Abstract
Background
Galectin-3 is a multivalent carbohydrate-binding protein involved in cell adhesion, cell cycle control, immunomodulation, and cancer progression, including prostate cancer. Galectin-3 function is regulated by proteolytic cleavage that destroys galectin-3 multivalency while preserving carbohydrate-binding activity. In human semen, galectin-3 is present in seminal plasma and is also associated with prostasomes, exosome-like vesicles secreted by the prostate. In the current study, we characterized the proteolytic activity that cleaves galectin-3 in human seminal plasma.
Methods
An in vitro assay was developed to investigate galectin-3 cleavage in seminal plasma. The effect of protease inhibitors, divalent ion chelators, and Zn2+ on the cleavage activity was determined. Proteases enriched from seminal plasma were tested for their ability to cleave galectin-3. Affinity purification and microsequence analysis were used to identify the cleavage site in galectin-3.
Results
Galectin-3 was identified in human seminal plasma in an intact and truncated form. Gelatinases enriched from seminal plasma did not cleave galectin-3. Inhibitor studies indicated that the galectin-3 cleavage activity in seminal plasma is a Zn2+ sensitive, serine protease. Prostate specific antigen (PSA) was demonstrated to cleave galectin-3 between tyrosine107-glycine108 and produce a functionally-active, monovalent lectin.
Conclusions
PSA is a chymotrypsin-like serine protease secreted by the prostatic epithelium and normally functions in liquefaction of semen following ejaculation. Furthermore, PSA is implicated in the promotion of localized prostate tumors and bone metastases by its roles in immunomodulation, invasion, and apoptosis. Our results indicate that PSA regulates galectin-3 in human semen and may regulate galectin-3 function during prostate cancer progression.
Keywords: lectin, serine protease, proteolysis, prostatic secretions, semen
INTRODUCTION
Galectin-3 is an ~30 kDa member of the galectin protein family, a group of conserved animal lectins that bind to β-galactoside moieties on glycoproteins [1]. The multiple extracellular and intracellular functions of galectin-3 include immunomodulation, pathogen-host interactions, mRNA splicing, cytoskeletal organization, regulation of cell proliferation and apoptosis, cell-cell adhesion, cell-extracellular matrix adhesion, and cancer progression [1–3]. The galectin-3 protein is composed of a carbohydrate recognition domain (CRD) linked to a non-lectin domain through a linker region [1]. The non-lectin domain can interact with proteins or lipid moieties while the CRD has a strong preference for the poly-lactosamine structure (Gal β1–4/3GlcNAc)n [4–6]. Galectin-3 also forms homodimers and homopentamers via self-association of the non-lectin domain, leaving the CRDs accessible for binding with multiple glycoconjugate ligands [7]. Extra- and intracellular galectin-3 functions are dependent on the multivalency of the galectin-3 monomer, dimer, and pentamer.
Galectin-3 function is regulated, in part, by proteolytic processing that cleaves the galectin-3 CRD from the N-terminal non-lectin domain [1,8–10]. The collagen-like linker sequence between the galectin-3 CRD and the non-lectin domain is subject to cleavage by multiple proteases including collagenases, matrix metalloproteases (MMP) -2, -9, and -13, neutrophil elastase, and leishmanolysin [8–12]. Proteolytic cleavage of galectin-3 results in the production of a functional galectin-3 CRD fragment and abolishes the ability of intact galectin-3 to crosslink its target ligands [10]. Galectin-3 proteolysis has been implicated in tumor progression in growing breast cancers [13], and an allelic variant of galectin-3 that exhibits increased susceptibility to MMP proteolysis has been associated with increased breast cancer risk [14,15]. Functional relevance for proteolytic processing of galectin-3 has also been proposed for prostate cancer progression [16], arthritis [8], regulation of neutrophil function [17], and pathogenesis of Leishmania major [12].
In the human male reproductive tract, galectin-3 expression was identified previously in the testis [18] and prostate [19]. In addition, multiple studies have associated galectin-3 with tumor progression in prostate cancer [16,19,20]. We previously reported galectin-3 immunoreactivity in the human epididymis, seminal vesicle, spermatozoa, and seminal plasma [21]. Proteomic analysis identified galectin-3 in prostasomes, which are exosome-like membranous vesicles that are secreted by the prostate into seminal plasma during ejaculation. In prostasomes, galectin-3 was identified as an intact form of the molecule and as a truncated form containing only the galectin-3 CRD [21]. Identification of the functional galectin-3 CRD fragment indicates proteolysis as a potential regulatory mechanism for galectin-3 function in prostasomes and seminal plasma. Furthermore, the previous identification of MMP-2 and -9 immunoreactivity in human seminal plasma [22] suggested that these enzymes may contribute to galectin-3 proteolysis in semen.
The present study focused on the characterization and identification of the protease(s) that proteolytically cleaves galectin-3 in human seminal plasma. An in vitro galectin-3 cleavage assay was developed to investigate the proteolytic processing of galectin-3, and galectin-3 was identified as a proteolytic substrate for prostate specific antigen (PSA). PSA is a chymotrypsin-like serine protease that is secreted by the prostatic glandular epithelium into the seminal plasma during ejaculation [23]. The implications of these findings for normal reproduction and prostate cancer are discussed.
MATERIAL AND METHODS
Recombinant galectin-3 and galectin-3 CRD
Human recombinant galectin-3 was expressed, purified, and biotinylated as described previously [21]. To generate a recombinant protein containing exclusively the galectin-3 CRD (amino acids 116–250), the human galectin-3 CRD cDNA sequence was amplified by polymerase chain reaction (PCR) from the pOTB7-galectin-3 plasmid construct (ATCC MGC-2058; American Type Culture Collection) using specific primers (IDT). The forward primer was 5′-ATATTTCATATGGTGCCTTATAACCTGCCT-3′, and the reverse primer was 5′-AGGATCCAGATTATATCATGGTATATG-3′; NdeI and BamHI restriction enzyme sites are underlined, respectively. PCR products were digested with restriction enzymes (New England Biolabs) and subcloned into the pET-11a expression vector (Promega). Recombinant galectin-3 CRD was expressed in E. coli BL21 (DE3) cells and purified from bacterial lysates by lactose affinity column chromatography [21].
Anti-galectin-3 CRD polyclonal antibodies
A female Hartley strain guinea pig (Charles River) was immunized subcutaneously with recombinant galectin-3 CRD (100 μg/ml) emulsified 1:1 with complete Freund’s adjuvant (Sigma). Booster doses were given at weeks 2 and 4 in incomplete Freund’s adjuvant. Pre- and post-immunization bleeds were collected at regular intervals and analyzed by immunoblot. All animal experiments were performed following protocols approved by the UAMS Institutional Animal Care and Use Committee.
