Abstract
The dorsal raphe nucleus (DR) is the major source of serotonin (5-hydroxytryptamine, 5-HT) in the forebrain and dysfunction of this midbrain structure is implicated in affective disorders. The DR is composed of several types of 5-HT and non-5-HT neurons and their excitable-membrane properties are heterogeneous and overlapping. In order to understand how these properties may be generated, we examined the mRNA expression patterns of voltage- and ligand-gated ion channels in the DR using the Allen Mouse Brain Atlas. Since DR cytoarchitecture is organized with respect to the midline, we sought to identify genes that were expressed in a pattern with respect to the midline, either enriched or depleted, rather than those that were homogenously expressed throughout the DR. Less than 10% of the screened genes for voltage-gated ion channels showed patterned expression within the DR. Identified genes included voltage-gated sodium channel beta subunits, potassium channels, P/Q-, N-type calcium channels, as well as the alpha2/delta-1 calcium channel. Several voltage-gated chloride channels were also identified, although these may function within intracellular compartments. Of the ligand-gated ion channels examined, 20% showed patterned expression. These consisted primarily of glutamate and GABA-A receptor subunits. The identified genes likely contribute to unique excitable properties of different groups of neurons in the DR and may include novel pharmacologic targets for affective disorders.
Keywords: Serotonin, Depression, Ion channel, Dorsal raphe
1. Introduction
Serotonin (5-hydroxytryptamine, 5-HT) neurotransmission modulates a variety of behavioral states such as mood, motivation and arousal. Reflecting its widespread behavioral influence, 5-HT producing neurons of the dorsal raphe nucleus (DR) widely innervate the brain (Peyron et al., 1998; Steinbusch et al., 1981). 5-HT neurons represent about a third of the neurons within the DR and are located primarily in clusters on the midline (Descarries et al., 1982; Jacobs and Azmitia, 1992). When neurons in the DR were initially characterized electrophysiologically, unique properties of putative 5-HT neurons were identified. In vivo extracellular recordings of rat midline DR identified characteristic pacemaker firing and biphasic extracellular waveforms (Aghajanian et al., 1968). Later work described a broad action potential, large slow afterhyperpolarization (AHP) potential, slow interspike depolarization, and high input resistance (Vandermaelen and Aghajanian, 1983).
Subsequently, electrophysiological recordings coupled with post-hoc immunohistochemical identification of 5-HT neurons revealed that 5-HT neurons themselves exhibit a range of characteristics, overlapping with those of non-5-HT neurons (Calizo et al., 2011; Kirby et al., 2003; Li et al., 2001; Marinelli et al., 2004). 5-HT neurons tended to have a broader action potential and greater input resistance than non-5-HT neurons, but these characteristics do not definitively identify 5-HT neurons. Moreover, features of the AHP are highly variable in DR neurons. Evidence suggests that fast-firing neurons in the DR are largely GABAergic (Allers and Sharp, 2003), however there may be diversity within GABAergic neuron populations as well.
In order to identify genes that could contribute to the membrane properties of DR neurons we sought to identify voltage- and ligand-gated ion channel genes that had a patterned distribution in the DR, rather than those that were present ubiquitously. The DR is a midline structure, with pronounced medio-lateral organization (Fig. 1). Therefore we screened to identify genes that exhibited patterns of expression with respect to the midline. Identification of the ion channels that influence the active membrane properties of various DR cell populations could enable future pharmacologic interventions for affective disorders. Additionally, mapping the topography of enriched ion channels provides future targets for genetic or pharmacological manipulation and should accelerate unraveling the electrochemical underpinnings of DR neuronal function.
Fig. 1.

Reciprocal midline and paramedial expression of two major cell types in the DR provided the basis for screening for genes expressed in medio-lateral patterns. A1. Expression of tryptophan hydroxylase 2 (Tph2), a marker for 5-HT neurons, is visible at the base of the aqueduct (arrows); Tph2 cells are outlined. The outline from this image is transferred to several other sagittal sections in subsequent figures as a reference for the distribution of 5-HT cells. Coronal sections at rostral (A2.), middle (A3.), and caudal (A4.) levels: note Tph2 expressing cells are enriched on the midline (bracketed area ‘M’), with less expression paramedially. B1–4. GABAergic neurons in the DR defined by the expression of the Vesicular GABA Transporter, Slc32a1 or VGAT. In sagittal section (B1.) note the low density of expression in the circled area where Tph2-expressing cells would lie. In coronal sections at rostral (B2.), middle (B3.), and caudal (B4.) levels, VGAT expressing neurons are more abundant paramedially (bracketed area, ‘PM’) than on the midline.
2. Results
All of the identified genes are summarized in Table 1.
Table 1.
Identified genes and their midline enrichment score (midline expression intensity–paramedial expression intensity). Positive numbers reflect genes enriched on the midline, and negative numbers reflect genes enriched paramedially.
| Sodium channels | Calcium channels | Glutamate receptors | |||
| Scn2b | 1.5 | Cacna1a | 2 | Gria3 | −1 |
| Scn3b | 2.5 | Cacna1b | 2 | Gria4 | 1 |
| Scn4b | −2 | Cacna1h | 0.5 | Grik1 | −1 |
| Cacna2d1 | 2.5 | Grik2 | 1 | ||
| Potassium channels | Cacnag3 | 2 | Grik5 | 2 | |
| Kcna1 | −2 | Cacnag5 | 1 | ||
| Kcna2 | −1 | GABA receptors | |||
| Kcna4 | 2 | Chloride channels | Gabra1 | −1 | |
| Kcnc1 | −1 | Ttyh3 | 2 | Gabrb1 | 1 |
| Kcnh8 | 1 | Clcn3 | 2 | Gabrb2 | −1 |
| Kcnj6 | 1 | Clcn5 | 1 | Gabrg2 | 1 |
| Kcnk1 | −1 | Clcn6 | 0.5 | Gabrg3 | 1 |
| Kcnk9 | 1 | Gabrq | −1 | ||
| Kcnq3 | 1 | Other | |||
| Chrna6 | 1 | ||||
| Chrnb2 | 0.5 | ||||
| Glra2 | 2 | ||||
| Glrb | –1 | ||||
2.1. Voltage gated ion channels
Of the 139 voltage gated ion channels listed by IUPHAR, 21 genes were not available on the Allen Mouse Brain Atlas. Of the remaining 118 genes, 51 (42%) had some expression visible in the DR but were ubiquitous throughout the DR and did not exhibit a pattern. Only 12 (10%) exhibited a patterned expression with respect to the midline. An additional 5 ion channels were identified using the NeuroBLAST tool to have selective expression patterns.
2.2. Voltage gated sodium channels (Nav)
9 sodium channel alpha subunits were searched, of these subunits 2 were not available, and among the remainder none exhibited a notable midline or paramedial expression pattern. However, one sodium channel beta subunit, Scn3b, was identified via a NeuroBLAST search, and subsequently we examined Scn1–4b. Of these SCN2b was enriched on the midline (Fig. 2) and Scn4b was enriched paramedially, just ventral to the DR.
Fig. 2.
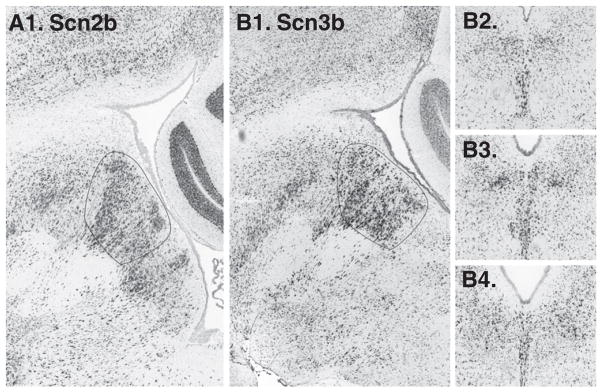
Voltage-dependent sodium channel beta subunits Scnb2 (A1. in a sagittal section, not available in coronal series) and Scn3b (B1. sagittal, B2–4. coronal) both appear enriched on the midline.
