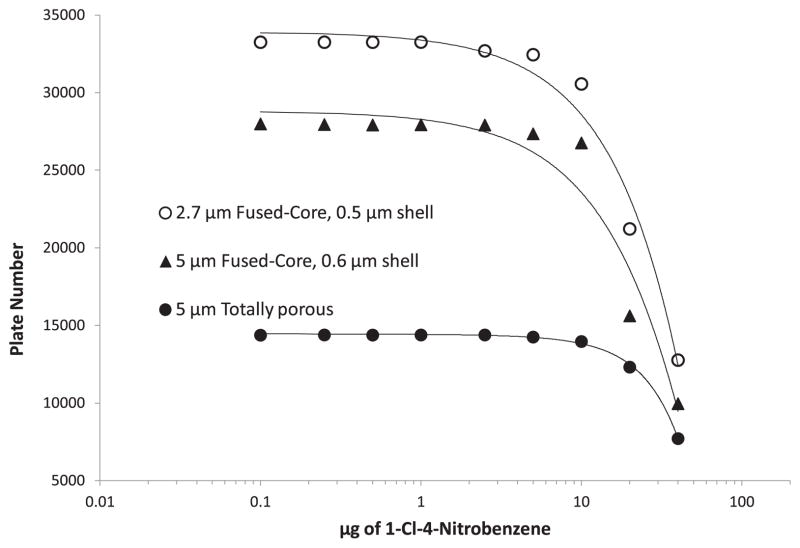
The level of sample loading on a column is largely a function of the surface area of the particles, if the solute is able to access the total particle pore volume. Fig. 7 shows the effect of the mass loading for a small molecule, 1-Cl-4-nitrobenzene, for columns of two Halo Fused-Core particles of different sizes and a totally porous particle. The totally porous particle of higher surface area shows slower change in column plate number with solute loading than the Fused-Core columns because of its higher surface area, although the difference is less than might be anticipated. In fact, the decrease in plate number for the column of Fused-Core particles with sample loading actually is magnified because of the sharper, lower volume peaks for these highly efficient columns.
Fig. 7.
Effect of particles on sample loading. Conditions as Fig. 2; solute: 1-Cl-4-nitrobenzene.
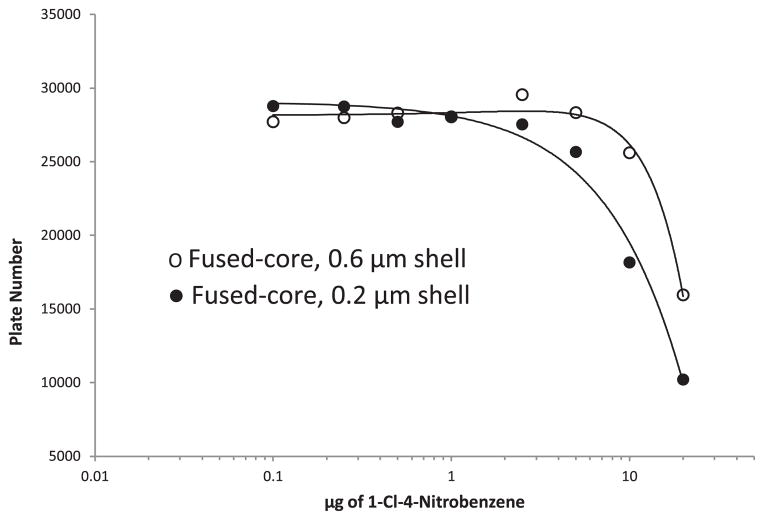
As previously mentioned, sample loading for columns directly depends on the available surface area for the particles in the column. This is illustrated in Fig. 8 for Fused-Core particles with 0.6 and 0.2 μm porous shell thicknesses. As expected, the particles with a thicker shell and a larger surface area (Table 1) show greater loading capacity for solutes without change in column plate number. Table 2 reports the sample mass loadings which result in an arbitrary practical 30% decrease in column plate number (15% loss in resolution) for some of the Fused-Core and totally porous particles studied. Totally porous particle C shows a somewhat lower loading than might be expected for the higher surface area. This effect is believed to be due to the fact that some of the pore volume (very small pores) in this particle could not be accessed by the solute, even though it was effectively probed by nitrogen for the BET surface area measurement.
