Abstract
Adenosine is a signaling nucleoside that is produced following tissue injury, particularly injury involving ischemia and hypoxia. The production of extracellular adenosine and its subsequent signaling through adenosine receptors plays an important role in orchestrating injury responses in multiple organs. There are four adenosine receptors that are widely distributed on immune, epithelial, endothelial, neuronal and stromal cells throughout the body. Interestingly, these receptors are subject to altered regulation following injury. Studies in mouse models and human cells and tissues have identified that the production of adenosine and its subsequent signaling through its receptors plays largely beneficial roles in acute disease states, with the exception of brain injury. In contrast, if elevated adenosine levels are sustained beyond the acute injury phase, adenosine responses can become detrimental by activating pathways that promote tissue injury and fibrosis. Understanding when during the course of disease adenosine signaling is beneficial as opposed to detrimental and defining the mechanisms involved will be critical for the advancement of adenosine based therapies for acute and chronic diseases. The purpose of this review is to discuss key observations that define the beneficial and detrimental aspects of adenosine signaling during acute and chronic disease states with an emphasis on cellular processes such as inflammatory cell regulation, vascular barrier function and tissue fibrosis.
Keywords: adenosine receptors, inflammation, fibrosis, vascular barrier function, CD73, ADORA2B, ADORA2A, ADORA3, ADORA1, acute lung injury, remodeling, anti-inflammatory
Introduction
Tissue responses to ischemia, acute inflammation or fibrosis involve severe levels of hypoxia [1]. Studies over the past decade provide strong evidence that cellular responses to hypoxia include robust increases in extracellular adenosine and signaling events through adenosine receptors. In acute injury settings, this hypoxic adenosine response activates pathways that promote tissue adaptation during hypoxia [1]. These pathways include restoration of normal oxygen levels, enhancing metabolic ischemia tolerance and dampening inflammation. Indeed, preclinical studies show that adenosine signaling is beneficial in ischemic acute injury in the lung [2–6], kidney [7–9], heart [10, 11], gastrointestinal track [12] and liver [13]. However, if elevated adenosine levels are sustained beyond the acute injury phase, hypoxic adenosine responses can become detrimental by activating pathways that promote tissue injury and fibrosis [14]. For example, chronic elevations of adenosine can contribute to tissue fibrosis in different organs including the lungs [15–17], liver [18, 19], skin [20], kidney [21] penis [22, 23] and following transplant [24]. Under these conditions of chronically elevated adenosine, blockade of adenosine signaling appears to be beneficial. Thus, adenosine signaling plays different roles in acute and chronic disease states.
Pharmaceutical companies are advancing adenosine-based therapies toward clinical trials for various diseases [25–27]. Understanding when during the course of disease adenosine signaling is beneficial as opposed to detrimental and defining the mechanisms involved will be critical for the advancement of such therapies. The purpose of this review is to discuss key observations that define the beneficial and detrimental aspects of adenosine signaling during acute and chronic disease states, with an emphasis on cellular processes such as inflammatory cell regulation, vascular barrier function and tissue fibrosis that may serve as biological readouts that can be targeted for the treatment of various diseases.
Adenosine Signaling in Acute Tissue Injury
Tissue Protective Roles of Adenosine
In response to injury, cells release ATP and other adenine nucleotides that are then converted to extracellular adenosine by ecto-nucleotidases [28]. Interestingly, these enzymes (CD39, which converts ATP to ADP/AMP and CD73, which converts AMP to adenosine) are regulated by transcriptional mechanisms involving the hypoxia-dependent transcription factor “hypoxia-induced factor” 1a (HIF1a) [29, 30]. Production of extracellular adenosine through these orchestrated pathways is the major source of extracellular adenosine following injury [28, 31, 32] (Figure 1). Increases in extracellular adenosine in turn elicit various responses on target cells by engaging cell surface adenosine receptors [33, 34]. There are four adenosine receptors (ADORA1, ADORA2A, ADORA2B, and ADORA3). All four of these receptors have been shown to be involved in cellular processes implicated in the regulation of tissue injury and are potential targets for the treatment of both acute and chronic diseases.
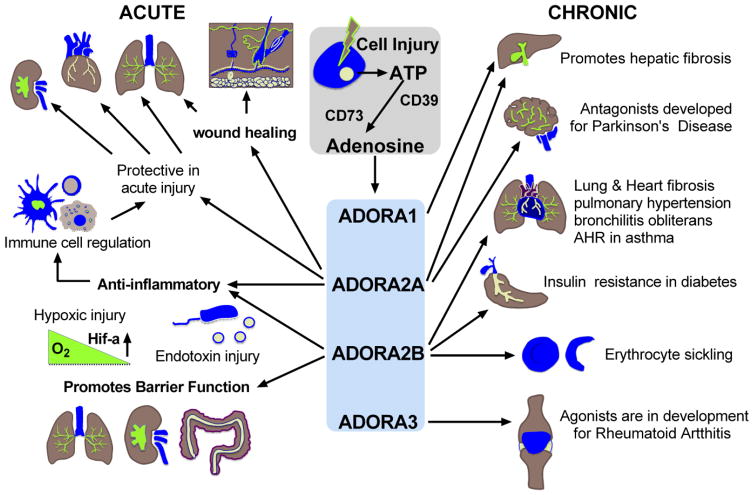
Figure 1. Adenosine Signaling in Acute and Chronic Disease.
Cellular injury associated with hypoxia and inflammation promote the release of ATP that is subsequently dephosphorylated by membrane bound CD39 and CD73. Extracellular adenosine then stimulates cell surface adenosine receptors (ADORA1, ADORA2A, ADORA2B, ADORA3) to influence tissue responses to injury. In acute disease states, adenosine largely contributes to anti-inflammatory and tissue protective responses such as the promotion of vascular barrier function. This signaling pathway also promotes wound healing. These adenosine responses are largely regulated by ADORA2A and ADORA2B signaling pathways. In chronic disease states, adenosine signaling can promote cellular processes such as fibrosis that contribute to disease progression. Depicted is fibrosis in the liver, lung and heart; however, findings suggest excessive adenosine signaling also contributes to fibrosis in the skin, kidney and penis. These responses are regulated by ADORA2A and ADORA2B signaling pathways. In addition, ADORA2B signaling promotes sickling of erythrocytes that can contribute to the progression of tissue injury, and promotes insulin resistance that can impact the development of diabetes.
