Abstract
Several reports indicate that the activity of the hypothalamic–pituitary–adrenal axis (HPA) is increased after a brain insult and that its down-regulation can improve detrimental outcomes associated with ischemic brain injuries. Granulocyte-colony stimulating factor (G-CSF) is a neuroprotective drug shown in the naïve rat to regulate hormones of the HPA axis. In this study we investigate whether G-CSF confers its neuroprotective properties by influencing the HPA response after neonatal hypoxia–ischemia (HI). Following the Rice–Vannucci model, seven day old rats (P7) were subjected to unilateral carotid ligation followed by 2.5 h of hypoxia. To test our hypothesis, metyrapone was administered to inhibit the release of rodent specific glucocorticoid, corticosterone, at the adrenal level. Dexamethasone, a synthetic glucocorticoid, was administered to agonize the effects of corticosterone. Our results show that both G-CSF and metyrapone significantly reduced infarct volume while dexamethasone treatment did not reduce infarct size even when combined with G-CSF. The protective effects of G-CSF do not include blood brain barrier preservation as suggested by the brain edema results. G-CSF did not affect the pituitary released adrenocorticotropic hormone (ACTH) levels in the blood plasma at 4 h, but suppressed the increase of corticosterone in the blood. The administration of G-CSF and metyrapone increased weight gain, and significantly reduced the Bax/Bcl-2 ratio in the brain while dexamethasone reversed the effects of G-CSF. The combination of G-CSF and metyrapone significantly decreased caspase-3 protein levels in the brain, and the effect was antagonized by dexamethasone. We report that G-CSF is neuroprotective in neonatal HI by reducing infarct volume, by suppressing the HI-induced increase of the Bax/Bcl-2 ratio, and by decreasing corticosterone in the blood. Metyrapone was able to confer similar neuroprotection as G-CSF while dexamethasone reversed the effects of G-CSF. In conclusion, we show that decreasing HPA axis activity is neuroprotective after neonatal HI, which can be conferred by administering G-CSF.
Keywords: Hypoxia–ischemia, G-CSF, HPA axis, Dexamethasone, Metyrapone, Caspase-3, Bax, Bcl-2
Introduction
Neonatal hypoxia ischemia (HI) remains the leading cause of perinatal brain injury, which ultimately leads to cerebral palsy, mental retardation, and epilepsy (Vannucci et al., 1999). It is a major public health concern with a prevalence of 60% in preterm infants and an incidence of 1–8 cases per 1000 births (Vannucci, 2000). In spite of its critical mortality and morbidity rate, current therapeutic avenues are still lacking; thus necessitating alternative strategies to amplify current therapeutic potential.
Several reports suggest that the activity of the hypothalamic–pituitary adrenal (HPA) axis is increased after HI, and that its down-regulation can reduce brain damage (Krugers et al., 1998, 2000). HPA axis activation involves the upregulation of the adrenocorticotropic hormone (ACTH) and glucocorticoids. ACTH is released from the pituitary gland in the blood stream where it acts on the adrenal glands to induce synthesis and release of glucocorticoids. Glucocorticoids are reported to play an important role in neurological function (Dumas et al., 2010; Sorrells et al., 2009), and neuronal damage in the hippocampus after ischemic insults (Roy and Sapolsky, 2003). Studies have shown that supraphysiological levels of glucocorticoids can exacerbate excitotoxic effects (Stein-Behrens et al., 1992), elevate levels of reactive oxygen species (McIntosh and Sapolsky, 1996), and increase neuroinflammation and apoptosis (Kuschinsky and Gillardon, 2000; MacPherson et al., 2005). This is of clinical relevance, since synthetic glucocorticoids are often administered systemically in premature infants (Thébaud et al., 2001). Clinical studies have shown that premature infants treated with synthetic glucocorticoids have reduced cerebral cortical gray matter volume (Murphy et al., 2001), and impaired long-term neuromotor and cognitive function (Yeh et al., 2004). Accordingly, targeting the HI induced elevation of glucocorticoids may be a promising therapeutic target.
A promising drug candidate, granulocyte-colony stimulating factor (G-CSF), is a glycoprotein, and a growth factor known to confer neuroprotection in various models of brain injury (Popa-Wagner et al., 2010; Yata et al., 2007). It is currently in Phase II clinical trial for adult ischemic stroke, and well tolerated at high doses (Schäbitz et al., 2010). In various animal studies, G-CSF has been reported to have anti-apoptotic, anti-inflammatory, excitoprotective, and neurotrophic properties (Gibson et al., 2005; Konishi et al., 1993; Schäbitz et al., 2003; Solaroglu et al., 2006). Furthermore, it has shown promise in neonatal HI studies where it protected neurons from apoptosis, and improved long term neurobehavior outcome (Fathali et al., 2010; Yata et al., 2007). A variety of intracellular cascades activated by G-CSF decrease apoptotic marker caspase-3, Bax (Solaroglu et al., 2009); these intracellular cascades are also regulated by glucocorticoids.
One study has shown that G-CSF stimulates ACTH and glucocorticoids synthesis in adult naïve rats (Mucha et al., 2000). However this study administered G-CSF chronically and was conducted in adult naïve rats, which have been reported to have a different HPA response and diurnal rhythm than neonates (Hindmarsh et al., 1989; Levine, 1994). In light of what this study highlights, it is probable that G-CSF may have differing activity on the HPA hormones in neonates. Considering the dearth of studies investigating G-CSF on HPA hormones, let alone in a brain injury model, we propose to investigate the latter. Whether G-CSF confers its neuroprotective effects by influencing the HPA axis in neonatal HI has yet to be determined.
On the basis of these observations, we hypothesize that G-CSF attenuates apoptosis partially by regulating the activity of the HPA axis. Herein we chose to focus on the pituitary–adrenal response by measuring ACTH and rodent specific glucocorticoid, corticosterone. To test this hypothesis we used metyrapone, an inhibitor of glucocorticoid synthesis, which suppresses circulating levels corticosterone. Metyrapone reduces corticosterone production by inhibiting the 11-β hydroxylation of 11-deoxycorticosterone. In addition, we used dexamethasone, a synthetic glucocorticoid that agonizes the effects of circulating corticosterone. Here we investigate whether G-CSF reduces neuronal apoptosis partially by regulating the ACTH and corticosterone response following experimental neonatal HI.
Materials and methods
Animal model
The Institutional Animal Care and Use Committee of Loma Linda University approved all the experiments used in this study. A modified Rice–Vannucci model was used as previously described (Chen et al., 2009; Rice et al., 1981). In brief, Sprague–Dawley 7-day old rat pups (P7) underwent unilateral right common carotid ligation under isoflurane anesthesia at 0300 h. Because P7 rats do not have an established diurnal rhythm of plasma corticosterone or ACTH, (Allen and Kendall, 1967; Leal et al., 1999) we chose a time that was in the dark cycle consistent with the nocturnal activities of rats (Levin and Levine, 1975). For consistent hormonal results, the ordered animals were allowed 3 days to acclimate to the new facility and the surgeries were all conducted at the same time. After recovery for 1 h, the animals were placed in a hypoxic chamber submerged in a 37 °C water bath, subjected to 8% O2 balanced in N2 for 2.5 h. Sham animals underwent anesthesia and neck incision, the carotid was exposed without the ligation and was exposed to normoxic conditions. All P7 rats were returned to their mothers at the same time after hypoxic exposure.