Human seminal plasma
Semen samples were provided by healthy human males following a protocol approved by the UAMS Institutional Review Board. The soluble fraction of seminal plasma was prepared as described previously [21]. Protein concentration was determined with the bicinchoninic acid (BCA) assay (Pierce) or the Bradford protein assay (Biorad).
Electrophoresis and electroblot analysis
Protein samples were separated by one dimensional SDS-polyacrylamide gel electrophoresis (SDS-PAGE) [24]. Immunoblot analysis and electroblot analysis of biotinylated protein were performed as described previously [21]. Dilution factors for immunoblots were anti-galectin-3 monoclonal antibody (mAb; clone 9C4; Fitzgerald) at 1:500, anti-galectin-3 CRD antibodies at 1:5000, anti-MMP-2 and -9 polyclonal antibodies (Calbiochem) at 1:300, and anti-PSA kallikrein loop polyclonal antibodies (Abcam) at 1:5000. Blots were developed by enhanced chemiluminescence (GE Healthcare) on X-ray film.
Galectin-3 cleavage assay
Biotinylated, recombinant galectin-3 was incubated at 37 °C with protease-containing samples for 2 hours, unless indicated otherwise, and subjected to electroblot analysis with streptavidin-horse radish peroxidase (Jackson ImmunoResearch). Heat-inactivated seminal plasma and Clostridium histolyticum collagenase Type VII (Sigma) were included as negative and positive controls, respectively. In some experiments, enzyme reactions were performed in the presence of EDTA, EGTA, 1,10-phenanthroline, ZnCl2, phenylmethylsulfonyl fluoride (PMSF), or benzamidine. Each experiment was performed at least in triplicate for quantitation and statistical analysis. Biotinylated protein bands representing cleaved (~16 kDa) and uncleaved galectin-3 (~30kDa) were quantitated using the ImageJ program. Percent cleavage of galectin-3 was calculated with the equation: % cleavage = cleaved galectin-3/(cleaved galectin-3 + uncleaved galectin-3).
Purification of gelatin-binding proteins from seminal plasma and zymography
Ten ml of the seminal plasma soluble fraction was diluted two-fold in PBS and applied to a gelatin Sepharose 4B (Pierce) affinity chromatography column. Gelatin-binding proteins were eluted with 10% DMSO. Gelatinolytic activity was assessed by gelatin zymography following non-reducing SDS-PAGE on a 10% acrylamide gel containing 2 mg/ml gelatin. Following electrophoresis, the gel was incubated for 30 minutes with renaturation buffer (2.5% Triton X-100), incubated for 20 hours at 37 °C in developing buffer (50 mM Tris, 200 mM NaCl, 5 mM CaCl2), and stained with Coomassie blue R-250 (Biorad). Bacterial collagenase was included as a positive control.
N-terminal microsequence analysis of endogenous galectin-3 CRD in human seminal plasma and of experimentally-cleaved recombinant galectin-3
Thirty ml seminal plasma soluble fraction was subjected to ammonium sulfate precipitation (35% – 60%) and centrifuged at 10,000 × g. The pellet was resuspended in PBS, dialyzed against 25 mM HEPES, pH 7.0 and ultracentrifuged at 100,000 × g. The supernatant was adjusted to 25 mM HEPES, 1 M sodium sulfate, pH 7.0 and applied to a thiophilic gel (T-gel) affinity chromatography column. The column was washed with 25 mM HEPES, 1M sodium sulfate, pH 7.0, and T-gel binding proteins were eluted using a stepwise gradient of decreasing Na2SO4 concentration. Fractions that exhibited galectin-3 immunoreactivity were pooled and subjected to lactose affinity chromatography. Lactose-binding fractions that exhibited galectin-3 immunoreactivity were pooled.
Biotinylated recombinant galectin-3 was proteolytically cleaved with clarified seminal plasma or with purified PSA (Calbiochem) for 24 hours at 37 °C in 200 mM EDTA. Biotinylated proteins were purified from cleavage reactions by avidin affinity column chromatography (Pierce).
Protein samples were subjected to SDS-PAGE, transferred onto PVDF membrane (Biorad), and stained with Ponceau S. For each sample, an ~16 kDa protein band was excised and the N-terminal end was sequenced by Edman degradation in the Microchemistry Facility at Harvard University.
T-gel chromatography and immunodepletion of PSA
The seminal plasma soluble fraction was subjected to T-gel column chromatography. Two μg of T-gel enriched material, which exhibited PSA immunoreactivity and galectin-3 cleavage activity, was treated with 20 μg anti-PSA mAb (clone 181827; Lab Vision) overnight at 4 °C. Protein G beads (Pierce) were added for 2 hours at room temperature and centrifuged at 2500 × g to remove the beads. Separate incubations with ChromPure irrelevant mouse IgG (Jackson ImmunoResearch) and Protein G beads alone were included as negative controls.
RESULTS
Identification of galectin-3 and galectin-3 CRD immunoreactivity in human seminal plasma
Total protein staining of β-galactoside-binding proteins from seminal identified multiple protein bands ranging from 10 kDa to 100 kDa (Fig. 1A). The anti-galectin-3 9C4 mAb, which recognizes an epitope in the galectin-3 N-terminal region [21], detected an immunoreactive band of ~30 kDa. The anti-galectin-3 CRD polyclonal antiserum identified bands of ~30 kDa and ~16 kDa corresponding to the molecular masses of intact galectin-3 and the galectin-3 CRD, respectively.
Figure 1.
Identification of a protease activity that cleaves galectin-3 in seminal plasma. A: Lactose-binding proteins from the soluble fraction of seminal plasma were analyzed by silver staining for total protein and by immunoblot. Anti-galectin-3 immunoreactivity was identified with a monoclonal antibody (Ab) against the N-terminal region of galectin-3 and with polyclonal antibodies generated against the galectin-3 CRD. B: Following incubation with aliquots of seminal plasma, cleavage of biotinylated recombinant galectin-3 was assessed by electroblot analysis with streptavidin. Bacterial collagenase and heat-inactivated seminal plasma were included as positive and negative controls, respectively. Molecular weight markers are indicated in kDa. C: Percent cleavage of galectin-3 was plotted against increasing concentrations of seminal plasma proteins. Each point represents the mean of three independent experiments and the value represents the mean ± s.e.
Characterization of galectin-3 proteolytic cleavage in human seminal plasma
A galectin-3 cleavage assay was developed to investigate the protease(s) in human seminal plasma that proteolytically cleaves galectin-3. Electroblot analysis identified biotinylated galectin-3 bands of ~30 kDa and ~16 kDa in samples incubated with bacterial collagenase and with clarified seminal plasma (Fig. 1B). Increasing intensity of the ~16 kDa band was observed with increasing concentrations of seminal plasma. Quantitation of the biotinylated protein bands and calculation of percent cleavage indicated that greater than 50% of the galectin-3 was cleaved with the highest amount of seminal plasma protein (400 μg) in the reaction mixture (Fig. 1C).