The importance of extracellular adenosine generation and signaling in acute tissue injury is highlighted by a series of studies using knockout mice for CD39 and CD73 and inhibitors of these enzymes that demonstrate enhanced inflammation and tissue injury in models of hypoxia and ischemic injury [29, 30, 32, 35–38] in association with diminished adenosine production. These studies demonstrate that elevations in extracellular adenosine play an important protective role following acute injury associated with hypoxia, and suggest that pharmacologic approaches to enhance adenosine elevations may have therapeutic benefit in acute injury settings. One mechanism for enhancing extracellular adenosine levels is through the prevention of adenosine uptake by equilibrative nucleoside transporters (ENTs) [39]. In a recent study, Almut Grenz and colleagues demonstrated that treatment with dipyridamole, an inhibitor of ENTs led to increased adenosine levels in association with tissue protection in a mouse model of ischemic acute kidney injury [40]. Collectively, these studies demonstrate that extracellular adenosine generation is a beneficial response in acute injury states.
The ADORA2A in Acute Injury Settings
Studies using adenosine receptor agonists, antagonist and knockout mice have assigned specific anti-inflammatory and tissue protective roles to the different adenosine receptors. One of the best characterized biological effects of adenosine is its ability to regulate immune responses [41, 42]. All immune cells express adenosine receptors and signaling through the various adenosine receptors can impact immune function in manners that directly influence tissue responses to injury. In acute injury settings, all of the adenosine receptors have been shown to serve anti-inflammatory roles [43] (Figure 1). Perhaps the best characterized are the anti-inflammatory actions on the ADORA2A. This receptor is coupled to G-stimulatory alpha subunits to increase cAMP levels in the cell [34]. Anti-inflammatory properties of this receptor have been demonstrated in T cells [44], NK T cells [45, 46], invariant NK T cells [46], macrophages [47], neutrophils [48], dendritic cells [49], and T regulatory cells [50] where ADOR2A signaling is associated with inhibition of proliferation, inflammatory cytokine production and increased production of anti-inflammatory cytokines. Based on these observations, preclinical studies, largely in mice, have shown that treatment with ADORA2A agonists is beneficial in models of ischemia reperfusion injury and other acute disease settings. These include reperfusion injury in the heart [11], liver [13], kidney [9] and lung [6]. In addition, treatment with the ADORA2A agonist ATL146e was shown to attenuate pulmonary dysfunction in a mouse model of sickle cell disease [46], a condition that can be viewed as a repetitive ischemia reperfusion disorder. The mechanism identified for this protection included the activation of up regulated ADORA2A receptors on invariant natural killer cells. These findings highlight the importance of heightened adenosine signaling following injury settings and the potential for targeting this pathway for the prevention and resolution of acute injuries, particularly those involving ischemia.
ADORA2A signaling is also protective in models that can be viewed as more chronic in nature, including the bleomycin model of acute lung injury and fibrosis [51], allergic models of asthma [52, 53], and a model of bronchiolitis obliterans [54]. In these settings, ADORA2A signaling appears to exert anti-inflammatory effects that are beneficial to the resolution of chronic aspects of these disorders. Other tissue protective effects of ADORA2A signaling include the promotion of wound healing in lung epithelial cells [55] and the skin [56] and pathological angiogenesis [57]. Collectively, these studies support the use of ADORA2A agonists for the treatment of various acute disease states and the promotion of wound healing.
The ADORA2B in Acute Injury Settings
ADORA2B signaling has also been shown to be anti-inflammatory. A study by Katia Ravid and colleagues demonstrated that ADORA2B knockout mice have increased baseline and LPS stimulated immune responses [58]. In addition, a series of studies by Holger Eltzschig and colleagues went on to use a combination of adenosine receptor knockout mice and ADORA2B agonists to demonstrate that this receptors serves anti-inflammatory and tissue protective roles in various acute injury tissue models associated with hypoxic or ischemic injury including the heart [10], lung [2, 4], intestine [12] and kidney [8, 40]. A recent study by Tobias Eckles and colleagues demonstrated that the protective effects of ADORA2B signaling in ischemic heart injury were linked to activation of the circadian transcription factor Per2 [59], providing an interesting and potentially clinically important connection to light induced activation of these ADORA2B mediated protective pathways in the heart. In acute injury in the intestine [12], lung [2, 5] and kidney [40], enhanced ADORA2B signaling is not only associated with diminished inflammation, but also with pronounced improvement in vascular barrier function (Figure 1). Moreover, the use of mice with conditional deletion of the ADORA2B specifically in endothelial cells demonstrated that the ADORA2B on endothelial cells plays an important role in regulating the protective vascular responses to ischemic injury during acute kidney injury [40]. Collectively, these studies demonstrate that the ADORA2B plays an important role in regulating adenosine’s anti-inflammation and tissue protective properties and suggests ADORA2B agonists may prove useful in the treatment of acute injuries to the heart, gastrointestinal tract, lung and kidney.
Adenosine Signaling in Chronic Tissue Injury
Converse to acute tissue injury, elevated levels of adenosine have been implicated in the progression of chronic disease states [14]. In such settings, the primary function of adenosine appears to be in promoting aberrant wound healing leading to fibrosis in organs including the lung [15, 60], skin [20, 61], kidney [18, 21], heart [62], liver [63] and penis [22, 23]. In addition, adenosine signaling has been implicated in wide array of chronic conditions including diabetes mellitus [64, 65], sickle cell disease [66], transplant rejection [24], Parkinson’s disease [67] and rheumatoid arthritis [68].
Adenosine in Patients with Chronic Disease
Several studies demonstrate elevated levels of adenosine in patients with chronic lung disease. Perhaps the most well-known effect of adenosine in chronic disease comes from its capacity to induce airway hyperresponsiveness in asthmatic but not normal individuals [69], a phenomenon reproduced in animal models [70]. However, it was not until 1993 that elevated levels of adenosine in the lavage fluid from asthmatic subjects were first documented [71]. These observations were later validated in exhaled breath condensate of patients with asthma [72]. In addition to asthma, increased levels of adenosine have been reported in sputum from patients with cystic fibrosis [73]. Recent studies have also demonstrated elevated adenosine levels in the exhaled breath condensate of patients with chronic obstructive pulmonary disease (COPD; [74]) that negatively correlated with lung function. In tandem with these observations, a reduced activity of adenosine deaminase (ADA), the major enzyme that breaks down adenosine, was observed in patients with COPD [75, 76], and in patients with idiopathic pulmonary fibrosis (IPF; [76]). In addition to the reduced levels of ADA activity in patients with chronic lung disease, increased levels of the enzyme CD73, the major enzyme of extracellular adenosine production, were observed in lung tissue from patients with COPD and IPF [76]. Together with these findings, heightened levels of ADORA2B were documented in both COPD and IPF patients [76] implicating a potential role of this receptor in the pathogenesis of chronic lung disease.
