Abstract
Background
Inhalation of diesel exhaust induces vascular effects including impaired endothelial function and increased atherosclerosis.
Objective
To examine the in vivo effects of subchronic diesel exhaust exposure on endothelial cell transcriptional responses in the presence of hypercholesterolemia.
Methods
ApoE (−/−) and ApoE (+/+) mice inhaled diesel exhaust diluted to particulate matter levels of 300 or 1000 μg/m3 vs. filtered air. After 30 days, endothelial cells were harvested from dispersed aortic cells by fluorescent-activated cell sorting (FACS). Relative mRNA abundance was evaluated by microarray analysis to measure strain-specific transcriptional responses in mice exposed to dilute diesel exhaust vs. filtered air.
Results
Forty-nine transcripts were significantly dysregulated by >2.8-fold in the endothelium of ApoE (−/−) mice receiving diesel exhaust at 300 or 1000 μg/m3. These included transcripts with roles in plasminogen activation, endothelial permeability, inflammation, genomic stability, and atherosclerosis; similar responses were not observed in ApoE (+/+) mice.
Conclusions
The potentiation of diesel exhaust-related endothelial gene regulation by hypercholesterolemia helps to explain air pollution-induced vascular effects in animals and humans. The observed regulated transcripts implicate pathways important in the acceleration of atherosclerosis by air pollution.
Keywords: Diesel engine exhaust, endothelium, gene expression, microarray, apolipoprotein E, hypercholesterolemia, air pollution
Introduction
Air pollution contributes to cardiovascular disease, including atherosclerosis (Künzli et al. 2005), acute myocardial infarction (Peters et al. 2004), and vascular endothelial dysfunction (Mills et al. 2005). Traffic-related emissions, as a major contributor to ambient air contamination, have been identified as a likely driver of vascular effects (Hoffmann et al. 2007). Pathophysiology of the endothelium (including impaired vasoregulation and anticoagulation, as well as enhanced cellular adhesivity and permeability) is exacerbated by atherosclerosis, hypertension, and diabetes; emerging evidence now shows a contribution from exposure to air pollution as well (Brook 2008). The proximity of endothelial cells to circulating, inhaled toxicants underscores their potential susceptibility. However, demonstrating the effects of inhaled particulate matter (PM) on endothelial cells has been difficult, owing to the tiny volume occupied by the endothelium in any tissue.
The effects of air pollution on atherosclerosis have been explored in animal models of hypercholesterolemia. These include ApoE (−/−) and LDL (−/−) mice and Watanabe hyper-lipidemic rabbits, which display accelerated atherosclerosis upon exposure to air pollutants, including diesel engine particulates (Yatera et al. 2008), concentrated ambient air nanoparticles (CAPS) (Araujo et al. 2008; Ying et al. 2009). Pollution-related expression of adhesion molecules in the rabbit model reveals a clear effect on the systemic vascular endothelium (Yatera et al. 2008) and the responses of ApoE (−/−) mice to CAPS include dysfunctional endothelial physiology (Ying et al. 2009). Importantly, exposure to CAPS results in augmented atherogenesis in a murine model of hypercholesterolemia (ApoE [−/−]) compared with wild type (C57BL/6) (Sun et al. 2005).
The responses of cultured endothelial cells to specific model particulates have been assessed in microarray studies (Yamawaki and Iwai 2006). However, cultured endothelium exposed to various components of air pollution represents an oversimplified model, given the complex set of responses that are likely to influence the vasculature in vivo. It has been shown that ultrafine PM from the lung is translocated to other organs, including the endothelium (Furuyama et al. 2009). However, the lung also acts to modify and amplify the response to PM by the secondary release of humoral mediators (Arimoto et al. 2007) and by the promotion of oxidative (Li et al. 2008) and nitrosative (Rundell et al. 2008) stress. Furthermore, PM exposures are coincident with exposure to pollutant gases, such as carbon monoxide, oxides of nitrogen, and volatile organic compounds, all of which have differing uptake and disposition. With the growing recognition of a systemic vascular effect of inhaled pollutants, ascertaining endothelial-specific transcriptional responses to complex “real-world” air pollutant mixtures will be useful. Our analysis of freshly isolated endothelial cells from ApoE (−/−) mice exposed to engine exhaust in vivo is a robust approach to discerning authentic vascular responses to air pollution.
Materials
Animals
BL/6 ApoE (−/−) and Tie2 GFP mice (strain name B6.129P2-Apoetm1Unc/J [stock #002052] and Tg[TIE2GFP]287Sato [stock #003658], from Jackson Labs, Bar Harbor, ME) were interbred and genetically selected to obtain Tie2 GFP/ApoE (−/−) mice on an approximately 1:1 mixed genetic background of C57Bl/6 and FVB/N. All mice received standard chow. A cholesterol assay (Cayman Chemical, Ann Arbor, MI; cat. #10007640) was used according to the manufacturer’s instructions to measure total cholesterol in the sera of male mice of the strain Tie2 GFP/ApoE (−/−) and both parental strains Tg(TIE2GFP)287Sato and B6.129P2-Apoetm1Unc. Tie2 GFP and Tie2 GFP/ApoE (−/−) mice were designated as ApoE (+/+) and ApoE (−/−) in this study for simplicity of nomenclature.
Diesel engine exhaust exposures
Four-month-old male mice underwent unrestrained (whole body) inhalation exposure in their cages to diesel exhaust generated from a Yanmar Diesel engine under controlled conditions of load. Exhaust particulate concentrations in a flow-through exposure chamber (model H2000; Hazleton Systems, Maywood, NJ) were monitored continuously with a nephelometer (model 8200; TSI Inc., Shoreview, MN) calibrated against determinations made gravimetrically with a Pallflex filter (Pall Inc., New York, NY) and were adjusted to 300 and 1000 μg PM/m3 by dilution with HEPA-filtered air. NOx levels in these atmospheres were 12 and 30 ppm, respectively, and CO levels were 3 and 100 ppm, respectively. Greater detail regarding volatile organic compounds, other pollutants, and uncertainty ranges have been previously published (McDonald et al. 2004). Exposures were performed 4 h/day, 7 days/week for 4 weeks in both ApoE (−/−) and ApoE (+/+) strains; littermates exposed to HEPA-filtered air were designated as controls. Separate groups containing four experimental and four control mice of each strain were utilized for each dose of PM. Mice were euthanized and aortic samples from each group were pooled within 24 h of the last exposure for subsequent processing and analysis.
All studies were approved by the Animal Care and Use Committees of the University of Texas Southwestern, Dallas, TX (the previous institution of JGM and RVS), and the Lovelace Respiratory Research Institute, Albuquerque, NM.