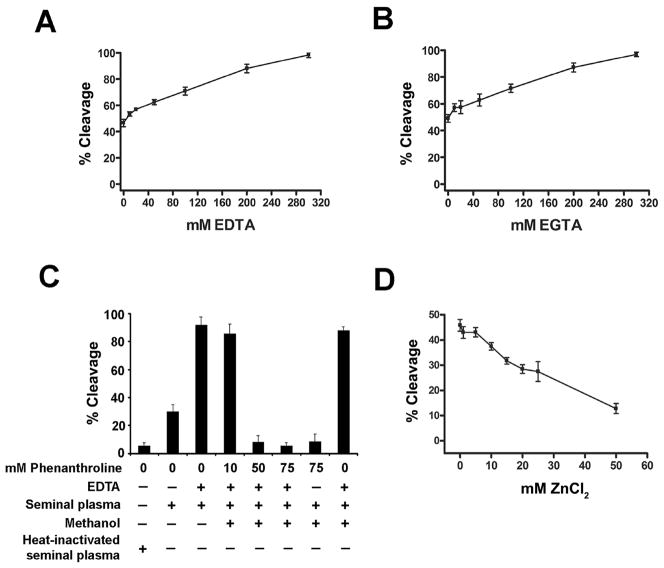
Galectin-3 cleavage increased up to 100% and 98% by pre-incubation of seminal plasma with increasing concentrations (0 to 300 mM) of EDTA (Fig. 2A) and EGTA (Fig. 2B), respectively. EDTA and EGTA alone did not cleave galectin-3 (data not shown). Following pre-treatment of seminal plasma with increasing concentrations (0 to 75 mM) of 1,10-phenanthroline, galectin-3 cleavage was inhibited by as much as 72% (Fig. 2C). Inhibition of galectin-3 cleavage by 1,10-phenanthroline was not affected by EDTA. Methanol, used as the solvent for 1,10-phenanthroline, did not inhibit galectin-3 cleavage. Pre-treatment of seminal plasma with increasing concentrations (0 to 50 mM) of ZnCl2 inhibited galectin-3 cleavage up to 72% (Fig. 2D).
Figure 2.
Effect of divalent cation concentration on the protease activity(ies) in seminal plasma that cleaves galectin-3. The galectin-3 cleavage assay was performed with 400 μg seminal plasma protein pretreated with increasing concentrations of the divalent cation chelators EDTA (A), EGTA (B), and 1,10-phenanthroline (C) and of ZnCl2 (D). A methanol-only negative control was included for cleavage reactions with 1,10-phenanthroline. Percent cleavage of galectin-3 was plotted against increasing concentrations of EDTA, EGTA, 1,10-phenanthroline and ZnCl2.
Gelatinases in human seminal plasma do not cleave galectin-3
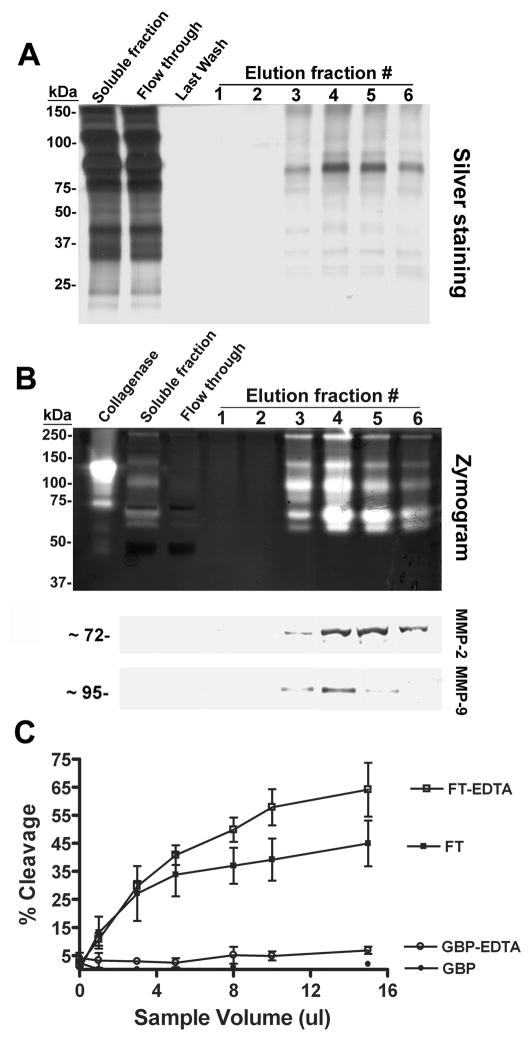
Gelatin-binding proteins were purified from seminal plasma by gelatin-affinity chromatography, and total protein staining of electrophoresed material identified a series of proteins eluted in fractions 3–6 that ranged in molecular weight from 25 kDa to 150 kDa (Fig. 3A). Gelatin zymography revealed multiple clear bands representative of gelatinolytic activity between 50 and 250 kDa in fractions 3–6 (Fig. 3B). Immunoblot analysis identified anti-MMP-2 and -9 immunoreactivity in fractions 3–6 (Fig. 3B). Neither gelatinolytic activity nor MMP-2 and -9 immunoreactivity were present in the flow-through from the gelatin-affinity column.
Figure 3.
Gelatinases in human seminal plasma do not cleave galectin-3. A: Eluted fractions from gelatin affinity column chromatography were subjected to SDS-PAGE and silver staining. B: Gelatinase activity was assessed by gelatin zymography. Bacterial collagenase and the soluble fraction of seminal plasma were included as positive controls (upper panel). Immunoblot analysis was performed with anti-MMP-2 and -9 polyclonal antibodies (lower panel). C: The galectin-3 cleavage assay was performed with increasing amounts (by volume) of purified gelatin-binding proteins and of the column flow-through, preincubated with or without 200 mM EDTA. Percent cleavage of galectin-3 was plotted against increasing amounts of flow-through (FT), flow through-EDTA (FT-EDTA), gelatin-binding proteins (GBP) and gelatin-binding protein-EDTA (GBP-EDTA).
The galectin-3 cleavage assay was performed with gelatin-binding proteins and the flow-through from the gelatin column purification. Galectin-3 was cleaved up to 45% with increasing concentration of the flow-through, and this cleavage was enhanced by 43% in the presence of EDTA (Fig. 3C). Galectin-3 cleavage was not observed in the presence of gelatin-binding proteins with or without EDTA (Fig. 3C). As a positive control, the previously reported cleavage of galectin-3 was confirmed with recombinant MMP-2 and -9 (Supplementary Fig. 1).
