Key Points
ATG induces monocyte TF procoagulant activity dependent on complement activation but independent of de novo protein synthesis.
TF decryption requires oxidation of cell surface PDI following C5 activation and phosphatidylserine membrane exposure following C7 insertion.
Abstract
Lymphocyte depletion with antithymocyte globulin (ATG) can be complicated by systemic coagulation activation. We found that ATG activated tissue factor procoagulant activity (TF PCA) on monocytic cells more potently than other stimuli that decrypt TF, including cell disruption, TF pathway inhibitor inhibition, and calcium ionophore treatment. Induction of TF PCA by ATG was dependent on lipid raft integrity and complement activation. We showed that ATG-mediated TF activation required complement activation until assembly of the C5b-7 membrane insertion complex, but not lytic pore formation by the membrane attack complex C5b-9. Consistently, induction of TF PCA by ATG did not require maximal phosphatidylserine membrane exposure and was not correlated with the magnitude of complement-induced lytic cell injury. Blockade of free thiols, an inhibitory monoclonal antibody to protein disulfide isomerase (PDI), and the small-molecule PDI antagonist quercetin-3-rutinoside prevented ATG-mediated TF activation, and C5 complement activation resulted in oxidation of cell surface PDI. This rapid and potent mechanism of cellular TF activation represents a novel connection between the complement system and cell surface PDI-mediated thiol-disulfide exchange. Delineation of this clinically relevant mechanism of activation of the extrinsic coagulation pathway during immunosuppressive therapy with ATG may have broader implications for vascular thrombosis associated with inflammatory disorders.
Introduction
Tissue factor (TF) initiates coagulation through complex formation with factor VIIa (FVIIa).1 TF is typically sequestered from the blood or exists in a noncoagulant (encrypted) form on hematopoietic cells.2 Although specific signaling pathways have been delineated that activate TF in murine thrombosis models,3,4 mechanisms controlling TF activation in human monocytes and other cell types remain incompletely understood.5
Several mechanisms contribute to the cellular procoagulant activity of TF. On certain cell membranes, TF is sequestered in specialized cholesterol-rich microdomains (ie, lipid rafts or caveolae),6 where it may form inactive homodimers or -oligomers.7 Reorganization of lipid rafts and dissociation of TF molecules may be necessary to expose a macromolecular binding site for factor X (FX).8 Similarly, membrane exposure of phosphatidylserine (PS) greatly enhances the activation of FX, and this may involve direct interactions of TF, FVIIa, and FX with the membrane to facilitate the association and dissociation of macromolecular substrate.9
Importantly, complement activation and deposition of the membrane attack complex is highly effective in mobilizing PS to the surface of platelets10 and inducing de novo TF expression in endothelial cells11 and leukocytes.12 Pathogen defense and clot formation are thus linked by simultaneous activation of the complement and coagulation systems, which may have evolved from a common embryonic enzyme cascade.13 However, cellular TF activity is not strictly correlated with PS exposure. For instance, TF activation by the rather nonphysiological agonists calcium ionophore,14,15 cell lysis,16 or HgCl217 is only inhibited by 50% to 80% using saturating concentrations of annexin V.
TF has a membrane-proximal Cys186-Cys209 disulfide that is solvent exposed and has the characteristic features of an allosteric disulfide bond.17-19 Because allosteric disulfide bonds control protein function in a redox-dependent manner, the Cys186-Cys209 disulfide has been implicated in TF activation. Mutagenesis of this disulfide showed that procoagulant activity of TF is high when the bond can form by oxidation and that the activity is low when it is broken (reflecting the reduced state),20,21 providing a mechanism to generate cryptic TF.
In this context, extracellular protein disulfide isomerase (PDI) has been found to be associated with TF and implicated in the regulation of TF activity.3,18,22 Of note, infusion of a blocking PDI antibody or small-molecule PDI antagonists into mice inhibited platelet deposition and fibrin formation in both micro- and macrovascular thrombosis models,3,23,24 indicating that PDI may be involved in TF activation in vivo. PDI is also expressed on cell surfaces,25 including monocytes and macrophages,4 and PDI-dependent thiol-disulfide exchange has been shown to switch TF function from coagulation to cell signaling.26 Furthermore, ATP-triggered activation of macrophages via the P2X7 receptor induces cellular TF activation and PDI-dependent release of TF-positive microparticles.4 However, it remains unclear whether PDI and thiol-disulfide exchange reactions play a pathophysiological role in a clinically relevant context.