Tissue collection and extraction
Animals were euthanized by CO2 asphyxiation. Aortae were excised by dissection and freed of adherent tissue from the iliac bifurcation to the aortic root. Luminal blood was rinsed and aortas were sliced into 1-mm segments. Segments pooled from four animals were suspended in 5 mL of Dulbecco’s phosphate-buffered saline (PBS) with 2 mg/mL of dextrose. The suspension was combined with 5 mL of prewarmed PBS containing 10 mg/mL type II collagenase (Worthington Biochemical, Lakewood, NJ), and 60 units/mL deoxyribonuclease I, agitated continuously at 37°C on a shaking platform, and triturated 10 times every 10 min for a total digestion period of 40 min to generate a single cell suspension. The cell suspension was maintained at 0–4°C throughout the remainder of the isolation, which lasted a total of 2–3 h. The suspension was then combined with 10 mL of 10% fetal bovine serum (FBS) in Dulbecco’s modified Eagle’s medium (DMEM) and cells were collected by centrifugation and resuspended in 10 mL of PBS followed by filtration through a sterile 40-μm mesh filter to remove undigested tissue fragments. Following centrifugation, the cellular pellet was resuspended in 0.3 mL PBS containing 0.5 mM ethylenediaminetetraacetic acid (EDTA), 30 U/mL deoxyribonuclease I, 3% FBS, and 2 mg/mL dextrose and once again filtered through a 40-μm mesh filter.
The resulting aortic cell suspensions were sorted using a MoFlo from Dako Cytomation (Carpinteria, CA). Cells were excited by a 488 nm laser and GFP signals collected at 510 to 550 nm. A pressure of up to 30 psi was utilized, generating 5000 to 10,000 events per second. Positive cells were collected directly into Trizol (Invitrogen, Carlsbad, CA) and RNA was isolated (with the addition of glycogen) according to the manufacturer’s protocol.
RNA amplification
RNA obtained from 10,000 endothelial cells obtained by FACS was amplified with the SPIA Aminoallyl™ system (Nugen, CA) according to the manufacturer’s instructions, producing at least 4 μg of cDNA.
cDNA hybridizations
Two-color array hybridizations were performed. For each hybridization, 2 μg of amplified cDNA was labeled with Cy3 and Cy5 with CyDye™ Post-Labeling Reactive Dye Pack (GE Healthcare, Piscataway, NJ) according to the manufacturer’s instructions. cDNA probes were combined, purified, and hybridized for 14 h at 42°C in a total volume of 35 μL of a hybridization solution containing 25% formamide, 3× SSC (0.45 M NaCl, 0.045 M Na citrate, pH 7.0), 0.2% sodium dodecyl sulfate (SDS), 1 μg of murine Cot1 DNA, 4 μg of polyA RNA, and 4 μg of tRNA. Microscopic slides pin-spotted with 32,000 long oligonucleotides of the Operon V3 set representing the entire mouse genome were produced by the UT Southwestern Microarray Core (Dallas, TX). Stringency washing at room temperature was sequentially performed for 10 min in each of the following solutions: 2× SSC containing 0.1% SDS, 0.1× SSC containing 0.1% SDS, and finally, 0.1× SSC. Performance of each experiment by microarray analysis was duplicated with the Cy3 and Cy5 labeling order reversed.
Data analysis
Slides were scanned with a Genepix 4000B dual channel scanner and the resultant images analyzed with Genepix and Acuity software (Molecular Devices, Sunnyvale, CA). Genepix 6.1 default settings were employed: median local background pixil intensity was subtracted, and features found “bad,” “absent,” “not found,” were flagged; features with a ratio of 635/532 <0.1 or >10 were excluded from normalization. Results were normalized by the ratio of medians method and filtered to exclude those with any of the following characteristics: a percentage of saturated pixils >3, a signal/noise ratio <3, a (regression ratio 635/532)2 <0.6, or a Genepix flag. Data passing the filter and exceeding threshold fold changes in replicate, dye-reversed analyses were considered to be dysregulated. For statistical analysis, the one sample T-test, with Benjamini–Hochberg correction, was applied to the combined results obtained at 300 and 1000 μg PM/m3 (4 microarrays per strain).
Immunolocalization
Tissue sections (5 μm) obtained from formalin-fixed, paraffin-embedded aortae were deparaffinized and exposed to the following: 95°C sodium citrate at 100 mM for 20 min, 0.3% (v/v) H2O2 for 5 min, and PBS containing 1% (w/v) bovine serum albumin and 5% (v/v) donkey serum for 20 min. Immunohistological localization was performed with goat anti-Terf1 (Santa Cruz Inc., Santa Cruz, CA) at 4 μg/mL for 16 h followed by biotinylated donkey anti-goat (Jackson Immunoresearch, West Grove, PA) at 1.0 μg/mL for 1 h. Slides were then incubated with ABC Elite™ and visualized with VIP™ substrate (both from Vector Labs, Burlingame, CA) according to the manufacturer’s instructions.
Results
ApoE (−/−) mice were hypercholesterolemic vs. ApoE (+/+), with cholesterol elevation comparable with the ApoE-deficient parental strain BL/6 ApoE (−/−) (see Figure 1). Furthermore, Tie2 GFP/ApoE (−/−) mice developed aortic atheroma (results not shown), as reported for the BL/6 ApoE (−/−) strain (Breslow 1996).
Figure 1.

Total serum cholesterol levels in ApoE (+/+), ApoE (−/−), and BL/6 ApoE (−/−) mice (n = 3). * indicates a P-value <0.05.
Microarray results were submitted to the Gene Expression Omnibus database (US NCBI) as series GSE13160. A total of 176 transcripts were found to be down-regulated (<0.35-fold) or up-regulated (>2.8-fold) in the ApoE (−/−) strain in at least one experimental dose; only two transcripts appeared dys-regulated in the ApoE (+/+) strain. A subset of transcripts possessing known RefSeq identities are shown in Tables A and B of the appendix. Thirty days of exposure to diesel exhaust resulted in >50 transcripts appearing dysregulated in ApoE (−/−) mice at both 300 and 1000 μg PM/m3 doses, with no transcripts appearing commonly dysregulated in ApoE (+/+) mice.
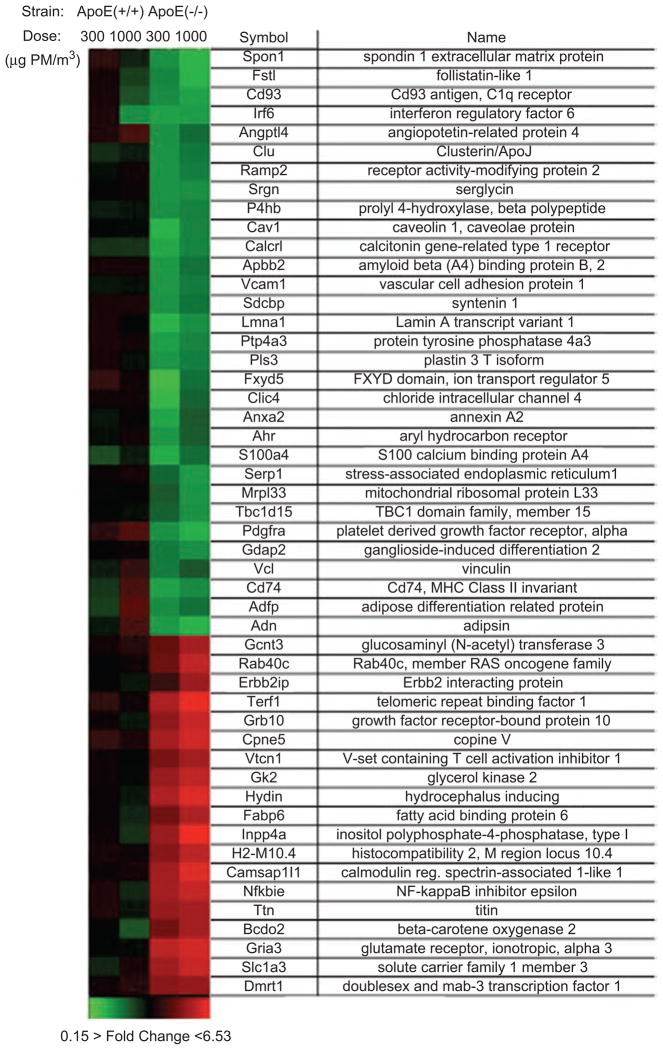
In the combined microarray results of ApoE (−/−) mice exposed to 300 and 1000 μg PM/m3, 49 of the highly dys-regulated transcripts appeared statistically significant (P-value <0.05); no transcripts appeared statistically significant in pollution-exposed ApoE (+/+) mice. These results were subjected to hierarchical cluster analysis, displayed as a heat map shown in Figure 2, which indicates a high level of concordance of responses to either concentration of diesel exhaust in ApoE (−/−) mice. Transcripts lacking RefSeq identity are not displayed.