2.3. Voltage-gated potassium channels (Kv) channels
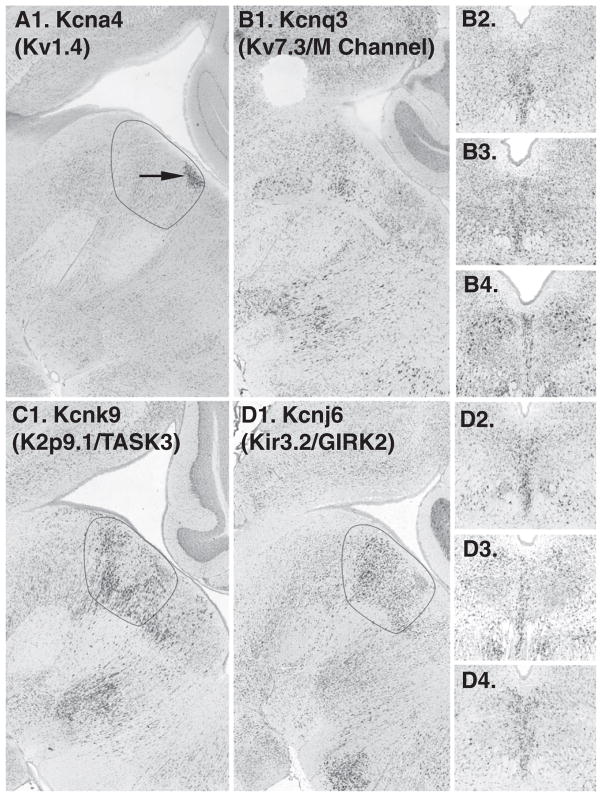
40 listed on the IUPHAR database were searched, of these 6 were not available. From the remainder, Kcna4 (Kv1.4, shaker family related) was present in a restricted area within the DR at the base of the aqueduct (Fig. 3A). In addition, Kcnq3 (Kv7.3, an M-channel) appeared modestly enriched on the midline in coronal series (Fig. 3B). In the sagittal series for this gene, the sections through the precise midline were not available, and the distribution was not confirmed (Fig. 3B1). Several additional genes had subtle expression patterns with respect to the midline and these included Kcnh8 (Kv12.1) or on the midline and Kcna1 (Kv1.1), kcna2 (Kv1.2), kcnc1 (Kv3.1) in paramedial areas (data not shown).
Fig. 3.
Voltage dependent potassium (Kv), inward-rectifying potassium (Kir), and two-pore potassium channels (K2P) with midline expression patterns. A1. In the sagittal plane Kcna4, (not available in coronal series), appears expressed in a cluster of cells on the midline (arrow). B1–4. Sagittal and coronal sections showing modest enrichment of Kcnq3 on the midline. C1. Kcnk9 is enriched on the midline of the DR. D1–4. Kcnj6 in sagittal and coronal series.
2.4. Two-pore-domain potassium channels (K2P)
15 were searched, and 2 were not available. Kcnk9 (K2P 9.1) was enriched on the midline (Fig. 3C). This channel is also known as TASK3. In addition, Kcnk1 (K2P 1.1) had a subtle paramedial expression pattern (not shown).
2.5. Inwardly rectifying potassium channels (Kir)
15 were examined, and 2 of these were not available. One gene, Kcnj6 (Kir 3.2), also known as GIRK2 (G protein-coupled inwardly rectifying potassium channel 2), had modest enriched on the midline (Fig. 3D).
2.6. Voltage gated calcium channels (Cav)
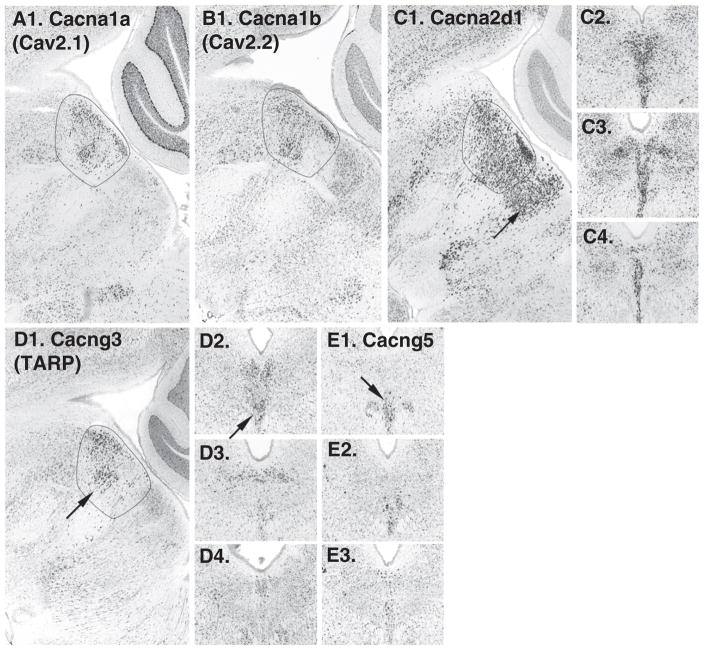
10 were examined and all were available. 3 were identified as enriched along the midline: Cacna1a (calcium channel, voltage-dependent, P/Q-type, alpha1A; Cav2.1; Fig. 4A); Cacna1b (calcium channel, voltage dependent, N-type, alpha1B; Cav2.2; Fig. 4B) and with a more subtle expression pattern, Cacna1h (calcium channel, voltage-dependent, T-type; Cav3.2). Neuroblast also identified Cacna2d1 (Calcium channel voltage-dependent alpha2/delta-1 subunit; Fig. 4C). Cacna2d1 expression seemed to extend in a broader area than TPH2 expression, extending caudally on the midline (Fig. 4C). Cacng3, voltage-dependent calcium channel gamma 3 subunit, which is also known as a type 1 transmembrane AMPA regulatory protein (TARP) was also identified using Neuroblast (Fig. 4D). In both coronal and sagittal image series Cacng3 had the highest expression levels in the rostral pole of the DR (Fig. 4D). Subsequently Cacng1–8 genes were examined, and Cacng5 was identified with a midline expression pattern in a ventral subset of cells (Fig. 4E).
Fig. 4.
Calcium channels. A1. Cacna1a and B1. Cacna1b in sagittal sections are enriched in the area of TPH2 expression (circled). Cacna2d1 in sagittal (C1.) and coronal series (C2–4.) series: expressing cells can be seen extending caudal to the DR (arrow). D1–4. In both sagittal and coronal series, Cacng3 appears preferentially expressed in the rostral pole of the DR on the midline (arrows in D1. and D2.). E1–3. Cacng5 appears enriched in ventrally located cells, particularly at rostral levels (arrow in E1.).
