Abstract
Objective
To determine relationship of echocardiographic measures of pulmonary hypertension to lung function and inflammatory biomarkers in HIV-infected individuals.
Design
Cross-sectional study of 116 HIV-infected outpatients.
Methods
Doppler-echocardiography and pulmonary function testing were performed. Induced sputum and plasma cytokines, sputum cell counts and differentials, markers of peripheral T cell activation, and serum N-terminal pro-brain natriuretic peptide (NT-proBNP) were measured. Univariate and multivariate analyses determined relationship of echocardiographic variables to pulmonary function, inflammation, and NT-proBNP.
Results
Mean estimated pulmonary artery systolic pressure (PASP) was 34.3 mmHg (SD 6.9) and mean tricuspid regurgitant jet velocity (TRV) was 2.5 m/sec (SD 0.32). Eighteen participants (15.5%) had PASP of at least 40 mmHg, and 9 (7.8%) had TRV of at least 3.0 m/sec. Elevated TRV was significantly associated with CD4 cell counts below 200 cells/μl and higher log HIV RNA levels. Forced expiratory volume in one second (FEV1) percent predicted, FEV1/forced vital capacity (FVC), and diffusing capacity for carbon monoxide (DLco) percent predicted were significantly lower in those with elevated PASP or TRV. Sputum interleukin-8, peripheral interleukin-8, peripheral interferon-γ levels, and CD8+ T-cell expression of CD69+ were associated increased with increasing PASP and TRV. Log NT-proBNP was significantly higher with increasing PASP and TRV. Left ventricular function was not associated with PASP or TRV.
Conclusions
Echocardiographic manifestations of pulmonary hypertension are common in HIV and are associated with respiratory symptoms, more advanced HIV disease, airway obstruction, abnormal DLco, and systemic and pulmonary inflammation. Pulmonary hypertension and COPD coexist in HIV and may arise secondary to common inflammatory mechanisms.
Keywords: HIV, pulmonary hypertension, emphysema, COPD, inflammation
Introduction
With the advent of combination antiretroviral therapy (ART), chronic pulmonary disorders such as obstructive lung disease and pulmonary arterial hypertension (PAH) have become a more frequent cause of morbidity and mortality in the HIV-infected population. Chronic obstructive pulmonary disease (COPD), defined as irreversible airflow obstruction, encompasses both airways obstruction and anatomic emphysema and is associated with an abnormal inflammatory response [1]. Several studies have shown that HIV is an independent risk factor for a diagnosis of COPD [2–4]. PAH is also increased in HIV infection with a pathological or right-heart catheterization diagnosis in 0.5% of HIV-infected individuals [5, 6] and with as many as 35–57 percent of HIV-infected individuals having pulmonary artery systolic pressures (PASP) above 30 mmHg on echocardiography [7, 8].
Pulmonary hypertension has been linked to COPD in the HIV-uninfected population, but the relationship between the two diseases has not been documented in HIV infection. In HIV-uninfected individuals with COPD, pulmonary hypertension has historically been thought to be primarily due to hypoxemia, but there is actually only a weak correlation of pulmonary artery pressures with hypoxemia. In addition, elevation of pulmonary artery pressures can be seen even in individuals with less severe COPD [9, 10]. Biomarkers such as interleukin (IL)-6, IL-8, and tumor necrosis factor (TNF)-α are increased in individuals with both COPD and pulmonary hypertension, and systemic and lung inflammation are associated with both COPD and pulmonary hypertension, suggesting that a common trigger of inflammation could link the two processes [11, 12].
Given the immune activation and persistent inflammation phenotype associated with HIV infection, similar changes might drive development of COPD and pulmonary hypertension in those infected with HIV. We investigated the relationship between non-invasive Doppler-echocardiographic measures of pulmonary artery systolic pressures and lung function parameters in a cohort of HIV-infected outpatients and tested the relationship of pulmonary artery pressures to pulmonary and systemic inflammation.
Methods
Participants
Individuals with documented HIV infection who were 18 years of age or older were recruited from the University of Pittsburgh HIV/AIDS clinic between July 1, 2007 and April 30, 2010. A description of a subset of the cohort has been published previously [13]. Participants were recruited using posted advertisements, word of mouth, and by use of a research registry. Exclusion criteria were new or increasing respiratory symptoms within the past four weeks, fever within the past four weeks, or a contraindication to performing pulmonary function testing. The University of Pittsburgh IRB approved the protocol, and all participants signed written informed consent.
Data collection
Demographic and clinical data were collected by participant interview and medical record review. Variables included age, race, gender, smoking history, alcohol use, intravenous drug use (IDU), and antiretroviral use. The most recent CD4+ T-lymphocyte cell count and plasma HIV ribonucleic acid (RNA) level within three months were recorded.. The lower limit of detection for the HIV RNA polymerase chain reaction assay was 50 copies/mL. Combination ART was defined as use of at least three antiretroviral agents from at least two classes of medications in the past 3 months. Respiratory symptoms were assessed using a modified version of the American Thoracic Society (ATS) DLD questionnaire [14].
Pulmonary function testing
All participants performed post-bronchodilator spirometry in accordance with ATS standards [15, 16]. Post-bronchodilator forced expiratory volume in 1 second (FEV1) and forced expiratory capacity (FVC) were calculated using Hankinson prediction equations [17]. Diffusing capacity for carbon monoxide (DLCO) percent predicted was calculated using Neas prediction equations and corrected for hemoglobin and carboxyhemoglobin [18].
Doppler-echocardiography
Echocardiography was performed by a single operator on the same echocardiography machine (GE-Vingmed Vivid 7, GE Vingmed Ultrasound, Horten, Norway) and read by one of three cardiologists blinded to participant status. Standard 2D views, pulsed and continuous wave Doppler measurements were obtained as per the American Echocardiography Association recommendations [19, 20]. Peak pulmonary artery systolic pressures (PASP) were estimated by calculating the systolic pressure gradient between the right ventricle and right atrium by the maximum velocity of the tricuspid regurgitant jet (TRV) using the modified Bernoulli equation, and then adding to this gradient an estimated right atrial pressure based on the size of the inferior vena cava and its variation with respiration [21]. Estimated PASP and TRV were the primary outcomes of interest and were examined as continuous variables and dichotomized as normal or elevated. We used a conservative estimate of ≥40 mmHg to define an elevated PASP [7]. An elevated TRV was defined as ≥3.0 m/sec. In cases where there was not a sufficient tricuspid jet to determine PASP and TRV and right ventricular size was normal (n=9), we classified PASP and TRV as normal as individuals with pulmonary hypertension would be expected to have measurable tricuspid regurgitation and/or increased right ventricle size [7, 22]. Left ventricular ejection fraction was determined by visual estimate, and diastolic function was estimated according to standard protocol [23].