Figure 2.
Microarray results of endothelial responses to diesel engine exhaust in vivo. Heat map of aortic endothelial transcripts dysregulated by >2.8- or <0.35-fold in ApoE (−/−) and ApoE (+/+) mice exposed for 30 days to fresh exhaust from a Yanmar diesel engine diluted with air to particulate concentrations of 300 or 1000 μg PM/m3. Transcripts shown were significantly dysregulated in the combined analyses of the ApoE (−/−) strain (n = 4, P < 0.05). Each rectangle represents the average results of two microarray analyses of pooled endothelium from four mice exposed to exhaust at 300 or 1000 μg PM/m3 vs. four control mice exposed to HEPA-filtered air. Red indicates up-regulation, green indicates down-regulation, and black indicates no change.
A literature search revealed important pathophysiological roles for a subset of these transcripts within the vasculature. Supporting references are provided in Table C of the appendix. The highly dysregulated transcripts observed within the ApoE (−/−) strain were subjected to analysis with the DAVID bioinformatics tool according to default settings (Huang da et al. 2009) to detect enrichment of annotation terms: Fisher’s exact t-test with Benjamini correction revealed no evidence of significant enrichment of annotation terms (all P-values >0.05).
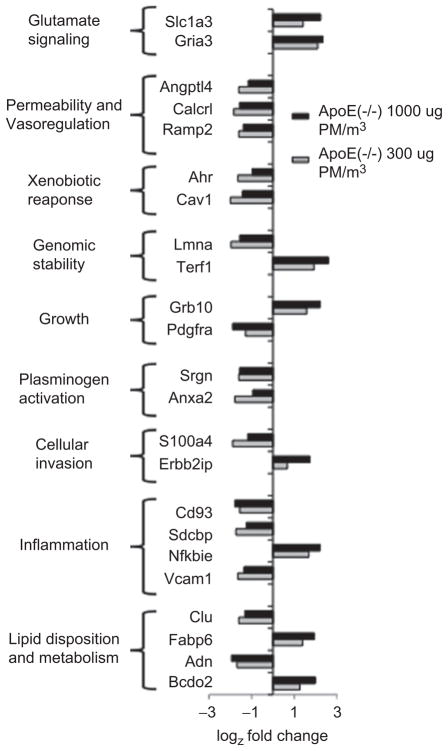
As displayed in Figure 3, a subset of transcripts appearing highly dysregulated in ApoE (−/−) mice exposed to diesel exhaust at 300 and 1000 μg PM/m3 possess important and overlapping roles in vascular function. Transcripts responding to diesel exhaust in Apo E (−/−) mice include molecules known to maintain normal vascular homeostasis or contribute to pathology in other models of vascular disease. Pdgfra, Vcam1, Cav1, and Clu/Clusterin appear down-regulated in response to diesel exhaust in our study. The progression of atherosclerosis in ApoE (−/−) mice can be suppressed pharmacologically with an inhibitor of PDGF signaling, Imatinib (Lassila et al. 2004) or by a peptide of Clusterin (Navab et al. 2005); atherogenesis in the ApoE (−/−) can be decreased by genetic deficiency of Cav1 (Frank et al. 2004) or Vcam1 (Dansky et al. 2001). Down-regulated transcripts, Anxa2/Annexin A2 and Srgn/serglycin, possess known roles in plasminogen activation and plasminogen intracellular trafficking, respectively (He et al. 2008; Kolset and Tveit 2008). Endothelial permeability has been shown to be increased by Angptl4 (Cazes et al. 2006) as well as both components of the adrenomedullin receptor complex, Ramp2 (Ichikawa-Shindo et al. 2008) and Calcrl (Temmesfeld-Wollbrück et al. 2007), all of which appear down-regulated by diesel exhaust. Transcripts of lipid metabolic enzymes dysregulated by diesel exhaust included up-regulated Bcdo2 and down-regulated Adipsin; lipid-binding proteins Clu and Fabp6 both appear down-regulated in our study. Transcripts up-regulated upon exposure to diesel exhaust included a glutamate receptor, Gria3, and a glutamate transporter, Slc1a3.
Figure 3.
Selected transcripts dysregulated by >2.8- or <0.35-fold in ApoE (−/−) mice exposed for 30 days to fresh exhaust from a Yanmar diesel engine diluted with HEPA-filtered air to engine exhaust at 300 or 1000 μg PM/m3. Transcripts shown have recognized roles in vascular pathophysiology. Fold changes are presented on a log2 scale with each bar representing the average results of two microarray determinations of pooled endothelium from four mice exposed to exhaust vs. four control mice exposed to air.
As shown in Figure 3, exposure of ApoE (−/−) mice to diesel exhaust resulted in dysregulation of inflammatory signaling transcripts within the aortic endothelium, including up-regulation of Nfkbie and down-regulation of Vcam1 and Sdcbp. Our observed increase in transcription of Nfkbie potentially reflects a compensatory mechanism to limit ongoing NF-κB signaling associated with responses to diesel engine particulate, similar to those observed in lung epithelium (Shukla et al. 2000).
As shown in Figure 4, immunolocalization revealed up-regulation of Terf1 in the aortic endothelium of ApoE (−/−) mice exposed to diesel exhaust at 1000 μg PM/m3, consistent with the 6-fold up-regulation indicated by our microarray analysis. Terf1 is an inhibitor of telomerase activity; telomere shortening has been observed in circulating endothelial progenitors obtained from patients with coronary artery disease (Satoh et al. 2008).
Figure 4.
Representative immunolocalization of Terf1, indicated by elevated staining (purple) in the aortic endothelium of ApoE (−/−) mice following exposure to dilute diesel exhaust at 1000 μg PM/m3 for 30 days vs. purified air. Arrows indicate endothelia; L indicates luminal space. Tissue sections lacking primary antibody displayed minimal background staining.