Galectin-3 is cleaved by a chymotrypsin-like protease in seminal plasma
The galectin-3 cleavage assay was performed with seminal plasma in the presence of increasing concentrations of PMSF and benzamidine. Seventy-five to 95% inhibition of galectin-3 cleavage was observed over a range of 1 to 20 mM PMSF (Fig. 4A). Inhibition of galectin-3 cleavage was not observed with isopropanol alone, which was used as the solvent for PMSF (data not shown). Benzamidine had no observable inhibitory effect on galectin-3 cleavage (Fig. 4B).
Figure 4.
Galectin-3 is cleaved by a chymotrypsin-like serine protease in human seminal plasma. A, B: The galectin-3 cleavage assay was performed with 400 μg seminal plasma proteins in the presence of increasing concentrations of the serine protease inhibitors PMSF (A) and benzamidine (B). Percent cleavage of galectin-3 was plotted against inhibitor concentration. C: Biotinylated proteins were purified by monomeric avidin column chromatography following cleavage with seminal plasma, and the pooled purified fractions were analyzed by silver (upper left panel) and Ponceau S staining (upper right panel). The N-terminal end of the ~16 kDa fragment was sequenced by Edman degradation. The identified peptide is underlined (lower panel) in the linker region of the galectin-3 sequence. Reported MMP-2 and MMP-9 cleavage sites are represented by an*. D: The arrow represents the identified cleavage site in the galectin-3 sequence. The P1 residue was identified as Y107 and the P1′ residue as G108.
To identify the cleavage site in recombinant galectin-3 recognized by the seminal plasma protease(s), biotinylated galectin-3 was incubated with seminal plasma, purified by avidin affinity chromatography, and subjected to SDS-PAGE. Total protein staining with silver and electroblot staining with Ponceau S identified a predominant band of ~16 kDa (Fig. 4C). Silver staining also identified faint bands at ~30 kDa, ~60 kDa, and ~90 kDa. The ten N-terminal amino acids of the ~16 kDa Ponceau S stained protein band were determined by Edman degradation as GAPAGPLIVP corresponding to amino acid residues 108–117 of the full length galectin-3 polypeptide sequence (Fig. 4C). Based on the identified N-terminal amino acid residues of the cleaved galectin-3, the enzyme cleavage site for the seminal plasma protease(s) was determined to be between the Y107 (P1) and G108 (P1′) residues (Fig. 4D) of the galectin-3 protein.
N-terminal microsequence analysis of the endogenous galectin-3 CRD in seminal plasma
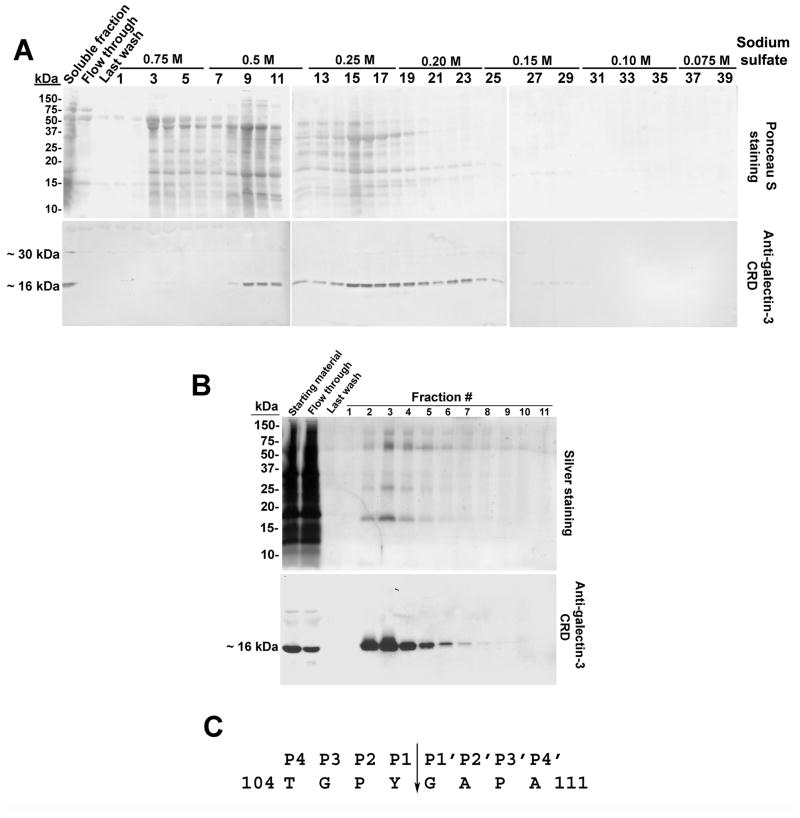
To enrich for the endogenous galectin-3 CRD fragment, seminal plasma proteins were subjected to ammonium sulfate precipitation followed by T-gel column chromatography. Total protein staining of electroblots identified a complex series of proteins eluted with decreasing concentrations of Na2SO4 (Fig. 5A). Immunoblot analysis with anti-galectin-3 CRD polyclonal antibodies identified a predominant immunoreactive band of ~16 kDa in fractions 8–28 (Fig. 5A). Less intense immunoreactivity was also observed at ~30 kDa in fractions 14–19. Immunoreactive fractions were pooled and further enriched on immobilized lactose. Silver staining identified specific protein bands of ~16, ~30, ~60, and ~90 kDa in the lactose affinity-purified fractions (Fig. 5B). An ~16 kDa immunoreactive band was detected with the anti-galectin-3 CRD polyclonal antibodies in the starting material, flow through, and in eluted fractions 2 to 9 (Fig. 5B). Pooled fractions 2–9 were subjected to SDS-PAGE and electroblot staining with Ponceau S. Microsequence analysis of the Ponceau S stained ~16 kDa protein band by Edman degradation identified the ten N-terminal amino acids as GAPAGPLIVP corresponding to amino acid residues 108–117 of the full length galectin-3 polypeptide sequence indicating that seminal plasma galectin-3 is cleaved between Y107 (P1) and G108 (P1′) (Fig. 5C).
Figure 5.
Microsequence analysis of the N-terminus of the endogenous galectin-3 CRD in seminal plasma. A: The endogenous galectin-3 CRD in seminal plasma was enriched by T-gel column chromatography. Eluted fractions were subjected to electroblot analysis for total protein and for anti-galectin-3 CRD immunoreactivity. B: T-gel purified fractions exhibiting galectin-3 CRD immunoreactivity were pooled and subjected to lactose affinity column chromatography. Eluted fractions were analyzed by silver staining and by immunoblot with anti-galectin-3 CRD polyclonal antibodies. C: Thiophilic, lactose-binding protein fractions that exhibited anti-galectin-3 CRD immunoreactivity were pooled and the ~16 kDa protein band was microsequenced by Edman degradation. The arrow represents the identified cleavage site in the galectin-3 sequence. The P1 residue was identified as Y107 and the P1′ residue as G108.