Induced sputum analyses
Sputum cell counts and percentages were determined from sputum samples induced with nebulized 3% saline [24]. Counts were performed as previously described, and samples with fewer than 30% squamous cells were considered acceptable for interpretation [25]. Cytokines and chemokines were analyzed in induced sputum supernatant using Luminex (Luminex Corporation, Austin, TX, USA). To minimize chance associations, cytokines and chemokines were selected based on relevance to pulmonary hypertension, HIV infection, or COPD and included IL-1β, IL-2, IL6, IL-8, IL-15, TNF-α, interferon (IFN)-γ, fractalkine, fibroblast growth factor (FGF), macrophage inflammatory protein (MIP)-1α, monocyte chemotactic protein (MCP)-1, vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and CC chemokine-ligand 5 (CCL-5) [11, 12].
Peripheral blood analyses
The same cytokines and chemokines were measured in plasma using Luminex (Luminex Corporation, Austin, TX, USA). Plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) was also analyzed using Roche ProBNP assay kit and Elecsys 2010 according to the manufacturer’s instructions. Because renal dysfunction can elevate NT-proBNP levels [26], we calculated the glomerular filtration rate (GFR) using the Modification of Diet in Renal Disease equation [27] and excluded participants with a GFR below 60 ml/min per 1.73 m2 from NT-proBNP analyses. In a subset of individuals in whom fresh peripheral blood mononuclear cells (PBMCs) were available, PBMCs were obtained by venipuncture and isolated by density gradient centrifugation. Cell surface staining was performed using fluorochrome-conjugated monoclonal antibodies including isotype controls: fluorescein isothiocyanate (FITC) anti-CD8; phycoerythrin (PE) anti-CD38, anti-CD25, and anti-CD69; phycoerythrin-cyanine 7 (PE-Cy7) anti-CD3; and allophycocyanin (APC) CD4 (BD Pharmingen, San Diego, CA). Flow cytometry was performed on ≥10,000 live cells after lymphocyte gating using a BD FACS Calibur (BD Biosciences, San Jose, CA).
Statistical analyses
Statistical analyses were performed using Stata version 10 (StataCorp, College Station, Texas, USA). Distributions of demographic and clinical characteristics were determined for the entire cohort. Prevalences of an elevated estimated PASP and TRV were calculated. Associations of demographic and clinical characteristics and respiratory symptoms with elevated PASP and TRV were then determined using Pearson correlation coefficient, Wilcoxon ranksum, or t-tests. Viral levels were log-transformed and a value of 49 copies/ml was used for continuous analyses. Pulmonary function variables (post-bronchodilator FEV1 percent predicted, FEV1/FVC, DLco percent predicted adjusted for hemoglobin and carboxyhemoglobin) were analyzed by t-tests by elevated PASP and TRV. Stepwise forward and reverse multivariable logistic regression was then performed to determine the independent relationship of pulmonary function to echocardiographic measures by including variables associated with either elevated PASP or TRV with a significance of at least p=0.05. If strongly correlated variables (i.e. CD4 and HIV RNA levels) were both found to be significant, the variable with the strongest association was included in multivariable models.
Relationship of sputum cell counts, sputum cytokines, and plasma cytokines to continuous values of PASP and TRV were determined using t-tests, Fisher’s exact test, or chi-square test. Continuous values of PASP and TRV were used in order to improve power to detect associations. Sputum cytokine levels and plasma cytokine levels were either log-transformed to approximate normality or dichotomized as detectable or undetectable if no normal transformation was possible. These values were available in a subset of the cohort (n=93 for sputum cell counts, n=87 for sputum cytokines, n=114 for plasma cytokines). Relationship of activated T cells (n=23) to PASP and TRV were performed using Pearson’s correlation.
To test the ability of the plasma cytokines and the presence of respiratory symptoms to predict an abnormal echocardiography result, we created a prediction variable that combined the presence of shortness of breath and either an elevated plasma IL-8 or IFN-γ level, defined as above the median level seen in the cohort. We then calculated the sensitivity, specificity, and positive and negative likelihood ratios for the presence of shortness of breath and elevated cytokines for an abnormal PASP or TRV. We also examined the prevalence of an abnormal PASP or TRV in those with shortness of breath and elevated cytokines compared to those without shortness of breath or elevated cytokines.
In order to determine the role of left-sided cardiac dysfunction in elevated right-sided heart pressures, we correlated the lower limit of the ejection fraction and the presence of diastolic dysfunction with elevated PASP and TRV and with continuous values of PASP and TRV. To determine if the echocardiographic findings of PASP and TRV were associated with cardiac strain, we also examined the correlation of the log of plasma NT-proBNP levels to absolute values of PASP and TRV using the Pearson correlation coefficient in individuals with GFR of at least 60 ml/min per 1.73 m2 (n=72).
Results
Description of cohort
One hundred and sixteen individuals completed both pulmonary function testing and echocardiography. Average age was 47.7 years and 30.2% were female (Table 1). Mean PASP was 34.3 mm Hg (standard deviation [SD] 6.9) and mean TRV was 2.5 m/sec (SD 0.32). Seventy-seven participants (66.4%) had a PASP (≥30 mmHg) with 18 (15.5%) meeting criteria for an elevated PASP (≥40 mmHg) and 9 (7.8%) having an elevated TRV (≥3 m/sec) (Table 1).
Table 1.