2.7. Chloride Channels
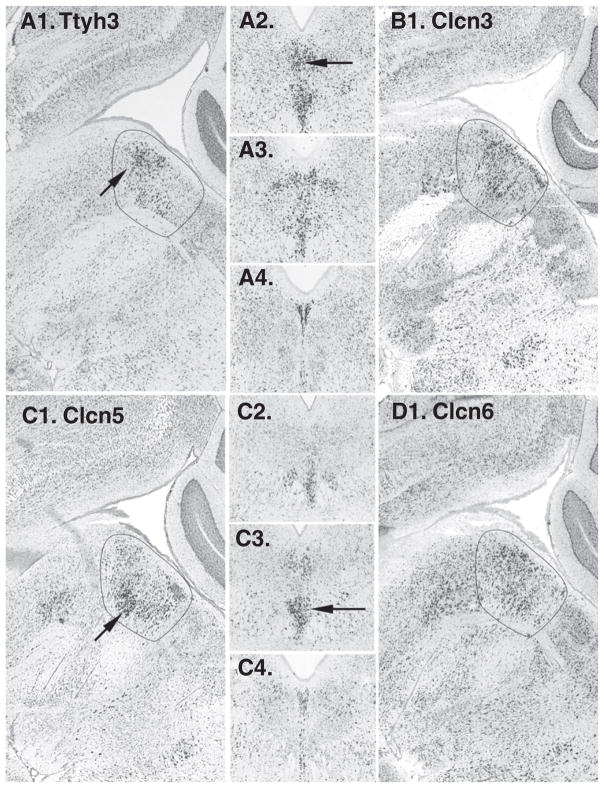
No Chloride Channels (Clc) were listed on the IUPHAR database. However, 2 were identified using NeuroBLAST. Ttyh3 (Tweety-like homolog 3) and Clcn5 (Chloride channel 5) (Fig. 5). Ttyh3 was distinctive in the rostral component of the DR whereas Clcn5 predominated ventrally (Fig. 5). Subsequently we searched additional voltage-gated chloride channels including members of the Ttyh, Clca, Clcn and Clcnk families and found Clcn3 and Clcn6 enriched on the midline (Fig. 5).
Fig. 5.
Voltage-dependent chloride channels. A1–4. Ttyh3 expression domain includes rostral and dorsal components of the DR, seen in both sagittal and coronal series (arrows). B1. Clcn3 in sagittal section is visible on the midline. C1–4. Clcn5 appears predominantly expressed in the ventral component of the DR on the midline (arrows). D1. Clcn6 in sagittal section is enriched on the midline.
2.8. Remaining voltage-gated ion channels
Several groups of voltage-gated ion channels had no members with a detected in a midline or paramedical expression pattern. These included Calcium-Activated Potassium channels (8 searched, 1 not available); CatSper and Two-Pore Calcium Channels (6 searched, 1 not available); Cyclic Nucleotide Regulated Channels (10 searched, 2 not available); and Transient Receptor Potential Channels (28 searched; 4 not available).
2.9. Ligand-gated ion channels
Of the 67 ligand-gated ion channels listed by IUPHAR, 3 were not available. Of the remaining 64, 18 (28%) appeared ubiquitously expressed in the DR. Another 14 (22%) showed patterned expressions. No additional genes were identified using NeuroBLAST searches.
2.10. Glutamate receptor subunits
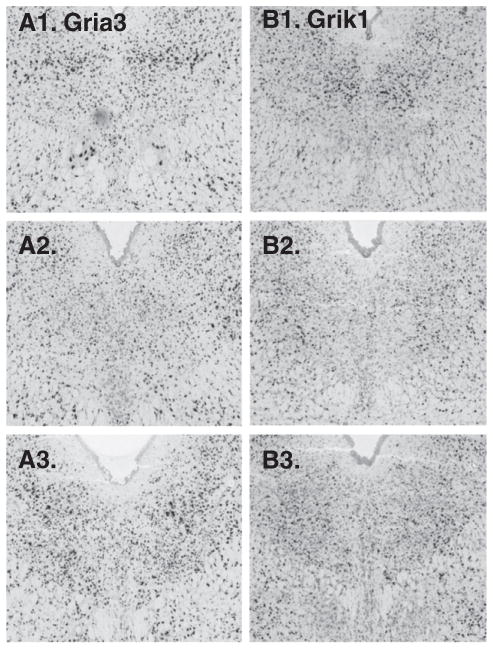
18 were searched, all available. Three showed enrichment on the midline: Gria4 (glutamate receptor, ionotropic AMPA 4), Grik2 (glutamate receptor, ionotropic, kainate 2) and Grik5 (glutamate receptor, ionotropic, kainite 5) (Fig. 6). Of these channels the Gria4 expression pattern was most pronounced. Two glutamate receptor subunits appeared more abundant paramedially than on the midline: Gria3 and Grik1 (Fig. 7).
Fig. 6.
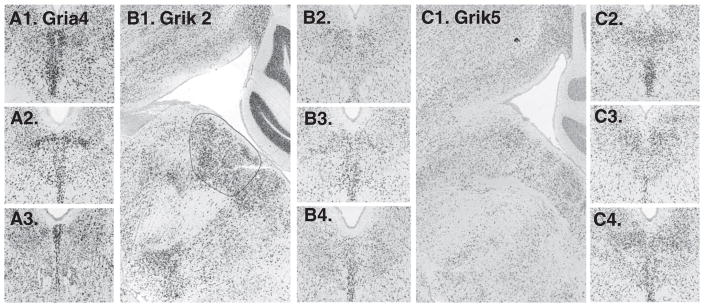
Glutamate receptor subunits with notable expression on the midline include A1–3. Gria4 (not available in sagittal series); B1–4. Grik2 and C1–4. Grik5.
Fig. 7.
Glutamate receptors showing notable paramedial expression patterns include: A1–3. Gria3 and B1–3. Grik1.
2.11. GABA-A receptor subunits
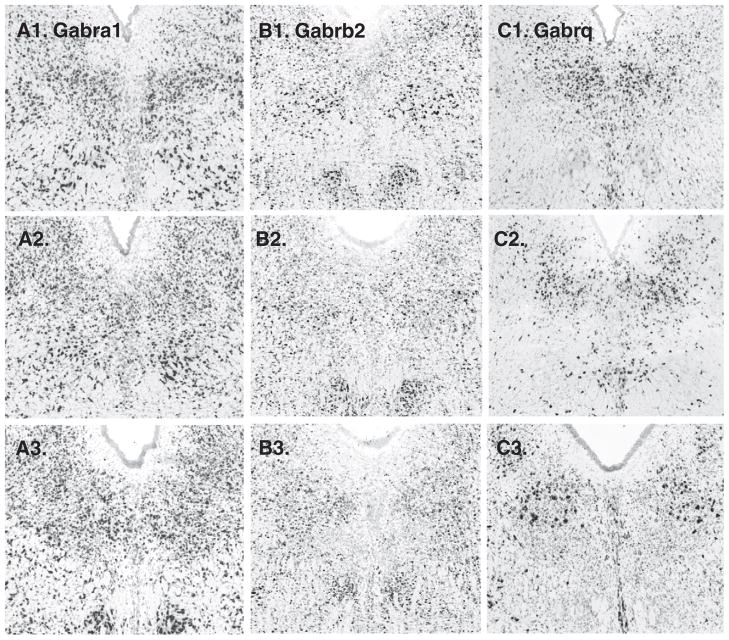
19 were searched, and 1 was not available. Among the remainder, 3 subunits appeared in a midline pattern, Garb1 (GABA-A receptor, beta 1) and Gabrg2 (GABA-A receptor, gamma 2), Gabrg3 (GABA-A receptor, gamma 3) (Fig. 8). Of these Garb1 and Gabrg2 were enriched on the midline throughout the rostro-caudal axis of the DR, whereas Gabrg3 was enriched in the ventral DR at mid-rostro-caudal levels. Three other subunits, Gabra1 (GABA-A receptor alpha1), Gabrb2 (GABA-A receptor, beta-2) ad Gabrq (GABA-A receptor, theta) appeared enriched in a paramedial distribution (Fig. 9).