Antithymocyte globulin (ATG) is a polyclonal horse or rabbit IgG with pleiotropic cellular effects that is used to prevent or treat allograft rejection and graft-versus-host disease.27 On the basis of our previous observation that ATG induces low-grade disseminated intravascular coagulation in patients undergoing hematopoietic stem cell transplantation,28 we were interested in further defining the effects of ATG on cells implicated in the initiation of coagulation in vivo. In this report, we show that binding of rabbit ATG to monocytic cells triggers a nonlytic complement cascade that is sufficient to rapidly activate TF procoagulant activity in a PDI-dependent manner.
Methods
Cell lines and culture
All cell lines were from the DSMZ (Braunschweig, Germany) except for G44 cells, which were a gift from the Department of Neurosurgery, University Medical Center Eppendorf, Hamburg, Germany.29 Cells were maintained in RPMI culture medium supplemented with 10% heat-inactivated fetal calf serum.
Antibodies
The following nonconjugated antibodies were obtained from commercial sources: anti-TF, #4509 (American Diagnostica, Pfungstadt, Germany); anti-PDI, RL90 (Novus Biologicals, Littleton, CO) and ab31811 (Abcam, Cambridge, United Kingdom); anti-C5, eculizumab (Alexion Pharmaceuticals, Cheshire, CT); anti-C7, 030-113.7.5.4 (AbD Serotec, Raleigh, NC); anti-C9, X197 (Hycult Biotech, Plymouth Meeting, PA); and IgG2a isotype control (R&D Systems, Minneapolis, MN). All antibodies containing preservatives were dialyzed against phosphate-buffered saline (PBS).
Isolation of peripheral blood monocytes
Peripheral blood monocytes were isolated from the first author and some of the coauthors who were directly involved in conducting the experiments. In accordance with the review board of our institution, all donors gave informed consent in accordance with the Declaration of Helsinki before the blood draw. Mononuclear cells were isolated from heparin-anticoagulated whole blood by Bicoll (Biochrom, Berlin, Germany) density gradient centrifugation and were further processed by magnetic-activated cell sorting (MACS) cell separation using CD14 MicroBeads (MiltenyiBiotec, Bergisch Gladbach, Germany) on an MS Column. Final preparations contained 2 × 106/mL monocytes as assessed by flow cytometry. Monocytes were maintained in suspension culture for 16 hours in RPMI supplemented with 20% fetal calf serum.
Single-stage clotting assay
Cells (1 × 106/mL) were incubated with 100 µg/mL rabbit IgG (Sigma, St Louis, MO) or ATG (Fresenius, Bad Homburg, Germany) for 15 minutes at room temperature (RT), washed, and resuspended in PBS at 2 × 106/mL. Samples were mixed 1:1 (v:v) with normal human plasma (NHP) (HemosIL; Instrumentation Laboratory, Kirchheim, Germany) for 5 minutes at 37°C. After recalcification, times until fibrin clot formation were recorded in a KC10 coagulation instrument (Amelung, Lemgo, Germany) and converted into procoagulant activity (PCA) units by reference to a standard curve obtained by serial dilutions (1:10-100 000) of relipidated recombinant human TF (Innovin; Dade Behring, Marburg, Germany).
Where indicated, IgG- or ATG-loaded cells were preincubated with TF, TF pathway inhibitor (TFPI), or PDI antibodies, ultrapure bacitracin (TOKU-E, Bellingham, WA), 2-chloroadenosine (2-CA), or the specific PDI inhibitor quercetin-3-rutinoside (both from Sigma) for 15 minutes at RT before being exposed to NHP. Alternatively, NHP was pretreated with complement antibodies or the cyclic C3-binding peptide compstatin (Bachem, Bubendorf, Switzerland). In experiments with the calcium ionophore A23187 (Sigma), cells were suspended in N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid–buffered saline (HBS) containing 12.5 mM CaCl2 (calcium-HBS). Free thiols on THP1 cells were blocked with 5 mM N-ethylmaleimide (Sigma) for 5 minutes at RT.
Thrombin generation assay
IgG- or ATG-loaded THP1 cells were exposed to NHP, washed, and resuspended in PBS at 3 × 106/mL. Thrombin generation was measured using the Technothrombin thrombin generation assay (TGA; Technoclone, Vienna, Austria) on a BioTek FLx800 TBI fluorometer: 20 μL of cell suspension was mixed in quadruplicate wells with 20 µL of NHP depleted of endogenous phospholipids and 60 μL of substrate solution containing 1 mM Z-G-G-R-AMC fluorogenic peptide and 15 mM CaCl2. Where indicated, THP1 cells were preincubated with 20 µg/mL of inhibitory anti-TF.