Demographic and clinical characteristics of HIV-infected participants.
| Characteristic | Overall, n=116 | Elevated PASP n=18 | Elevated TRV n=9 |
|---|---|---|---|
|
| |||
| Age, mean years (SD) | 47.7 (9.3) | 50.8 (4.1) | 46.8 (4.1) |
|
| |||
| Male, n (%) | 81 (69.8) | 11 (61.1) | 5 (55.6) |
|
| |||
| Race/ethnicity, n (%) | |||
| White | 60 (51.7) | 9 (50.0) | 3 (33.3) |
| African-American | 51 (44.0) | 9 (50.0) | 6 (66.6) |
| Hispanic | 5 (4.3) | 0 | 0 |
|
| |||
| HIV risk factor, n (%) | |||
| MSM | 54 (46.6) | 8 (44.4) | 4 (44.4) |
| Heterosexual | 38 (32.8) | 4 (22.2) | 3 (33.3) |
| Other or unknown | 24 (20.7) | 6 (33.3) | 2 (22.2) |
|
| |||
| Hepatitis ever, n (%) | 15 (13.0) | 2 (11.1) | 1 (11.1) |
|
| |||
| ART use, n (%) | 103 (88.8) | 15 (83.3) | 7 (77.8) |
|
| |||
| Ever smoker, n (%) | 95 (81.9) | 14 (77.8) | 45 (86.5) |
|
| |||
| Current smoker, n (%) | 64 (55.2) | 11 (61.1) | 7 (77.8) |
|
| |||
| IDU in past 6 months, n (%) | 1 (0.86) | 0 | 0 |
|
| |||
| CD4 cell count, median cells/μl, (range) | 578 (24–1798) | 644 (24–1493) | 606 (24–1213) |
|
| |||
| CD4 cell count below 200 cells/μl, n (%) | 9 (7.8) | 3 (16.7) | 3 (33.3)* |
|
| |||
| Mean log10 HIV viral level, (SD) | 2.18 (1.04) | 2.55 (1.36) | 3.15 (0.95)* |
|
| |||
| Detectable HIV viral level, n (%) | 32 (27.6) | 6 (33.3) | 5 (55.6) |
Abbreviations: ART, antiretroviral therapy; IDU, intravenous drug use; MSM, men who have sex with men; PASP, pulmonary artery systolic pressure; SD, standard deviation; TRV, tricuspid regurgitant velocity. Abnormal PASP defined as at least 40 mm Hg and abnormal TRV defined as at least 3.0m/sec. Detectable HIV viral level defined as less than 50 copies/ml.
p=0.022 for comparison of CD4 cell count below 200 cells/μl in elevated and normal TRV; p=0.0032 for comparison of log10 HIV RNA level in elevated and normal TRV.
Clinical correlates of PASP and TRV
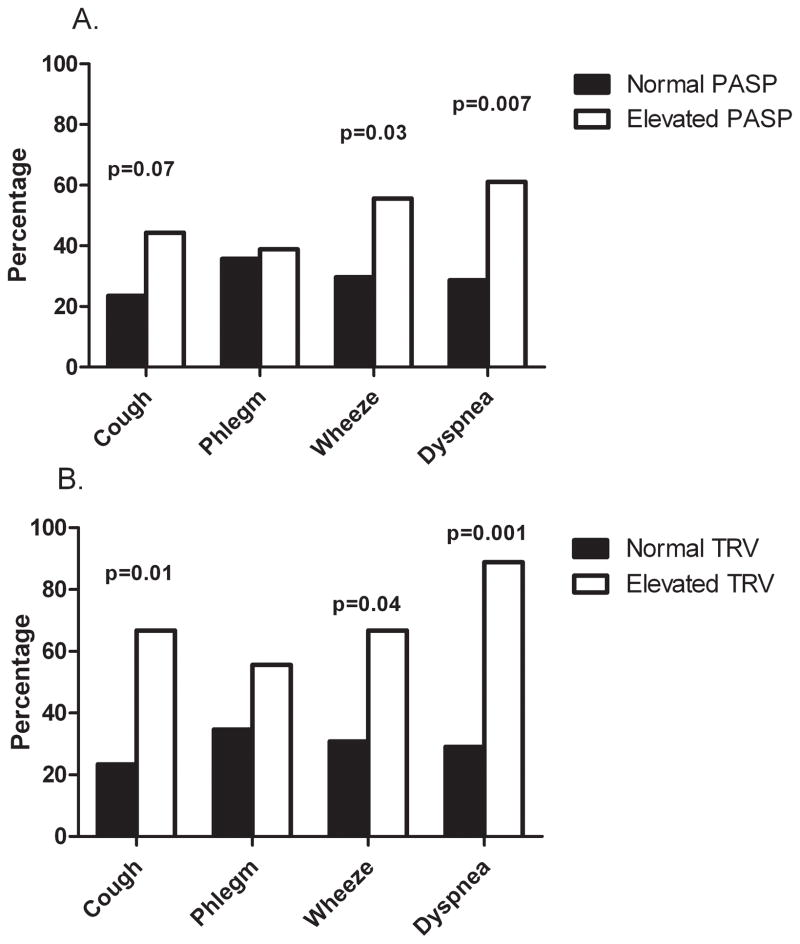
We examined clinical predictors of elevated PASP and TRV. Variables such as age, gender, race, intravenous drug use, cocaine use, hepatitis, and smoking history were not significantly associated with elevated PASP or TRV levels (Table 1). Absolute CD4 cell count did not differ in those with elevated PASP or TRV, but the percentage of individuals with CD4 cell counts below 200 cells/μl was significantly higher in those with an elevated TRV (33.3% vs. 5.7%, p=0.022). HIV RNA levels were similar in those with an elevated PASP (2.55 vs. 2.10 log10 copies/ml, p=0.10), but were significantly greater in participants with an elevated TRV (3.15 vs. 2.11 log10 copies/ml, p=0.0032). Having a detectable HIV RNA level tended to be more common in those with an elevated TRV (55.6% vs. 25.2%, p=0.051). ART use and use of ritonavir or abacavir were not associated with elevated PASP or TRV. Individuals with elevated PASP or TRV reported more respiratory symptoms except for phlegm production (Figure 1).
Figure 1.
Percentage of participants reporting respiratory symptoms compared by A. Normal or elevated pulmonary artery systolic pressures (≥40 mm Hg) and B. Normal or elevated tricuspid regurgitant velocity (≥3.0 m/sec).