Adenosine also plays a role in other chronic diseases. In the context of ethanol-induced liver cirrhosis, increased adenosine levels, as a consequence of ethanol metabolism [77, 78], contribute to the development of cirrhosis [19, 63]. In support of these findings is the observation that many of the drugs used to treat rheumatoid arthritis, most notably methotrexate (MTX), cause increases in extracellular adenosine that appear to be pivotal for the beneficial effects of MTX [68, 79, 80]; however, a well-documented side effect of MTX is its capacity to induce liver fibrosis [81], a phenomenon consistent with the heightened levels of extracellular adenosine.
In chronic diseases affecting the skin, high levels of adenosine have been reported in dermal lesions obtained from patients with psoriasis [82], where treatment with caffeine, a non-selective adenosine receptor antagonist, was considered an effective therapy [83]. In scleroderma and systemic scleroris (SSc) skin biopsies from patients with SSc presented with higher levels of IL-6 in fibroblasts following exposure to ATP [84]. Recently, increased levels of ADORA2A were evident and treatment with CGS21680 (a selective ADORA2A agonist) resulted in increased collagen production and myo-fibroblast differentiation [85] in fibroblasts from patients with SSc. Involvement of ADORA2A is also observed in patients with chronic heart failure where increased ADORA2A receptor expression, density, was found in both circulating cells and in the explanted hearts of heart failure patients [86].
In the framework of other chronic disease affecting the circulatory system, adenosine has been recently shown to be elevated in patients with sickle cell disease where elevated levels of adenosine are postulated to promote sickling of erythrocytes [66]. In addition to these observations, ADORA2B gene expression has been postulated as a biomarker for patients with elevated tricuspid regurgitation velocity in sickle cell disease [87]. These findings suggest that agents that lower adenosine levels or block the ADORA2B receptor may prove beneficial in the treatment of sickle cell disease.
Adenosine Signaling in Chronic Disease Models
The use of experimental models of chronic disease has led to a much better understanding of the role of adenosine in orchestrating the pathophysiology of chronic diseases [14]. In chronic lung disease, the use of ADA knockout mice demonstrated that persistently high levels of adenosine can lead to changes in lung pathology similar to those seen in chronic lung injury including airspace enlargement and fibrosis, cardinal signs of COPD and IPF [15, 16, 88]. In addition to these experiments, chronic exposure of mice to bleomycin was shown to lead to extensive fibrosis [16, 89, 90] altered lung function and gas exchange and the development of hallmarks of pulmonary hypertension [89] a fatal complication of IPF [91]. In these experiments, the ADORA2B was found to be up regulated in association with mediators involved in remodeling such as IL-6 and matrix metalloproteins, and molecules such as endothelin-1 that are involved in the development of pulmonary hypertension [16, 89]. In both ADA-knockout mice and the bleomycin model, treatment with an ADORA2B antagonist, or genetic removal of ADORA2B was able to abrogate the development of lung injury, including fibrosis [15, 16], airspace enlargement [16] and pulmonary hypetension [89]. Similarly, in allergic models of asthma, genetic deletion of the ADORA2B [17] or treatment with ADORA2B antagonists [92] were associated with decreased airway disease including airway remodeling.
The ability of the ADORA2B to regulate the differentiation of immune effector cells may represent a major mechanism by which adenosine contributes to the development or progression of chronic lung disease. In a model of bronchiolitis obliterans, a form of chronic allograft rejection in the lung, the ADORA2B was found to contribute to fibrosis associated with transplant rejection [24]. In this study, ADORA2B knockout mice exhibited decreased fibrosis associated with elevations in the numbers of T regulatory cells, suggesting ADORA2B signaling may promote transplant rejection by inhibiting regulatory T cell infiltration. The ADORA2B has also been shown to promote the differentiation of myeloid suppressor cells that could contribute to cancer progression [93] and alternatively activated macrophages [94] that can produce remodeling mediators that drive the progression of fibrotic diseases such as IPF [14]. Along these lines, alternatively activated macrophages isolated from the airways of patients with IPF have been shown to produce pro-fibrotic mediators in response to ADORA2B stimulation [14]. Continued efforts to understand the role of adenosine signaling through the ADORA2B to regulate the appearance of immune effector cells that impact tissue remodeling could be helpful in identifying when ADORA2B antagonist are most effective in attenuating aspects of chronic disease (Figure 1).
The use of ADA knockout mice has also provided further understanding of the role of adenosine in other chronic diseases (Figure 1). Using this model, Yang Xia and colleagues identified a role for adenosine and ADORA2B in chronic kidney disease [21] as well as a role in penile fibrosis [95]. In experiments looking at the lung and kidney in ADA knockout mice, the activation of ADORA2B has been demonstrated to contribute to the development of remodeling processes through the release of mediators such as IL-6 [21, 89, 96] and osteopontin [97]. Similarly, studies looking at cardiac remodeling following a model of myocardial infarction in mice demonstrated that blockade of ADORA2B lead to improved remodeling of the heart via inhibition of IL-6 and TNF-α [62].
Models have also been useful in examining the observation that ADORA2B signaling promotes erythrocyte sickling and tissue injury. In a transgenic model of SCD, elevated levels of adenosine promoted sickling and hemolytic damage to several organs and treatment with ADA enzyme therapy to lower adenosine or an with and ADORA2B antagonist proved beneficial for the treatment of SCD [98]. Activation of ADORA2B has recently been shown to contribute to insulin resistance via the enhanced production of IL-6 from macrophages and endothelial cells of diabetic animals [65]. Taken together these results point at ADORA2B as a key mediator in chronic disease that is not restricted to tissue remodeling but that is implicated in other functions such as erythrocyte integrity and metabolic disease.
Similar to the role of ADORA2B in fibrosis, several studies demonstrate that engagement of ADORA2A contributes to tissue remodeling (Figure 1). In a model of carbon tetrachloride (CCl 4) or thioacetamide-induced hepatic fibrosis, ADORA2A but not ADORA3 knockout mice were protected from the development of fibrosis [63]. However, in a separate mouse model of liver damage resulting from ethanol ingestion, investigators showed that ADORA1, ADORA2B or CD73-deficient mice were protected from ethanol-induced hepatic steatosis, consistent with mice treated with an ADORA1 or ADORA2B antagonist [19]. In the skin, studies using ADA-deficient mice demonstrated that elevated levels of adenosine contribute to dermal fibrosis via IL-13, TGF-beta and connective tissue growth factor production that is abrogated in ADORA2A knockout mice or following treatment with the ADORA2A antagonist ZM241385 [61]. These observations are consistent with studies where deletion or blockade of ADORA2A inhibits bleomycin-induced dermal fibrosis by preventing infiltration of fibrocytes [99]. These data support the use of ADORA2A antagonists for the treatment of chronic disease, particularly those affecting the skin or liver.