Fig. 8.
Effect of shell thickness on sample loading. Conditions as Fig. 2; solute: 1-Cl-4-nitrobenzene.
Table 2.
Effect of sample mass on column efficiency loss.
| Particle type | Surface area, m2/g | Sample mass for 30% loss in plate number, μga |
|---|---|---|
| 5 μm Halo Fused-Core, 0.6 μm shell | 90 | 18 |
| 4 μm Halo Fused-Core, 0.6 μm shell | 98 | 17 |
| 4 μm Halo Fused-Core, 0.2 μm shell | 30 | 10 |
| 2.7 μm Halo Fused-Core, 0.5 μm shell | 135 | 20 |
| 5 μm Totally porous B | 170 | 30 |
| 5 μm Totally porous C | 450 | 43 |
Columns: 4.6 mm × 150 mm; sample solute: 1-CI-4-nitrobenzene.
3.4. Comparison with totally porous particles
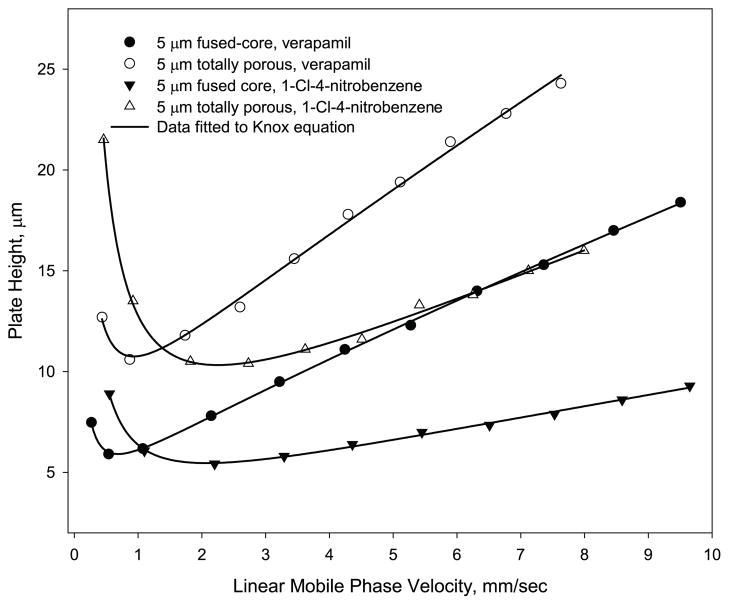
As previously noted, an unforeseen advantage of the 5 μm Fused-Core particles is the high column efficiency that can be attained with this material. Fig. 9 compares van Deemter data for columns of 5 μm Fused-Core particles with that of a high-quality 5 μm totally porous particle column using two solutes of different molecular weights, 1-Cl-4-nitrobenzene (MW = 158) and verapamil (MW = 491). For the smaller solute, 1-Cl-4-nitrobenzene, the reduced plate height of the totally porous particle column at the plate height minimum is 2.1, while the column of Fused-Core particles shows a reduced plate height of 1.2, even when corrections for extra-column band broadening are not applied. For the larger solute, verapamil (MW = 491), the Fused-Core particles show a reduced plate height of 1.3 at the plate height minimum for the separation conditions used. The totally porous column was found to have a reduced plate height of 2.2 under the same operating conditions. Both solutes show a 60–70% increase in column efficiency for the Fused-Core particles over the column of totally porous particles, representing a large improvement for separations with no increase in pressure requirements. The data show that the larger solute has highest performance (h at the plate height minimum) at a lower flow rate than the smaller solute.
Fig. 9.