Abbreviations: PASP, pulmonary artery systolic pressure; TRV, tricuspid regurgitant velocity
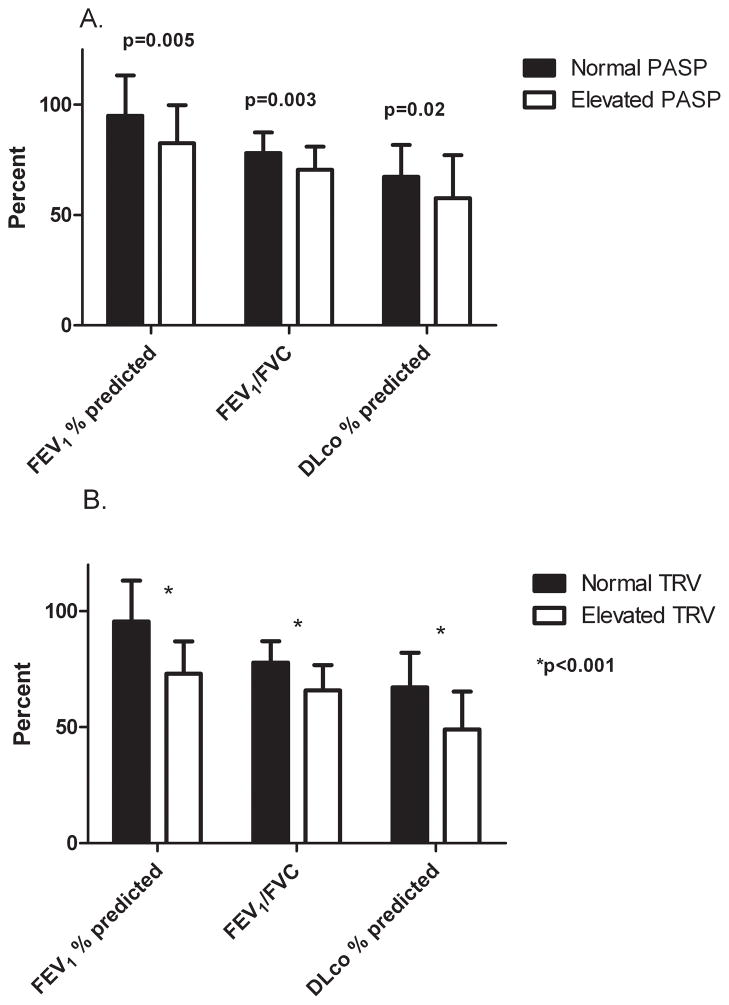
Relationship of echocardiographic measurements to pulmonary function
Elevated PASP and TRV were significantly associated with worse airflow obstruction by post-bronchodilator FEV1 percent predicted and FEV1/FVC and with decreased DLco values in univariate analyses (Figure 2). Multivariate modeling was not performed for PASP as no other variables were significantly associated with elevated values. In multivariate analyses of elevated TRV adjusting for CD4 cell count below 200 cells/μl, elevated TRV was independently associated with FEV1 percent predicted (odds ratio [OR]=0.0007 for each % decrease, 95% confidence interval [CI]=6.2×10−6 to 0.14, p=0.003), FEV1/FVC (OR=0.00003 for each % decrease, 95% CI=4.3×10−8 to 0.023, p=0.002, and DLco (OR= 0.001 for each percent decrease, 95% CI=8.4×10−6 to 0.14, p=0.006).
Figure 2.
Comparison of pulmonary function values for HIV-infected individuals with A. Normal or elevated pulmonary artery systolic pressures (≥40 mm Hg) and B. Normal or elevated tricuspid regurgitant velocity (≥3.0 m/sec) showing significantly worse pulmonary function in those with elevated echocardiographic pressures.
Abbreviations: PASP, pulmonary artery systolic pressure; TRV, tricuspid regurgitant velocity
Relationship of inflammation to PASP and TRV
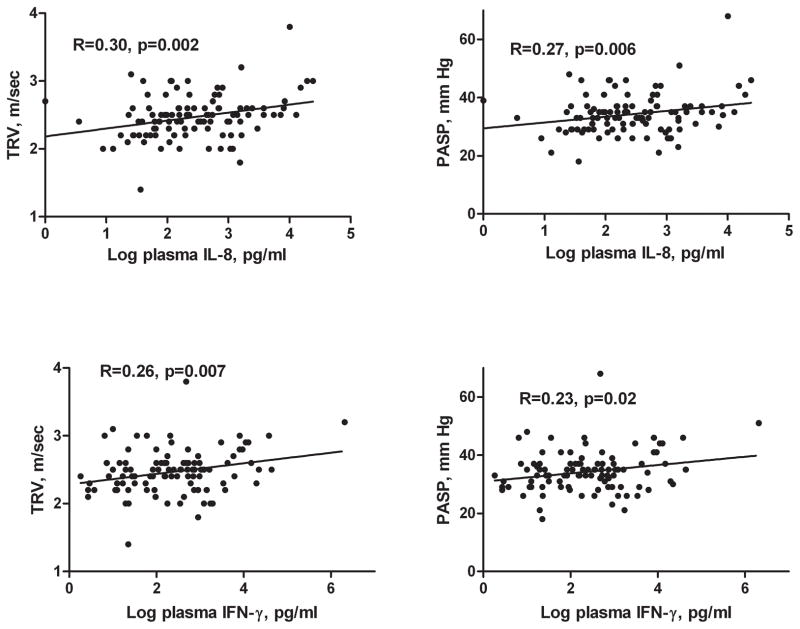
We then examined the relationship of pulmonary artery pressure measurements to pulmonary and systemic inflammation. Percent sputum neutrophils tended to increase with increasing TRV (R=0.19, p=0.08), but did not reach statistical significance. Several sputum and plasma cytokines were associated with pulmonary pressures. Increasing log sputum IL-8 was significantly associated with higher PASP and TRV (R=0.25, p=0.03 for PASP; R=0.24, p=0.02 for TRV). Log plasma IL-8 was also significantly associated with increasing PASP and TRV (Figure 3). Log plasma interferon-γ levels were a significant predictor of higher PASP and TRV (Figure 3). Other sputum and plasma cytokines were not significantly associated with PASP or TRV (Table 1S). There was a significant correlation between increasing percentage of CD8+CD69+ T cells and higher PASP (R=0.45, p=0.037) and higher TRV (R=0.42, p=0.044). There was no relationship between percentages of CD8+CD38+ or CD8+CD25+ T cells and echocardiographic variables.
Figure 3.
Relationship of peripheral cytokines to PASP and TRV.
Abbreviations: IL, interleukin; IFN, interferon; PASP, pulmonary artery systolic pressure; TRV, tricuspid regurgitant velocity.