An exciting area of adenosine signaling is the involvement of ADORA2A in Parkinson’s disease (PD) where several clinical trials have been initiated to analyze the therapeutic potential of adenosine ADORA2A antagonists in the treatment of this chronic neurodegenerative disease [27]. In the brain, postsynaptic activation of ADORA2A neutralizes the inhibitory effect of dopamine [100]; as such blockade of ADORA2A in conditions of dopamine scarcity is hypothesized to have therapeutic benefits in PD. These observations were first made clinically where a reduced risk of developing PD following consumption of caffeinated coffee was reported [101]. Since then a vast library of ADORA2A antagonists have been tested in animal models of PD and clinically. Interestingly, Jiang-Fan Chen and colleagues have conducted a series of studies that suggest that ADORA2A stimulation may also be detrimental in other aspects of brain injury. For an extensive review see Armentero et al.[27]
Not all the effects of adenosine are detrimental in chronic diseases. As discussed earlier, increases in adenosine are thought to play an important role in the mechanism of action of MTX, a drug commonly prescribed to treat RA [68, 79, 80]. Experiments characterizing the role of adenosine receptors in lymphocytes from patients with RA have also shown increases in ADORA2A and ADORA3 levels [102]. Interestingly, in these studies, activation of either ADORA2A or ADORA3 inhibited the NF-kB pathway and diminished inflammatory cytokines such as TNF-α, IL-1β and IL-6 [102]. These results and the development of ADORA3 agonists for the treatment of chronic disease such as RA or dry eye syndrome [103] demonstrate the vast effects that adenosine can have through activation of its membrane-bound receptors in chronic disease and point at the necessity of further dissecting the signaling pathways in chronic disease in order to better understand its function in disease. This review has largely focused on the effects of ADORA2A and ADORA2B in the processes due to the large amount of literature supporting the involvement of these receptors; however, the ADORA1 and ADORA3 have also been implicated in many of the same processes. Please see the following reviews for more information on the functions of these receptors in acute and chronic disease processes [25, 42, 43].
Conclusions
The production of extracellular adenosine has emerged as a major cellular process for orchestrating tissue responses to injury. The rapid release of ATP from cells, its conversion to adenosine and subsequent stimulation of adenosine receptors has likely evolved as a mechanism to protect tissues from stress, particularly stress associated with hypoxia. Harnessing these protective aspects of adenosine signaling will likely prove beneficial in the treatment of acute injuries where approaches to elevate extracellular adenosine, such as dipyridamole treatment, or stimulate adenosine receptors with selective agonists, will promote vascular barrier function, decrease inflammation and enhance aspects of wound healing. Interestingly, many chronic diseases display histopathological changes such as fibrosis that can be viewed as an overactive wound healing response. The observation that excessive adenosine elevations are associated with chronic disease progression suggests that adenosine may activate unremitting wound healing processes in chronic environments. Accordingly, adenosine receptor antagonism, may prove beneficial in treating various chronic diseases.
It will be critical to decipher when during the course of disease progression adenosine signaling is beneficial or detrimental. Clarity of this issue will emerge as the specific mechanisms of adenosine regulated responses in specific diseases become better understood. Aspects to consider include the observation that the levels of adenosine receptors, particularly the ADORA2A and ADORA2B, are substantially up regulated on immune and stromal cells in injured environments. Moreover, the ability of adenosine to regulate the differentiation of effector cells that directly impact tissue remodeling, such as alternatively activated macrophages, dendritic cells or regulatory T cells may provide mechanisms for screening when blockade or activation of the adenosine signaling pathway is beneficial.
Acknowledgments
We would like to thank Tingting Weng and Kelly Volcik for their assistance in preparing and reviewing this manuscript. We would also like to acknowledge the following grant support: National Institute of Health Grants HL070952 to M.R.B. and DK083559 to Y.X. and American Heart Association Grant 12IRG9150001 to Y.X..
Footnotes
Conflict of interest: The authors have declared no conflict of interest
DISCLOSURE
The authors declare that they have no conflict of interests.
References
- 1.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111:2024–2035. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schingnitz U, Hartmann K, Macmanus CF, Eckle T, Zug S, Colgan SP, Eltzschig HK. Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J Immunol. 2010;184:5271–5279. doi: 10.4049/jimmunol.0903035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest. 2008;118:3301–3315. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Y, Schneider DJ, Morschl E, Song L, Pedroza M, Karmouty-Quintana H, Le T, Sun CX, Blackburn MR. Distinct roles for the A2B adenosine receptor in acute and chronic stages of bleomycin-induced lung injury. J Immunol. 2010;186:1097–1106. doi: 10.4049/jimmunol.1002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma AK, Linden J, Kron IL, Laubach VE. Protection from pulmonary ischemia-reperfusion injury by adenosine A2A receptor activation. Respir Res. 2009;10:58. doi: 10.1186/1465-9921-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okusa MD, Linden J, Macdonald T, Huang L. Selective A2A adenosine receptor activation reduces ischemia-reperfusion injury in rat kidney. The American journal of physiology. 1999;277:F404–412. doi: 10.1152/ajprenal.1999.277.3.F404. [DOI] [PubMed] [Google Scholar]