Comparison of Fused-Core and totally porous particles. Columns: 4.6 mm × 150 mm; instrument: Agilent 1100 with autosampler; 5 μm Halo Fused-Core with 0.6 μm shell and 5 μm totally porous particles. Conditions for 1-Cl-4-nitrobenzene as in Fig. 2; capacity factor k = 2.7 for Fused-Core, k = 4.3 for totally porous. Conditions for verapamil – Mobile phase: 35% acetonitrile/65% aqueous 0.1% trifluoroacetic acid; temperature: 40 °C; injection: 0.5 μL; Fused-Core k = 2.8; totally porous k = 6.3.
Reduced plate heights for the Fused-Core columns in Fig. 9 also show a smaller increase with higher mobile phase velocities than for the totally porous particles. This effect is due to the improved mass transfer (van Deemter C term) of the Fused-Core particle. However, this mass transfer difference is relatively small because diffusion of both solutes for the conditions used is sufficient to minimize mass transfer differences in both particles. However, the end result is that the Fused-Core column can be operated more efficiently at higher mobile phase velocities for faster separations than the column of totally porous particles. Based on the data in Fig. 9, a 50 mm Fused-Core column could be used for a faster, essentially equivalent separation to that for the 150 mm totally porous column, resulting in about a three-fold decrease in separation time. In this case, the operating pressure requirement would be even further reduced by the shorter column.
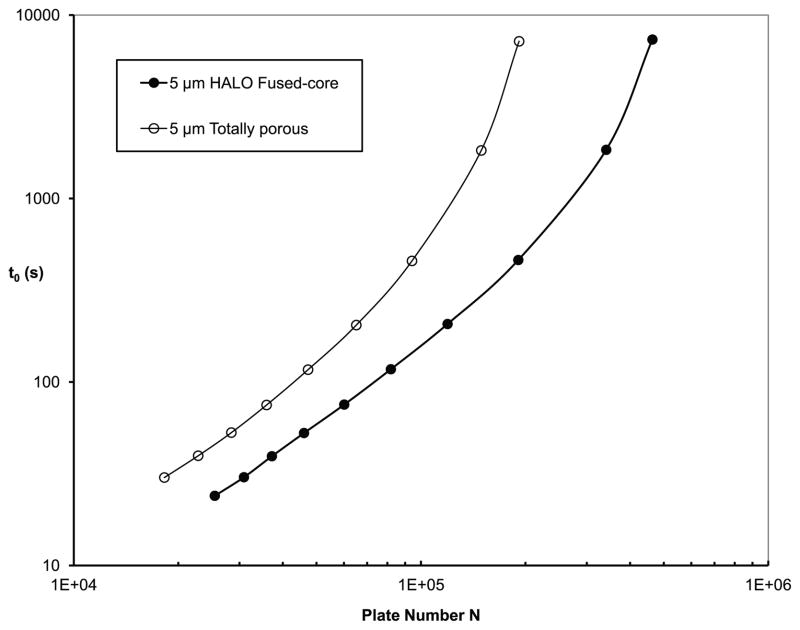
The kinetic plots in Fig. 10 for columns of 5 μm Fused-Core and totally porous particles show that Fused-Core particles outperform totally porous particles of the same particle size at both short and long analysis times. Data in this figure were calculated for instruments limited to 400 bar. Most routine applications of HPLC are still performed within this pressure limit because of practical reasons such as instrument cost and reliability, data reproducibility, and convenience of operation.
Fig. 10.
Comparative kinetic plots: Fused-Core vs. totally porous. Conditions as Fig. 2.
3.5. Column stability
The stability of the 5 μm Fused-Core particles with a C18 stationary phase is demonstrated in Fig. 11. Here, the plate number for two small solutes was measured after 450 sequential injections using a mobile phase flow rate of 1.8 mL/min with 50% acetonitrile–50% water/0.1% aqueous trifluoroacetic acid at a temperature of 60 °C. No change in column efficiency was seen after almost 30,000 column volumes of mobile phase passed though the column under these conditions.
Fig. 11.