Fig. 8.
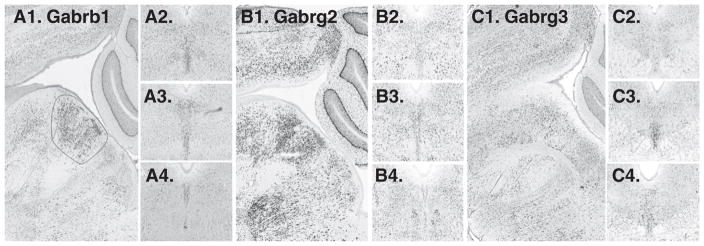
GABA receptor subunits with expression on the midline. A1. sagittal and A2–A4. cononal series of Gabrb1. B1–B4. Gabrg2 is expressed along the midline. C1–C4. Gabrg3 appears enriched, particularly in the ventral part of the DR, in mid-rostro-caudal location (C3. arrow).
Fig. 9.
GABA receptor subunits with paramedial expression patterns. A1–3. Gabra1, B1–3. Gabrb2, and C1–3. Gabrq.
2.12. Other ligand gated ion channels
Additional receptor subunits searched include 5-HT3 (2 searched), glycine subunits (5 searched), nicotinic acetylcholine receptor subunits (16 searched, 1 was not available) and P2X (7 searched, 1 was not available). Of these, Glra2 was present on the midline, Glrb paramedially, Chrna6 appeared expressed in cells contiguous with the A10 dorsal–caudal cell group and Chrnb2 was modestly enriched on the midline (not shown).
3. Discussion
In this study we analyzed gene expression of voltage- and ligand-gated ion channels in the DR and identified several genes with medio-lateral expression patterns. Selective expression of particular genes may underlie the unique electro-physiological characteristics of cell populations within the DR. Few of the identified genes appeared to precisely recapitulate the pattern of either 5-HT or GABAergic neurons in the DR (Fig. 1), consistent with the observations that 5-HT and non-5-HT neurons have overlapping excitable properties. Some genes exhibited dorsal–ventral or rostral–caudal gradients of expression within the DR, suggesting association with functional subgroups of 5-HT and/or non-5-HT neurons. Although there are important species differences, some of the identified channels could be relevant to human disorders associated with 5-HT dysfunction.
3.1. Methodological considerations
The Allen Brain Atlas is high-throughput and therefore has the potential to produce both false negative and false positive findings, based upon the known characteristics of the database (Lein et al., 2007). In addition, the method of colorimetric detection of in situ hybridization signal also has limited quantitative validity and indeed, it is not unusual for different series of in situ hybridization results on the Allen Mouse Brain Atlas to have different signal intensity. Relative expression levels that generate patterns however were more consistent, and often replicated in both coronal and sagittal series, as shown in this report. However, there is the potential that genes with patterned expression were not detected, in particular when genes were only available in sagittal series where medio-lateral patterns may be harder to detect. Likewise, some genes may be regulated at a post-transcriptional level, therefore some of the detected genes may not be present as functional protein. Furthermore, it deserves emphasis that while we focused on genes with patterned expression, there are many additional voltage-and ligand-gated channel genes are expressed in the DR more ubiquitously.
Supporting the validity of the approach we used, we were able to identify two genes that have already been established to have high expression levels in the DR, and coincidentally within 5-HT neurons. These include Kcnj6 also known as ‘weaver gene’, Kir 3.2 or GIRK2. Expressed in DR 5-HT neurons, this channel is implicated in coupling to 5-HT1A receptors (Costa et al., 2005; Penington et al., 1993; Saenz del Burgo et al., 2008). The second gene previously identified as highly expressed in 5-HT neurons is Kcnk9 (K2P9.1) or TASK-3 a “leak” channel that influences the resting membrane potential (Berg et al., 2004; Lesage and Lazdunski, 2000; Marinc et al., 2010). TASK3 contributes to sleep behavior and response to anesthetics (Linden et al., 2007; Pang et al., 2009). TASK3 channels account for the pH sensitivity of 5-HT neurons, are modified by Gq second messenger signaling and appear to be located somato-dendritically on 5-HT neurons (Marinc et al., 2010; Veale et al., 2007; Washburn et al., 2002).
We found that sodium channel beta subunits Scn2b and more so, Scn3b, are expressed on the midline of the DR. Beta subunits encoded by Scn2b and Scn3b function to regulate Nav alpha subunits, influencing the activation and inactivation kinetics of sodium channels. Scn3b, when expressed with the alpha subunit Nav1.3, promotes slower channel inactivation, which would increase the duration of the action potential (Cusdin et al., 2010). This finding raises the possibility that expression of these subunits may contribute to the broad action potential associated primarily, but not exclusively, with 5-HT neurons (Calizo et al., 2011; Kirby et al., 2003; Li et al., 2001; Marinelli et al., 2004).
Despite the fact that voltage-gated potassium channels accounted for the most numerous type of channels screened, few had pronounced patterns of expression. Kcnq3, or Kv7.3, a member of the KQT-like subfamily, was enhanced on the midline although the intensity of the pattern was not very robust. Kcnq3 is thought to function as a heteromer in conjunction with either Kcnq2, Kcnq4 or Kcnq5 to mediate M-currents, which are voltage dependent, slowly activating and deactivating potassium currents (Wang et al., 1998; Yang et al., 1998). Indeed, Kcnq4 or Kv7.4 has been reported in 5-HT neurons in the rat DR, although little expression is detectable in the Allen Mouse Brain Atlas (Hansen et al., 2008; Kharkovets et al., 2000). The late phase of the afterhyperpolarization (AHP) is mediated in part by a calcium dependent potassium conductance (Crunelli et al., 1983; Pan et al., 1994) and in the hippocampus, Kcnq3 channels contribute to the calcium activated medium and slow AHP (Tzingounis and Nicoll, 2008). Therefore, this channel could contribute to some features of the AHP in DR neurons. However, there is considerable variability within the AHP in both 5-HT and non-5-HT neurons in the DRN (Kirby et al., 2003; Li et al., 2001; Marinelli et al., 2004), and this is consistent with our finding that the majority of potassium channels that could contribute to these characteristics did not have robustly patterned expression.
We found Kv1.4 expressed in a subgroup of cells within the DR, perhaps corresponding to the B6 cluster of neurons. However, immunohistochemical localization of Kv1.4 does not confirm this distribution (Lujan et al., 2003) thus this finding awaits further evaluation.
A few calcium channels exhibited interesting patterns of expression in the DR. One of them is Cacna1a, which is a P/Q type calcium channel, important for presynaptic neurotransmitter release (Catterall, 1999). The Rolling mouse Nagoya has a mutation in this gene and these mice have age related alterations in markers of 5-HT neuron function and behavioral abnormalities in tests for anxiety and depression-like behavior, suggesting that this gene may be important for 5-HT neuron function (Takahashi et al., 2009). Mice lacking Cacna1b have altered activity, arousal and are hyperaggresive (Beuckmann et al., 2003; Kim et al., 2009). These mutants also display increased firing of 5-HT neurons, suggesting this channel participates in controlling the excitability of 5-HT neurons.
Cacna2d1, the L-type voltage-dependent calcium channel alpha2/delta-1 subunit, was expressed in a subset of DR cells. The alpha2/delta-1 subunit possesses a stereoselective, high-affinity binding site for gabapentin and pregabalin, which are primarily used to treat chronic pain (Dooley et al., 2007; Gee et al., 1996; Marais et al., 2001). Gabapentin has been associated with increased suicidality, however pregabalin may have positive effects for anxiety (Pande et al., 2003). While alpha2/delta-1 is expressed in many brain areas (Taylor and Garrido, 2008) the expression pattern we found in the DR raises the possibility that the DR, and perhaps 5-HT, may contribute to the physiological effects of gabapentin and pregabalin.