- 8.Grenz A, Osswald H, Eckle T, Yang D, Zhang H, Tran ZV, Klingel K, Ravid K, Eltzschig HK. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 2008;5:e137. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day YJ, Huang L, McDuffie MJ, Rosin DL, Ye H, Chen JF, Schwarzschild MA, Fink JS, Linden J, Okusa MD. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest. 2003;112:883–891. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 11.Yang Z, Day YJ, Toufektsian MC, Xu Y, Ramos SI, Marshall MA, French BA, Linden J. Myocardial infarct-sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation. 2006;114:2056–2064. doi: 10.1161/CIRCULATIONAHA.106.649244. [DOI] [PubMed] [Google Scholar]
- 12.Eltzschig HK, Rivera-Nieves J, Colgan SP. Targeting the A2B adenosine receptor during gastrointestinal ischemia and inflammation. Expert opinion on therapeutic targets. 2009;13:1267–1277. doi: 10.1517/14728220903241666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Day YJ, Marshall MA, Huang L, McDuffie MJ, Okusa MD, Linden J. Protection from ischemic liver injury by activation of A2A adenosine receptors during reperfusion: inhibition of chemokine induction. American journal of physiology Gastrointestinal and liver physiology. 2004;286:G285–G293. doi: 10.1152/ajpgi.00348.2003. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y, Schneider DJ, Blackburn MR. Adenosine signaling and the regulation of chronic lung disease. Pharmacol Ther. 2009;123:105–116. doi: 10.1016/j.pharmthera.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chunn JL, Molina JG, Mi T, Xia Y, Kellems RE, Blackburn MR. Adenosine-dependent pulmonary fibrosis in adenosine deaminase-deficient mice. J Immunol. 2005;175:1937–1946. doi: 10.4049/jimmunol.175.3.1937. [DOI] [PubMed] [Google Scholar]
- 16.Sun CX, Zhong H, Mohsenin A, Morschl E, Chunn JL, Molina JG, Belardinelli L, Zeng D, Blackburn MR. Role of A2B adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. J Clin Invest. 2006;116:2173–2182. doi: 10.1172/JCI27303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaynagetdinov R, Ryzhov S, Goldstein AE, Yin H, Novitskiy SV, Goleniewska K, Polosukhin VV, Newcomb DC, Mitchell D, Morschl E, et al. Attenuation of chronic pulmonary inflammation in A2B adenosine receptor knockout mice. Am J Respir Cell Mol Biol. 2010;42:564–571. doi: 10.1165/rcmb.2008-0391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chunn JL, Mohsenin A, Young HW, Lee CG, Elias JA, Kellems RE, Blackburn MR. Partially adenosine deaminase-deficient mice develop pulmonary fibrosis in association with adenosine elevations. Am J Physiol Lung Cell Mol Physiol. 2006;290:L579–587. doi: 10.1152/ajplung.00258.2005. [DOI] [PubMed] [Google Scholar]
- 19.Peng Z, Borea PA, Varani K, Wilder T, Yee H, Chiriboga L, Blackburn MR, Azzena G, Resta G, Cronstein BN. Adenosine signaling contributes to ethanol-induced fatty liver in mice. J Clin Invest. 2009;119:582–594. doi: 10.1172/JCI37409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan ES, Fernandez P, Merchant AA, Montesinos MC, Trzaska S, Desai A, Tung CF, Khoa DN, Pillinger MH, Reiss AB, et al. Adenosine A2A receptors in diffuse dermal fibrosis: pathogenic role in human dermal fibroblasts and in a murine model of scleroderma. Arthritis Rheum. 2006;54:2632–2642. doi: 10.1002/art.21974. [DOI] [PubMed] [Google Scholar]
- 21.Dai Y, Zhang W, Wen J, Zhang Y, Kellems RE, Xia Y. A2B adenosine receptor-mediated induction of IL-6 promotes CKD. J Am Soc Nephrol. 2011;22:890–901. doi: 10.1681/ASN.2010080890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen J, Jiang X, Dai Y, Zhang Y, Tang Y, Sun H, Mi T, Phatarpekar PV, Kellems RE, Blackburn MR, Xia Y. Increased adenosine contributes to penile fibrosis, a dangerous feature of priapism, via A2B adenosine receptor signaling. FASEB journal. 2011;24:740–749. doi: 10.1096/fj.09-144147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mi T, Abbasi S, Zhang H, Uray K, Chunn JL, Xia LW, Molina JG, Weisbrodt NW, Kellems RE, Blackburn MR, Xia Y. Excess adenosine in murine penile erectile tissues contributes to priapism via A2B adenosine receptor signaling. J Clin Invest. 2008;118:1491–1501. doi: 10.1172/JCI33467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, LaPar DJ, Steidle J, Emaminia A, Kron IL, Ailawadi G, Linden J, Lau CL. Adenosine signaling via the adenosine 2B receptor is involved in bronchiolitis obliterans development. J Heart Lung Transplant. 2010;29:1405–1414. doi: 10.1016/j.healun.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nature reviews Drug discovery. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalla RV, Zablocki J. Progress in the discovery of selective, high affinity A(2B) adenosine receptor antagonists as clinical candidates. Purinergic signalling. 2009;5:21–29. doi: 10.1007/s11302-008-9119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armentero MT, Pinna A, Ferre S, Lanciego JL, Muller CE, Franco R. Past, present and future of A(2A) adenosine receptor antagonists in the therapy of Parkinson’s disease. Pharmacol Ther. 2011;132:280–299. doi: 10.1016/j.pharmthera.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5′-nucleotidase (CD73) Purinergic signalling. 2006;2:351–360. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hart ML, Gorzolla IC, Schittenhelm J, Robson SC, Eltzschig HK. SP1-dependent induction of CD39 facilitates hepatic ischemic preconditioning. J Immunol. 2010;184:4017–4024. doi: 10.4049/jimmunol.0901851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson EK, Ren J, Mi Z. Extracellular 2′,3′-cAMP is a source of adenosine. J Biol Chem. 2009;284:33097–33106. doi: 10.1074/jbc.M109.053876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volmer JB, Thompson LF, Blackburn MR. Ecto-5′-nucleotidase (CD73)-mediated adenosine production is tissue protective in a model of bleomycin-induced lung injury. J Immunol. 2006;176:4449–4458. doi: 10.4049/jimmunol.176.7.4449. [DOI] [PubMed] [Google Scholar]