Drug administration
A total of two hundred and fourteen animals were used in this study. Sixteen animals expired in the hypoxic chamber giving this study a mortality of 7.47%. The remaining one hundred and ninety-eight animals were randomly divided into the following groups: Sham (n=24), Vehicle (n=27), G-CSF 50 μg/kg (n=29), metyrapone 10 mg/kg (n=8), metyrapone 30 mg/kg (n=29), metyrapone 30 mg/kG + G-CSF (n=27), dexamethasone 0.1 mg/kg (n=7), dexamethasone 0.5 mg/kg (n=23), and dexamethasone 0.5 mg/kg + G-CSF (n=24). The drugs were administered subcutaneously in a total volume of 30 μL 1 h after hypoxia. The stock solution of G-CSF was a 300 μg/mL, the metyrapone stock solution was 100 mM, and the dexamethasone stock solution was 4 mg/mL. Appropriate dilutions for treatment were made in saline from these stock solutions based on the weight and quantity of animals that needed to be treated.
Blood and collection
Blood was sampled 3 h after drug administration and 24 h after HI. Since the drugs were administered 1 h after HI, and blood was measured 3 h after their administration, a total of 4 h will have elapsed after ischemia. Briefly the animals were deeply anesthetized with isoflurane, and the blood was collected via cardiac puncture with a 22-gauge needle. Blood was transferred in EDTA coated tubes, and centrifuged at 3000 rpm for 5 min. Blood plasma was immediately removed and stored at −80 °C until assayed (Mucha et al., 2000).
Infarct volume and body weight
At 24 h, brains were collected and infarct volume was determined with 2,3,5-triphenyltetrazoliumchloride monohydrate (TTC) (Sigma Aldrich, St-Louis, MO USA) staining and analyzed by Image J software as previously described (Zhou et al., 2009). Briefly, the brains were sectioned in 2 mm slices, incubated in 2% TTC solution for 5 min in the dark, washed in phosphate buffered saline (PBS), and fixed in 10% formaldehyde. The infarct volume was traced and analyzed with Image J Software (Version 1.43u; National Institutes of Health, Bethesda, MD, USA). The animals were weighed on a high precision balance before surgery and at 24 h immediately before being euthanized. The weight difference was calculated as (weight 24 h after HI — weight before surgery).
Brain water content
Pups were euthanized 24 h after HI and the brains were divided in three parts (ipsilateral and contralateral hemispheres, and cerebellum). Each part was immediately weighed (wet weight) on a high precision balance (Denver Instrument, sensitivity ± 0.001 g) and again after drying in a 100 °C oven for 24 h (dry weight) as previously described (Chen et al., 2008). The percentage was calculated as [(wet weight − dry weight)/wet weight]×100.
Hormone assay
ACTH was measured with a two-site enzyme-linked immunosorbent assay (ELISA) kit (MD Bioproducts, St.-Paul, MN, USA) following the manufacturer’s protocol. This assay used a goat polyclonal antibody to ACTH, and a mouse monoclonal antibody to ACTH that respectively bound the C-terminal (34–39) and the N-terminal of ACTH (1–24). The minimum detection limit of the assay was 0.22 pg/mL. Corticosterone was measured with an ELISA kit (Enzo Life Sciences, Plymouth Meeting, PA, USA) with a sensitivity of 27.0 pg/ml according to the manufacturer’s instructions.
Western blot
Animals were euthanized at 24 h after HI. The brain hemispheres were immediately collected and snap frozen in liquid nitrogen and stored at −80 °C until analyzed. Whole cell protein extracts were obtained from brain samples by homogenizing in RIPA lysis buffer (Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA) and centrifuging for 25 min in 4 °C at 14,000 g. Ten percent SDS-PAGE gels were used, and 50 μg of denatured protein extracts was loaded in each well. The gel was electrophoresed and transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). The membrane was blocked with 5% non-fat blocking grade milk (Bio-Rad) and probed with appropriate dilution of primary antibody overnight. The following primary antibodies used were: cleaved caspase-3 (Santa Cruz Biotechnology, 1: 1000), Bax (Cell Signaling Technology, Danvers, MA, USA, 1:1000), Bcl-2 (Cell Signaling Technology, 1:1000), and actin (Santa Cruz Biotechnology, 1:1000). After washing the membranes three times, they were probed with specie-specific secondary antibodies (Santa Cruz Biotechnology, Inc) at 1:1000 dilutions for 1 h at room temperature and visualized using ECL Plus, Chemiluminescence (GE Healthcare and Life Sciences, Piscataway, NJ). The optical densities of the bands were analyzed with Image J Software (Version 1.43u; National Institutes of Health, Bethesda, MD) and normalized to actin as the loading control.
Statistical analysis
The results are presented as the mean ± standard error mean (SEM). Statistical differences amongst groups were analyzed by using one-way analysis of variance (ANOVA) followed by the Tukey multiple comparison test (Graph Pad Prism Version 5.0 d). Probability value<0.05 was considered statistically significant.
Results
Determining the optimal dose of metyrapone and dexamethasone
The metyrapone clinical dose for adrenal insufficiency is 30 mg/ kg (Giordano et al., 2008) hence the starting dose of choice. We used two doses 10 mg/kg and 30 mg/kg to determine which dose would optimally reduce infarct volume compared to the control groups. Dexamethasone on the other hand has previously been studied in neonatal HI using the two following doses: 0.1 mg/kg and 0.5 mg/kg (Ikeda et al., 2002; Tuor et al., 1993b). However, these reports use dexamethasone as pretreatment, our study will use it as post-treatment. Our infarct volume results indicate that metyrapone reduces infarct volume and the effect is significant with 30 mg/kg (Fig. 1). The Vehicle group has a mean of 29.42% ± 3.40 and metyrapone 30 mg/kg significantly reduced the infarct percentage to 16.08% ± 2.57 (p<0.01 vs HI + Vehicle). The administration of dexamethasone did not significantly reduce infarct volume when compared to Vehicle; low dose (0.1 mg/kg) mean infarct percentage was 23.99%±1.25 and high dose (0.5 mg/ kg) was 27.69%±1.69 (Fig. 1). Dexamethasone 0.5 mg/kg produced a significantly larger infarct volume compared to that produced by metyrapone 30 mg/kg treatment (p<0.05, Fig. 1). Based on these results metyrapone 30 mg/kg and dexamethasone 0.5 mg/kg were considered as the appropriate dosages for all the following experiments and molecular studies. The F value for the ANOVA analysis conducted was 22.36.