Cacng3 and Cacng5, members of the TARP family, were found enriched in different subregions of the DR. Cacng3 was located rostral and dorsal in the nucleus, whereas Cacng5 was distinguished ventrally. Related to Stargazin, TARP proteins selectively bind to AMPA receptor subunits and are implicated in both gating and trafficking of AMPA receptors (reviewed by: Jackson and Nicoll, 2011; Kato et al., 2010; Payne, 2008). TARPs are enriched in postsynaptic densities and may change biophysical channel properties by stabilizing specific confirmations of AMPA receptors. Therefore Cacng3 and Cacng5 expression may confer differential AMPA receptor function within the expressed cells. Cacng5 is reported to be a susceptibility locus for schizophrenia and bipolar disorder (Curtis et al., 2011), and function within the DR could be relevant to this observation.
The role of voltage-dependent chloride channels in neurons is poorly understood, however several were identified with a patterned distribution in the DR. Ttyh3, is a homolog of the drosophila gene tweety, located in the flightless locus. Ttyh3 encodes a large conductance calcium-activated chloride current (Suzuki, 2006), whose functional importance may be interesting to pursue since this gene had a particularly clear expression pattern. Clcn3, Clcn5 and Clcn6 are associated with acidifying intracellular compartments, including synaptic vesicles and endosome–lysosome compartments (Riazanski et al., 2011; Smith and Lippiat, 2010). Loss of all three chloride channels is associated with a neurodegenerative disorder, neuronal ceroid lipofuscinosis (NCL), indicating their importance to neuron function (Poet et al., 2006; Pontikis et al., 2004; Pressey et al., 2010; von Schantz et al., 2008).
Glutamate neurotransmission is of particular interest in the DR due to the potential use of glutamate receptor antagonists for treatment-resistant depression (Machado-Vieira et al., 2009; Pittenger et al., 2007; Yilmaz et al., 2002; Zarate et al., 2004). Among AMPA receptor subunits, Gria4 was the most abundantly expressed in the midline region showing a clear pattern of expression that overlaps with the TPH2 pattern, suggesting an association with 5-HT neurons. Interestingly, Gria4-KO mice show increased anxiety-like behavior in response to light (Sagata et al., 2010). Gria3 showed a paramedial pattern of expression and polymorphism of this gene has been identified as a genetic risk factor for loss of libido in citalopram-treated patients (Perlis et al., 2009) as well as with suicidal ideation emerging during citalopram treatment of major depression, together with Grik2 (Laje et al., 2007).
Two kainate receptor subunits appear particularly enriched on the midline in the DR, and this could contribute to some of the behavioral effects associated with these receptors. Specifically, polymorphisms of Grik2 have been associated with obsessive–compulsive disorder and sexual dysfunction in citalopram-treated patients (Perlis et al., 2009; Sampaio et al., 2010). Grik5, which contributes to slowly-deactivating currents, has been associated with bipolar disorder (Barberis et al., 2008; Yosifova et al., 2011). Grik1, which has paramedial expression, is expressed in GABAergic interneurons in the hippocampus (Segerstrale et al., 2010) and the Grik1-KO mice have increased anxiety-related behaviors (Wu et al., 2007). Taken together these observations would suggest the possibility that Grik1 could be selectively associated with GABAergic neurons.
GABA-A subunits that displayed enriched midline expression include Gabrb1, Gabrg2, and Gabrg3 genes while those associated with lateral expressions are Gabra1, Gabrb2, and Gabrq genes. Some of the midline-enriched genes have been reported to be associated with drug dependence and mood disorders. For instance, possible genetic linkage in humans has been reported between Gabrb1 gene and alcohol dependence as well as bipolar disorder (Craddock et al., 2008; Kertes et al., 2011; Parsian and Zhang, 1999). In mice, Gabrg2 receptor deficits result in anxiety-like, depressive-like, and anhedonia-like behavior, which are ameliorated by antidepressants (Shen et al., 2010). Genes that are enriched laterally have been implicated in mediating actions of anesthetics or alcohols. A few studies showed that point mutations of Gabra1 and Gabrb2 reduce currents potentiated by isoflurane or alcohols (Borghese et al., 2006; McCracken et al., 2010; Nishikawa et al., 2002).
Overall, ligand-gated ion channels, primarily glutamate and GABA receptors, were twice as likely as the voltage-gated ion channels to show patterned expression in the DR. This finding suggests that DR neurons in particular vary in how they receive and integrate synaptic glutamatergic and GABAergic neuro-transmission. This conclusion would be consistent with electrophysiological evidence suggesting differences in the regulation of glutamatergic and GABAergic innervation of 5-HT and non-5-HT neurons in the DR (Kirby et al., 2007).
In this study we identified several voltage- and ligand-gated ion channels that have patterned expression through the DR, and therefore may contribute to the heterogeneity of membrane properties of neurons recorded in this nucleus. Additional studies are required to determine how these genes distribute to 5-HT and non-5-HT cell populations in the DR, and the functional importance of each gene. However, the current results have the capacity to drive hypotheses in this regard.
4. Experimental procedures
The source of data for this project was the Allen Mouse Brain Atlas (ABA) (http://mouse.brain-map.org), a genome-wide database of high-throughput in-situ hybridization data for over 20,000 genes (Lein et al., 2007). Two approaches were used to identify genes with expression patterns that could be selective for different groups of neurons within the DR. The first entailed a manual examination of a list of ion channel genes provided by the International Union of Basic and Clinical Pharmacology (IUPHAR) (http://www.iuphar-db.org/) (Sharman et al., 2010). IUPHAR’s list comprised 142 voltage-gated channels and 72 ligand-gated channels. We previously reported a preliminary analysis of glutamate receptor genes (Soiza-Reilly and Commons, 2011), but include this group into the current report to complete the data set. The second approach was to identify genes using the Allen Brain Atlas’ NeuroBLAST tool.
For the IUPHAR database search, individual genes were searched. If the gene had a coronal series, this was examined. If there were two or more coronal series, the one with greater signal strength was examined. Sections were chosen from the center of the rostro-caudal extent of the DR, between 3450 and 3850 μm ABA coordinates. On the selected section, gene expression was scored in two zones: one on the midline, and the other paramedial to the midline. The location of these zones were defined by reference sections for the serotonin biosynthetic enzyme tryptophan hydroxylase 2 (TPH2) and the Vesicular GABA Transporter (Slc6a1, VGAT; Fig. 1), which exhibit reciprocal patterns with respect to the midline: TPH2 is enriched on the midline, whereas VGAT is present in a paramedial distribution. In each of these two zones, expression level was evaluated by visual inspection of the ABA generated heatmap; ranging from absent (black, 0), sparse green (low, 1) to green/yellow (medium, 2) to orange/red (high, 3) with 0.5 increments used for intermediate values. Once one series of sections was evaluated, a second series was evaluated to confirm the observations. The pattern of expression was then defined as the midline score subtracted from the paramedial score. For genes that were only available in a sagittal series, the section closest to the midline was evaluated, and genes were given one score for expression intensity within the area where 5-HT cells are located, based on TPH2 expression in an analogous section. Subsequently, more lateral sections were inspected to determine if there was a patterned expression. All the genes identified in these screens were subsequently reevaluated by a second observer to reduce the incidence of false-positive identification. Some genes in the IUPHAR list were either not available on the ABA or were present only in sagittal series that did not include a section close enough to the midline to capture the location of 5-HT cells. The frequency of these events is noted in results as the number of genes ‘not available’ for each family of ion channels.