- 33.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 34.Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Muller CE. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors--an update. Pharmacol Rev. 2011;63:1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedman DJ, Kunzli BM, AR YI, Sevigny J, Berberat PO, Enjyoji K, Csizmadia E, Friess H, Robson SC. From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci U S A. 2009;106:16788–16793. doi: 10.1073/pnas.0902869106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. The Journal of experimental medicine. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hart ML, Grenz A, Gorzolla IC, Schittenhelm J, Dalton JH, Eltzschig HK. Hypoxia-inducible factor-1alpha-dependent protection from intestinal ischemia/reperfusion injury involves ecto-5′-nucleotidase (CD73) and the A2B adenosine receptor. J Immunol. 2011;186:4367–4374. doi: 10.4049/jimmunol.0903617. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Eckle T, Fullbier L, Wehrmann M, Khoury J, Mittelbronn M, Ibla J, Rosenberger P, Eltzschig HK. Identification of ectonucleotidases CD39 and CD73 in innate protection during acute lung injury. J Immunol. 2007;178:8127–8137. doi: 10.4049/jimmunol.178.12.8127. [DOI] [PubMed] [Google Scholar]
- 39.Loffler M, Morote-Garcia JC, Eltzschig SA, Coe IR, Eltzschig HK. Physiological roles of vascular nucleoside transporters. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:1004–1013. doi: 10.1161/ATVBAHA.106.126714. [DOI] [PubMed] [Google Scholar]
- 40.Grenz A, Bauerle JD, Dalton JH, Ridyard D, Badulak A, Tak E, McNamee EN, Clambey E, Moldovan R, Reyes G, et al. Equilibrative nucleoside transporter 1 (ENT1) regulates postischemic blood flow during acute kidney injury in mice. J Clin Invest. 2012;122:693–710. doi: 10.1172/JCI60214. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Sitkovsky MV, Ohta A. The ‘danger’ sensors that STOP the immune response: the A2 adenosine receptors? Trends in immunology. 2005;26:299–304. doi: 10.1016/j.it.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 42.Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends in immunology. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 43.Blackburn MR, Vance CO, Morschl E, Wilson CN. Adenosine receptors and inflammation. Handb Exp Pharmacol. 2009:215–269. doi: 10.1007/978-3-540-89615-9_8. [DOI] [PubMed] [Google Scholar]
- 44.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 45.Raskovalova T, Lokshin A, Huang X, Jackson EK, Gorelik E. Adenosine-mediated inhibition of cytotoxic activity and cytokine production by IL-2/NKp46-activated NK cells: involvement of protein kinase A isozyme I (PKA I) Immunol Res. 2006;36:91–99. doi: 10.1385/IR:36:1:91. [DOI] [PubMed] [Google Scholar]
- 46.Wallace KL, Linden J. Adenosine A2A receptors induced on iNKT and NK cells reduce pulmonary inflammation and injury in mice with sickle cell disease. Blood. 2010;116:5010–5020. doi: 10.1182/blood-2010-06-290643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hasko G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, Marton A, Szabo C. Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. FASEB journal. 2000;14:2065–2074. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 48.Cronstein BN, Kramer SB, Weissmann G, Hirschhorn R. Adenosine: a physiological modulator of superoxide anion generation by human neutrophils. The Journal of experimental medicine. 1983;158:1160–1177. doi: 10.1084/jem.158.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Panther E, Corinti S, Idzko M, Herouy Y, Napp M, la Sala A, Girolomoni G, Norgauer J. Adenosine affects expression of membrane molecules, cytokine and chemokine release, and the T-cell stimulatory capacity of human dendritic cells. Blood. 2003;101:3985–3990. doi: 10.1182/blood-2002-07-2113. [DOI] [PubMed] [Google Scholar]
- 50.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. The Journal of experimental medicine. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Collins SL, Black KE, Chan-Li Y, Ahn YH, Cole PA, Powell JD, Horton MR. Hyaluronan fragments promote inflammation by down-regulating the anti-inflammatory A2a receptor. Am J Respir Cell Mol Biol. 2011;45:675–683. doi: 10.1165/rcmb.2010-0387OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fozard JR, Ellis KM, Villela Dantas MF, Tigani B, Mazzoni L. Effects of CGS 21680, a selective adenosine A2A receptor agonist, on allergic airways inflammation in the rat. European journal of pharmacology. 2002;438:183–188. doi: 10.1016/s0014-2999(02)01305-5. [DOI] [PubMed] [Google Scholar]
- 53.Nadeem A, Ponnoth DS, Ansari HR, Batchelor TP, Dey RD, Ledent C, Mustafa SJ. A2A adenosine receptor deficiency leads to impaired tracheal relaxation via NADPH oxidase pathway in allergic mice. J Pharmacol Exp Ther. 2009;330:99–108. doi: 10.1124/jpet.109.151613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lau CL, Zhao Y, Kron IL, Stoler MH, Laubach VE, Ailawadi G, Linden J. The role of adenosine A2A receptor signaling in bronchiolitis obliterans. Ann Thorac Surg. 2009;88:1071–1078. doi: 10.1016/j.athoracsur.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allen-Gipson DS, Wong J, Spurzem JR, Sisson JH, Wyatt TA. Adenosine A2A receptors promote adenosine-stimulated wound healing in bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L849–855. doi: 10.1152/ajplung.00373.2005. [DOI] [PubMed] [Google Scholar]
- 56.Montesinos MC, Desai A, Chen JF, Yee H, Schwarzschild MA, Fink JS, Cronstein BN. Adenosine promotes wound healing and mediates angiogenesis in response to tissue injury via occupancy of A(2A) receptors. The American journal of pathology. 2002;160:2009–2018. doi: 10.1016/S0002-9440(10)61151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mohsenin A, Mi T, Xia Y, Kellems RE, Chen JF, Blackburn MR. Genetic removal of the A2A adenosine receptor enhances pulmonary inflammation, mucin production, and angiogenesis in adenosine deaminase-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L753–761. doi: 10.1152/ajplung.00187.2007. [DOI] [PubMed] [Google Scholar]