Fig 1.
Dose response of metyrapone and dexamethasone. Representative TTC stained coronal brain section of the dose response analysis for metyrapone (MET) and dexamethasone (DEX) at 24 h. (*=p<0.01 vs Vehicle, #=p<0.05 vs MET 30 mg/kg, all HI groups p<0.01 vs Sham). Each line on the left hand side of the brain images demarks 1 mm.
Infarct volume and body weight after G-CSF, metyrapone and dexamethasone administration
The administration of G-CSF (50 μg/kg) 1 h after HI was able to significantly reduce infarct percentage at 24 h from 29.42%±3.40 to 14.70%±3.63 (p<0.05 vs. HI + Vehicle, Fig. 2A). Metyrapone (30 mg/ kg) and the metyrapone + G-CSF groups similarly conferred neuroprotection by significantly reducing infarct volume compared to the Vehicle group to 16.08%±2.57 and 15.08%±3.47 respectively (p<0.05 vs. HI + Vehicle). Furthermore, agonizing the HPA axis with dexamethasone (0.5 mg/kg) administration did not significantly affect infarct volume compared to the Vehicle group (27.69%±1.69 infarct percentage) and G-CSF lost its neuroprotective effect when co-administered with dexamethasone yielding an infarct percentage of 26.36%±2.78 (Fig. 2A). The F value for the ANOVA analysis conducted was 5.27.
Fig 2.
Infarct volume and body weight at 24 h after G-CSF, metyrapone and dexamethasone administration. A. TTC stained coronal brain section of Sham, and G-CSF, MET, DEX treated groups after HI. (*=p<0.05 vs Vehicle, #=p<0.05 vs G-CSF, all HI groups p<0.01 vs Sham). B. Body weight gain was measured on a high precision balance scale and calculated as such: (Weight 24 h post-HI)−(Weight before HI). (*=p<0.01 vs Vehicle, #=p<0.05, **=p<0.001 vs Sham, ##=p<0.01 vs MET + G-CSF). Each line on the left hand side of the brain images demarks 1 mm.
Since a characteristic of HI injury is weight loss (Chen et al., 2011), the weight difference at 24 h after HI was measured. The sham animals gained a mean weight of 2.11 g±0.22 after 24 h while the HI Vehicle treated pups lost an average 0.78 g±0.42. The animals treated with G-CSF lost 0.63 g±0.49 and the group treated with metyrapone lost 1.07 g±0.26 which were not significantly different compared to the Vehicle treated group. Co-administration of G-CSF and metyrapone significantly reversed the weight loss compared to all the other HI groups, the animals gained 1.25 g±0.39 (P<0.01, Fig. 2B). The administration of dexamethasone significantly exacerbated weight loss compared to Vehicle treated group by losing a mean 2.73 g±0.23 (p<0.01, Fig. 2B). Even when co-administered with G-CSF, the animals lost an average of 2.135±0.42 (p<0.01, Fig. 2B). All the groups that underwent HI significantly lost weight compared to the Sham group (p<0.001) except for the G-CSF + metyrapone treated group. The F value for the ANOVA analysis conducted was 20.11.
Measuring the effect of G-CSF, metyrapone and dexamethasone on brain edema after HI
Brain edema was assessed by measuring brain water content, which was elevated in the ipsilateral hemisphere of the Vehicle treated to 89.47%±0.407 group compared to the Sham mean 87.66%±0.08 (p<0.05, Fig. 3). G-CSF treated group had a brain edema of 88.49%± 0.34, which was not significantly different compared to the Vehicle treated group. However, the brain water content of G-CSF treated animals was significantly different than the dexamethasone treated group whose brain water content averaged 90.50%±0.48 (p<0.01, Fig. 3). The dexamethasone treated group also had elevated brain edema when compared to Sham (87.66%±0.08, p<0.01, Fig. 3). Dexamethasone increased brain water content even when co-administered with G-CSF; the average brain water content measured was 90.08%±0.61 (p<0.05 vs. Sham) but was not significantly different to the Vehicle treated group. The administration of metyrapone alone or in combination with G-CSF did not significantly alter brain water content compared to all the groups. The F value for the ANOVA analysis conducted for the ipsilateral hemisphere was 3.97. No significant changes in the brain water content amongst all the groups were observed in the contralateral hemisphere and the cerebellum (Fig. 3).
Fig 3.
Brain water content after G-CSF, metyrapone, and dexamethasone treatment after HI. Quantification of brain water content 24 h after HI in the cerebellum, contra-lateral, and ipsilateral brain hemisphere. Brain water content was markedly increased by HI + Vehicle, DEX and DEX + G-CSF treated groups. (*=p<0.05). No significance was observed in G-CSF, MET and MET + G-CSF. DEX treated group had higher brain water content than the G-CSF treated group (##=p<0.05 vs G-CSF). No significant difference was observed amongst the groups in the cerebellum and contralateral hemisphere.
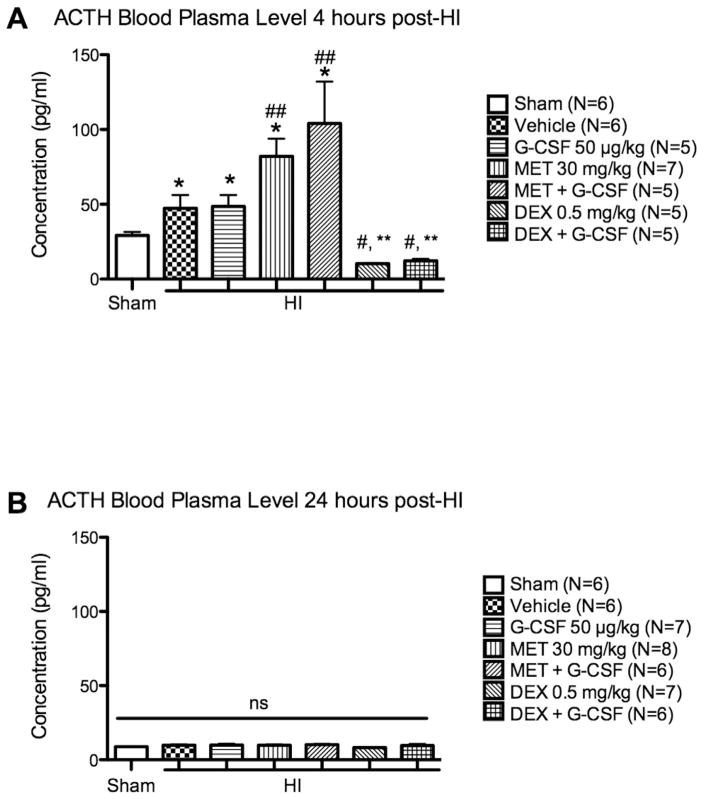
ACTH blood plasma levels 4 h and 24 h after HI
The ACTH level in the blood was increased after HI at 4 h from 24.14 pg/mL±2.42 as seen in Sham to 47.36 pg/mL±8.83 (p<0.05 vs. Sham, Fig. 4A). Administering G-CSF did not influence ACTH levels compared to Vehicle; 48.61 pg/ml±7.63 (Fig. 4A). The administration of metyrapone increased ACTH levels to 82.02 pg/mL±11.93, even when co-administered with G-CSF to 111.5 pg/mL±22.93 (p<0.05 vs. HI + Vehicle, Fig. 4A). Dexamethasone significantly reduced ACTH levels to 10.16 pg/mL ± 0.32, and the combination of dexamethasone with G-CSF dropped the levels to 12.22 pg/mL ± 1.24 compared to the metyrapone and G-CSF + metyrapone treated groups (p<0.01, Fig. 4A). The interaction of metyrapone and dexamethasone with the negative feedback mechanism of the HPA axis is what caused the significant increase and drop of ACTH levels. The F value for the ANOVA analysis conducted was 10.84. At 24 h, no significant differences were observed amongst all the groups (Fig. 4B).