For the NeuroBLAST search, we selected coronal and sagittal series of TPH2 gene expression and designated the area ‘midbrain’. The output list was then examined using NIH’s DAVID gene identification tool (http://david.abcc.ncifcrf.gov/) to identify ion channels. Image series of these genes were subsequently evaluated to eliminate false-positives. If we found a gene using NeuroBLAST search, we subsequently searched other members of the same class or gene family.
To generate figures, all the images were downloaded from the ABA website (Allen Mouse Brain Atlas [Internet]. Seattle (WA): Allen Institute for Brain Science. ©2009. Available from: http://mouse.brain-map.org) and assembled using Adobe Photoshop. Images were adjusted for contrast and brightness using ‘image–adjust–brightness–contrast’ or ‘image–adjust–curves’ functions.
Acknowledgments
This work was supported by a grant from NIH, DA-021801, and the Sara Page Mayo Endowment for Pediatric Pain Research. We appreciate the comments of Drs. Lynn Kirby and Chris Vaughan on draft versions of the manuscript.
References
- Aghajanian GK, Foote WE, Sheard MH. Lysergic acid diethylamide: sensitive neuronal units in the midbrain raphe. Science. 1968;161:706–708. doi: 10.1126/science.161.3842.706. [DOI] [PubMed] [Google Scholar]
- Allers KA, Sharp T. Neurochemical and anatomical identification of fast- and slow-firing neurones in the rat dorsal raphe nucleus using juxtacellular labelling methods in vivo. Neuroscience. 2003;122:193–204. doi: 10.1016/s0306-4522(03)00518-9. [DOI] [PubMed] [Google Scholar]
- Barberis A, Sachidhanandam S, Mulle C. GluR6/KA2 kainate receptors mediate slow-deactivating currents. J Neurosci. 2008;28:6402–6406. doi: 10.1523/JNEUROSCI.1204-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AP, Talley EM, Manger JP, Bayliss DA. Motoneurons express heteromeric TWIK-related acid-sensitive K+ (TASK) channels containing TASK-1 (KCNK3) and TASK-3 (KCNK9) subunits. J Neurosci. 2004;24:6693–6702. doi: 10.1523/JNEUROSCI.1408-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuckmann CT, Sinton CM, Miyamoto N, Ino M, Yanagisawa M. N-type calcium channel alpha1B subunit (Cav2.2) knock-out mice display hyperactivity and vigilance state differences. J Neurosci. 2003;23:6793–6797. doi: 10.1523/JNEUROSCI.23-17-06793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese CM, Werner DF, Topf N, Baron NV, Henderson LA, Boehm SL, II, Blednov YA, Saad A, Dai S, Pearce RA, Harris RA, Homanics GE, Harrison NL. An isoflurane- and alcohol-insensitive mutant GABA(A) receptor alpha(1) subunit with near-normal apparent affinity for GABA: characterization in heterologous systems and production of knockin mice. J Pharmacol Exp Ther. 2006;319:208–218. doi: 10.1124/jpet.106.104406. [DOI] [PubMed] [Google Scholar]
- Calizo LH, Akanwa A, Ma X, Pan YZ, Lemos JC, Craige C, Heemstra LA, Beck SG. Raphe serotonin neurons are not homogenous: electrophysiological, morphological and neurochemical evidence. Neuropharmacology. 2011;61:524–543. doi: 10.1016/j.neuropharm.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. Interactions of presynaptic Ca2+ channels and snare proteins in neurotransmitter release. Ann N Y Acad Sci. 1999;868:144–159. doi: 10.1111/j.1749-6632.1999.tb11284.x. [DOI] [PubMed] [Google Scholar]
- Costa AC, Stasko MR, Stoffel M, Scott-McKean JJ. G-protein-gated potassium (GIRK) channels containing the GIRK2 subunit are control hubs for pharmacologically induced hypothermic responses. J Neurosci. 2005;25:7801–7804. doi: 10.1523/JNEUROSCI.1699-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock N, Jones L, Jones IR, Kirov G, Green EK, Grozeva D, Moskvina V, Nikolov I, Hamshere ML, Vukcevic D, Caesar S, Gordon-Smith K, Fraser C, Russell E, Norton N, Breen G, St Clair D, Collier DA, Young AH, Ferrier IN, Farmer A, McGuffin P, Holmans PA, Donnelly P, Owen MJ, O’Donovan MC. Strong genetic evidence for a selective influence of GABAA receptors on a component of the bipolar disorder phenotype. Mol Psychiatry. 2008;15:146–153. doi: 10.1038/mp.2008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V, Forda S, Brooks PA, Wilson KC, Wise JC, Kelly JS. Passive membrane properties of neurones in the dorsal raphe and periaqueductal grey recorded in vitro. Neurosci Lett. 1983;40:263–268. doi: 10.1016/0304-3940(83)90049-6. [DOI] [PubMed] [Google Scholar]
- Curtis D, Vine AE, McQuillin A, Bass NJ, Pereira A, Kandaswamy R, Lawrence J, Anjorin A, Choudhury K, Datta SR, Puri V, Krasucki R, Pimm J, Thirumalai S, Quested D, Gurling HM. Case–case genome-wide association analysis shows markers differentially associated with schizophrenia and bipolar disorder and implicates calcium channel genes. Psychiatr Genet. 2011;21:1–4. doi: 10.1097/YPG.0b013e3283413382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusdin FS, Nietlispach D, Maman J, Dale TJ, Powell AJ, Clare JJ, Jackson AP. The sodium channel {beta}3-subunit induces multiphasic gating in NaV1.3 and affects fast inactivation via distinct intracellular regions. J Biol Chem. 2010;285:33404–33412. doi: 10.1074/jbc.M110.114058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L, Watkins KC, Garcia S, Beaudet A. The serotonin neurons in nucleus raphe dorsalis of adult rat: a light and electron microscope radioautographic study. J Comp Neurol. 1982;207:239–254. doi: 10.1002/cne.902070305. [DOI] [PubMed] [Google Scholar]
- Dooley DJ, Taylor CP, Donevan S, Feltner D. Ca2+ channel alpha2delta ligands: novel modulators of neurotransmission. Trends Pharmacol Sci. 2007;28:75–82. doi: 10.1016/j.tips.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem. 1996;271:5768–5776. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- Hansen HH, Waroux O, Seutin V, Jentsch TJ, Aznar S, Mikkelsen JD. Kv7 channels: interaction with dopaminergic and serotonergic neurotransmission in the CNS. J Physiol. 2008;586:1823–1832. doi: 10.1113/jphysiol.2007.149450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AC, Nicoll RA. The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron. 2011;70:178–199. doi: 10.1016/j.neuron.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Kato AS, Gill MB, Yu H, Nisenbaum ES, Bredt DS. TARPs differentially decorate AMPA receptors to specify neuropharmacology. Trends Neurosci. 2010;33:241–248. doi: 10.1016/j.tins.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Kertes DA, Kalsi G, Prescott CA, Kuo PH, Patterson DG, Walsh D, Kendler KS, Riley BP. Neurotransmitter and neuromodulator genes associated with a history of depressive symptoms in individuals with alcohol dependence. Alcohol Clin Exp Res. 2011;35:496–505. doi: 10.1111/j.1530-0277.2010.01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharkovets T, Hardelin JP, Safieddine S, Schweizer M, El-Amraoui A, Petit C, Jentsch TJ. KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proc Natl Acad Sci U S A. 2000;97:4333–4338. doi: 10.1073/pnas.97.8.