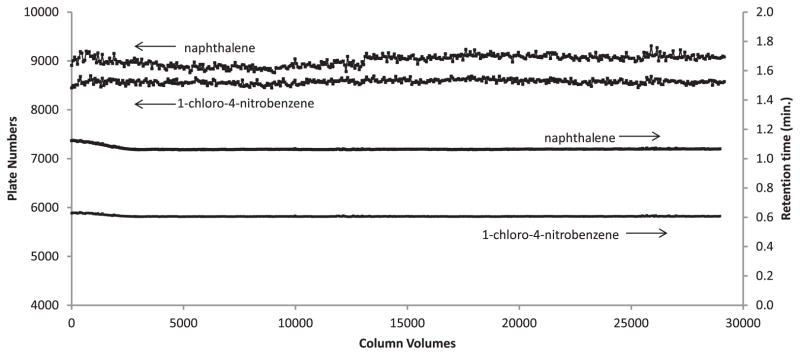
Stability testing for C18 Fused-Core column. Column: 4.6 mm × 50 mm; temperature: 60 °C; mobile phase: 50% acetonitrile/50% aqueous 0.1% trifluoroacetic acid; flow rate: 1.8 mL/min; instrument: Shimadzu Prominence UFLC XR with autosampler; injection: 1.0 μL; solutes: 1-Cl-4-nitrobenzene, naphthalene, k = 1.7 and 3.8, respectively.
3.6. Applications
Comparison of a typical application for 50 mm columns of 5 μm Halo Fused-Core and 5 μm totally porous particles is shown in Fig. 12. This 2.5 min gradient separation shows slightly less retention with the Fused-Core column because of smaller particle surface area compared to the totally porous particles C (Table 2). However, the peaks have a smaller volume (sharper peaks) for the Fused-Core column, representing higher efficiency, and better peak resolution. A different separation selectivity occurs for the 2- and 4-chlorophenol pair for the totally porous column with these separating conditions, causing severe peak overlap. For these short columns the peak capacity Pc for the Fused-Core and totally porous particles is 59 and 46, respectively, where [26]
Fig. 12.
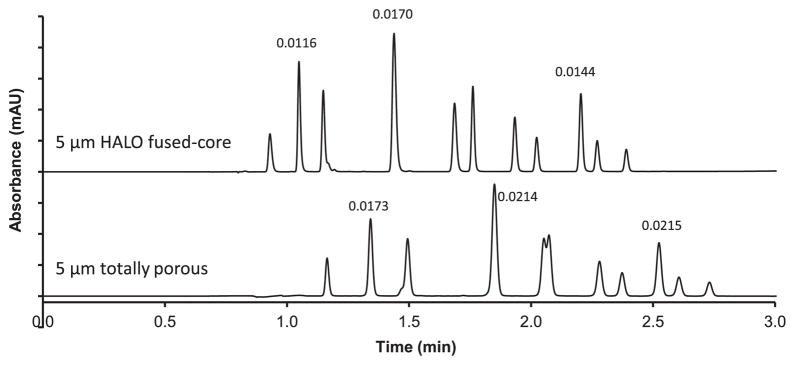
Separation of phenolics with Fused-Core and totally porous particles. Columns: 4.6 mm × 50 mm; mobile phase gradient: 3–70% acetonitrile/water with 0.1% formic acid in 2.7 min; instrument: Agilent 1100; injection delay: 0.41 min; temperature: 45 °C; peak identities (in elution order): hydroquinone, resorcinol, catechol, phenol, 4-nitrophenol, 4,4′-biphenol, 2-chlorophenol, 4-chlorophenol, 2,2′-biphenol, 2,6-dichlorophenol, 2,4-dichlorophenol. Peak widths in minutes for selected analytes.
| (4) |
and tz and ta are the retention times for two peaks that elute, respectively, at the start and finish of the gradient, and W is the base width (4 sigma) of the average peak widths.
Separation comparison for columns of 5 μm Fused-Core and totally porous particles is shown in Fig. 13. The Fused-Core separation of this drug mixture required about half the time as for the column of totally porous particles with a column efficiency of better than 60% greater with these operating conditions. Peak capacities Pc for these isocratic separations were 85 and 81, respectively, for the Fused-Core and totally porous columns [18].