FXa generation assay
IgG- or ATG-loaded THP1 cells were exposed to PBS, NHP or heat-inactivated (15 minutes at 60°C) NHP, or normal or C7- or C8-deficient human serum (Sigma) for 5 minutes at 37°C, washed, and resuspended in HBS at 2 × 106/mL. FXa generation was measured using a two-stage chromogenic end point assay: 25 µL of cell suspension was incubated with 50 µL of HBS containing 300 nM FX (Calbiochem, Darmstadt, Germany), 20 nM recombinant FVIIa (Novo Nordisk, Bagsvaerd, Denmark), and 10 mM CaCl2 for 2 hours at 37°C. FXa generation was stopped by 25 µL of EDTA buffer (25 mM). Following incubation with 25 µL of 4 mM FXa substrate Biophen CS-11(65) (Hyphen Biomed, Neuville-sur-Oise, France) for 15 minutes at 37°C, absorbance was read at 405 nm and referred to an Innovin (1:50-1:5000) standard curve.
PDI activity assay
A fluorescence-based insulin reduction assay (ProteoStat PDI Assay Kit; Enzo Life Sciences, Farmingdale, NY) was used to measure cell surface PDI activity.
Analysis of cellular ATG binding
IgG- or ATG-loaded cells (2 × 106/mL) were stained with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG (Beckman Coulter, Krefeld, Germany), washed, and analyzed by flow cytometry (see below). In some experiments, IgG and ATG were preincubated with recombinant human PDI (Enzo Life Sciences) before cell loading.
TF, TFPI, and PDI flow cytometry
TF expression was analyzed by direct flow cytometry using phycoerythrin (PE)-conjugated monoclonal antibody HTF-1 (BD Biosciences, San Jose, CA) in comparison with an isotype-matched control (Beckman Coulter). For TFPI analysis, cells were incubated with 50 µg/mL polyclonal anti-TFPI30 or control IgG, washed, and stained with secondary FITC-conjugated goat anti-rabbit IgG. For PDI analysis, cells were incubated with 50 µg/mL monoclonal antibody RL90 or IgG2a control antibody, washed, and stained with PE-conjugated goat anti-mouse IgG (Beckman Coulter). Alternatively, cells were directly stained with DyLight 488-conjugated PDI monoclonal antibody 1D3 (Enzo Life Sciences) or isotype control (Beckman Coulter). Cells were analyzed on a FACSCalibur counting 20 000 events.
Alteration of membrane lipid raft structure
Cells (2 × 106/mL in HBS) were incubated with 5 µg/mL filipin III or 10 mM methyl-β-cyclodextrin (both from Sigma) at 37°C for 15 or 45 minutes, respectively. Filipin controls received vehicle only (0.5% ethanol, final concentration). Following washes, cells were loaded with IgG or ATG, exposed to NHP, and analyzed for PCA.
PS membrane exposure and propidium iodide uptake
IgG- or ATG-loaded cells were exposed to NHP for 5 minutes at 37°C. Following washes, cells were resuspended in ice-cold binding buffer containing 25 mM CaCl2 and incubated with annexin V-FITC (BD Biosciences) or lactadherin-FITC (Cambridge Bioscience, Cambridge, United Kingdom) for 15 minutes on ice before analyzed by flow cytometry. When time course experiments were performed, ATG-loaded THP1 cells were mixed with NHP supplemented with propidium iodide (PI; BD Biosciences). After the indicated times (0-300 seconds), 200-µL aliquots were diluted into 5 mL of ice-cold PBS. Cells were washed and analyzed for annexin V-FITC binding and PI uptake by flow cytometry.
Solid-phase PDI binding assay
Microtiter plates were coated overnight at 4°C with 1 µg/mL recombinant human PDI (GenWay Biotech, San Diego, CA), washed, and blocked with 1% bovine serum albumin (Sigma) for 1 hour at RT. Increasing concentrations (0.01-5 µg/mL) of rabbit IgG, ATG, or polyclonal anti-PDI were added to separate duplicate wells for 90 minutes. Bound immunoglobulins were detected using a 1:10 000 dilution of biotin-conjugated goat anti-rabbit IgG for 90 minutes, a 1:1000 dilution of ExtrAvidin peroxidase for 30 minutes, and 2,2′-azino-bis(3-ethylbenz-thiazoline-6-sulfonic acid) liquid substrate (all from Sigma) for 20 minutes, after which absorbance was read at 405 nm.
Statistical analysis
All experiments were repeated at least 3 times, and the results are expressed as mean ± standard deviation. Data were analyzed using the two-sided Student t test for paired observations. A P value of <.05 was considered statistically significant.