Discussion
We report substantial transcriptional responses in the aortic endothelium of ApoE (−/−) mice exposed to diesel engine exhaust. Importantly, our analysis revealed a much lower degree of dysregulation in identically exposed wild-type mice, suggesting that: (1) healthy endothelial cells are able to maintain homeostasis over a prolonged exposure to diesel emissions and (2) hypercholesterolemia increases the sensitivity of the endothelial transcriptional response to air pollution. The high degree of concordance of the responses of ApoE (−/−) mice exposed to either 300 or 1000 μg PM/m3 implies a threshold of activation below 300 μg PM/m3, a concentration of diesel particulate observed in mining and other occupational settings (Pronk et al. 2009); due to their similarity, these data sets were combined for statistical evaluation.
Interestingly, studies of ApoE (−/−) mice exposed to gasoline or diesel emissions for up to 50 days in the presence of a high-fat diet revealed enhancement of matrix metalloproteinase (MMP), heme oxygenase-1, and endothelin-1 gene expression in the whole aorta, accompanied by evidence of enhanced remodeling and monocyte invasion of the vascular wall (Lund et al. 2007, 2009). The results of the present study of normally fed mice, wherein gene regulation in non-endothelial cell types has been minimized by FACS isolation, did not reveal MMPs or other previously reported transcripts. Furthermore, serum cholesterol levels of ApoE (−/−) mice receiving normal chow used in our study were >2-fold lower than those receiving a high fat (BioServ #S3282, results not shown), thus more closely approximating levels found in clinical hypercholesterolemia. These differences highlight the importance of understanding cell-specific responses that drive whole-organ outcomes.
In an earlier study, acute exposure of rats to diesel exhaust at 300 μg PM/m3 for 5 h resulted in endothelial-dependent changes in coronary responsiveness to endothelin-1 (Cherng et al. 2009). One might expect prolonged exposure of wild-type mice to diesel exhaust at 300 μg PM/m3 would lead to transcriptional changes as well; however, it is possible that biochemical effects in the absence of gene regulation, or homeostatic mechanisms counterbalanced these effects upon 30 days of exposure. In the present study, substantial transcriptional responses in the endothelium to the effects of diesel exhaust were apparent only in the ApoE (−/−) strain. This differential response is attributable to dyslipidemia, impaired macrophage lipid scavenging activity (Atkinson et al. 2008), or other metabolic differences characteristic of the ApoE (−/−) strain. Such susceptibility in the ApoE (−/−) strain further demonstrates diesel exhaust and other forms of air pollution as cardiovascular risk factors, which may become manifest only in the added presence of impaired lipid disposition.
Our data indicate that chronic exposure of ApoE (−/−) mice to diesel exhaust affects regulators of endothelial adhesion (Nfkbie and Vcam1), permeability (Angptl4, Calcrl, Ramp2), invasion (Erbb2ip), and genomic stability (Terf1 and Lmna). Not all of these transcripts were up-regulated; the complex response may reflect homeostatic regulation of these processes or selective survival of endothelial cells that exhibit these changes. Our findings of responses to diesel exhaust among modulators of endothelial function are consistent with reports of accelerated atherogenesis in the aortae of ApoE (−/−) mice exposed to air pollution (Sun et al. 2005; Araujo et al. 2008).
A subset of dysregulated transcripts identified in our analysis possesses known vascular function and thus provides a molecular foundation for the epidemiological association of air pollution with accelerated atherogenesis. The present study combines realistic exposure and cell-specific genomic inquiry to obtain robust clues to in vivo mechanisms underlying the augmentation of atherosclerosis by diesel exhaust. Future work using similar methods will enable a greater understanding of the biological outcomes following exposures to the complex mixtures that comprise combustion-source air pollution.
Acknowledgments
This work was supported by the NIEHS ES013395, NCRR RR016453, and NHLBI HL073449 (to RVS).
Appendix
Table A.
Transcripts down-regulated in aortic endothelium in ApoE (+/+) and ApoE (−/−) mice in response to exposure to diesel exhaust for 30 days.
| Marker symbol | Name | Ref Seq | Average fold changea
|
|||
|---|---|---|---|---|---|---|
| ApoE (+/+)
|
ApoE (−/−)
|
|||||
| 300 μg/m3 | 1000 μg/m3 | 300 μg/m3 | 1000 μg/m3 | |||
| Anxa2 | Annexin A2 | NM_007585 | 0.88 | 1.0 | 0.29 | 0.52 |
| Cd93 | Cd93 antigen, C1q receptor | NM_010740 | 1.2 | 0.77 | 0.34 | 0.29 |
| Vcam1 | Vascular cell adhesion protein 1 | NM_011693 | 0.88 | 0.91 | 0.32 | 0.39 |
| Fxyd5 | FXYD domain-containing ion transport regulator 5 | NM_008761 | 1.5 | 1.12 | 0.21 | 0.43 |
| Pdgfra | Platelet-derived growth factor receptor, alpha polypeptide | NM_011058 | 1.4 | 1.9 | 0.41 | 0.27 |
| Lmna | Lamin A | NM_019390 | 1.2 | 1.0 | 0.25 | 0.34 |
| Irf6 | Interferon regulatory factor 6 | NM_016851 | 1.3 | 0.34 | 0.30 | 0.30 |
| Ahr | Aryl hydrocarbon receptor | NM_013464 | 0.92 | 1.1 | 0.32 | 0.51 |
| Cxcl2 | Chemokine (C-X-C motif) ligand 2 | NM_009140 | 1.2 | 3.9 | 0.80 | 0.22 |
| Apbb2 | Amyloid beta (A4) precursor protein-binding, family B, member 2 | NM_009686 | 1.2 | 1.12 | 0.31 | 0.43 |
| Angptl4 | Angiopoietin-related protein 4 | NM_020581 | 1.3 | 1.7 | 0.33 | 0.45 |
| Vcl | Vinculin | NM_009502 | 1.1 | 1.2 | 0.36 | 0.32 |
| Sdcbp | Syntenin 1 | NM_016807 | 1.2 | 0.87 | 0.30 | 0.42 |
| Gdap2 | Ganglioside-induced differentiation-associated protein 2 | NM_010269 | 1.0 | 1.1 | 0.39 | 0.34 |
| Srgn | Serglycin | NM_011157 | 1.0 | 1.10 | 0.33 | 0.34 |
| Tbc1d15 | TBC1 domain family, member 15 | NM_025706 | 0.81 | 0.91 | 0.49 | 0.33 |
| Clic4 | Chloride intracellular channel 4 | NM_013885 | 1.1 | 1.1 | 0.23 | 0.48 |
| Cav1 | Caveolin 1, caveolae protein | NM_007616 | 0.98 | 0.95 | 0.25 | 0.37 |
| Pls3 | Plastin 3 T isoform | NM_145629 | 1.2 | 0.92 | 0.32 | 0.38 |
| Serp1 | Stress-associated endoplasmic reticulum protein 1 | NM_030685 | 1.1 | 1.2 | 0.47 | 0.31 |
| Spon1 | Spondin 1 extracellular matrix protein | NM_145584 | 1.4 | 0.81 | 0.33 | 0.24 |
| Adamts8 | A disintegrin and metalloproteinase with thrombospondin motifs 8 | NM_013906 | 0.75 | 2.0 | 0.29 | 1.1 |
| Clu | Clusterin | NM_013492 | 0.73 | 0.85 | 0.33 | 0.40 |
| Ramp2 | Receptor activity-modifying protein 2 | NM_019444 | 0.91 | 0.84 | 0.33 | 0.38 |
| Calcrl | Calcitonin gene-related peptide type 1 receptor | NM_018782 | 0.70 | 0.69 | 0.28 | 0.34 |
| S100a4 | S100 calcium-binding protein A4 | NM_011311 | 0.57 | 0.85 | 0.27 | 0.44 |
| Adn | Adipsin | NM_013459 | 0.86 | 1.8 | 0.31 | 0.26 |
| Ptp4a3 | Protein tyrosine phosphatase 4a3 | NM_008975 | 1.2 | 1.1 | 0.30 | 0.37 |
| Fstl | Follistatin-like 1 | NM_008047 | 1.2 | 0.64 | 0.36 | 0.25 |
| Cd74 | Cd74 antigen (invariant polypeptide of major histocompatibility complex, class II antigen-associated) | NM_001042605 | 0.68 | 1.8 | 0.29 | 0.35 |
| P4hb | Prolyl 4-hydroxylase, beta-polypeptide | NM_011032 | 0.84 | 0.89 | 0.34 | 0.37 |
| Adfp | Adipose differentiation-related protein | NM_007408 | 0.63 | 2.0 | 0.34 | 0.43 |
| Mrpl33 | Mitochondrial ribosomal protein L33 | NM_025796 | 0.95 | 0.98 | 0.52 | 0.32 |
| Rbm7 | RNA-binding motif protein 7 | NM_144948 | 0.93 | 0.88 | 0.37 | 0.31 |
Values in bold were down-regulated ≤0.35.