Fig. 13.
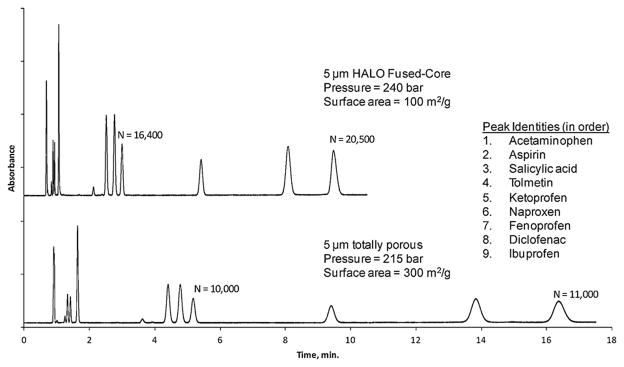
Comparative separations with 5 μm particles: Fused-Core vs. totally porous. Columns: 4.6 mm × 150 mm; temperature: 35 °C; mobile phase – A: 20 mM potassium phosphate, pH 2.5; B: 50% acetonitrile/50% methanol; A:B = 48% A:52% B; flow rate: 2.0 mL/min; instrument: Shimadzu Prominence UFLC XR; injection: 2 μL.
4. Conclusions
As predicted by theory, a reduction in the particle size of Fused-Core particles results in increased column efficiency, and this is verified by results with Fused-Core particles. However, also as predicted, operating pressure increases drastically, as a square of the particle size. A surprising find of this study was that the reduced plate heights of 4 and 5 μm Fused-Core columns are smaller than for smaller Fused-Core particles. This result suggests that larger Fused-Core particles can be packed with even greater packed bed homogeneity than the already excellent homogeneity of beds packed with smaller Fused-Core particles (e.g., 2.7 μm) [1,2]. As a result, for example, columns of 5 μm Fused-Core particles can replace columns of 5 μm totally porous particles with efficiency that equates or exceeds that of 3 μm totally porous particles. This opportunity provides increased separation speed and/or resolution exceeding that of 3 μm totally porous particles while maintaining the low back pressure and robustness of columns with 5 μm totally porous particles.
As might also be expected from theory, a reduction in shell thickness for superficially porous particles results in a flatter van Deemter plot than for totally porous particles, showing lower loss in column efficiency as the mobile phase velocity increases. This effect is particularly evident for higher molecular weight solutes, where differences in the reduced plate height versus mobile phase velocity plots are larger. However, reducing the shell thickness on a Fused-Core particle initiates a compromise, as thinner porous shells result in reduced retention and smaller sample loading capability (due to lower surface area). Particles with porous shell thicknesses in the 0.5–0.7 μm range have surface areas and sample loading capabilities that are quite practical for the chromatography of small molecules. Compared to columns of smaller particles, a practical advantage of columns with the 5 μm Fused-Core particles is that extra-column band broadening effects are somewhat less important. As with totally porous particles, the use of larger porosity frits (2 μm porosity) for retaining the 5 μm Fused-Core particles in the column also represents a practical advantage in maintaining column performance and minimizing operating difficulties.
Acknowledgments
We appreciate partial support of this study furnished by the NIH with SBIR grant NIGMS GM099355. We also thank Robert Moran for his assistance in chromatographic measurements.
Footnotes
Presented at the 38th International Symposium on High Performance Liquid Phase Separations and Related Techniques, Anaheim, CA, USA, 16–21 June 2012.