Results
ATG activates surface-expressed TF on THP1 cells
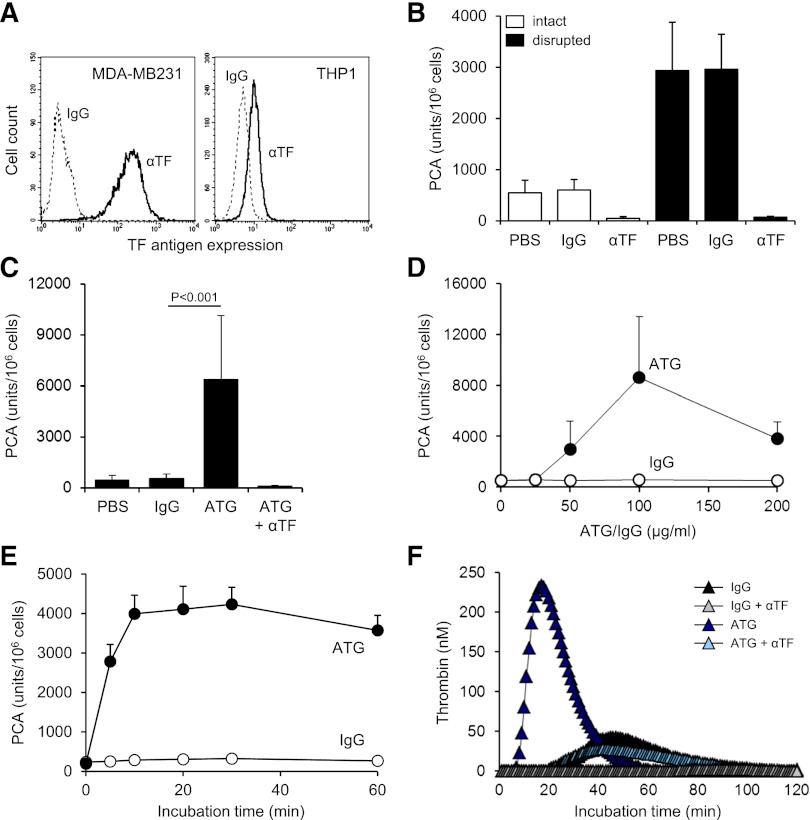
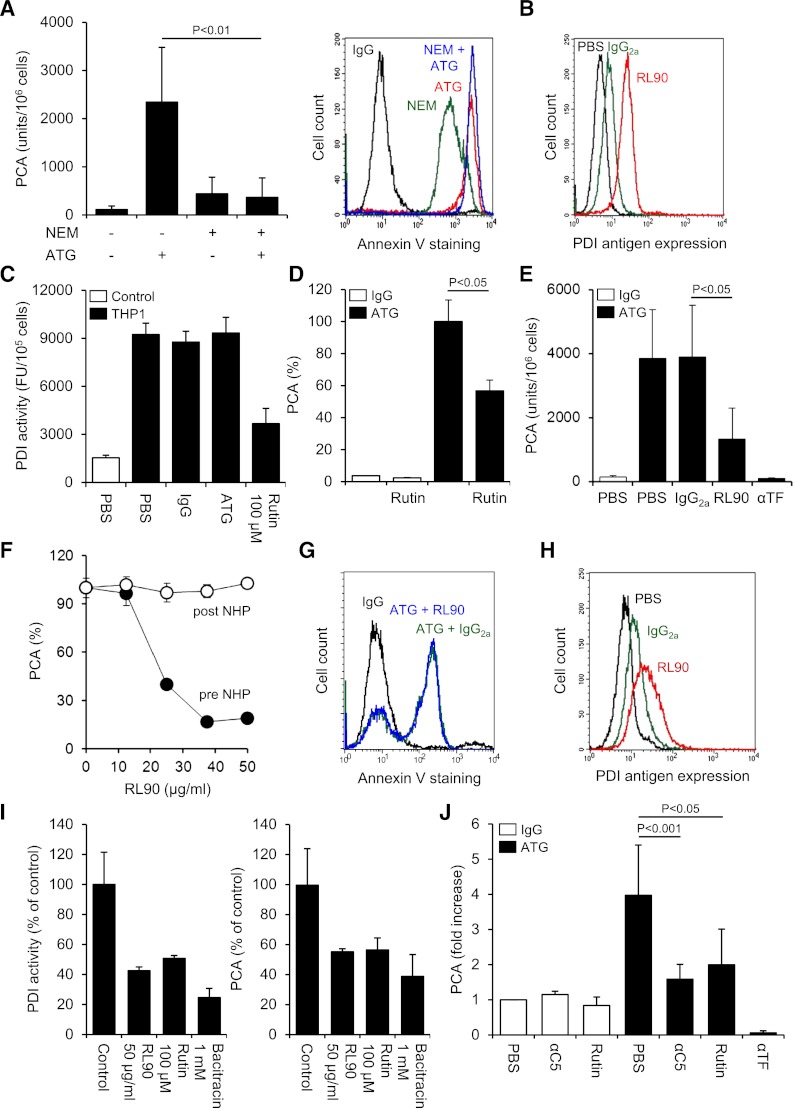
TF expression was significantly less on monocytic THP1 compared with breast cancer MDA-MB231 cells as analyzed by flow cytometry (Figure 1A) and clotting assay (supplemental Figure 1A). Cell disruption increased TF PCA of THP1 cells fivefold (Figure 1B). This effect was completely abolished by preincubation with inhibitory anti-TF followed by removal of unbound antibody, confirming that encrypted TF was entirely surface expressed before activation.2 After exposure of ATG-loaded THP1 cells to citrated plasma (NHP), a significant and concentration-dependent increase in cell-associated TF-specific PCA was observed (Figure 1C-D). Importantly, ATG had no effect on clotting induced by relipidated recombinant TF (supplemental Figure 1B), excluding effects on the coagulation reaction. Furthermore, ATG at saturating concentrations also rapidly activated THP1 cells resuspended in NHP (Figure 1E), supporting activation of preformed TF rather than de novo synthesis. A marked increase in TF PCA on ATG-loaded THP1 cells was also documented by TGA (Figure 1F). Collectively, these findings show that ATG is a potent and specific inducer of TF PCA on monocytic THP1 cells in the presence of plasma.