Fig 4.
ACTH blood plasma level 4 h and 24 h after HI. A. At 4 h, ACTH levels were significantly increased by HI (*=p<0.05 vs Sham). MET and MET + G-CSF groups had markedly increased ACTH level compared to Vehicle (##=p<0.05). DEX and DEX + G-CSF had significantly decreased ACTH levels compared to MET and MET + G-CSF groups (#=p<0.01 vs MET 30 mg/kg, **=p<0.01 vs MET + G-CSF). B. ACTH blood plasma level is normalized to Sham at 24 h after HI.
Corticosterone blood plasma levels 4 h and 24 h after HI
The levels of corticosterone in the blood significantly increased at 4 h after HI from the Sham value of 2.97×103 pg/mL ± 1323 to 33.63×103 pg/mL ± 2943 (p<0.05 vs. Sham), and the administration of G-CSF reduced corticosterone to 9.65×103 pg/mL ± 3479 (p<0.01 vs. HI + Vehicle, Fig. 5A). Metyrapone did not significantly reduce corticosterone levels at 4 h, but the co-administration with G-CSF significantly reduced it to 26.07×103 pg/mL ± 2454 (p<0.05 vs. HI + Vehicle). The administration of dexamethasone alone or in combination with G-CSF increased corticosterone levels 4 h after HI. The dexamethasone + G-CSF treated group significantly increased the levels to 51.05×103 pg/mL ± 14,918 compared to Sham (p<0.05) and compared to G-CSF treated group (P<0.05). The F value for the ANOVA analysis conducted was 1.498. At 24 h, corticosterone levels decreased in all the groups. It must be noted that the groups that had G-CSF in their treatment regimen had lower corticosterone levels than their control groups (Fig. 5B), which is significant between the dexamethasone and dexamethasone + G-CSF treated group (p<0.05). The levels respectively dropped from 22.10×103 pg/mL ± 4895 in the dexamethasone group to 3.94×103 pg/mL ± 397.4. The F value for the ANOVA analysis conducted was 3.491.
Fig 5.
Corticosterone blood plasma level 4 h and 24 h after HI. A. HI considerably increases CORT levels and G-CSF robustly decreases CORT levels at 4 h (# = p<0.05 vs Sham, * = p <0.01 vs Vehicle). MET +G-CSF decreased CORT compared to Vehicle group while the DEX + G-CSF group increased CORT levels significantly (** = p <0.05). B. CORT levels remain higher in Vehicle and DEX treated groups (* = p <0.05 vs Sham). All groups with G-CSF in their regimen have lower CORT levels than their control group (# = p<0.05 vs DEX 0.5 mg/kg).
Apoptotic marker expression in the brain after treatment
The western blot results indicate that G-CSF reduced pro-apoptotic marker Bax (Fig. 6A), and increases Bcl-2 (Fig. 6B) in the ipsilateral hemisphere however the data did not yield statistical significance. HI induced an increase in the Bax/Bcl-2 ratio (p<0.05); this ratio was significantly lowered by the treatment of G-CSF, metyrapone and the combination of metyrapone and G-CSF (p<0.05) (Fig. 6C). The HI-induced increase of cleaved caspase-3 was significantly reduced by the combination of G-CSF and metyrapone treatment. Dexamethasone and dexamethasone + G-CSF co-treatment further increased cleaved caspase-3 levels (p<0.05 vs. Sham, p<0.05 vs. HI + MET+G-CSF).
Fig 6.
The expression of apoptotic markers in the ipsilateral hemisphere 24 h post-HI. A. HI increased Bax protein expression. No significant difference is reported amongst all groups. B. Bcl-2 relative density normalized to actin in decreased by HI but no significant difference is observed between groups. C. Bax/Bcl-2 ratio was significantly increased by HI (# = p<0.05 vs Sham). G-CSF, MET and MET + G-CSF treated groups significantly reduced Bax/Bcl-2 ratio compared to Vehicle treated groups (* = p<0.05). D. Cleaved caspase-3 levels are markedly increased by HI (# = p<0.05 vs Sham), and relatively lowered by G-CSF and MET groups (no significance observed). MET + G-CSF significantly decreased caspase-3 levels (* = p<0.05 vs Vehicle), and DEX + G-CSF group antagonized the effects (** = p<0.05 vs MET + G-CSF).
Discussion
In the present study, we show that targeting the elevation of corticosterone in the blood with G-CSF and metyrapone can protect the neonatal brain from HI injury, increase development, and reduce apoptosis. We also show that administering dexamethasone after HI impairs development, and worsen apoptosis. These results support the hypothesis that elevated corticosteroids are detrimental to the functioning and recovery of neurons after an insult (McIntosh and Sapolsky, 1996; Tombaugh and Sapolsky, 1992).
G-CSF reduced infarct volume 24 h after HI, and metyrapone similarly reduced infarct volume. When G-CSF was co-administered with metyrapone, no further reduction on infarct size was observed. As metyrapone could not potentiate the lowering effect of G-CSF on the infarct volume it may be probable that both G-CSF and metyrapone share a common mechanism of neuroprotective action. However this remains to be elucidated. We postulate that the combination of G-CSF + metyrapone treatment maximally reduced the progression of HI in the penumbra where milder injury is sustained as detected by TTC for that specific time point of 24 h. Because HI injury is more severe and necrotic at its core (Nakajima et al., 2000; Pulera et al., 1998), the necrotic cells are irreversibly damaged and are beyond rescue. The penumbra, the area surrounding the ischemic core, is not exposed to the same intensity of energy failure, these neurons are in a critical stage where they can be recovered. It is with that premise that combined therapy, may have maximally rescued the cells in the penumbra but could not reverse the damage caused at the core. Although no synergistic effect was observed in the infarct volume analysis, the animals treated with G-CSF and metyrapone gained weight in comparison to all the other groups that were subjected to HI.