- 58.Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, St Hilaire C, Seldin DC, Toselli P, et al. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest. 2006;116:1913–1923. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, Lowes BD, Han J, Borchers CH, Buttrick PM, et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nature medicine. 2012;18:774–782. doi: 10.1038/nm.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun CX, Zhong H, Mohsenin A, Morschl E, Chunn JL, Molina JG, Belardinelli L, Zeng D, Blackburn MR. Role of A2B receptor signaling in adenosine-dependent pulmonary inflammation and injury. J Clin Invest. 2006;116:1–10. doi: 10.1172/JCI27303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fernandez P, Trzaska S, Wilder T, Chiriboga L, Blackburn MR, Cronstein BN, Chan ES. Pharmacological blockade of A2A receptors prevents dermal fibrosis in a model of elevated tissue adenosine. The American journal of pathology. 2008;172:1675–1682. doi: 10.2353/ajpath.2008.070952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Toldo S, Zhong H, Mezzaroma E, Van Tassell B, Kannan H, Zeng D, Belardinelli L, Voelkel N, Abbate A. GS-6201, a selective blocker of the A2B adenosine receptor, attenuates cardiac remodeling following acute myocardial infarction in the mouse. J Pharmacol Exp Ther. 2012 doi: 10.1124/jpet.111.191288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chan ES, Montesinos MC, Fernandez P, Desai A, Delano DL, Yee H, Reiss AB, Pillinger MH, Chen JF, Schwarzschild MA, et al. Adenosine A(2A) receptors play a role in the pathogenesis of hepatic cirrhosis. British journal of pharmacology. 2006;148:1144–1155. doi: 10.1038/sj.bjp.0706812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Awad AS, Huang L, Ye H, Duong ET, Bolton WK, Linden J, Okusa MD. Adenosine A2A receptor activation attenuates inflammation and injury in diabetic nephropathy. American journal of physiology Renal physiology. 2006;290:F828–837. doi: 10.1152/ajprenal.00310.2005. [DOI] [PubMed] [Google Scholar]
- 65.Figler RA, Wang G, Srinivasan S, Jung DY, Zhang Z, Pankow JS, Ravid K, Fredholm B, Hedrick CC, Rich SS, et al. Links between insulin resistance, adenosine A2B receptors, and inflammatory markers in mice and humans. Diabetes. 2011;60:669–679. doi: 10.2337/db10-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y, Dai Y, Wen J, Zhang W, Grenz A, Sun H, Tao L, Lu G, Alexander DC, Milburn MV, et al. Detrimental effects of adenosine signaling in sickle cell disease. Nature Medicine. 2011;17:79–86. doi: 10.1038/nm.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwarzschild MA, Agnati L, Fuxe K, Chen JF, Morelli M. Targeting adenosine A2A receptors in Parkinson’s disease. Trends Neurosci. 2006;29:647–654. doi: 10.1016/j.tins.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 68.Cronstein BN. Low-dose methotrexate: a mainstay in the treatment of rheumatoid arthritis. Pharmacol Rev. 2005;57:163–172. doi: 10.1124/pr.57.2.3. [DOI] [PubMed] [Google Scholar]
- 69.Cushley MJ, Tattersfield AE, Holgate ST. Inhaled adenosine and guanosine on airway resistance in normal and asthmatic subjects. Br J Clin Pharmacol. 1983;15:161–165. doi: 10.1111/j.1365-2125.1983.tb01481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hannon JP, Tigani B, Williams I, Mazzoni L, Fozard JR. Mechanism of airway hyperresponsiveness to adenosine induced by allergen challenge in actively sensitized Brown Norway rats. British journal of pharmacology. 2001;132:1509–1523. doi: 10.1038/sj.bjp.0703961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Driver AG, Kukoly CA, Ali S, Mustafa SJ. Adenosine in bronchoalveolar lavage fluid in asthma. The American review of respiratory disease. 1993;148:91–97. doi: 10.1164/ajrccm/148.1.91. [DOI] [PubMed] [Google Scholar]
- 72.Huszar E, Vass G, Vizi E, Csoma Z, Barat E, Molnar VG, Herjavecz I, Horvath I. Adenosine in exhaled breath condensate in healthy volunteers and in patients with asthma. Eur Respir J. 2002;20:1393–1398. doi: 10.1183/09031936.02.00005002. [DOI] [PubMed] [Google Scholar]
- 73.Li Y, Wang W, Parker W, Clancy JP. Adenosine regulation of cystic fibrosis transmembrane conductance regulator through prostenoids in airway epithelia. Am J Respir Cell Mol Biol. 2006;34:600–608. doi: 10.1165/rcmb.2005-0421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Esther CR, Jr, Lazaar AL, Bordonali E, Qaqish B, Boucher RC. Elevated airway purines in COPD. Chest. 2011;140:954–960. doi: 10.1378/chest.10-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goodarzi MT, Abdi M, Tavilani H, Nadi E, Rashidi M. Adenosine deaminase activity in COPD patients and healthy subjects. Iran J Allergy Asthma Immunol. 2010;9:7–12. [PubMed] [Google Scholar]
- 76.Zhou Y, Murthy JN, Zeng D, Belardinelli L, Blackburn MR. Alterations in adenosine metabolism and signaling in patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. PloS one. 5:e9224. doi: 10.1371/journal.pone.0009224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nagy LE, Diamond I, Casso DJ, Franklin C, Gordon AS. Ethanol increases extracellular adenosine by inhibiting adenosine uptake via the nucleoside transporter. J Biol Chem. 1990;265:1946–1951. [PubMed] [Google Scholar]
- 78.Puig JG, Fox IH. Ethanol-induced activation of adenine nucleotide turnover. Evidence for a role of acetate. J Clin Invest. 1984;74:936–941. doi: 10.1172/JCI111512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chan ES, Cronstein BN. Molecular action of methotrexate in inflammatory diseases. Arthritis Res. 2002;4:266–273. doi: 10.1186/ar419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stamp LK, Hazlett J, Roberts RL, Frampton C, Highton J, Hessian PA. Adenosine receptor expression in rheumatoid synovium: a basis for methotrexate action. Arthritis Res Ther. 2012;14:R138. doi: 10.1186/ar3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bjorkman DJ, Boschert M, Tolman KG, Clegg DO, Ward JR. The effect of long-term methotrexate therapy on hepatic fibrosis in rheumatoid arthritis. Arthritis Rheum. 1993;36:1697–1701. doi: 10.1002/art.1780361208. [DOI] [PubMed] [Google Scholar]
- 82.Duell EA. Adenosine-induced alterations in the adenosine 3′:5′-monophosphate levels in mammalian epidermis. Mol Pharmacol. 1980;18:49–52. [PubMed] [Google Scholar]
- 83.Vali A, Asilian A, Khalesi E, Khoddami L, Shahtalebi M, Mohammady M. Evaluation of the efficacy of topical caffeine in the treatment of psoriasis vulgaris. J Dermatolog Treat. 2005;16:234–237. doi: 10.1080/09546630510011801. [DOI] [PubMed] [Google Scholar]
- 84.Lo Monaco A, Gulinelli S, Castellino G, Solini A, Ferrari D, La Corte R, Trotta F, Di Virgilio F. Increased sensitivity to extracellular ATP of fibroblasts from patients affected by systemic sclerosis. Ann Rheum Dis. 2007;66:1124–1125. doi: 10.1136/ard.2006.065078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lazzerini PE, Natale M, Gianchecchi E, Capecchi PL, Montilli C, Zimbone S, Castrichini M, Balistreri E, Ricci G, Selvi E, Garcia-Gonzalez E, Galeazzi M, Laghi-Pasini F. Adenosine A2A receptor activation stimulates collagen production in sclerodermic dermal fibroblasts either directly and through a cross-talk with the cannabinoid system. J Mol Med (Berl) 2012;90:331–342. doi: 10.1007/s00109-011-0824-5. [DOI] [PubMed] [Google Scholar]