Relationship of respiratory symptoms and cytokine levels to PASP and TRV
In individuals with an abnormal PASP, the sensitivity and specificity of the presence of shortness of breath combined with either elevated plasma IL-8 or IFN-γ levels were 38.9% and 81.6%, respectively. The positive likelihood ratio was 2.11 and the negative likelihood ratio was 0.75. For an abnormal TRV, the sensitivity was 55.6% with a specificity of 81.3%. The positive likelihood ratio was 2.97, and the negative likelihood ratio was 0.55. In individuals reporting shortness of breath who had either an elevated plasma IL-8 or IFN-γ, 28.0% had an abnormal PASP and 20.0% had an abnormal TRV. In those without shortness of breath or elevated plasma cytokines, only 6.9% had an abnormal PASP and 3.5% had an abnormal TRV.
Measurements of cardiac function
Because HIV infection has previously been associated with increased left-sided heart failure that might in part lead to elevated pulmonary artery pressures [28–30], we examined the relationship of ejection fraction and diastolic dysfunction to PASP and TRV. Only 2 participants had an ejection fraction of 40% or lower, and there was no association of the lower limit of the ejection fraction with absolute values of PASP (p=0.53) and TRV (p=0.61) or elevated PASP and TRV (mean ejection fraction 57% vs. 57%, p=0.85 for PASP and 57% vs. 57%, p=0.90 for TRV). There was no relationship between either absolute values of PASP or TRV and presence of diastolic dysfunction (mean PASP 34.5 mmHg without diastolic dysfunction vs. 32.7 mmHg with diastolic dysfunction, p=0.32; mean TRV 2.5 m/sec without diastolic dysfunction vs. 2.4 m/sec with diastolic dysfunction, p=0.37). Individuals with elevated PASP or TRV were no more likely to have diastolic dysfunction than those without (11.1% with elevated PASP vs. 14.3% with normal PASP, p=0.72; 0% with elevated TRV vs. 15.0% with normal TRV, p=0.21)
To determine if right-sided echocardiographic pressures indicated the presence of cardiac strain, we measured systemic NT-proBNP levels in participants with a GFR greater than 60 mL/min per 1.73 m2 (n=72). There was a significant positive correlation of the log of the NT-proBNP levels with both PASP (R=0.29, p=0.01) and TRV (R=0.25, p=0.03). Mean log NT-proBNP levels were not associated with the lower level of the ejection fraction (p=0.48) or with diastolic dysfunction (p=0.36).
Discussion
This study documents a high prevalence of echocardiographic manifestations of pulmonary hypertension in a cohort of HIV-infected outpatients and is the first to link signs of pulmonary hypertension with impaired lung function in HIV infection. We found that increasing pulmonary pressures were significantly associated with both worsening airflow and lower DLco. In addition, we determined that both sputum and peripheral inflammation are related to higher PASP and TRV and that NT-proBNP increases with increasing pressures. Individuals with an elevated PASP or TRV were also more likely to report respiratory symptoms and those with an elevated TRV were also more likely to have lower CD4 cell counts and higher HIV RNA levels. Signs of left-sided heart failure were not correlated to signs of right-sided pressure.
There was a high prevalence of elevated PASP in our cohort. The prevalence of pulmonary hypertension in HIV has been estimated to be 0.5 percent based on pathology and catheterization studies [5, 6], but a study examining echocardiography in a clinic-based cohort of HIV-infected individuals found that 35 percent had a PASP of at least 30 mmHg and 6.6% of at least 40 mmHg [7]. Another recent study reported that 57 percent of HIV-infected individuals had pulmonary artery pressures greater or equal to 30 mmHg and 7 percent had pressures greater than or equal to 40 mmHg [8]. In the current study, we found an even greater prevalence of elevated PASP. This increase was not due to other risk factors such as intravenous drug use, hepatitis, or smoking as these characteristics were either comparable or less common in our cohort. It is possible that other differences between the cohorts accounted for the increased prevalence.
We also discovered a high prevalence of elevated TRV. Few studies of echocardiography in HIV infection have reported the TRV, but a TRV of 2.5 m/second or greater is a marker for mortality in at-risk populations such as those with sickle cell disease [31]. Using the conservative estimate of an elevated TRV as at least 3.0 m/second, we had a similar prevalence as that seen in some sickle cell populations [31]. Although we do not have mortality data in our cohort, participants with an elevated TRV had increased respiratory symptoms suggesting that this finding is clinically relevant. Our findings indicate that the presence of shortness of breath in association with inflammation has a reasonable specificity for the presence of an elevated TRV on echocardiogram and that abnormal echocardiographic findings are uncommon in those without shortness of breath and low cytokine levels.
The relationship of pulmonary hypertension to various HIV-related variables has been debated. HIV-associated pulmonary hypertension appears to develop at all stages of HIV. Although not all studies have found a relationship of CD4 cell counts or HIV RNA levels to pulmonary hypertension, one study found that pulmonary hypertension documented by right heart catheterization was increased in individuals with CD4 cell counts below 200 cells/μl [6, 7]. We also found that participants with elevated TRV were more likely to have a CD4 cell count below 200 cells/μl. Individuals with an elevated TRV also had significantly higher HIV RNA levels, suggesting that more advanced immunodeficiency may play a role in disease development, possibly secondary to chronic immune activation. Data on the relationship of pulmonary hypertension to ART use has been conflicting. While some studies have found improvements in pulmonary hemodynamics with ART or no relationship with ART, others have found increased risk with certain agents [6, 32–34]. We did not find any associations of PASP and TRV with antiretroviral therapy in general or with specific use of abacavir or ritonavir as previously reported [8].
This study is the first to demonstrate that elevations in pulmonary artery pressures measured by echocardiography are independently associated with lung function in HIV-infected individuals. This association included both spirometry measurements and DLco values. The findings linking pulmonary pressures and lung function abnormalities have direct clinical implications for the care of HIV-infected individuals. Pulmonary hypertension in the setting of COPD in the HIV-uninfected population is related to an increased risk of severe exacerbations, increased utilization of health resources, and decreased life expectancy [35–37]. In addition, studies have documented that elevated pulmonary artery pressures in COPD are associated with an increased mortality, independent of severity of lung disease [38]. These studies suggest that HIV-infected individuals with both obstructive lung disease and elevated pulmonary artery pressures are a high-risk group.