Galectin-3 is a substrate for PSA
The galectin-3 cleavage assay was performed with commercially-purified PSA. Calculation of percent cleavage indicated that 75% of the galectin-3 was cleaved with the highest amount of PSA (500 ng) in the reaction mixture (Fig. 6A). PSA cleavage of galectin-3 was inhibited by PMSF, 1,10-phenanthroline and ZnCl2 and resistant to inhibition by benzamidine (Supplementary Fig. 2). The ~16 kDa biotinylated cleavage product generated by PSA proteolysis was affinity purified on immobilized avidin, and microsequence analysis identified the ten N-terminal amino acids as GAPAGPLIVP corresponding to amino acid residues 108–117 of the full length galectin-3 polypeptide sequence. These results indicated that PSA cleaved galectin-3 between Y107 (P1) and G108 (P1′) (Fig. 6B).
Figure 6.
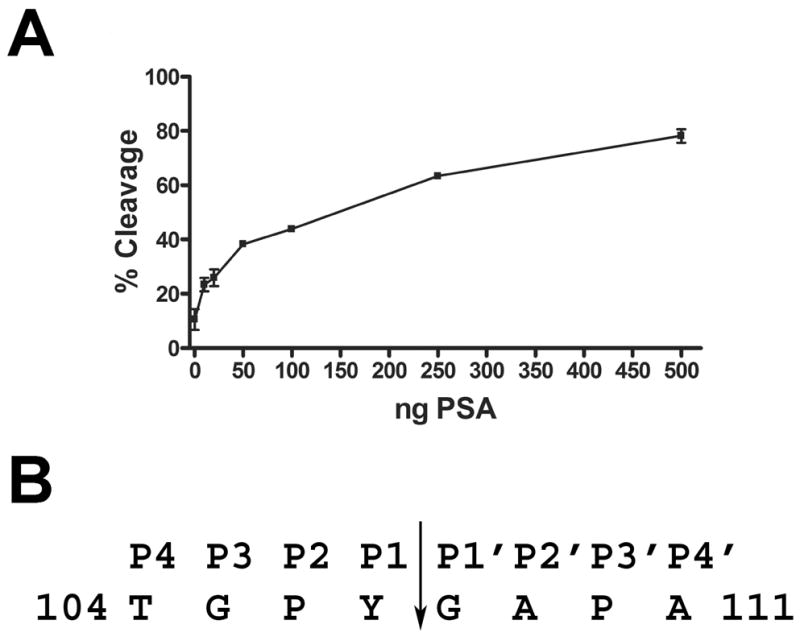
Galectin-3 is proteolytically cleaved by PSA. A: Biotinylated galectin-3 was incubated for 16 hours with increasing concentrations of PSA purified from seminal plasma. Percent cleavage of galectin-3 was plotted against increasing concentrations of PSA. B: The PSA-cleaved, biotinylated galectin-3 CRD fragment was purified by monomeric avidin column chromatography, and its N-terminus was microsequenced by Edman degradation. The arrow represents the identified cleavage site in the galectin-3 sequence. The P1 residue was identified as Y107 and the P1′ as G108.
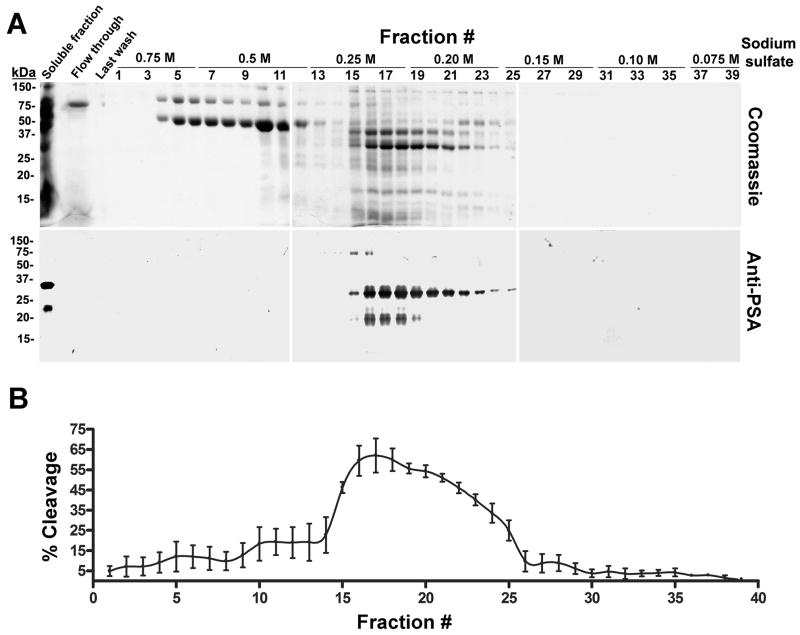
To confirm the proteolytic cleavage of galectin-3 by PSA, PSA was enriched from seminal plasma by T-gel column chromatography. Total protein staining of electroblots identified a complex series of proteins eluted with decreasing concentration of Na2SO4 from the T-gel column (Fig. 7A). Anti-PSA immunoreactivity (~60 kDa, ~30 kDa, and ~22 kDa) was detected in the starting material and fractions 15–25 (Fig. 7A). A broad peak of proteolytic activity was identified in purified fractions 15–25. Galectin-3 cleavage activity peaked (~65%) in T-gel fractions 16–18. Thus, the galectin-3 cleavage activity in seminal plasma co-eluted with anti-PSA immunoreactivity from the T-gel affinity column (Fig. 7B). Furthermore, immunodepletion of T-gel fraction #18 with an anti-PSA mAb and Protein G beads decreased the galectin-3 cleavage activity by ~75% as compared to pretreatment with Protein G beads alone (P < 0.005; Fig. 8). Irrelevant mouse IgG did not deplete galectin-3 cleavage activity (data not shown).
Figure 7.
PSA and the proteolytic activity that cleaves galectin-3 in seminal plasma exhibit identical thiophilic properties. A: Thiophilic proteins were purified from the soluble fraction of human seminal plasma and analyzed by Coomassie blue staining and immunoblot with anti-PSA polyclonal antibodies. B: The galectin-3 cleavage assay was performed for 16 hours with the purified fractions, and percent cleavage of galectin-3 was plotted against fraction numbers.
Figure 8.