Figure 1.
ATG induces TF activation on THP1 cells. (A) Flow cytometric analysis of TF antigen expression on monocytic THP1 in comparison with breast cancer MDA-MB231 cells using PE-conjugated TF monoclonal (αTF) and isotype-matched control antibody (IgG). (B) THP1 cells were incubated with PBS, mouse IgG, or inhibitory TF monoclonal antibody (αTF), washed twice, and analyzed for PCA both before (intact) and after repeated freeze-thawing (disrupted) (mean ± standard deviation [SD], n = 3). (C) THP1 cells were incubated with PBS, polyclonal rabbit IgG, or ATG (100 µg/mL) for 15 minutes at RT, washed, and mixed 1:1 (v:v) with NHP for 5 minutes at 37°C. Cell-associated PCA was subsequently measured by a single-stage clotting assay in the presence or absence of inhibitory anti-TF (mean ± SD, n = 4-14). (D) THP1 cells were incubated with increasing concentrations (0-200 µg/mL) of IgG or ATG. Following washing, cells were exposed to NHP and analyzed for PCA as described above (mean ± SD, n = 4). (E) THP1 cells were mixed 1:1 (v:v) with NHP. Following the addition of IgG or ATG (100 µg/mL), cell-associated PCA was measured after indicated time points. (F) THP1 cells were treated with IgG or ATG (100 µg/mL) as described above. Following exposure to NHP, cells were washed and analyzed by thrombin generation assay in the presence or absence of inhibitory anti-TF. Representative experiments are shown in panels A, E, and F.
TF activation by ATG is dependent on lipid rafts but independent of TFPI inhibition
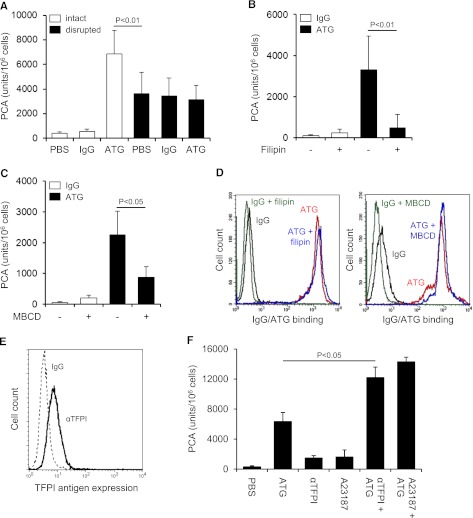
ATG induced TF PCA more potently than freeze-thawing but had no effect after cell disruption (Figure 2A), indicating that it changed the inhibitory balance of an intact plasma membrane. Consistently, lipid raft alteration by filipin (Figure 2B) or methyl-β-cyclodextrin (Figure 2C) abolished TF activation by ATG, a finding that could not be explained by impaired ATG surface binding (Figure 2D). Importantly, prolonged cholesterol depletion with methyl-β-cyclodextrin did not disturb cell integrity as assessed by annexin V binding and PI uptake (supplemental Figure 2A). Furthermore, when lipid rafts were perturbed by filipin after plasma exposure, TF PCA was not significantly reduced (supplemental Figure 2B), demonstrating that functional cholesterol-rich microdomains were specifically required for the TF activation process.
Figure 2.