The causal direction of the association of pulmonary hypertension and COPD is unknown. While pulmonary hypertension is associated with decreases in DLco, the thickening and obliteration of vasculature that lead to these decreases would not be expected to result in decrements of the airway measures of FEV1 and FEV1/FVC. Alternatively, it is possible that COPD leads to elevation of pulmonary pressures. In the HIV-uninfected population, airway obstruction has been associated with elevation in pulmonary artery pressures [39]. Furthermore, treatment of acute COPD exacerbations has been associated with improvement in right ventricular dysfunction [40]. In the HIV-uninfected population, the relationship of COPD to pulmonary hypertension has historically been thought to be primarily due hypoxemia. The participants in our cohort generally had mild obstructive disease and none were using oxygen, so this mechanism seems unlikely. In addition, the link of pulmonary hypertension with hypoxemia in COPD has been questioned; and in fact, vascular changes of pulmonary hypertension can be seen in HIV-uninfected COPD patients with milder lung function impairment [9, 10]. It has been suggested that a common underlying process could drive pathogenesis of these processes.
One possible link of COPD and pulmonary hypertension that is particularly relevant to HIV is inflammation. Both systemic and pulmonary inflammation have been implicated in the pathogenesis of pulmonary hypertension as well as COPD. In addition, chronic immune activation is common in HIV and has been associated with progression to AIDS and with end-organ damage such as cardiovascular disease [41–46]. Although ART lessens immune activation, levels do not return to normal, particularly in persons with a suboptimal response [47].
We found increased pulmonary and systemic inflammation correlated with measures of pulmonary hypertension in our cohort. The findings of elevated sputum IL-8, plasma IL-8, and plasma IFN-γ levels in association with higher pulmonary artery pressures suggest that inflammation could be involved in the pathogenesis of pulmonary hypertension in HIV infection. Lung and peripheral concentrations of IL-8 have been linked to severity of airway obstruction in the HIV-uninfected population, and plasma levels are also associated with cardiovascular disease and pulmonary hypertension [48–50]. IFN-γ overproduction results in emphysema in a mouse model [51], and IFN-γ is also increased in human COPD [52]. We also found that CD8+ T cell expression of CD69, an early activation marker in HIV, was associated with increasing PASP and TRV. Although CD38+ and CD25+ activation markers were not upregulated, we may have lacked power to detect these associations. Activated T cells, particularly CD8+ cells, are seen during the chronic immune activation associated with HIV, but have not previously been reported in HIV-associated pulmonary hypertension. Triggers of this inflammation in HIV-infected individuals with pulmonary hypertension could include the virus itself, bacterial antigens from microbial translocation, or presence of co-infections such as cytomegalovirus or hepatitis. Our findings suggest that immune activation and inflammation might link pulmonary hypertension and COPD in HIV infection, and that inflammatory pathways or triggers of inflammation could be targeted as novel treatments of pulmonary hypertension in HIV.
One limitation of our study is that pulmonary artery pressures were measured by echocardiography. Although the correlation of echocardiographic measurements with right heart catheterization findings may be variable, it is likely that the findings are clinically significant. HIV-infected individuals with elevated estimated PASP and TRV had more complaints of respiratory symptoms despite similar smoking histories as those with normal values, and the correlation of echocardiographic findings with pulmonary function, inflammation, and elevated NT-proBNP suggests that these variables are clinically relevant. In addition, in the sickle cell population, a TRV above 2.5 m/second has been shown to predict mortality [31]. Taken together, these findings suggest that HIV-infected individuals with echocardiographic signs of pulmonary hypertension may be at risk not only for progression of pulmonary hypertension, but also potentially for other serious outcomes.
Other limitations include the possibility that we may have lacked sufficient power to detect additional significant associations with sputum and peripheral cytokines or with activated peripheral T cells, and it is possible that other aspects of inflammation are related to pulmonary artery pressures in this population. Nonetheless, our data are the first to link pulmonary vascular disease to an inflammatory phenotype in HIV, and future work should investigate the detailed immune response associated with pulmonary vascular disease as well as potential triggers. There may also have been other covariates that affected pulmonary pressures that we could not detect that might also have been related to pulmonary function. Finally, this analysis is a cross-sectional study, and we do not know how elevated pulmonary pressures in the setting of COPD influence long-term outcomes.
In summary, this study is the first to link echocardiographic signs of pulmonary hypertension to lung function impairment in HIV-infected individuals. We found that abnormal estimated PASP and TRV are common in an HIV-infected outpatient cohort and associated with more advanced HIV disease. There was a strong association of PASP and TRV values with both airway obstruction and diffusing capacity abnormalities. The association with peripheral and pulmonary inflammation implies that a common trigger of inflammatory pathways might drive progression of both PAH and COPD. Given data in HIV-uninfected populations linking pulmonary hypertension in COPD to excess mortality and morbidity, the current findings in HIV-infected individuals indicate that this population may be particularly at risk and future studies should examine the effects of these diseases on health outcomes.
Supplementary Material
Acknowledgments
Sources of funding: R01 HL083461, HL083461S, and Gilead Sciences (AM); NIH T32 HL007563 (MG); P50 HL084948, N01 HR76193 (FS); P01 HL103455 (MTG, AM).
Footnotes
Conflicts of interest: For the remaining authors, none were declared.
Author contributions:
Alison Morris designed the study, analyzed the data, and wrote the manuscript. Matthew R. Gingo performed data analyses and provided critical review of the manuscript. M. Patricia George assisted with performance and interpretation of flow cytometry analyses and provided critical review of the manuscript. Lorrie Lucht assisted with laboratory assays, data analysis, and critical review of the manuscript. Cathy Kessinger was responsible for execution of the study, data entry, and critical review of the manuscript. Vikas Singh performed and analyzed flow cytometry data and provided critical review of the manuscript. Maria Hillenbrand assisted with laboratory assays, data analysis, and critical review of the manuscript. Michelle Busch assisted with laboratory assays, data analysis, and critical review of the manuscript. Deborah McMahon assisted with conception and execution of the study design and critical review of the manuscript. Karen Norris assisted with performance and interpretation of flow cytometry analyses and provided critical review of the manuscript. Hunter Champion assisted with performance and interpretation of echocardiographic data and with writing of the manuscript. Mark Gladwin assisted with interpretation of echocardiographic data NT-proBNP levels and with critical review of the manuscript. Yingzhe Zhang performed NT-proBNP analyses, assisted with interpretations of NT-proBNP levels, and provided critical review of the manuscript. Chad Steele performed cytokine analyses, assisted with interpretations of cytokine levels, and provided critical review of the manuscript. Frank Sciurba assisted with conception and execution of the study design and critical review of the manuscript.