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Jeon D, Kim YH, Lee CJ, Kim H, Shin HS. Deletion of N-type Ca(2+) channel Ca(v)2.2 results in hyperaggressive behaviors in mice. J Biol Chem. 2009;284:2738–2745. doi: 10.1074/jbc.M807179200. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Pernar L, Valentino RJ, Beck SG. Distinguishing characteristics of serotonin and non-serotonin-containing cells in the dorsal raphe nucleus: electrophysiological and immunohistochemical studies. Neuroscience. 2003;116:669–683. doi: 10.1016/s0306-4522(02)00584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Pan YZ, Freeman-Daniels E, Rani S, Nunan JD, Akanwa A, Beck SG. Cellular effects of swim stress in the dorsal raphe nucleus. Psychoneuroendocrinology. 2007;32:712–723. doi: 10.1016/j.psyneuen.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laje G, Paddock S, Manji H, Rush AJ, Wilson AF, Charney D, McMahon FJ. Genetic markers of suicidal ideation emerging during citalopram treatment of major depression. Am J Psychiatry. 2007;164:1530–1538. doi: 10.1176/appi.ajp.2007.06122018. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Lesage F, Lazdunski M. Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol Renal Physiol. 2000;279:F793–F801. doi: 10.1152/ajprenal.2000.279.5.F793. [DOI] [PubMed] [Google Scholar]
- Li YQ, Li H, Kaneko T, Mizuno N. Morphological features and electrophysiological properties of serotonergic and non-serotonergic projection neurons in the dorsal raphe nucleus. An intracellular recording and labeling study in rat brain slices. Brain Res. 2001;900:110–118. doi: 10.1016/s0006-8993(01)02272-7. [DOI] [PubMed] [Google Scholar]
- Linden AM, Sandu C, Aller MI, Vekovischeva OY, Rosenberg PH, Wisden W, Korpi ER. TASK-3 knockout mice exhibit exaggerated nocturnal activity, impairments in cognitive functions, and reduced sensitivity to inhalation anesthetics. J Pharmacol Exp Ther. 2007;323:924–934. doi: 10.1124/jpet.107.129544. [DOI] [PubMed] [Google Scholar]
- Lujan R, de Cabo de la Vega C, Dominguez del Toro E, Ballesta JJ, Criado M, Juiz JM. Immunohistochemical localization of the voltage-gated potassium channel subunit Kv1.4 in the central nervous system of the adult rat. J Chem Neuroanat. 2003;26:209–224. doi: 10.1016/j.jchemneu.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Salvadore G, Diazgranados N, Zarate CA., Jr Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacol Ther. 2009;123:143–150. doi: 10.1016/j.pharmthera.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais E, Klugbauer N, Hofmann F. Calcium channel alpha(2)delta subunits—structure and gabapentin binding. Mol Pharmacol. 2001;59:1243–1248. doi: 10.1124/mol.59.5.1243. [DOI] [PubMed] [Google Scholar]
- Marinc C, Preisig-Muller R, Pruss H, Derst C, Veh RW. Immunocytochemical localization of TASK-3 (K(2P)9.1) channels in monoaminergic and cholinergic neurons. Cell Mol Neurobiol. 2010;31:323–335. doi: 10.1007/s10571-010-9625-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli S, Schnell SA, Hack SP, Christie MJ, Wessendorf MW, Vaughan CW. Serotonergic and nonserotonergic dorsal raphe neurons are pharmacologically and electrophysiologically heterogeneous. J Neurophysiol. 2004;92:3532–3537. doi: 10.1152/jn.00437.2004. [DOI] [PubMed] [Google Scholar]
- McCracken ML, Borghese CM, Trudell JR, Harris RA. A transmembrane amino acid in the GABAA receptor beta2 subunit critical for the actions of alcohols and anesthetics. J Pharmacol Exp Ther. 2010;335:600–606. doi: 10.1124/jpet.110.170472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa K, Jenkins A, Paraskevakis I, Harrison NL. Volatile anesthetic actions on the GABAA receptors: contrasting effects of alpha 1(S270) and beta 2(N265) point mutations. Neuropharmacology. 2002;42:337–345. doi: 10.1016/s0028-3908(01)00189-7. [DOI] [PubMed] [Google Scholar]
- Pan ZZ, Grudt TJ, Williams JT. Alpha 1-adrenoceptors in rat dorsal raphe neurons: regulation of two potassium conductances. J Physiol. 1994;478 (Pt. 3):437–447. doi: 10.1113/jphysiol.1994.sp020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande AC, Crockatt JG, Feltner DE, Janney CA, Smith WT, Weisler R, Londborg PD, Bielski RJ, Zimbroff DL, Davidson JR, Liu-Dumaw M. Pregabalin in generalized anxiety disorder: a placebo-controlled trial. Am J Psychiatry. 2003;160:533–540. doi: 10.1176/appi.ajp.160.3.533. [DOI] [PubMed] [Google Scholar]
- Pang DS, Robledo CJ, Carr DR, Gent TC, Vyssotski AL, Caley A, Zecharia AY, Wisden W, Brickley SG, Franks NP. An unexpected role for TASK-3 potassium channels in network oscillations with implications for sleep mechanisms and anesthetic action. Proc Natl Acad Sci U S A. 2009;106:17546–17551. doi: 10.1073/pnas.0907228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsian A, Zhang ZH. Human chromosomes 11p15 and 4p12 and alcohol dependence: possible association with the GABRB1 gene. Am J Med Genet. 1999;88:533–538. doi: 10.1002/(sici)1096-8628(19991015)88:5<533::aid-ajmg18>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Payne HL. The role of transmembrane AMPA receptor regulatory proteins (TARPs) in neurotransmission and receptor trafficking (Review) Mol Membr Biol. 2008;25:353–362. doi: 10.1080/09687680801986480. [DOI] [PubMed] [Google Scholar]
- Penington NJ, Kelly JS, Fox AP. Whole-cell recordings of inwardly rectifying K+ currents activated by 5-HT1A receptors on dorsal raphe neurones of the adult rat. J Physiol. 1993;469:387–405. doi: 10.1113/jphysiol.1993.sp019819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis RH, Laje G, Smoller JW, Fava M, Rush AJ, McMahon FJ. Genetic and clinical predictors of sexual dysfunction in citalopram-treated depressed patients. Neuropsychopharmacology. 2009;34:1819–1828. doi: 10.1038/npp.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Sanacora G, Krystal JH. The NMDA receptor as a therapeutic target in major depressive disorder. CNS Neurol Disord Drug Targets. 2007;6:101–115. doi: 10.2174/187152707780363267. [DOI] [PubMed] [Google Scholar]
- Poet M, Kornak U, Schweizer M, Zdebik AA, Scheel O, Hoelter S, Wurst W, Schmitt A, Fuhrmann JC, Planells-Cases R, Mole SE, Hubner CA, Jentsch TJ. Lysosomal storage disease upon disruption of the neuronal chloride transport protein ClC-6. Proc Natl Acad Sci U S A. 2006;103:13854–13859. doi: 10.1073/pnas.0606137103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontikis CC, Cella CV, Parihar N, Lim MJ, Chakrabarti S, Mitchison HM, Mobley WC, Rezaie P, Pearce DA, Cooper JD. Late onset neurodegeneration in the Cln3−/− mouse model of juvenile neuronal ceroid lipofuscinosis is preceded by low level glial activation. Brain Res. 2004;1023:231–242. doi: 10.1016/j.brainres.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Pressey SN, O’Donnell KJ, Stauber T, Fuhrmann JC, Tyynela J, Jentsch TJ, Cooper JD. Distinct neuropathologic phenotypes after disrupting the chloride transport proteins ClC-6 or ClC-7/Ostm1. J Neuropathol Exp Neurol. 2010;69:1228–1246. doi: 10.1097/NEN.0b013e3181ffe742. [DOI] [PubMed] [Google Scholar]