When the activity of corticosterone was agonized with dexamethasone no difference in infarct volume was detected when compared to the Vehicle treated group. The protection observed with G-CSF treatment was completely lost when combined with dexamethasone. These results suggest that G-CSF may also exert its protection through lowering the corticosterone level. We understand that G-CSF has pleiotropic effects that are not limited to targeting the HPA axis, however our data show that dexamethasone obliterates the protective effect of G-CSF seen in infarct volume and apoptosis (Fig. 2, Fig. 6). Since glucocorticoids regulate glucose metabolism (Vinson, 2009), it is probable that the effect observed is due to the latter; particularly since glucose has been reported to influence brain injury outcomes (Hattori and Wasterlain, 1990; Tuor et al., 1993a). However, it has been reported that hyperglycemia associated with glucocorticoid treatment does not fully account for the effect of dexamethasone in neonatal rats (Tuor et al., 1993a). Additionally, there are previous studies showing that G-CSF does not influence blood glucose in a middle cerebral artery occlusion rat model (Schneider et al., 2005). Nevertheless, this remains to be verified.
Multiple reports suggest that dexamethasone treatment is neuroprotective in HI studies (Ekert et al., 1997; Felszeghy et al., 2004; Feng et al., 2011; Ikeda et al., 2005; Tuor and Del Bigio, 1996). In those reports, dexamethasone was administered before inducing experimental HI. Since most incidences of HI can occur in utero thus making the time occurrence difficult to detect (Perlman, 2006; Wörle and Versmold, 1984), the translatability of these findings is extremely difficult. Especially since pretreatment would imply indentifying children who are at risk for hypoxia ischemia, which can be a difficult task to assess (Butt et al., 2008; Perlman, 2006). Additionally, a long-term study reports that preterm infants treated with dexamethasone for bronchopulmonary dysplasia or chronic lung disease (Halliday et al., 2009) were at increased risk for cerebral palsy and adverse neurological effects, all of which are outcomes caused by HI (Vannucci, 2000; Volpe, 2001). Although pre-treatment of dexamethasone does reduce HI injury, and facilitates extubation, (Davis and Henderson-Smart, 2000; Tuor, 1995) it could be detrimental for proper neurological development in preterm infants. Our results show that dexamethasone administration after HI does not reduce infarct volume, and further exacerbates development as indicated by weight loss. If G-CSF was to be administered clinically in a preterm child, the administration of dexamethasone may antagonize its effects. This postulate would require human studies to be authenticated.
The occurrence of apoptosis prevails in the penumbra where milder injury has been sustained after the HI insult (Pulera et al., 1998). Apoptosis peaks from 24 to 72 h; since its occurrence is delayed it is an important and suitable target for treatment. Our results show that the expression of apoptotic markers is influenced by the hormones involved in the pituitary–adrenal response. The Bax/Bcl-2 ratio was significantly lowered by the administration of metyrapone alone and G-CSF. Additionally, the combined G-CSF + metyrapone treated group had significantly lower caspase-3 than G-CSF and metyrapone alone. These results suggest that the synergistic effect of both drugs could be attributed to other pathways all directed at lowering caspase-3. The lowering of caspase-3 by the combination of metyrapone and G-CSF is not reflective in the reduction of infarct volume. Markedly, we suggest that this is due to the irreversible necrotic core, which is beyond rescue. Therefore at 24 h it appears that the reduction of cell death as detected by TTC is maximally optimized. When dexamethasone was administered, higher expression of caspase-3 was observed, thus inferring that the progression of the disease is worsened by dexamethasone administration.
The pathophysiology of HI also involves an increased vascular permeability, which leads to brain edema. It is well documented clinically that the administration of corticosteroid can reduce brain edema (Betz and Coester, 1990; Heiss et al., 1996). The integrity of the blood brain barrier is usually compromised within hours of ischemic injuries (Zhang et al., 2000). Administering corticosteroid to target brain edema has been shown to reduce the pathological sequelae involved in brain swelling (Fishman, 1982). Our results however indicate that brain edema was not significantly affected compared to Vehicle when dexamethasone 0.5 mg/kg was administered. The contradiction of our results to what is typically observed clinically could be due to the inverted-U shape dose effect curve of corticosteroids (Baldi and Bucherelli, 2005). The non-linear effect of corticosteroids ought to be considered when interpreting our results. Understanding the exact dose response of dexamethasone and treatment in neonatal hypoxia ischemia therapy for brain edema is an area necessitating further studies. Also, whether targeting brain edema is sufficient to reduce neuronal cell death in neonates is another area granting further investigation. When it came to G-CSF and metyrapone, these drugs did not significantly reduce brain edema. The interpretation of this data suggests that their mechanism of action may not involve preserving blood brain barrier integrity.
It is clear that the pituitary–adrenal response is increased after HI, and that modulating its effects may prove beneficial in reducing infarct volume in the acute phase of injury. Considerable reports have shown that elevated plasma corticosterone can exacerbate neuronal damage in adult brain injury models of HI (Krugers et al., 2000; Stein and Sapolsky, 1988). Our results show that administering metyrapone and G-CSF reduced corticosterone levels in neonates after HI. It is important to note however that metyrapone alone did not significantly reduce corticosterone 3 h after it was administered. We account this incongruity to the time-point chosen, not to the efficacy of the drug. When considering the pharmacokinetic profile of the other drugs used in this study we chose 3 h after treatment as previously reported (Mucha et al., 2000). Correspondingly, 4 h after HI since treatment started 1 h after HI. With this approach, it is highly probable to overlook pertinent plasma changes that occur before 3 h and after 3 h of the drug administration. Nevertheless, it is clear that corticosterone inhibition occurred with metyrapone treatment otherwise the spike in ACTH levels indicating that the negative feedback was inhibited would not be observed (Fig. 4A). The administration of G-CSF reduced corticosterone levels more robustly at the 4 h time point compared to metyrapone (Fig. 5A). At 24 h, corticosterone levels decreased in all the groups. Metyrapone has a short half-life of 1.9 h±0.7, therefore no effect is expected at 24 h as previously reported (Krugers et al., 2000). All the animals that had G-CSF in their treatment regimen had lower corticosterone levels than their control group at 24 h; this was significant only between the dexamethasone and dexamethasone + G-CSF treated groups. What is perplexing is that ACTH levels were not affected by the administration of G-CSF. This implies that G-CSF signaling is able to either directly affect steroidogenesis at the adrenal level, or decrease the free corticosterone in the blood by increasing corticosteroid-binding protein in the blood. In the same token, G-CSF may also interact with ACTH modulation since the decrease in corticosterone did not interfere with the negative feedback mechanism. Unlike metyrapone, no spike in ACTH was observed in the G-CSF treated group (Fig. 4A).