References
- 1.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 2.Crothers K, Butt AA, Gibert CL, Rodriguez-Barradas MC, Crystal S, Justice AC. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest. 2006;130:1326–1333. doi: 10.1378/chest.130.5.1326. [DOI] [PubMed] [Google Scholar]
- 3.Crothers K, Huang L, Goulet JL, Goetz MB, Brown ST, Rodriguez-Barradas MC, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183:388–395. doi: 10.1164/rccm.201006-0836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diaz PT, King MA, Pacht ER, Wewers MD, Gadek JE, Nagaraja HN, et al. Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Ann Intern Med. 2000;132:369–372. doi: 10.7326/0003-4819-132-5-200003070-00006. [DOI] [PubMed] [Google Scholar]
- 5.Speich R, Jenni R, Opravil M, Pfab M, Russi EW. Primary pulmonary hypertension in HIV infection. Chest. 1991;100:1268–1271. doi: 10.1378/chest.100.5.1268. [DOI] [PubMed] [Google Scholar]
- 6.Sitbon O, Lascoux-Combe C, Delfraissy JF, Yeni PG, Raffi F, De Zuttere D, et al. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med. 2008;177:108–113. doi: 10.1164/rccm.200704-541OC. [DOI] [PubMed] [Google Scholar]
- 7.Hsue PY, Deeks SG, Farah HH, Palav S, Ahmed SY, Schnell A, et al. Role of HIV and human herpesvirus-8 infection in pulmonary arterial hypertension. AIDS. 2008;22:825–833. doi: 10.1097/QAD.0b013e3282f7cd42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mondy KE, Gottdiener J, Overton ET, Henry K, Bush T, Conley L, et al. High prevalence of echocardiographic abnormalities among HIV-infected persons in the era of highly active antiretroviral therapy. Clin Infect Dis. 2011;52:378–386. doi: 10.1093/cid/ciq066. [DOI] [PubMed] [Google Scholar]
- 9.Chaouat A, Bugnet AS, Kadaoui N, Schott R, Enache I, Ducolone A, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:189–194. doi: 10.1164/rccm.200401-006OC. [DOI] [PubMed] [Google Scholar]
- 10.Chaouat A, Naeije R, Weitzenblum E. Pulmonary hypertension in COPD. Eur Respir J. 2008;32:1371–1385. doi: 10.1183/09031936.00015608. [DOI] [PubMed] [Google Scholar]
- 11.Joppa P, Petrasova D, Stancak B, Tkacova R. Systemic inflammation in patients with COPD and pulmonary hypertension. Chest. 2006;130:326–333. doi: 10.1378/chest.130.2.326. [DOI] [PubMed] [Google Scholar]
- 12.Chaouat A, Savale L, Chouaid C, Tu L, Sztrymf B, Canuet M, et al. Role for interleukin-6 in COPD-related pulmonary hypertension. Chest. 2009;136:678–687. doi: 10.1378/chest.08-2420. [DOI] [PubMed] [Google Scholar]
- 13.Gingo MR, George MP, Kessinger CJ, Lucht L, Rissler B, Weinman R, et al. Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. Am J Respir Crit Care Med. 2010;182:790–796. doi: 10.1164/rccm.200912-1858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comstock GW, Tockman MS, Helsing KJ, Hennesy KM. Standardized respiratory questionnaires: comparison of the old with the new. Am Rev Respir Dis. 1979;119:45–53. doi: 10.1164/arrd.1979.119.1.45. [DOI] [PubMed] [Google Scholar]
- 15.Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 16.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 17.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 18.Neas LM, Schwartz J. The determinants of pulmonary diffusing capacity in a national sample of U.S adults. Am J Respir Crit Care Med. 1996;153:656–664. doi: 10.1164/ajrccm.153.2.8564114. [DOI] [PubMed] [Google Scholar]
- 19.Henry WL, DeMaria A, Gramiak R, King DL, Kisslo JA, Popp RL, et al. Report of the American Society of Echocardiography Committee on nomenclature and standards in two-dimensional echocardiography. Circulation. 1980;62:212–217. doi: 10.1161/01.cir.62.2.212. [DOI] [PubMed] [Google Scholar]
- 20.Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: A report from the doppler quantification task force of the nomenclature and standards committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–184. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 21.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70:657–662. doi: 10.1161/01.cir.70.4.657. [DOI] [PubMed] [Google Scholar]
- 22.Borgeson DD, Seward JB, Miller FA, Jr, Oh JK, Tajik AJ. Frequency of Doppler measurable pulmonary artery pressures. J Am Soc Echocardiogr. 1996;9:832–837. doi: 10.1016/s0894-7317(96)90475-7. [DOI] [PubMed] [Google Scholar]
- 23.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 24.Gershman NH, Wong HH, Liu JT, Mahlmeister MJ, Fahy JV. Comparison of two methods of collecting induced sputum in asthmatic subjects. Eur Respir J. 1996;9:2448–2453. doi: 10.1183/09031936.96.09122448. [DOI] [PubMed] [Google Scholar]
- 25.Fahy JV, Boushey HA, Lazarus SC, Mauger EA, Cherniack RM, Chinchilli VM, et al. Safety and reproducibility of sputum induction in asthmatic subjects in a multicenter study. Am J Respir Crit Care Med. 2001;163:1470–1475. doi: 10.1164/ajrccm.163.6.9901105. [DOI] [PubMed] [Google Scholar]
- 26.Tsutamoto T, Wada A, Sakai H, Ishikawa C, Tanaka T, Hayashi M, et al. Relationship between renal function and plasma brain natriuretic peptide in patients with heart failure. J Am Coll Cardiol. 2006;47:582–586. doi: 10.1016/j.jacc.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 27.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 28.Himelman RB, Chung WS, Chernoff DN, Schiller NB, Hollander H. Cardiac manifestations of human immunodeficiency virus infection: a two-dimensional echocardiographic study. J Am Coll Cardiol. 1989;13:1030–1036. doi: 10.1016/0735-1097(89)90256-8. [DOI] [PubMed] [Google Scholar]