- 86.Varani K, Laghi-Pasini F, Camurri A, Capecchi PL, Maccherini M, Diciolla F, Ceccatelli L, Lazzerini PE, Ulouglu C, Cattabeni F, Borea PA, Abbracchio MP. Changes of peripheral A2A adenosine receptors in chronic heart failure and cardiac transplantation. FASEB journal. 2003;17:280–282. doi: 10.1096/fj.02-0543fje. [DOI] [PubMed] [Google Scholar]
- 87.Desai AA, Zhou T, Ahmad H, Zhang W, Mu W, Trevino S, Wade MS, Raghavachari N, Kato GJ, Peters-Lawrence MH, Thiruvoipati T, Turner K, Artz N, Huang Y, Patel AR, Yuan JX, Gordeuk VR, Lang RM, Garcia JG, Machado RF. A novel molecular signature for elevated tricuspid regurgitation velocity in sickle cell disease. American journal of respiratory and critical care medicine. 2012;186:359–368. doi: 10.1164/rccm.201201-0057OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blackburn MR, Volmer JB, Thrasher JL, Zhong H, Crosby JR, Lee JJ, Kellems RE. Metabolic consequences of adenosine deaminase deficiency in mice are associated with defects in alveogenesis, pulmonary inflammation, and airway obstruction. The Journal of experimental medicine. 2000;192:159–170. doi: 10.1084/jem.192.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karmouty-Quintana H, Zhong H, Acero L, Weng T, Melicoff E, West JD, Hemnes A, Grenz A, Eltzschig HK, Blackwell TS, Xia Y, Johnston RA, Zeng D, Belardinelli L, Blackburn MR. The A2B adenosine receptor modulates pulmonary hypertension associated with interstitial lung disease. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2012;26:2546–2557. doi: 10.1096/fj.11-200907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou Y, Schneider DJ, Morschl E, Song L, Pedroza M, Karmouty-Quintana H, Le T, Sun CX, Blackburn MR. Distinct roles for the A2B adenosine receptor in acute and chronic stages of bleomycin-induced lung injury. J Immunol. 2011;186:1097–1106. doi: 10.4049/jimmunol.1002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Farkas L, Gauldie J, Voelkel NF, Kolb M. Pulmonary hypertension and idiopathic pulmonary fibrosis: a tale of angiogenesis, apoptosis, and growth factors. Am J Respir Cell Mol Biol. 2011;45:1–15. doi: 10.1165/rcmb.2010-0365TR. [DOI] [PubMed] [Google Scholar]
- 92.Mustafa SJ, Nadeem A, Fan M, Zhong H, Belardinelli L, Zeng D. Effect of a specific and selective A(2B) adenosine receptor antagonist on adenosine agonist AMP and allergen-induced airway responsiveness and cellular influx in a mouse model of asthma. J Pharmacol Exp Ther. 2007;320:1246–1251. doi: 10.1124/jpet.106.112250. [DOI] [PubMed] [Google Scholar]
- 93.Ryzhov S, Novitskiy SV, Goldstein AE, Biktasova A, Blackburn MR, Biaggioni I, Dikov MM, Feoktistov I. Adenosinergic regulation of the expansion and immunosuppressive activity of CD11b+Gr1+ cells. J Immunol. 2011;187:6120–6129. doi: 10.4049/jimmunol.1101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Csoka B, Selmeczy Z, Koscso B, Nemeth ZH, Pacher P, Murray PJ, Kepka-Lenhart D, Morris SM, Jr, Gause WC, Leibovich SJ, Hasko G. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2012;26:376–386. doi: 10.1096/fj.11-190934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wen J, Jiang X, Dai Y, Zhang Y, Tang Y, Sun H, Mi T, Phatarpekar PV, Kellems RE, Blackburn MR, Xia Y. Increased adenosine contributes to penile fibrosis, a dangerous feature of priapism, via A2B adenosine receptor signaling. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2010;24:740–749. doi: 10.1096/fj.09-144147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pedroza M, Schneider DJ, Karmouty-Quintana H, Coote J, Shaw S, Corrigan R, Molina JG, Alcorn JL, Galas D, Gelinas R, Blackburn MR. Interleukin-6 contributes to inflammation and remodeling in a model of adenosine mediated lung injury. PloS one. 2011;6:e22667. doi: 10.1371/journal.pone.0022667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schneider DJ, Lindsay JC, Zhou Y, Molina JG, Blackburn MR. Adenosine and osteopontin contribute to the development of chronic obstructive pulmonary disease. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2010;24:70–80. doi: 10.1096/fj.09-140772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang Y, Dai Y, Wen J, Zhang W, Grenz A, Sun H, Tao L, Lu G, Alexander DC, Milburn MV, Carter-Dawson L, Lewis DE, Zhang W, Eltzschig HK, Kellems RE, Blackburn MR, Juneja HS, Xia Y. Detrimental effects of adenosine signaling in sickle cell disease. Nature medicine. 2011;17:79–86. doi: 10.1038/nm.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Katebi M, Fernandez P, Chan ES, Cronstein BN. Adenosine A2A receptor blockade or deletion diminishes fibrocyte accumulation in the skin in a murine model of scleroderma, bleomycin-induced fibrosis. Inflammation. 2008;31:299–303. doi: 10.1007/s10753-008-9078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Azdad K, Gall D, Woods AS, Ledent C, Ferre S, Schiffmann SN. Dopamine D2 and adenosine A2A receptors regulate NMDA-mediated excitation in accumbens neurons through A2A-D2 receptor heteromerization. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2009;34:972–986. doi: 10.1038/npp.2008.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ross GW, Abbott RD, Petrovitch H, White LR, Tanner CM. Relationship between caffeine intake and parkinson disease. JAMA: the journal of the American Medical Association. 2000;284:1378–1379. [PubMed] [Google Scholar]
- 102.Varani K, Padovan M, Vincenzi F, Targa M, Trotta F, Govoni M, Borea PA. A2A and A3 adenosine receptor expression in rheumatoid arthritis: upregulation, inverse correlation with disease activity score and suppression of inflammatory cytokine and metalloproteinase release. Arthritis Res Ther. 2011;13:R197. doi: 10.1186/ar3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fishman P, Bar-Yehuda S, Liang BT, Jacobson KA. Pharmacological and therapeutic effects of A3 adenosine receptor agonists. Drug Discov Today. 2012;17:359–366. doi: 10.1016/j.drudis.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]