Table B.
Transcripts up-regulated in aortic endothelium in ApoE (+/+) and ApoE (−/−) mice in response to exposure to diesel exhaust for 30 days.
| Marker symbol | Name | Ref Seq | Average fold changea
|
|||
|---|---|---|---|---|---|---|
| ApoE (+/+)
|
Apo E (−/−)
|
|||||
| 300 μg/m3 | 1000 μg/m3 | 300 μg/m3 | 1000 μg/m3 | |||
| Terf1 | Telomeric repeat-binding factor 1 | NM_009352 | 1.4 | 1.1 | 3.8 | 6.1 |
| Fabp6 | Fatty acid-binding protein 6, ileal (gastrotropin) | NM_008375 | 0.87 | 0.53 | 2.61 | 3.8 |
| Gria3 | Glutamate receptor, ionotropic, alpha 3 | NM_016886 | 0.97 | 0.94 | 4.3 | 5.0 |
| Nfkbie | NF-κB inhibitor epsilon | NM_008690 | 1.3 | 0.78 | 3.2 | 4.6 |
| Gk2 | Glycerol kinase 2 | NM_010294 | 1.1 | 0.95 | 3.2 | 4.4 |
| Bcdo2 | Beta-carotene oxygenase 2 | NM_133217 | 0.98 | 0.55 | 2.4 | 4.0 |
| Gcnt3 | Glucosaminyl (N-acetyl) transferase 3, mucin type | NM_028087 | 1.2 | 1.4 | 2.4 | 4.3 |
| Dmrt1 | Double sex and mab-3-related transcription factor 1 | NM_015826 | 1.1 | 1.3 | 2.9 | 3.1 |
| Hydin | Hydrocephalus-inducing | NM_172916 | 1.1 | 0.88 | 3.8 | 5.5 |
| Grb10 | Growth factor receptor-bound protein 10 | NM_010345 | 1.1 | 0.88 | 3.0 | 4.6 |
| H2-M10.4 | Histocompatibility 2, M region locus 10.4 | NM_177634 | 1.2 | 1.1 | 2.8 | 4.3 |
| Cpne5 | Copine V | NM_153166 | 1.5 | 1.2 | 3.6 | 5.0 |
| Rab40c | Rab40c, member RAS oncogene family | NM_139154 | 1.0 | 1.1 | 2.3 | 4.4 |
| Inpp4a | Inositol polyphosphate-4-phosphatase, type I | NM_030266 | 1.1 | 0.71 | 3.3 | 6.5 |
| Camsap1l1 | Calmodulin-regulated spectrin-associated protein 1-like 1 | NM_001081360 | 1.1 | 1.2 | 3.5 | 5.6 |
| Ttn | Titin | NM_176926 | 1.3 | 0.88 | 3.2 | 3.6 |
| Erbb2ip | Erbb2-interacting protein | NM_001005868 | 1.0 | 0.87 | 1.6 | 3.3 |
| Vtcn1 | V-set domain-containing T-cell activation inhibitor 1 | NM_178594 | 1.1 | 0.98 | 2.5 | 3.5 |
| Slc1a3 | Solute carrier family 1 (glial high-affinity glutamate transporter), member 3 | NM_148938 | 0.78 | 1.0 | 2.7 | 4.7 |
Values in bold were up-regulated ≥2.8.
Table C.
Transcripts with known vascular functions, which appear dysregulated in aortic endothelium of ApoE (−/−) mice in response to 30 days of diesel exhaust.