It can be argued that G-CSF affects the pituitary–adrenal response as a by-standard effect of its capacity to reduce injury (Yata et al., 2007) and thus reducing the stress on the animal. However, we oppose this precept since G-CSF did not affect ACTH levels. Furthermore a previous report has shown that G-CSF can modulate the pituitary–adrenal response in naïve rats (Mucha et al., 2000). Although the study reported by Mucha and colleagues indicate a direct interaction between G-CSF, and the modulation of ACTH and corticosterone there are certain parameters that ought to be considered. Mucha and colleagues used naïve adult rats, and administered G-CSF daily for seven days before measuring the hormones (2000). The age, the pathophysiological state, and the time point of hormone measurement are factors that may explain why our results are opposite to what Mucha and colleagues observed (2000). This study used neonatal rats that underwent hypoxia–ischemia and only received one dose of G-CSF. The mechanism by which G-CSF affects corticosterone level in neonates after HI should be a new area of interest to further understand its application clinically.
We conclude that both G-CSF and metyrapone reduce infarct volume, HI-induced corticosterone elevation, pro-apoptotic markers and increase body weight. Additionally, the nature of G-CSF neuroprotection may involve corticosterone suppression in the blood. Lastly, we also demonstrate that administering dexamethasone after HI did not significantly reduce infarct volume, impaired development and exacerbated apoptosis.
Acknowledgments
This study was partially supported by grants NS060936 to Dr. Jiping Tang and NS054685 to Dr. John H. Zhang.
Abbreviations
- G-CSF
Granulocyte-colony stimulating factor
- MET
Metyrapone
- DEX
Dexamethasone
- ACTH
Adrenocorticotropic hormone
- HPA
Hypothalamic–pituitary adrenal
- Bcl-2
B cell lymphoma 2
- Bax
Bcl-2 associated protein X
References
- Allen C, Kendall JW. Maturation of the circadian rhythm of plasma corticosterone in the rat. Endocrinology. 1967 May;80(5):926–930. doi: 10.1210/endo-80-5-926. [DOI] [PubMed] [Google Scholar]
- Baldi E, Bucherelli C. The inverted “u-shaped” dose–effect relationships in learning and memory: modulation of arousal and consolidation. Nonlinearity Biol Toxicol Med. 2005 Jan;3(1):9–21. doi: 10.2201/nonlin.003.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz AL, Coester HC. Effect of steroids on edema and sodium uptake of the brain during focal ischemia in rats. Stroke. 1990 Aug;21(8):1199–1204. doi: 10.1161/01.str.21.8.1199. [DOI] [PubMed] [Google Scholar]
- Butt TK, Farooqui R, Khan MA. Risk factors for hypoxic ischemic encephalopathy in children. J Coll Physicians Surg Pak. 2008 Jul;18(7):428–432. [PubMed] [Google Scholar]
- Chen W, Jadhav V, Tang J, Zhang JH. HIF-1alpha inhibition ameliorates neonatal brain injury in a rat pup hypoxic–ischemic model. Neurobiol Dis. 2008 Sep;31(3):433–441. doi: 10.1016/j.nbd.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Hartman R, Ayer R, Marcantonio S, Kamper J, Tang J, Zhang JH. Matrix metalloproteinases inhibition provides neuroprotection against hypoxia-ischemia in the developing brain. J Neurochem. 2009 Nov;111(3):726–736. doi: 10.1111/j.1471-4159.2009.06362.x. [DOI] [PubMed] [Google Scholar]
- Chen W, Ma Q, Suzuki H, Hartman R, Tang J, Zhang JH. Osteopontin reduced hypoxia-ischemia neonatal brain injury by suppression of apoptosis in a rat pup model. Stroke. 2011 Mar;42(3):764–769. doi: 10.1161/STROKEAHA.110.599118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PG, Henderson-Smart DJ. Intravenous dexamethasone for extubation of newborn infants. Cochrane Database Syst Rev. 2000;(2):CD000308. doi: 10.1002/14651858.CD000308. Review. Update in: Cochrane Database Syst Rev. 2001;(4):CD000308. [DOI] [PubMed] [Google Scholar]
- Dumas TC, Gillette T, Ferguson D, Hamilton K, Sapolsky RM. Anti-glucocorticoid gene therapy reverses the impairing effects of elevated corticosterone on spatial memory, hippocampal neuronal excitability, and synaptic plasticity. J Neurosci. 2010 Feb 3;30(5):1712–1720. doi: 10.1523/JNEUROSCI.4402-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekert P, MacLusky N, Luo XP, Lehotay DC, Smith B, Post M, Tanswell AK. Dexamethasone prevents apoptosis in a neonatal rat model of hypoxic-ischemic encephalopathy (HIE) by a reactive oxygen species-independent mechanism. Brain Res. 1997 Jan 30;747(1):9–17. doi: 10.1016/s0006-8993(96)01201-2. [DOI] [PubMed] [Google Scholar]
- Fathali N, Lekic T, Zhang JH, Tang J. Long-term evaluation of granulocyte-colony stimulating factor on hypoxic–ischemic brain damage in infant rats. Intensive Care Med. 2010 Sep;36(9):1602–1608. doi: 10.1007/s00134-010-1913-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felszeghy K, Banisadr G, Rostène W, Nyakas C, Haour F. Dexamethasone down regulates chemokine receptor CXCR4 and exerts neuroprotection against hypoxia/ischemia-induced brain injury in neonatal rats. Neuroimmunomodulation. 2004;11 (6):404–413. doi: 10.1159/000080151. [DOI] [PubMed] [Google Scholar]
- Feng Y, Rhodes PG, Bhatt AJ. Dexamethasone pre-treatment protects brain against hypoxic-ischemic injury partially through up-regulation of vascular endothelial growth factor A in neonatal rats. Neuroscience. 2011 Apr 14;179:223–232. doi: 10.1016/j.neuroscience.2011.01.050. [DOI] [PubMed] [Google Scholar]
- Fishman RA. Steroids in the treatment of brain edema. N Engl J Med. 1982 Feb 11;306(6):359–360. doi: 10.1056/NEJM198202113060609. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Jones NC, Prior MJ, Bath PM, Murphy SP. G-CSF suppresses edema formation and reduces interleukin-1beta expression after cerebral ischemia in mice. J Neuropathol Exp Neurol. 2005 Sep;64(9):763–769. doi: 10.1097/01.jnen.0000179196.10032.dd. [DOI] [PubMed] [Google Scholar]