TF activation by ATG requires lipid raft integrity, but does not involve TFPI inhibition. (A) THP1 cells were incubated with PBS, IgG, or ATG (100 µg/mL), washed, and either left intact or disrupted by repeated freeze-thawing. Intact and disrupted cells were mixed 1:1 (v:v) with NHP for 5 minutes at 37°C and analyzed for PCA by a single-stage clotting assay (mean ± SD, n = 3). THP1 cells were pretreated with (B) filipin or (C) methyl-β-cyclodextrin (MBCD) before being loaded with rabbit IgG or ATG (100 µg/mL) and analyzed for PCA as described above (mean ± SD, n = 5). (D) Binding of IgG or ATG (100 µg/mL) to THP1 cells was evaluated using indirect flow cytometry with or without (left) filipin or (right) MBCD pretreatment. Representative histograms are shown. (E) Analysis of TFPI antigen expression on THP1 cells by indirect flow cytometry using polyclonal anti-TFPI (αTFPI) in comparison with control IgG. A representative experiment is shown. (F) THP1 cells were suspended in calcium-HBS and incubated with PBS, ATG (100 µg/mL), inhibitory anti-TFPI (50 µg/mL), and/or calcium ionophore A23187 (20 µM) for 15 minutes at 37°C. Cells were washed, resuspended in PBS, and exposed to NHP before PCA was measured as described above (mean ± SD, n = 3).
Because THP1 cells express TFPI (Figure 2E), a raft-localized protein,31 we asked whether ATG activated TF PCA by inhibiting TFPI. PCA of THP1 cells was moderately increased in the presence of inhibitory anti-TFPI and to a similar extent by A23187 stimulation (Figure 2F). Both anti-TFPI and the calcium ionophore further increased PCA when added to ATG, providing evidence that ATG stimulated TF activation largely independent of TFPI inhibition (Figure 2F).
Role of complement in ATG-mediated TF activation
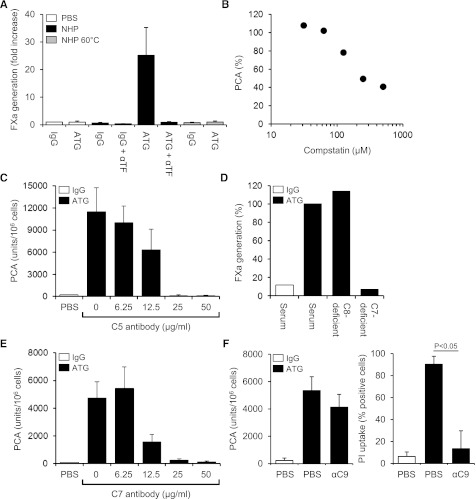
By FXa generation assay, ATG had no effect on cellular TF PCA (Figure 3A). However, when ATG-loaded THP1 cells were first exposed to NHP and then washed, TF-dependent FXa generation was markedly increased. TF activation was completely abolished by heat-inactivating the plasma (Figure 3A), suggesting that heat-labile plasma components were required. Implicating complement activation, PCA was not increased when either ATG-derived F(ab′)2 fragments were used or when the ATG Fc portion was blocked with F(ab′)2 to rabbit Fc (supplemental Figure 3A-B). Although binding of ATG to Fc-γ receptors I-III was not required (supplemental Figure 3C), Fc-dependent complement activation was directly demonstrated by the C3-binding peptide compstatin, which dose-dependently inhibited ATG-mediated TF activation in the clotting assay (Figure 3B). TF activation by ATG was also completely inhibited by eculizumab, which blocks C5 cleavage and activation (Figure 3C).
Figure 3.
TF activation by ATG is dependent on complement activation and C5b-7 membrane insertion. (A) IgG- or ATG-loaded THP1 cells were mixed 1:1 (v:v) with PBS, NHP, or heat-inactivated NHP for 5 minutes at 37°C. Following washes, cell-associated FXa generation was measured using a chromogenic end point assay in the presence or absence of inhibitory anti-TF. Results are expressed as fold increase over IgG-loaded cells mixed with PBS (mean ± SD, n = 3). (B) ATG-loaded THP1 cells were exposed to NHP in the presence of increasing concentrations (31.25-500 µM) of compstatin. Results are expressed as percent PCA of ATG-loaded cells exposed to NHP in the absence of compstatin. A representative experiment is shown. (C) Inhibition of ATG-mediated TF activation on THP1 cells by C5 monoclonal antibody eculizumab (mean ± SD, n = 3). (D) ATG-loaded THP1 cells were exposed to normal or C7- or C8-deficient serum. Following washes, cell-associated FXa generation was measured as described above. Results are presented as percent FXa generation of ATG-loaded cells exposed to normal human serum. A representative experiment is shown. (E) Inhibition of ATG-mediated TF activation on THP1 cells by C7 monoclonal antibody (mean ± SD, n = 3). (F) Effect of C9 monoclonal antibody X (also see Materials, Antibodies section)197 (αC9) on ATG-mediated (left) TF activation and (right) PI uptake. The antibody was used at 50 µg/mL (mean ± SD, n = 3-6).