| Marker symbola | Ref Seq | Role in vasculature |
|---|---|---|
| Anxa2 (↓) | NM_007585 | Annexin A2 heterotetramer with S100A10/p11 is endothelial locus for tPA and plasmin activation (He et al. 2008) |
| Cd93 (↓) | NM_010740 | Cd93 is prominently expressed on endothelium and contributes to removal of apoptotic cells (Fonseca et al. 2001; Norsworthy et al. 2004) |
| Vcam1 (↓) | NM_011693 | Vcam1 domain 4-deficient (D4D) +/+ mice crossed with Apo E (−/−) mice exhibit decreased atherogenesis (Dansky et al. 2001) |
| Pdgfra (↓) | NM_011058 | Imatinib/Gleevec (blockade of PDGFr) down-regulates atherogenesis induced by type I diabetes model in Apo E mice (Lassila et al. 2004) |
| Terf1 (↑) | NM_009352 | Terf1 regulates telomere length. Endothelial progenitor cell telomere length is shorter in CAD patients and in vitro EPC treated with oxidative treatments (Okamoto et al. 2008; Satoh et al. 2008) |
| Ahr (↓) | NM_013464 | Benzopyrene leads to up-regulation of ICAM-1, which is disrupted by Cav1 deficiency (Oesterling et al. 2008) |
| Apbb2 (↓) | NM_009686 | Amyloid precursor cleavage by gamma secretase is inhibited by Apo E (Irizarry et al. 2004) |
| Angptl4 (↓) | NM_020581 | Angiopoietin-like 4 inhibits endothelial permeabilization (Cazes et al. 2006) |
| Sdcbp (↓) | NM_016807 | TNF-inducible in cultured endothelium (Stier et al. 2000) |
| Srgn (↓) | NM_011157 | Serglycin binds plasminogen activator in endothelium (Kolset and Tveit 2008) |
| Cav1 (↓) | NM_007616 | Knockout of Cav1 confers resistance to atherogenesis in the Apo E (−/−) mouse (Frank et al. 2004) |
| Spon1 (↓) | NM_145584 | F-spondin (an ECM protein) affects amyloid precursor processing by interacting with Apo E receptor (Hoe et al. 2005) |
| Fabp6 (↑) | NM_008375 | Fabp6 levels are regulated by sterol receptors FXR, LXR, and SREBP1c (Besnard et al. 2004) |
| Clu (↓) | NM_013492 | Potentially protects against the effects of modified LDL. Clu/ApoJ peptide mimetic suppresses atherosclerosis in Apo E mice (Navab et al. 2005) |
| Ramp2 (↓) | NM_019444 | Ramp2 underexpression is associated with increased vascular permeability (Ichikawa-Shindo et al. 2008) |
| Gria3 (↑) | NM_016886 | Vasodilation by glutamate is mediated by carbon monoxide produced by heme oxygenase (Parfenova et al. 2003) |
| Nfkbie (↑) | NM_008690 | I kappa B epsilon is involved in c-Rel-associated ICAM-1 induction in endothelium (Spiecker et al. 2000) |
| Bcdo2 (↑) | NM_133217 | Asymmetric cleavage of beta-carotene |
| Calcrl (↓) | NM_018782 | Calcrl knockout resembles adrenomedullin knockout; adrenomedullin protects against endothelial barrier breakdown; AM AMBP instillation protects against endothelial dysfunction in rat model of sepsis (Dackor et al. 2006; Temmesfeld-Wollbrück et al. 2007; Zhou et al. 2007) |
| S100a4 (↓) | NM_011311 | S100a is required for metastasis (Grigorian et al. 2008) |
| Adn (↓) | NM_013459 | Up-regulated in endothelium of model of Type I diabetes (Maresh and Shohet 2008) |
| Grb10 (↑) | NM_010345 | Grb10 inhibits Vegf-r2 internalization (Murdaca et al. 2004) |
| Ptp4a3 (↓) | NM_008975 | Expression in endothelium of tumors (Bardelli et al. 2003) |
| Fstl (↓) | NM_008047 | Fstl/Tsc-36 promotes endothelial function in ischemic tissue (Ouchi et al. 2008) |
| H2-M10.4 (↑) | NM_177634 | MHC Class II, M region locus 10.4 |
| Cpne5 (↑) | NM_153166 | Ca2+-dependent phospholipid-binding protein (Tomsig and Creutz 2002) |
| Cd74 (↓) | NM_001042605 | MHC Class II invariant chain |
| Inpp4a (↑) | NM_030266 | PI3 kinase signal regulation (Ivetac et al. 2005) |
| Adfp (↓) | NM_007408 | Adfp antisense oligo decreases hepatic insulin resistance (Varela et al. 2008) |
| Mrpl33 (↓) | NM_025796 | Mitochondrial ribosomal protein with nuclear code |
| Erbb2ip (↑) | NM_001005868 | Endothelium from aged mice susceptible to tumor cell invasion in in vitro assay and this process requires Erbb EGF signaling cross talk (Price et al. 2004) |
| Vtcn1 (↑) | NM_178594 | Negative modulator of T-cell response (Suh et al. 2006) |
| Lmna (↓) | NM_001002011 | Mutant lamin A (progerin) accumulation in dermal vascular cells of Hutchinson–Gilford progeria (McClintock et al. 2006) |
Arrows indicate direction of dysregulation in the Apo E (−/−) strain; n.c. indicates no change upon exposure to exhaust at 300 μg/m3.
Footnotes
Declaration of interest
The authors report no declaration of interest.
References
- Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, Nel AE. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res. 2008;102:589–596. doi: 10.1161/CIRCRESAHA.107.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimoto T, Takano H, Inoue K, Yanagisawa R, Yoshino S, Yamaki K, Yoshikawa T. Pulmonary exposure to diesel exhaust particle components enhances circulatory chemokines during lung inflammation. Int J Immunopathol Pharmacol. 2007;20:197–201. doi: 10.1177/039463200702000124. [DOI] [PubMed] [Google Scholar]
- Atkinson RD, Coenen KR, Plummer MR, Gruen ML, Hasty AH. Macrophage-derived apolipoprotein E ameliorates dyslipidemia and atherosclerosis in obese apolipoprotein E-deficient mice. Am J Physiol Endocrinol Metab. 2008;294:E284–E290. doi: 10.1152/ajpendo.00601.2007. [DOI] [PubMed] [Google Scholar]
- Bardelli A, Saha S, Sager JA, Romans KE, Xin B, Markowitz SD, Lengauer C, Velculescu VE, Kinzler KW, Vogelstein B. PRL-3 expression in meta-static cancers. Clin Cancer Res. 2003;9:5607–5615. [PubMed] [Google Scholar]
- Besnard P, Landrier JF, Grober J, Niot I. Is the ileal bile acid-binding protein (I-BABP) gene involved in cholesterol homeostasis? Med Sci (Paris) 2004;20:73–77. doi: 10.1051/medsci/200420173. [DOI] [PubMed] [Google Scholar]
- Breslow JL. Mouse models of atherosclerosis. Science. 1996;272:685–688. doi: 10.1126/science.272.5262.685. [DOI] [PubMed] [Google Scholar]
- Brook RD. Cardiovascular effects of air pollution. Clin Sci. 2008;115:175–187. doi: 10.1042/CS20070444. [DOI] [PubMed] [Google Scholar]