- Giordano R, Picu A, Bonelli L, Balbo M, Berardelli R, Marinazzo E, Corneli G, Ghigo E, Arvat E. Hypothalamus–pituitary–adrenal axis evaluation in patients with hypothalamo-pituitary disorders: comparison of different provocative tests. Clin Endocrinol (Oxf) 2008 Jun;68(6):935–941. doi: 10.1111/j.1365-2265.2007.03141.x. [DOI] [PubMed] [Google Scholar]
- Halliday HL, Ehrenkranz RA, Doyle LW. Early (<8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2009 Jan;21(1):CD001146. doi: 10.1002/14651858.CD001146.pub2. [DOI] [PubMed] [Google Scholar]
- Hattori H, Wasterlain CG. Posthypoxic glucose supplement reduces hypoxic–ischemic brain damage in the neonatal rat. Ann Neurol. 1990;28 (2):122–128. doi: 10.1002/ana.410280203. [DOI] [PubMed] [Google Scholar]
- Heiss JD, Papavassiliou E, Merrill MJ, Nieman L, Knightly JJ, Walbridge S, Edwards NA, Oldfield EH. Mechanism of dexamethasone suppression of brain tumor-associated vascular permeability in rats. Involvement of the glucocorticoid receptor and vascular permeability factor. J Clin Invest. 1996 Sep 15;98(6):1400–1408. doi: 10.1172/JCI118927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindmarsh KW, Tan L, Sankaran K, Laxdal VA. Diurnal rhythms of cortisol, ACTH, and beta-endorphin levels in neonates and adults. West J Med. 1989 Aug;151(2):153–156. [PMC free article] [PubMed] [Google Scholar]
- Ikeda T, Mishima K, Yoshikawa T, Iwasaki K, Fujiwara M, Xia YX, Ikenoue T. Dexamethasone prevents long-lasting learning impairment following neonatal hypoxic-ischemic brain insult in rats. Behav Brain Res. 2002 Oct 17;136(1):161–170. doi: 10.1016/s0166-4328(02)00107-9. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Mishima K, Aoo N, Liu AX, Egashira N, Iwasaki K, Fujiwara M, Ikenoue T. Dexamethasone prevents long-lasting learning impairment following a combination of lipopolysaccharide and hypoxia–ischemia in neonatal rats. Am J Obstet Gynecol. 2005 Mar;192(3):719–726. doi: 10.1016/j.ajog.2004.12.048. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Chui DH, Hirose H, Kunishita T, Tabira T. Trophic effect of erythropoietin and other hematopoietic factors on central cholinergic neurons in vitro and in vivo. Brain Res. 1993 Apr 23;609(1–2):29–35. doi: 10.1016/0006-8993(93)90850-m. [DOI] [PubMed] [Google Scholar]
- Krugers HJ, Kemper RH, Korf J, Ter Horst GJ, Knollema S. Metyrapone reduces rat brain damage and seizures after hypoxia–ischemia: an effect independent of modulation of plasma corticosterone levels? J Cereb Blood Flow Metab. 1998;18 (4):386–390. doi: 10.1097/00004647-199804000-00006. [DOI] [PubMed] [Google Scholar]
- Krugers HJ, Maslam S, Korf J, Joëls M, Holsboer F. The corticosterone synthesis inhibitor metyrapone prevents hypoxia/ischemia-induced loss of synaptic function in the rat hippocampus. Stroke. 2000;31 (5):1162–1172. doi: 10.1161/01.str.31.5.1162. [DOI] [PubMed] [Google Scholar]
- Kuschinsky W, Gillardon F. Apoptosis and cerebral ischemia. Cerebrovasc Dis. 2000 May-Jun;10(3):165–169. doi: 10.1159/000016052. [DOI] [PubMed] [Google Scholar]
- Leal AM, Carvalho J, Moreira AC. Ontogenetic diurnal variation of adrenal responsiveness to ACTH and stress in rats. Horm Res. 1999;52 (1):25–29. doi: 10.1159/000023428. [DOI] [PubMed] [Google Scholar]
- Levin R, Levine S. Development of circadian periodicity in base and stress levels of corticosterone. Am J Physiol. 1975 Nov;229(5):1397–1399. doi: 10.1152/ajplegacy.1975.229.5.1397. [DOI] [PubMed] [Google Scholar]
- Levine S. The ontogeny of the hypothalamic–pituitary–adrenal axis: the influence of maternal factors. Ann N Y Acad Sci. 1994;30 (746):289–293. doi: 10.1111/j.1749-6632.1994.tb39245.x. [DOI] [PubMed] [Google Scholar]
- MacPherson A, Dinkel K, Sapolsky R. Glucocorticoids worsen excitotoxin-induced expression of pro-inflammatory cytokines in hippocampal cultures. Exp Neurol. 2005 Aug;194(2):376–383. doi: 10.1016/j.expneurol.2005.02.021. [DOI] [PubMed] [Google Scholar]
- McIntosh LJ, Sapolsky RM. Glucocorticoids may enhance oxygen radical-mediated neurotoxicity. Neurotoxicology. 1996;17 (3–4):873–882. [PubMed] [Google Scholar]
- Mucha S, Zylińska K, Pisarek H, Komorowski J, Robak T, Korycka A, Stepień H. Pituitary–adrenocortical responses to the chronic administration of granulocyte colony-stimulating factor in rats. J Neuroimmunol. 2000 Jan 3;102(1):73–78. doi: 10.1016/s0165-5728(99)00143-5. [DOI] [PubMed] [Google Scholar]
- Murphy BP, Inder TE, Huppi PS, Warfield S, Zientara GP, Kikinis R, Jolesz FA, Volpe JJ. Impaired cerebral cortical gray matter growth after treatment with dexamethasone for neonatal chronic lung disease. Pediatrics. 2001 Feb;107(2):217–221. doi: 10.1542/peds.107.2.217. [DOI] [PubMed] [Google Scholar]
- Nakajima W, Ishida A, Lange MS, Gabrielson KL, Wilson MA, Martin LJ, Blue ME, Johnston MV. Apoptosis has a prolonged role in the neurodegeneration after hypoxic ischemia in the newborn rat. J Neurosci. 2000 Nov 1;20(21):7994–8004. doi: 10.1523/JNEUROSCI.20-21-07994.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman JM. Summary proceedings from the neurology group on hypoxic-ischemic encephalopathy. Pediatrics. 2006 Mar;117(3 Pt 2):S28–S33. doi: 10.1542/peds.2005-0620E. [DOI] [PubMed] [Google Scholar]
- Popa-Wagner A, Stöcker K, Balseanu AT, Rogalewski A, Diederich K, Minnerup J, Margaritescu C, Schäbitz WR. Effects of granulocyte-colony stimulating factor after stroke in aged rats. Stroke. 2010 May;41(5):1027–1031. doi: 10.1161/STROKEAHA.109.575621. [DOI] [PubMed] [Google Scholar]
- Pulera MR, Adams LM, Liu H, Santos DG, Nishimura RN, Yang F, Cole GM, Wasterlain CG. Apoptosis in a neonatal rat model of cerebral hypoxia-ischemia. Stroke. 1998 Dec;29(12):2622–2630. doi: 10.1161/01.str.29.12.2622. [DOI] [PubMed] [Google Scholar]