Immunodepletion of galectin-3 cleavage activity with an anti-PSA mAb identified galectin-3 as a substrate for PSA in human seminal plasma. T-gel purified material that exhibited anti-PSA immunoreactivity and galectin-3 cleavage activity was immunodepleted with an anti-PSA mAb and tested in the galectin-3 cleavage assay using an incubation time of 16 hours (left panel). Non-immunodepleted material and protein G beads alone were used as positive and negative controls, respectively. Percent cleavage of galectin-3 was calculated for the immunodepleted T-gel purified fraction #18 and controls (right panel). Each bar represents the average of three independent experiments and the value represents the mean ± s.e. (* P < 0.005).
The galectin-3 CRD retains its carbohydrate binding activity after PSA cleavage
Biotinylated recombinant galectin-3 protein was cleaved with purified PSA, and cleavage products were purified by lactose affinity column chromatography. Electroblot analysis identified biotinylated bands of ~30 kDa and ~16 kDa corresponding to the intact and cleaved galectin-3 CRD, respectively, in purified fractions 2–8 (Fig. 9).
Figure 9.
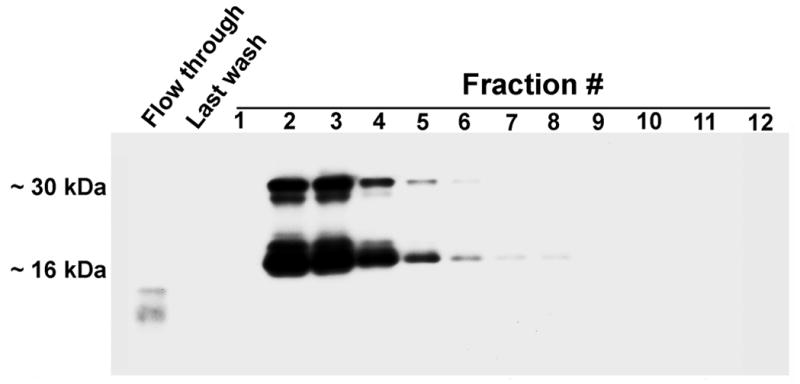
Galectin-3 cleaved by PSA retains its β-galactoside-binding activity. Biotinylated recombinant galectin-3 was cleaved with PSA for 16 hours and subjected to lactose affinity column chromatography. Eluted fractions were analyzed by electroblot analysis with streptavidin to identify biotinylated proteins with β-galactoside-binding activity.
DISCUSSION
Galectin-3 function is regulated by proteolytic processing that destroys galectin-3 multivalency while preserving its carbohydrate-binding activity. The functional relevance of galectin-3 cleavage has been indicated in immunomodulation, cancer, and pathogenic infection [1–3]. We previously identified galectin-3 in its intact form and in a truncated form containing the CRD in human prostasomes isolated from seminal plasma, suggesting that galectin-3 in semen is proteolytically cleaved [21]. In the current study, β-galactoside-binding proteins were purified from the soluble fraction of human seminal plasma, and the identification of galectin-3 immunoreactive protein bands at ~30 kDa and ~16 kDa indicated that intact galectin-3 and a truncated galectin-3 form containing a functional CRD are present in human seminal plasma. N-terminal microsequence analysis confirmed the identity of the ~16 kDa immunoreactive band as galectin-3 and identified the N-terminal amino acid as G108, indicating that galectin-3 was cleaved between Y107-G108 within the galectin-3 collagen-like linker region. Cleavage in the linker region indicates that the truncated galectin-3 form contains an intact CRD. Significantly, the galectin-3 linker region contains multiple cleavage sites for proteases that mediate galectin-3 function [8–12].
A galectin-3 cleavage assay was developed with biotinylated, recombinant galectin-3 to test the hypothesis that a protease activity(ies) that cleaves galectin-3 is present in human seminal plasma. The percentage of galectin-3 cleavage was found to increase with increasing concentration of seminal plasma proteins in the reaction mixture. Moreover, recombinant galectin-3 was cleaved between Y107-G108, the identical cleavage site identified in endogenous galectin-3. Collectively, these results suggested that the ~16 kDa truncated form of galectin-3 in seminal plasma results from proteolytic cleavage of the intact galectin-3 polypeptide between Y107-G108 in the collagen-like linker region.
Galectin-3 is known to be a proteolytic substrate for the gelatinolytic metalloproteases MMP-2 and -9 [11]. Gelatinolytic activity and MMP-2 and -9 immunoreactivity were previously identified in human seminal plasma [22,25]. Furthermore, MMP activity was detected on the surface of prostasomes isolated from seminal plasma, but the specific metalloprotease(s) involved was not determined [26]. In the current study, galectin-3 cleavage in seminal plasma was inhibited by the Zn2+/Fe2+ chelator 1,10-phenanthroline, which inhibits metalloprotease activity. To test the ability of gelatinases in seminal plasma to cleave galectin-3, gelatin-binding proteins were purified from seminal plasma. Fractions containing purified gelatin-binding proteins exhibited gelatinolytic activity and MMP-2 and -9 immunoreactivity. However, the purified gelatin-binding proteins did not cleave galectin-3, while commercially-purchased MMP-2 and -9 did cleave galectin-3. Galectin-3 cleavage activity was identified in the flow-through of the gelatin affinity column; the flow-through represented a sample of seminal plasma proteins depleted of gelatinase activity. Furthermore, galectin-3 cleavage in seminal plasma was inhibited by PMSF, which does not inhibit MMP-2 or -9 [27]. Galectin-3 cleavage was enhanced by EDTA and EGTA, which inhibit MMP-2 and -9. Moreover, MMP-2 and -9 reportedly cleave galectin-3 between A62-Y63 and G32-A33 residues [11,13] and not between Y107-G108, the cleavage site identified in this study. Although MMP-2 and -9 immunoreactivity can be demonstrated in seminal plasma, the gelatinases present in seminal plasma have not been definitively identified or characterized for their specific activities. Therefore, seminal plasma may contain an immunochemically cross-reactive metalloprotease(s) that does not cleave galectin-3. Nevertheless, our results indicate that gelatinases present in human seminal plasma are not responsible for the identified proteolytic cleavage of galectin-3 at Y107-G108.
Cleavage of galectin-3 after a tyrosine residue and inhibition of this cleavage by the serine protease inhibitor PMSF indicated that galectin-3 is cleaved by a chymotrypsin-like serine protease in seminal plasma. The proteolytic activity was not affected by benzamidine; however, benzamidine does not inhibit all serine proteases as it is a reversible inhibitor that binds through weak, non-covalent interactions. Galectin-3 cleavage in seminal plasma was also inhibited in the presence of ZnCl2, suggesting that the serine protease activity is regulated by the divalent zinc cation. Furthermore, galectin-3 cleavage in seminal plasma was inhibited by 1,10-phenanthroline and enhanced by the divalent ion chelators EDTA and EGTA. Significantly, PSA is a chymotrypsin-like serine protease in seminal plasma with an activity that is inhibited by ZnCl2, PMSF and 1,10-phenanthroline and unaffected by benzamidine [28,29]. EDTA has been shown to reverse the inhibition of PSA activity by Zn2+ in seminal plasma. Furthermore, in this study PSA was found to cleave galectin-3 between Y107-G108, which is the identical site in galectin-3 that is cleaved in seminal plasma. To confirm galectin-3 as a substrate for PSA, PSA was enriched from seminal plasma by T-gel column chromatography, and PSA immunoreactivity was found to co-elute with galectin-3 cleavage activity. Immunodepletion of the PSA-enriched material with a specific anti-PSA mAb significantly decreased the ability to generate the ~16 kDa galectin-3 cleavage product.