References
- 1.Kirkland J, Langlois T, DeStefano J. Am Lab. 2007;39:18. [Google Scholar]
- 2.DeStefano J, Langlois T, Kirkland J. J Chromatogr Sci. 2008;46:254. doi: 10.1093/chromsci/46.3.254. [DOI] [PubMed] [Google Scholar]
- 3.Fekete S, Fekete J, Ganzler K. J Pharm Biomed Anal. 2009;50:703. doi: 10.1016/j.jpba.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 4.Song W, Pabbisetty D, Groeber E, Steenwyk R, Fast D. J Pharm Biomed Anal. 2009;50:491. doi: 10.1016/j.jpba.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Yang P, McCabe T, Pursch M. J Sep Sci. 2011;34:2975. doi: 10.1002/jssc.201100530. [DOI] [PubMed] [Google Scholar]
- 6.Guiochon G, Gritti F. J Chromatogr A. 2011;1218:1915. doi: 10.1016/j.chroma.2011.01.080. [DOI] [PubMed] [Google Scholar]
- 7.Gritti F, Cavazzini A, Marchetti N, Guiochon G. J Chromatogr A. 2007;1157:289. doi: 10.1016/j.chroma.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 8.Schuster SA, Boyes BE, Wagner BM, Kirkland JJ. J Chromatogr A. 2012;1228:232. doi: 10.1016/j.chroma.2011.07.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Barber WE, Long WJ. J Chromatogr A. 2012;1228:72. doi: 10.1016/j.chroma.2011.07.083. [DOI] [PubMed] [Google Scholar]
- 10.Fekete S, Berky R, Fekete J, Veuthey JL, Guillarme D. J Chromatogr A. 2012;1236:177. doi: 10.1016/j.chroma.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Schuster SA, Wagner BM, Boyes BE, Kirkland JJ. HPLC 2012. Anaheim, CA: 2012. [Google Scholar]
- 12.Kirkland J, Truszkowski F, Dilks C, Jr, Engel G. J Chromatogr A. 2000;890:3. doi: 10.1016/s0021-9673(00)00392-7. [DOI] [PubMed] [Google Scholar]
- 13.Cunliffe J, Maloney T. J Sep Sci. 2007;30:3104. doi: 10.1002/jssc.200700260. [DOI] [PubMed] [Google Scholar]
- 14.Oláh E, Fekete S, Fekete J, Ganzler K. J Chromatogr A. 2010;1217:3642. doi: 10.1016/j.chroma.2010.03.052. [DOI] [PubMed] [Google Scholar]
- 15.Omamogho JO, Hanrahan JP, Tobin J, Glennon JD. J Chromatogr A. 2011;1218:1942. doi: 10.1016/j.chroma.2010.11.067. [DOI] [PubMed] [Google Scholar]
- 16.Gritti F, Omamogho J, Guiochon G. J Chromatogr A. 2011;1218:7078. doi: 10.1016/j.chroma.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 17.Fekete S, Oláh E, Fekete J. J Chromatogr A. 2012;1228:57. doi: 10.1016/j.chroma.2011.09.050. [DOI] [PubMed] [Google Scholar]
- 18.Snyder LR, Kirkland JJ, Dolan JW. Introduction to Modern Liquid Chromatography. Vol. 2 John Wiley; Hoboken, NJ: 2010. [Google Scholar]
- 19.Fekete S. PhD thesis. Department of Inorganic and Analytical Chemistry, Budapest University of Technology and Economics; Budapest: 2011. [Google Scholar]
- 20.Gritti F, Guiochon G. J Chromatogr A. 2011;1218:1592. doi: 10.1016/j.chroma.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 21.Desmet G, Clicq D, Gzil P. Anal Chem. 2005;77:4058. doi: 10.1021/ac050160z. [DOI] [PubMed] [Google Scholar]
- 22.Broeckhoven K, Cabooter D, Eeltink S, Desmet G. J Chromatogr A. 2012;1228:20. doi: 10.1016/j.chroma.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Barber WE, Carr PW. J Chromatogr A. 2006;1107:139. doi: 10.1016/j.chroma.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 24.van Deemter JJ, Zuiderweg FJ, Klinkenberg A. Chem Eng Sci. 1956;5:271. [Google Scholar]
- 25.Bristow P, Knox JH. Chromatographia. 1977;10:279. [Google Scholar]
- 26.Snyder LR, Kirkland JJ, Dolan JW. Introduction to Modern Liquid Chromatography. Vol. 9 John Wiley; Hoboken, NJ: 2010. [Google Scholar]