- Rice JE, III, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic–ischemic brain damage in the rat. Ann Neurol. 1981 Feb;9(2):131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- Roy M, Sapolsky RM. The exacerbation of hippocampalexcitotoxicity by glucocorticoids is not mediated by apoptosis. Neuroendocrinology. 2003;77 (1):24–31. doi: 10.1159/000068337. [DOI] [PubMed] [Google Scholar]
- Schäbitz WR, Kollmar R, Schwaninger M, Juettler E, Bardutzky J, Schölzke MN, Sommer C, Schwab S. Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke. 2003 Mar;34(3):745–751. doi: 10.1161/01.STR.0000057814.70180.17. [DOI] [PubMed] [Google Scholar]
- Schäbitz WR, Laage R, Vogt G, Koch W, Kollmar R, Schwab S, Schneider D, Hamann GF, Rosenkranz M, Veltkamp R, Fiebach JB, Hacke W, Grotta JC, Fisher M, Schneider A. AXIS: a trial of intravenous granulocyte colony-stimulating factor in acute ischemic stroke. Stroke. 2010 Nov;41(11):2545–2551. doi: 10.1161/STROKEAHA.110.579508. [DOI] [PubMed] [Google Scholar]
- Schneider A, Krüger C, Steigleder T, Weber D, Pitzer C, Laage R, Aronowski J, Maurer MH, Gassler N, Mier W, Hasselblatt M, Kollmar R, Schwab S, Sommer C, Bach A, Kuhn HG, Schäbitz WR. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005 Aug;115(8):2083–2098. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaroglu I, Tsubokawa T, Cahill J, Zhang JH. Anti-apoptotic effect of granulocyte-colony stimulating factor after focal cerebral ischemia in the rat. Neuroscience. 2006 Dec 28;143(4):965–974. doi: 10.1016/j.neuroscience.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaroglu I, Cahill J, Tsubokawa T, Beskonakli E, Zhang JH. Granulocyte colony-stimulating factor protects the brain against experimental stroke via inhibition of apoptosis and inflammation. Neurol Res. 2009 Mar;31(2):167–172. doi: 10.1179/174313209X393582. [DOI] [PubMed] [Google Scholar]
- Sorrells SF, Caso JR, Munhoz CD, Sapolsky RM. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron. 2009 Oct;64(1):33–39. doi: 10.1016/j.neuron.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BA, Sapolsky RM. Chemical adrenalectomy reduces hippocampal damage induced by kainic acid. Brain Res. 1988 Nov 8;473(1):175–180. doi: 10.1016/0006-8993(88)90332-0. [DOI] [PubMed] [Google Scholar]
- Stein-Behrens BA, Elliott EM, Miller CA, Schilling JW, Newcombe R, Sapolsky RM. Glucocorticoids exacerbate kainic acid-induced extracellular accumulation of excitatory amino acids in the rat hippocampus. J Neurochem. 1992;58 (5):1730–1735. doi: 10.1111/j.1471-4159.1992.tb10047.x. [DOI] [PubMed] [Google Scholar]
- Thébaud B, Lacaze-Masmonteil T, Watterberg K. Postnatal glucocorticoids in very preterm infants: “the good, the bad, and the ugly”? Pediatrics. 2001 Feb;107(2):413–415. doi: 10.1542/peds.107.2.413. [DOI] [PubMed] [Google Scholar]
- Tombaugh GC, Sapolsky RM. Corticosterone accelerates hypoxia- and cyanide-induced ATP loss in cultured hippocampalastrocytes. Brain Res. 1992 Aug 14;588(1):154–158. doi: 10.1016/0006-8993(92)91356-j. [DOI] [PubMed] [Google Scholar]
- Tuor UI. Dexamethasone and the prevention of neonatal hypoxic–ischemic brain damage. Ann N Y Acad Sci. 1995 Sep 15;765:179–195. doi: 10.1111/j.1749-6632.1995.tb16574.x. [DOI] [PubMed] [Google Scholar]
- Tuor UI, Del Bigio MR. Protection against hypoxic-ischemic damage with corticosterone and dexamethasone: inhibition of effect by a glucocorticoid antagonist RU38486. Brain Res. 1996 Dec 16;743(1–2):258–262. doi: 10.1016/s0006-8993(96)01054-2. [DOI] [PubMed] [Google Scholar]
- Tuor UI, Simone CS, Arellano R, Tanswell K, Post M. Glucocorticoid prevention of neonatal hypoxic–ischemic damage: role of hyperglycemia and antioxidant enzymes. Brain Res. 1993a Feb 26;604(1–2):165–172. doi: 10.1016/0006-8993(93)90364-s. [DOI] [PubMed] [Google Scholar]
- Tuor UI, Simone CS, Barks JD, Post M. Dexamethasone prevents cerebral infarction without affecting cerebral blood flow in neonatal rats. Stroke. 1993b Mar;24(3):452–457. doi: 10.1161/01.str.24.3.452. [DOI] [PubMed] [Google Scholar]
- Vannucci RC. Hypoxic–ischemic encephalopathy. Am J Perinatol. 2000;17 (3):113–120. doi: 10.1055/s-2000-9293. [DOI] [PubMed] [Google Scholar]
- Vannucci RC, Connor JR, Mauger DT, Palmer C, Smith MB, Towfighi J, Vannucci SJ. Rat model of perinatal hypoxic–ischemic brain damage. J Neurosci Res. 1999;55 (2):158–163. doi: 10.1002/(SICI)1097-4547(19990115)55:2<158::AID-JNR3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Vinson GP. The adrenal cortex and life. Mol Cell Endocrinol. 2009 Mar 5;300(1–2):2–6. doi: 10.1016/j.mce.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Perinatal brain injury: from pathogenesis to neuroprotection. Ment Retard Dev Disabil Res Rev. 2001;7 (1):56–64. doi: 10.1002/1098-2779(200102)7:1<56::AID-MRDD1008>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Wörle H, Versmold H. Hypoxic-ischemic encephalopathy. Clinical considerations Padiatr Padol. 1984;19 (4):355–364. [PubMed] [Google Scholar]
- Yata K, Matchett GA, Tsubokawa T, Tang J, Kanamaru K, Zhang JH. Granulocyte-colony stimulating factor inhibits apoptotic neuron loss after neonatal hypoxia–ischemia in rats. Brain Res. 2007 May 11;1145:227–238. doi: 10.1016/j.brainres.2007.01.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh TF, Lin YJ, Lin HC, Huang CC, Hsieh WS, Lin CH, Tsai CH. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med. 2004 Mar 25;350(13):1304–1313. doi: 10.1056/NEJMoa032089. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M. VEGF enhances angiogenesis and promotes blood–brain barrier leakage in the ischemic brain. J Clin Invest. 2000 Oct;106(7):829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Fathali N, Lekic T, Tang J, Zhang JH. Glibenclamide improves neurological function in neonatal hypoxia-ischemia in rats. Brain Res. 2009 May 13;1270:131–139. doi: 10.1016/j.brainres.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]