Collectively, our results indicate that galectin-3 is a proteolytic substrate for PSA. Although PSA may not be the only protease in semen that cleaves galectin-3, the results demonstrate that the predominant galectin-3 cleavage product in human seminal plasma is generated by PSA proteolysis. Thus, PSA likely represents the major proteolytic activity for galectin-3 cleavage in seminal plasma. To the best of our knowledge, this is the first report that demonstrates galectin-3 as a substrate for PSA in human seminal plasma, specifically, and as a substrate for a chymotrypsin-like serine protease, in general.
The human PSA glycoprotein, also designated as kallikrein 3, is a chymotrypsin-like serine protease that is secreted by the prostate into seminal plasma during ejaculation [23]. PSA cleaves the proteins that form the semen coagulum to promote semen liquefaction and facilitate the release of spermatozoa in the female genital tract. The known substrates of PSA in the semen coagulum include the seminogelins and fibronectin [30]. The results discussed herein now add galectin-3 to this list. Furthermore, the known interaction of galectin-3 with fibronectin [31] and the identification of galectin-3 as a substrate for PSA suggest that galectin-3 is involved in the formation of the semen coagulum. The conserved lectin activity of the galectin-3 CRD indicates the functional significance of this cleavage and suggests that the truncated galectin-3 may have a physiological function in semen.
PSA and galectin-3 have each been indicated independently in the molecular mechanisms of prostate cancer. Although the study of PSA has focused on its use as a prostate cancer biomarker, a growing body of literature implicates a role for PSA cleavage of its substrates in multiple aspects of prostate cancer progression [32]. For example, PSA secreted by metastatic cells is implicated in the degradation of extracellular matrix proteins such as fibronectin and laminin during invasion. Proteolytic cleavage of insulin-like growth factor binding protein-3 and latent TGFβ by PSA are indicated in prostate cancer cell proliferation and detachment, respectively. PSA cleavage of PTH-related protein may contribute to bone remodeling in metastatic tumors. Furthermore, Yoshida et al. [33] suggested that PSA cleavage of urokinase-type plasminogen activator initiates a protease cascade involved in prostate cancer metastasis and invasion. Thus, it is tempting to speculate that PSA cleavage of galectin-3 is involved in prostate cancer progression.
Galectin-3 is involved in immunomodulation, apoptotic regulation, angiogenesis, and metastatic cell adhesion in multiple cancers including prostate cancer [5,34]. Significantly, Raz and co-workers demonstrated that the proteolytic cleavage of galectin-3 by MMP-9 in breast tissue and by an unidentified protease in the prostate was associated with local tumor progression [13,16]. Our results here identify PSA as a candidate protease for the observed galectin-3 cleavage in prostate tumors. The relevance of galectin-3 cleavage to prostate cancer progression remains to be determined.
CONCLUSIONS
Galectin-3 is a proteolytic substrate for PSA in human seminal plasma. We anticipate that the identification of galectin-3 as a PSA substrate will provide critical insight into the molecular mechanisms involved in prostate tumor progression, metastasis, and invasion. The results described herein will also lay the groundwork for future studies to better understand the role of galectin-3 and PSA in the normal physiology of human reproduction.
Supplementary Material
Acknowledgments
The authors wish to thank Anne K. Bowlin for her assistance in the production of the polyclonal anti-serum. This work was supported by NIH HD50540.
References
- 1.Dumic J, Dabelic S, Flogel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006;1760:616–635. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 2.Vasta GR. Roles of galectins in infection. Nat Rev Microbiol. 2009;7:424–438. doi: 10.1038/nrmicro2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabinovich GA, Toscano MA. Turning ‘sweet’ on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol. 2009;9:338–352. doi: 10.1038/nri2536. [DOI] [PubMed] [Google Scholar]
- 4.Bachhawat-Sikder K, Thomas CJ, Surolia A. Thermodynamic analysis of the binding of galactose and poly-N-acetyllactosamine derivatives to human galectin-3. FEBS Lett. 2001;500:75–79. doi: 10.1016/s0014-5793(01)02586-8. [DOI] [PubMed] [Google Scholar]
- 5.Ochieng J, Furtak V, Lukyanov P. Extracellular functions of galectin-3. Glycoconj J. 2004;19:527–535. doi: 10.1023/B:GLYC.0000014082.99675.2f. [DOI] [PubMed] [Google Scholar]
- 6.Zhuang T, Lee HS, Imperiali B, Prestegard JH. Structure determination of a Galectin-3-carbohydrate complex using paramagnetism-based NMR constraints. Protein Sci. 2008;17:1220–1231. doi: 10.1110/ps.034561.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmad N, Gabius HJ, Andre S, Kaltner H, Sabesan S, Roy R, Liu B, Macaluso F, Brewer CF. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J Biol Chem. 2004;279:10841–10847. doi: 10.1074/jbc.M312834200. [DOI] [PubMed] [Google Scholar]
- 8.Guevremont M, Martel-Pelletier J, Boileau C, Liu FT, Richard M, Fernandes JC, Pelletier JP, Reboul P. Galectin-3 surface expression on human adult chondrocytes: a potential substrate for collagenase-3. Ann Rheum Dis. 2004;63:636–643. doi: 10.1136/ard.2003.007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karlsson A, Follin P, Leffler H, Dahlgren C. Galectin-3 activates the NADPH-oxidase in exudated but not peripheral blood neutrophils. Blood. 1998;91:3430–3438. [PubMed] [Google Scholar]
- 10.Ochieng J, Green B, Evans S, James O, Warfield P. Modulation of the biological functions of galectin-3 by matrix metalloproteinases. Biochim Biophys Acta. 1998;1379:97–106. doi: 10.1016/s0304-4165(97)00086-x. [DOI] [PubMed] [Google Scholar]