- Riazanski V, Deriy LV, Shevchenko PD, Le B, Gomez EA, Nelson DJ. Presynaptic CLC-3 determines quantal size of inhibitory transmission in the hippocampus. Nat Neurosci. 2011;14:487–494. doi: 10.1038/nn.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz del Burgo L, Cortes R, Mengod G, Zarate J, Echevarria E, Salles J. Distribution and neurochemical characterization of neurons expressing GIRK channels in the rat brain. J Comp Neurol. 2008;510:581–606. doi: 10.1002/cne.21810. [DOI] [PubMed] [Google Scholar]
- Sagata N, Iwaki A, Aramaki T, Takao K, Kura S, Tsuzuki T, Kawakami R, Ito I, Kitamura T, Sugiyama H, Miyakawa T, Fukumaki Y. Comprehensive behavioural study of GluR4 knockout mice: implication in cognitive function. Genes Brain Behav. 2010;9:899–909. doi: 10.1111/j.1601-183X.2010.00629.x. [DOI] [PubMed] [Google Scholar]
- Sampaio AS, Fagerness J, Crane J, Leboyer M, Delorme R, Pauls DL, Stewart SE. Association between polymorphisms in GRIK2 gene and obsessive–compulsive disorder: a family-based study. CNS Neurosci Ther. 2010;17:141–147. doi: 10.1111/j.1755-5949.2009.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrale M, Juuri J, Lanore F, Piepponen P, Lauri SE, Mulle C, Taira T. High firing rate of neonatal hippocampal interneurons is caused by attenuation of afterhyperpolarizing potassium currents by tonically active kainate receptors. J Neurosci. 2010;30:6507–6514. doi: 10.1523/JNEUROSCI.4856-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharman JL, Mpamhanga CP, Spedding M, Germain P, Staels B, Dacquet C, Laudet V, Harmar AJ. IUPHAR-DB: new receptors and tools for easy searching and visualization of pharmacological data. Nucleic Acids Res. 2010;39:D534–D538. doi: 10.1093/nar/gkq1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Lal R, Luellen BA, Earnheart JC, Andrews AM, Luscher B. Gamma-aminobutyric acid-type A receptor deficits cause hypothalamic–pituitary–adrenal axis hyperactivity and antidepressant drug sensitivity reminiscent of melancholic forms of depression. Biol Psychiatry. 2010;68:512–520. doi: 10.1016/j.biopsych.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AJ, Lippiat JD. Direct endosomal acidification by the outwardly rectifying CLC-5 Cl(−)/H(+) exchanger. J Physiol. 2010;588:2033–2045. doi: 10.1113/jphysiol.2010.188540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soiza-Reilly M, Commons KG. Glutamatergic drive of the dorsal raphe nucleus. J Chem Neuroanat. 2011;41:247–255. doi: 10.1016/j.jchemneu.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbusch HW, Nieuwenhuys R, Verhofstad AA, Van der Kooy D. The nucleus raphe dorsalis of the rat and its projection upon the caudatoputamen. A combined cytoarchitectonic, immunohistochemical and retrograde transport study. J Physiol Paris. 1981;77:157–174. [PubMed] [Google Scholar]
- Suzuki M. The Drosophila tweety family: molecular candidates for large-conductance Ca2+-activated Cl− channels. Exp Physiol. 2006;91:141–147. doi: 10.1113/expphysiol.2005.031773. [DOI] [PubMed] [Google Scholar]
- Takahashi E, Niimi K, Itakura C. Emotional behavior in heterozygous rolling mouse Nagoya Ca v 2.1 channel mutant mice. Neurobiol Aging. 2009;32:486–496. doi: 10.1016/j.neurobiolaging.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Taylor CP, Garrido R. Immunostaining of rat brain, spinal cord, sensory neurons and skeletal muscle for calcium channel alpha2-delta (alpha2-delta) type 1 protein. Neuroscience. 2008;155:510–521. doi: 10.1016/j.neuroscience.2008.05.053. [DOI] [PubMed] [Google Scholar]
- Tzingounis AV, Nicoll RA. Contribution of KCNQ2 and KCNQ3 to the medium and slow afterhyperpolarization currents. Proc Natl Acad Sci U S A. 2008;105:19974–19979. doi: 10.1073/pnas.0810535105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermaelen CP, Aghajanian GK. Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Res. 1983;289:109–119. doi: 10.1016/0006-8993(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Veale EL, Kennard LE, Sutton GL, MacKenzie G, Sandu C, Mathie A. G(alpha)q-mediated regulation of TASK3 two-pore domain potassium channels: the role of protein kinase C. Mol Pharmacol. 2007;71:1666–1675. doi: 10.1124/mol.106.033241. [DOI] [PubMed] [Google Scholar]
- von Schantz C, Saharinen J, Kopra O, Cooper JD, Gentile M, Hovatta I, Peltonen L, Jalanko A. Brain gene expression profiles of Cln1 and Cln5 deficient mice unravels common molecular pathways underlying neuronal degeneration in NCL diseases. BMC Genomics. 2008;9:146. doi: 10.1186/1471-2164-9-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- Washburn CP, Sirois JE, Talley EM, Guyenet PG, Bayliss DA. Serotonergic raphe neurons express TASK channel transcripts and a TASK-like pH- and halothane-sensitive K+ conductance. J Neurosci. 2002;22:1256–1265. doi: 10.1523/JNEUROSCI.22-04-01256.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LJ, Ko SW, Toyoda H, Zhao MG, Xu H, Vadakkan KI, Ren M, Knifed E, Shum F, Quan J, Zhang XH, Zhuo M. Increased anxiety-like behavior and enhanced synaptic efficacy in the amygdala of GluR5 knockout mice. PLoS One. 2007;2:e167. doi: 10.1371/journal.pone.0000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WP, Levesque PC, Little WA, Conder ML, Ramakrishnan P, Neubauer MG, Blanar MA. Functional expression of two KvLQT1-related potassium channels responsible for an inherited idiopathic epilepsy. J Biol Chem. 1998;273:19419–19423. doi: 10.1074/jbc.273.31.19419. [DOI] [PubMed] [Google Scholar]
- Yilmaz A, Schulz D, Aksoy A, Canbeyli R. Prolonged effect of an anesthetic dose of ketamine on behavioral despair. Pharmacol Biochem Behav. 2002;71:341–344. doi: 10.1016/s0091-3057(01)00693-1. [DOI] [PubMed] [Google Scholar]
- Yosifova A, Mushiroda T, Kubo M, Takahashi A, Kamatani Y, Kamatani N, Stoianov D, Vazharova R, Karachanak S, Zaharieva I, Dimova I, Hadjidekova S, Milanova V, Madjirova N, Gerdjikov I, Tolev T, Poryazova N, O’Donovan MC, Owen MJ, Kirov G, Toncheva D, Nakamura Y. Genome-wide association study on bipolar disorder in the Bulgarian population. Genes Brain Behav. 2011;10:789–797. doi: 10.1111/j.1601-183X.2011.00721.x. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Payne JL, Quiroz J, Sporn J, Denicoff KK, Luckenbaugh D, Charney DS, Manji HK. An open-label trial of riluzole in patients with treatment-resistant major depression. Am J Psychiatry. 2004;161:171–174. doi: 10.1176/appi.ajp.161.1.171. [DOI] [PubMed] [Google Scholar]