Additional experiments in the FXa generation assay showed a marked increase in TF activity after incubation of ATG-loaded cells with normal or C8-deficient serum (Figure 3D). In contrast, C7-deficient serum did not support ATG-induced FXa generation, suggesting that C7 membrane insertion, but not full assembly of the C5b-9 membrane-attack complex (MAC), was required. Consistently, a monoclonal C7 antibody, which prevents recruitment of C7 to the fluid-phase C5b6 complex, dose-dependently inhibited ATG-mediated TF activation in the clotting assay (Figure 3E). In contrast, a monoclonal C9 antibody, which interferes with the incorporation of C9 into the C5b-8 complex and thus inhibits full MAC assembly, had no significant effect on cellular TF PCA, but completely abolished pore formation as measured by PI uptake (Figure 3F). Collectively, these findings show that formation of the membrane insertion complex C5b-7 is a critical step in cellular TF activation by ATG.
TF activation by ATG is not correlated with extracellular PS
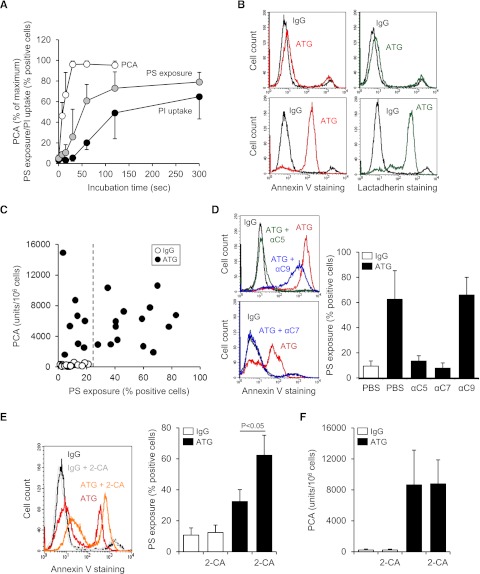
Because ATG-mediated TF activation occurred upstream of full MAC assembly, we performed time course experiments to establish the temporal correlation between TF activation and complement-induced cell injury. TF PCA was maximally induced 30 seconds after exposure of ATG-loaded THP1 cells to NHP, whereas PS membrane externalization followed a more delayed time course, reaching saturation after 120 seconds (Figure 4A). Uptake of PI reached a plateau even later after 120 to 300 seconds. These data showed that ATG-mediated TF activation preceded maximal PS externalization and pore formation by the MAC.
Figure 4.
ATG-induced TF activation is not correlated with PS membrane exposure. (A) Time course of cell-associated PCA, PS exposure, and PI uptake after exposing ATG-loaded THP1 cells to NHP. PCA is presented as percent of maximum, whereas PS exposure and PI uptake are presented as percent positive cells (mean ± SD, n = 5-7). (B) Similar binding of (left) annexin V-FITC and (right) lactadherin-FITC to ATG-loaded and NHP-exposed THP1 cells. Histograms are representative of experiments with (top) low and (bottom) high PS exposure. (C) IgG- (○) or ATG-loaded THP1 cells (●) were exposed to NHP for 5 minutes at 37°C. Samples were analyzed for both PCA (clotting assay) and PS exposure (annexin V-FITC binding). The dashed line indicates the upper limit of PS exposure on IgG-loaded cells (n = 22). (D) Effect of C5, C7, and C9 antibodies on PS externalization on ATG-loaded THP1 cells exposed to NHP. Representative (left) histograms and (right) summary statistics indicating percent annexin V-positive cells are shown (mean ± SD, n = 3-7). (E) Coincubation with 20 µM 2-CA enhances PS externalization on ATG-loaded and NHP-exposed THP1 cells. (Left) Representative histograms and (right) summary statistics are shown (mean ± SD, n = 4). (F) 2-CA does not affect TF activation on ATG-loaded and NHP-exposed THP1 cells (mean ± SD, n = 6).
ATG-induced PS exposure measured by annexin V staining was highly variable. Virtually identical results were obtained when extracellular PS was detected with lactadherin (Figure 4B), excluding that annexin V staining was of limited value to detect relevant procoagulant PS.32 Importantly, there was no correlation between TF PCA and PS exposure on ATG-treated cells (r = −0.03; Figure 4C).