- 11.Ochieng J, Fridman R, Nangia-Makker P, Kleiner DE, Liotta LA, Stetler-Stevenson WG, Raz A. Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and -9. Biochemistry. 1994;33:14109–14114. doi: 10.1021/bi00251a020. [DOI] [PubMed] [Google Scholar]
- 12.Pelletier I, Sato S. Specific recognition and cleavage of galectin-3 by Leishmania major through species-specific polygalactose epitope. J Biol Chem. 2002;277:17663–17670. doi: 10.1074/jbc.M201562200. [DOI] [PubMed] [Google Scholar]
- 13.Nangia-Makker P, Raz T, Tait L, Hogan V, Fridman R, Raz A. Galectin-3 cleavage: a novel surrogate marker for matrix metalloproteinase activity in growing breast cancers. Cancer Res. 2007;67:11760–11768. doi: 10.1158/0008-5472.CAN-07-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balan V, Nangia-Makker P, Schwartz AG, Jung YS, Tait L, Hogan V, Raz T, Wang Y, Yang ZQ, Wu GS, Guo Y, Li H, Abrams J, Couch FJ, Lingle WL, Lloyd RV, Ethier SP, Tainsky MA, Raz A. Racial disparity in breast cancer and functional germ line mutation in galectin-3 (rs4644): a pilot study. Cancer Res. 2008;68:10045–10050. doi: 10.1158/0008-5472.CAN-08-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lotz MM, Andrews CW, Jr, Korzelius CA, Lee EC, Steele GD, Jr, Clarke A, Mercurio AM. Decreased expression of Mac-2 (carbohydrate binding protein 35) and loss of its nuclear localization are associated with the neoplastic progression of colon carcinoma. Proc Natl Acad Sci U S A. 1993;90:3466–3470. doi: 10.1073/pnas.90.8.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Nangia-Makker P, Tait L, Balan V, Hogan V, Pienta KJ, Raz A. Regulation of Prostate Cancer Progression by Galectin-3. Am J Pathol. 2009 doi: 10.2353/ajpath.2009.080816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nieminen J, St-Pierre C, Sato S. Galectin-3 interacts with naive and primed neutrophils, inducing innate immune responses. J Leukoc Biol. 2005;78:1127–1135. doi: 10.1189/jlb.1204702. [DOI] [PubMed] [Google Scholar]
- 18.Wollina U, Schreiber G, Gornig M, Feldrappe S, Burchert M, Gabius HJ. Sertoli cell expression of galectin-1 and -3 and accessible binding sites in normal human testis and Sertoli cell only-syndrome. Histol Histopathol. 1999;14:779–784. doi: 10.14670/HH-14.779. [DOI] [PubMed] [Google Scholar]
- 19.Pacis RA, Pilat MJ, Pienta KJ, Wojno K, Raz A, Hogan V, Cooper CR. Decreased galectin-3 expression in prostate cancer. Prostate. 2000;44:118–123. doi: 10.1002/1097-0045(20000701)44:2<118::aid-pros4>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 20.Ellerhorst J, Troncoso P, Xu XC, Lee J, Lotan R. Galectin-1 and galectin-3 expression in human prostate tissue and prostate cancer. Urol Res. 1999;27:362–367. doi: 10.1007/s002400050164. [DOI] [PubMed] [Google Scholar]
- 21.Jones JL, Saraswati S, Block AS, Lichti CF, Mahadevan M, Diekman AB. Galectin-3 is associated with prostasomes in human semen. Glycoconjugate Journal. 2009 doi: 10.1007/s10719-009-9262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimokawa K, Katayama M, Matsuda Y, Takahashi H, Hara I, Sato H. Complexes of gelatinases and tissue inhibitor of metalloproteinases in human seminal plasma. J Androl. 2003;24:73–77. [PubMed] [Google Scholar]
- 23.Veveris-Lowe TL, Kruger SJ, Walsh T, Gardiner RA, Clements JA. Seminal fluid characterization for male fertility and prostate cancer: kallikrein-related serine proteases and whole proteome approaches. Semin Thromb Hemost. 2007;33:87–99. doi: 10.1055/s-2006-958467. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Baumgart E, Lenk SV, Loening SA, Jung K. Quantitative differences in matrix metalloproteinase (MMP)-2, but not in MMP-9, tissue inhibitor of metalloproteinase (TIMP)-1 or TIMP-2, in seminal plasma of normozoospermic and azoospermic patients. Hum Reprod. 2002;17:2919–2923. doi: 10.1093/humrep/17.11.2919. [DOI] [PubMed] [Google Scholar]
- 26.Bellezza I, Aisa MC, Palazzo R, Costanzi E, Mearini E, Minelli A. Extracellular matrix degrading enzymes at the prostasome surface. Prostate Cancer Prostatic Dis. 2005;8:344–348. doi: 10.1038/sj.pcan.4500828. [DOI] [PubMed] [Google Scholar]
- 27.Song L, Porter DG, Coomber BL. Production of gelatinases and tissue inhibitors of matrix metalloproteinases by equine ovarian stromal cells In vitro. Biol Reprod. 1999;60:1–7. doi: 10.1095/biolreprod60.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Cohen P, Graves HC, Peehl DM, Kamarei M, Giudice LC, Rosenfeld RG. Prostate-specific antigen (PSA) is an insulin-like growth factor binding protein-3 protease found in seminal plasma. J Clin Endocrinol Metab. 1992;75:1046–1053. doi: 10.1210/jcem.75.4.1383255. [DOI] [PubMed] [Google Scholar]
- 29.Robert M, Gibbs BF, Jacobson E, Gagnon C. Characterization of prostate-specific antigen proteolytic activity on its major physiological substrate, the sperm motility inhibitor precursor/semenogelin I. Biochemistry. 1997;36:3811–3819. doi: 10.1021/bi9626158. [DOI] [PubMed] [Google Scholar]
- 30.Lilja H. Structure, function, and regulation of the enzyme activity of prostate-specific antigen. World J Urol. 1993;11:188–191. doi: 10.1007/BF00185066. [DOI] [PubMed] [Google Scholar]
- 31.Ochieng J, Leite-Browning ML, Warfield P. Regulation of cellular adhesion to extracellular matrix proteins by galectin-3. Biochem Biophys Res Commun. 1998;246:788–791. doi: 10.1006/bbrc.1998.8708. [DOI] [PubMed] [Google Scholar]
- 32.Williams SA, Singh P, Isaacs JT, Denmeade SR. Does PSA play a role as a promoting agent during the initiation and/or progression of prostate cancer? Prostate. 2007;67:312–329. doi: 10.1002/pros.20531. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida E, Ohmura S, Sugiki M, Maruyama M, Mihara H. Prostate-specific antigen activates single-chain urokinase-type plasminogen activator. Int J Cancer. 1995;63:863–865. doi: 10.1002/ijc.2910630618. [DOI] [PubMed] [Google Scholar]
- 34.Califice S, Castronovo V, Van Den Brule F. Galectin-3 and cancer (Review) Int J Oncol. 2004;25:983–992. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.