- Cazes A, Galaup A, Chomel C, Bignon M, Bréchot N, Le Jan S, Weber H, Corvol P, Muller L, Germain S, Monnot C. Extracellular matrix-bound angiopoietin-like 4 inhibits endothelial cell adhesion, migration, and sprouting and alters actin cytoskeleton. Circ Res. 2006;99:1207–1215. doi: 10.1161/01.RES.0000250758.63358.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherng TW, Campen MJ, Knuckles TL, Gonzalez Bosc L, Kanagy NL. Impairment of coronary endothelial cell ET(B) receptor function after short-term inhalation exposure to whole diesel emissions. Am J Physiol Regul Integr Comp Physiol. 2009;297:R640–R647. doi: 10.1152/ajpregu.90899.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackor RT, Fritz-Six K, Dunworth WP, Gibbons CL, Smithies O, Caron KM. Hydrops fetalis, cardiovascular defects, and embryonic lethality in mice lacking the calcitonin receptor-like receptor gene. Mol Cell Biol. 2006;26:2511–2518. doi: 10.1128/MCB.26.7.2511-2518.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansky HM, Barlow CB, Lominska C, Sikes JL, Kao C, Weinsaft J, Cybulsky MI, Smith JD. Adhesion of monocytes to arterial endothelium and initiation of atherosclerosis are critically dependent on vascular cell adhesion molecule-1 gene dosage. Arterioscler Thromb Vasc Biol. 2001;21:1662–1667. doi: 10.1161/hq1001.096625. [DOI] [PubMed] [Google Scholar]
- Fonseca MI, Carpenter PM, Park M, Palmarini G, Nelson EL, Tenner AJ. C1qR(P), a myeloid cell receptor in blood, is predominantly expressed on endothelial cells in human tissue. J Leukoc Biol. 2001;70:793–800. [PubMed] [Google Scholar]
- Frank PG, Lee H, Park DS, Tandon NN, Scherer PE, Lisanti MP. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:98–105. doi: 10.1161/01.ATV.0000101182.89118.E5. [DOI] [PubMed] [Google Scholar]
- Furuyama A, Kanno S, Kobayashi T, Hirano S. Extrapulmonary translocation of intratracheally instilled fine and ultrafine particles via direct and alveolar macrophage-associated routes. Arch Toxicol. 2009;83:429–437. doi: 10.1007/s00204-008-0371-1. [DOI] [PubMed] [Google Scholar]
- Grigorian M, Ambartsumian N, Lukanidin E. Metastasis-inducing S100A4 protein: implication in non-malignant human pathologies. Curr Mol Med. 2008;8:492–496. doi: 10.2174/156652408785747942. [DOI] [PubMed] [Google Scholar]
- He KL, Deora AB, Xiong H, Ling Q, Weksler BB, Niesvizky R, Hajjar KA. Endothelial cell annexin A2 regulates polyubiquitination and degradation of its binding partner S100A10/p11. J Biol Chem. 2008;283:19192–19200. doi: 10.1074/jbc.M800100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoe HS, Wessner D, Beffert U, Becker AG, Matsuoka Y, Rebeck GW. F-Spondin interaction with the apolipoprotein E receptor ApoEr2 affects processing of amyloid precursor protein. Mol Cell Biol. 2005;25:9259–9268. doi: 10.1128/MCB.25.21.9259-9268.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann B, Moebus S, Möhlenkamp S, Stang A, Lehmann N, Dragano N, Schmermund A, Memmesheimer M, Mann K, Erbel R, Jöckel KH Heinz Nixdorf Recall Study Investigative Group. Residential exposure to traffic is associated with coronary atherosclerosis. Circulation. 2007;116:489–496. doi: 10.1161/CIRCULATIONAHA.107.693622. [DOI] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Ichikawa-Shindo Y, Sakurai T, Kamiyoshi A, Kawate H, Iinuma N, Yoshizawa T, Koyama T, Fukuchi J, Iimuro S, Moriyama N, Kawakami H, Murata T, Kangawa K, Nagai R, Shindo T. The GPCR modulator protein RAMP2 is essential for angiogenesis and vascular integrity. J Clin Invest. 2008;118:29–39. doi: 10.1172/JCI33022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry MC, Deng A, Lleo A, Berezovska O, Von Arnim CA, Martin-Rehrmann M, Manelli A, LaDu MJ, Hyman BT, Rebeck GW. Apolipoprotein E modulates gamma-secretase cleavage of the amyloid precursor protein. J Neurochem. 2004;90:1132–1143. doi: 10.1111/j.1471-4159.2004.02581.x. [DOI] [PubMed] [Google Scholar]
- Ivetac I, Munday AD, Kisseleva MV, Zhang XM, Luff S, Tiganis T, Whisstock JC, Rowe T, Majerus PW, Mitchell CA. The type I alpha inositol polyphosphate 4-phosphatase generates and terminates phosphoinositide 3-kinase signals on endosomes and the plasma membrane. Mol Biol Cell. 2005;16:2218–2233. doi: 10.1091/mbc.E04-09-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolset SO, Tveit H. Serglycin—structure and biology. Cell Mol Life Sci. 2008;65:1073–1085. doi: 10.1007/s00018-007-7455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künzli N, Jerrett M, Mack WJ, Beckerman B, LaBree L, Gilliland F, Thomas D, Peters J, Hodis HN. Ambient air pollution and atherosclerosis in Los Angeles. Environ Health Perspect. 2005;113:201–206. doi: 10.1289/ehp.7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassila M, Allen TJ, Cao Z, Thallas V, Jandeleit-Dahm KA, Candido R, Cooper ME. Imatinib attenuates diabetes-associated atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:935–942. doi: 10.1161/01.ATV.0000124105.39900.db. [DOI] [PubMed] [Google Scholar]
- Li YJ, Takizawa H, Azuma A, Kohyama T, Yamauchi Y, Takahashi S, Yamamoto M, Kawada T, Kudoh S, Sugawara I. Disruption of Nrf2 enhances susceptibility to airway inflammatory responses induced by low-dose diesel exhaust particles in mice. Clin Immunol. 2008;128:366–373. doi: 10.1016/j.clim.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Lund AK, Knuckles TL, Obot Akata C, Shohet R, McDonald JD, Gigliotti A, Seagrave JC, Campen MJ. Gasoline exhaust emissions induce vascular remodeling pathways involved in atherosclerosis. Toxicol Sci. 2007;95:485–494. doi: 10.1093/toxsci/kfl145. [DOI] [PubMed] [Google Scholar]
- Lund AK, Lucero J, Lucas S, Madden MC, McDonald JD, Seagrave JC, Knuckles TL, Campen MJ. Vehicular emissions induce vascular MMP-9 expression and activity associated with endothelin-1-mediated pathways. Arterioscler Thromb Vasc Biol. 2009;29:511–517. doi: 10.1161/ATVBAHA.108.176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresh JG, Shohet RV. In vivo endothelial gene regulation in diabetes. Cardiovasc Diabetol. 2008;7:8. doi: 10.1186/1475-2840-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock D, Gordon LB, Djabali K. Hutchinson–Gilford progeria mutant lamin A primarily targets human vascular cells as detected by an anti-Lamin A G608G antibody. Proc Natl Acad Sci USA. 2006;103:2154–2159. doi: 10.1073/pnas.0511133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JD, Barra EB, White RK. Design, characterization, and evaluation of a small-scale diesel exhaust exposure system. Aerosol Sci Technol. 2004;38:62–78. [Google Scholar]
- Mills NL, Törnqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T, Newby DE. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112:3930–3936. doi: 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- Murdaca J, Treins C, Monthouël-Kartmann MN, Pontier-Bres R, Kumar S, Van Obberghen E, Giorgetti-Peraldi S. Grb10 prevents Nedd4-mediated vascular endothelial growth factor receptor-2 degradation. J Biol Chem. 2004;279:26754–26761. doi: 10.1074/jbc.M311802200. [DOI] [PubMed] [Google Scholar]
- Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Wagner AC, Hama S, Hough G, Bachini E, Garber DW, Mishra VK, Palgunachari MN, Fogelman AM. An oral apoJ peptide renders HDL antiinflammatory in mice and monkeys and dramatically reduces atherosclerosis in apolipo-protein E-null mice. Arterioscler Thromb Vasc Biol. 2005;25:1932–1937. doi: 10.1161/01.ATV.0000174589.70190.e2. [DOI] [PubMed] [Google Scholar]
- Norsworthy PJ, Fossati-Jimack L, Cortes-Hernandez J, Taylor PR, Bygrave AE, Thompson RD, Nourshargh S, Walport MJ, Botto M. Murine CD93 (C1qRp) contributes to the removal of apoptotic cells in vivo but is not required for C1q-mediated enhancement of phagocytosis. J Immunol. 2004;172:3406–3414. doi: 10.4049/jimmunol.172.6.3406. [DOI] [PubMed] [Google Scholar]
- Oesterling E, Toborek M, Hennig B. Benzo[a]pyrene induces intercellular adhesion molecule-1 through a caveolae and aryl hydrocarbon receptor mediated pathway. Toxicol Appl Pharmacol. 2008;232:309–316. doi: 10.1016/j.taap.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Iwano T, Tachibana M, Shinkai Y. Distinct roles of TRF1 in the regulation of telomere structure and lengthening. J Biol Chem. 2008;283:23981–23988. doi: 10.1074/jbc.M802395200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N, Oshima Y, Ohashi K, Higuchi A, Ikegami C, Izumiya Y, Walsh K. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J Biol Chem. 2008;283:32802–32811. doi: 10.1074/jbc.M803440200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfenova H, Fedinec A, Leffler CW. Ionotropic glutamate receptors in cerebral microvascular endothelium are functionally linked to heme oxygenase. J Cereb Blood Flow Metab. 2003;23:190–197. doi: 10.1097/01.WCB.000004823561824.C4. [DOI] [PubMed] [Google Scholar]
- Peters A, von Klot S, Heier M, Trentinaglia I, Hörmann A, Wichmann HE, Löwel H Cooperative Health Research in the Region of Augsburg Study Group. Exposure to traffic and the onset of myocardial infarction. N Engl J Med. 2004;351:1721–1730. doi: 10.1056/NEJMoa040203. [DOI] [PubMed] [Google Scholar]
- Price DJ, Avraham S, Jiang S, Fu Y, Avraham HK. Role of the aging vasculature and Erb B-2 signaling in epidermal growth factor-dependent intra-vasation of breast carcinoma cells. Cancer. 2004;101:198–205. doi: 10.1002/cncr.20340. [DOI] [PubMed] [Google Scholar]
- Pronk A, Coble J, Stewart PA. Occupational exposure to diesel engine exhaust: a literature review. J Expo Sci Environ Epidemiol. 2009;19:443–457. doi: 10.1038/jes.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundell KW, Slee JB, Caviston R, Hollenbach AM. Decreased lung function after inhalation of ultrafine and fine particulate matter during exercise is related to decreased total nitrate in exhaled breath condensate. Inhal Toxicol. 2008;20:1–9. doi: 10.1080/08958370701758593. [DOI] [PubMed] [Google Scholar]
- Satoh M, Ishikawa Y, Takahashi Y, Itoh T, Minami Y, Nakamura M. Association between oxidative DNA damage and telomere shortening in circulating endothelial progenitor cells obtained from metabolic syndrome patients with coronary artery disease. Atherosclerosis. 2008;198:347–353. doi: 10.1016/j.atherosclerosis.2007.09.040. [DOI] [PubMed] [Google Scholar]
- Shukla A, Timblin C, BeruBe K, Gordon T, McKinney W, Driscoll K, Vacek P, Mossman BT. Inhaled particulate matter causes expression of nuclear factor (NF)-kappaB-related genes and oxidant-dependent NF-kappaB activation in vitro. Am J Respir Cell Mol Biol. 2000;23:182–187. doi: 10.1165/ajrcmb.23.2.4035. [DOI] [PubMed] [Google Scholar]
- Spiecker M, Darius H, Liao JK. A functional role of I kappa B-epsilon in endothelial cell activation. J Immunol. 2000;164:3316–3322. doi: 10.4049/jimmunol.164.6.3316. [DOI] [PubMed] [Google Scholar]
- Stier S, Totzke G, Grünewald E, Neuhaus T, Fronhoffs S, Sachinidis A, Vetter H, Schulze-Osthoff K, Ko Y. Identification of syntenin and other TNF-inducible genes in human umbilical arterial endothelial cells by suppression subtractive hybridization. FEBS Lett. 2000;467:299–304. doi: 10.1016/s0014-5793(00)01177-7. [DOI] [PubMed] [Google Scholar]
- Suh WK, Wang S, Duncan GS, Miyazaki Y, Cates E, Walker T, Gajewska BU, Deenick E, Dawicki W, Okada H, Wakeham A, Itie A, Watts TH, Ohashi PS, Jordana M, Yoshida H, Mak TW. Generation and characterization of B7-H4/B7S1/B7x-deficient mice. Mol Cell Biol. 2006;26:6403–6411. doi: 10.1128/MCB.00755-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, Aguinaldo JG, Fayad ZA, Fuster V, Lippmann M, Chen LC, Rajagopalan S. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA. 2005;294:3003–3010. doi: 10.1001/jama.294.23.3003. [DOI] [PubMed] [Google Scholar]
- Temmesfeld-Wollbrück B, Hocke AC, Suttorp N, Hippenstiel S. Adrenomedullin and endothelial barrier function. Thromb Haemost. 2007;98:944–951. doi: 10.1160/th07-02-0128. [DOI] [PubMed] [Google Scholar]
- Tomsig JL, Creutz CE. Copines: a ubiquitous family of Ca(2+)-dependent phospholipid-binding proteins. Cell Mol Life Sci. 2002;59:1467–1477. doi: 10.1007/s00018-002-8522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela GM, Antwi DA, Dhir R, Yin X, Singhal NS, Graham MJ, Crooke RM, Ahima RS. Inhibition of ADRP prevents diet-induced insulin resistance. Am J Physiol Gastrointest Liver Physiol. 2008;295:G621–G628. doi: 10.1152/ajpgi.90204.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki H, Iwai N. Mechanisms underlying nano-sized air-pollution-mediated progression of atherosclerosis: carbon black causes cytotoxic injury/inflammation and inhibits cell growth in vascular endothelial cells. Circ J. 2006;70:129–140. doi: 10.1253/circj.70.129. [DOI] [PubMed] [Google Scholar]
- Yatera K, Hsieh J, Hogg JC, Tranfield E, Suzuki H, Shih CH, Behzad AR, Vincent R, van Eeden SF. Particulate matter air pollution exposure promotes recruitment of monocytes into atherosclerotic plaques. Am J Physiol Heart Circ Physiol. 2008;294:H944–H953. doi: 10.1152/ajpheart.00406.2007. [DOI] [PubMed] [Google Scholar]
- Ying Z, Kampfrath T, Thurston G, Farrar B, Lippmann M, Wang A, Sun Q, Chen LC, Rajagopalan S. Ambient particulates alter vascular function through induction of reactive oxygen and nitrogen species. Toxicol Sci. 2009;111:80–88. doi: 10.1093/toxsci/kfp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Maitra SR, Wang P. Adrenomedullin and adrenomedullin binding protein-1 protect endothelium-dependent vascular relaxation in sepsis. Mol Med. 2007;13:488–494. doi: 10.2119/2007-00113.Zhou. [DOI] [PMC free article] [PubMed] [Google Scholar]