- 29.Hsue PY, Hunt PW, Ho JE, Farah HH, Schnell A, Hoh R, et al. Impact of HIV infection on diastolic function and left ventricular mass. Circ Heart Fail. 2010;3:132–139. doi: 10.1161/CIRCHEARTFAILURE.109.854943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butt AA, Chang CC, Kuller L, Goetz MB, Leaf D, Rimland D, et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med. 2011;171:737–743. doi: 10.1001/archinternmed.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 32.Opravil M, Pechere M, Speich R, Joller-Jemelka HI, Jenni R, Russi EW, et al. HIV-associated primary pulmonary hypertension. A case control study. Swiss HIV Cohort Study. Am J Respir Crit Care Med. 1997;155:990–995. doi: 10.1164/ajrccm.155.3.9117037. [DOI] [PubMed] [Google Scholar]
- 33.Zuber JP, Calmy A, Evison JM, Hasse B, Schiffer V, Wagels T, et al. Pulmonary arterial hypertension related to HIV infection: improved hemodynamics and survival associated with antiretroviral therapy. Clin Infect Dis. 2004;38:1178–1185. doi: 10.1086/383037. [DOI] [PubMed] [Google Scholar]
- 34.Degano B, Guillaume M, Savale L, Montani D, Jais X, Yaici A, et al. HIV-associated pulmonary arterial hypertension: survival and prognostic factors in the modern therapeutic era. AIDS. 2010;24:67–75. doi: 10.1097/QAD.0b013e328331c65e. [DOI] [PubMed] [Google Scholar]
- 35.Weitzenblum E, Chaouat A, Canuet M, Kessler R. Pulmonary hypertension in chronic obstructive pulmonary disease and interstitial lung diseases. Semin Respir Crit Care Med. 2009;30:458–470. doi: 10.1055/s-0029-1233315. [DOI] [PubMed] [Google Scholar]
- 36.Kessler R, Faller M, Fourgaut G, Mennecier B, Weitzenblum E. Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:158–164. doi: 10.1164/ajrccm.159.1.9803117. [DOI] [PubMed] [Google Scholar]
- 37.Oswald-Mammosser M, Weitzenblum E, Quoix E, Moser G, Chaouat A, Charpentier C, et al. Prognostic factors in COPD patients receiving long-term oxygen therapy. Importance of pulmonary artery pressure. Chest. 1995;107:1193–1198. doi: 10.1378/chest.107.5.1193. [DOI] [PubMed] [Google Scholar]
- 38.Stone AC, Machan JT, Mazer J, Casserly B, Klinger JR. Echocardiographic evidence of pulmonary hypertension is associated with increased 1-year mortality in patients admitted with chronic obstructive pulmonary disease. Lung. 2011;189:207–212. doi: 10.1007/s00408-011-9293-4. [DOI] [PubMed] [Google Scholar]
- 39.Fayngersh V, Drakopanagiotakis F, Dennis McCool F, Klinger JR. Pulmonary hypertension in a stable community-based COPD population. Lung. 2011;189:377–382. doi: 10.1007/s00408-011-9315-2. [DOI] [PubMed] [Google Scholar]
- 40.Akcay M, Yeter E, Durmaz T, Keles T, Akar Bayram N, Uyar M, et al. Treatment of acute chronic obstructive pulmonary disease exacerbation improves right ventricle function. Eur J Echocardiogr. 2010;11:530–536. doi: 10.1093/ejechocard/jeq013. [DOI] [PubMed] [Google Scholar]
- 41.Deeks SG, Kitchen CM, Liu L, Guo H, Gascon R, Narvaez AB, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 42.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 43.Liu Z, Cumberland WG, Hultin LE, Prince HE, Detels R, Giorgi JV. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 44.van Lelyved S, Gras L, Kesselring A, Zhang S, deWolf F, Wensing A, et al. Incomplete immune recovery on HAART is associated with significantly more cardiovascular events and a trend towards more non-AIDS related malignancies in Dutch ATHENA Cohort. 17th Conference on Retroviruses and Opportunistic Infections; San Francisco. 2010. p. 714. [Google Scholar]
- 45.Guzman-Fulgencio M, Medrano J, Rallon N, Echeverria-Urabayen A, Miguel Benito J, Restrepo C, et al. Soluble markers of inflammation are associated with Framingham scores in HIV-infected patients on suppressive antiretroviral therapy. J Infect. 2011;63:382–390. doi: 10.1016/j.jinf.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 46.Plaeger SF, Collins BS, Musib R, Deeks SG, Read S, Embry A. Immune activation in the pathogenesis of treated chronic HIV Disease: A workshop summary. AIDS Res Hum Retroviruses. 2011 Sep; doi: 10.1089/aid.2011.0213. epublished. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hunt PW, Deeks SG, Rodriguez B, Valdez H, Shade SB, Abrams DI, et al. Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy. AIDS. 2003;17:1907–1915. doi: 10.1097/00002030-200309050-00009. [DOI] [PubMed] [Google Scholar]
- 48.Stockley RA. Progression of chronic obstructive pulmonary disease: impact of inflammation, comorbidities and therapeutic intervention. Curr Med Res Opin. 2009;25:1235–1245. doi: 10.1185/03007990902868971. [DOI] [PubMed] [Google Scholar]
- 49.Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996;153:530–534. doi: 10.1164/ajrccm.153.2.8564092. [DOI] [PubMed] [Google Scholar]
- 50.Soon E, Holmes AM, Treacy CM, Doughty NJ, Southgate L, Machado RD, et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation. 2010;122:920–927. doi: 10.1161/CIRCULATIONAHA.109.933762. [DOI] [PubMed] [Google Scholar]
- 51.Wang Z, Zheng T, Zhu Z, Homer RJ, Riese RJ, Chapman HA, Jr, et al. Interferon gamma induction of pulmonary emphysema in the adult murine lung. J Exp Med. 2000;192:1587–1600. doi: 10.1084/jem.192.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shirai T, Suda T, Inui N, Chida K. Correlation between peripheral blood T-cell profiles and clinical and inflammatory parameters in stable COPD. Allergol Int. 2010;59:75–82. doi: 10.2332/allergolint.09-OA-0126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.