Binding of annexin V to ATG-loaded and plasma-activated THP1 cells was completely abolished by C5 and C7, but not by C9, inhibition (Figure 4D), demonstrating that PS exposure required C5b-7 membrane insertion but not full MAC assembly. However, the staining intensity on annexin V–positive cells was somewhat reduced by C9 inhibition (Figure 4D), indicating that MAC-mediated pore formation partly contributed to PS exposure. To further evaluate the role of lytic PS exposure, we used 2-CA to inhibit MAC removal from the cell surface.33 As expected, 2-CA increased PS exposure (Figure 4E) and PI uptake (supplemental Figure 4) but had no effect on cellular PCA (Figure 4F), further demonstrating that TF activation by ATG did not primarily result from MAC-induced damage to the cell membrane.
Role of thiol-disulfide exchange in TF activation by ATG
We asked whether ATG-mediated TF activation involved PDI-dependent thiol-disulfide exchange. Blocking free thiols on THP1 cells with N-ethylmaleimide slightly increased basal TF activity but completely abolished further TF activation by ATG, demonstrating that thiol exchange reactions were critically involved (Figure 5A). Importantly, thiol blockade with N-ethylmaleimide alone resulted in significant annexin V binding but did not interfere with maximal ATG-induced PS exposure, indicating that PS externalization in itself was insufficient and that additional thiol-dependent molecular events were required for TF activation by ATG.
Figure 5.
Role of PDI in cellular TF activation by ATG. (A) Effect of thiol blockade by N-ethylmaleimide (NEM) on ATG-mediated (left) TF activation and (right) PS exposure. Summary statistics (mean ± SD, n = 8) and representative histograms are shown. (B) Indirect flow cytometric analysis of PDI antigen expression on THP1 cells using the monoclonal antibody RL90. A representative experiment is shown. (C) THP1 cells were treated with PBS, IgG, or ATG (100 µg/mL) or rutin (100 µM) before cell-associated insulin reductase activity was measured. Results are presented as fluorescence units (FUs) per 105 cells. A representative experiment is shown. (D) IgG- or ATG-loaded THP1 cells were incubated with 100 µM rutin or vehicle for 10 minutes at RT, mixed with NHP, and analyzed for PCA as described above. Results are presented as percent of maximum PCA (mean ± SD, n = 3). (E) ATG-loaded THP1 cells received either PBS or 50 µg/mL control IgG2a, RL90, or inhibitory anti-TF for 15 minutes at RT before being exposed to NHP and analyzed for PCA (mean ± SD, n = 9). (F) ATG-loaded THP1 cells received RL90 either before (●) or 5 minutes after mixing with NHP (○). The PDI antibody was allowed to incubate for 15 minutes. Results are presented as percent of maximum PCA. A representative experiment is shown. (G) Flow cytometric analysis of annexin V-FITC binding to ATG-loaded and NHP-exposed THP1 cells preincubated with IgG2a or RL90. A representative experiment is shown. (H) Flow cytometric analysis of PDI antigen expression on HL60 cells using the monoclonal antibody RL90. (I) (Left) Effect of RL90 and PDI inhibitors on insulin reductase activity on HL60 cells. Results are presented as percent PDI activity of untreated cells (mean ± SD, n = 2-5). (Right) Effect of RL90 and PDI inhibitors on ATG-mediated TF activation on HL60 cells. Results are presented as percent PCA of ATG-loaded cells exposed to NHP (mean ± SD, n = 2-5). (J) Following loading of isolated monocytes with rabbit IgG or ATG (100 µg/mL), cell-associated PCA was measured in the presence of PBS, 50 µg/mL eculizumab (αC5), 100 µM rutin, or 20 µg/mL inhibitory anti-TF. Results are presented as fold increase over IgG-treated monocytes in the presence of PBS (mean ± SD, n = 5-11).
We detected PDI antigen expression on untreated THP1 cells (Figure 5B), suggesting that this thiol-disulfide isomerase contributed to TF activation. Recently, the flavonol quercetin-3-rutinoside (rutin) has been identified as a specific PDI inhibitor.24 Using a fluorescence-based insulin reduction assay, we confirmed that rutin inhibited the activity of recombinant human PDI with an IC50 of 14 µM and maximum inhibition at >50 µM (supplemental Figure 5A). Significant insulin reductase activity was detected on the surface of THP1 cells (Figure 5C), and this activity was largely abolished by 100 µM rutin. Importantly, ATG had no effect on cell-associated PDI activity, excluding that ATG directly modulated PDI function. However, in the presence of rutin, ATG-mediated TF activation on THP1 cells was inhibited by 43% (Figure 5D), implicating PDI and no other thiol isomerases in the TF activation process. Similarly, anti-PDI RL90 significantly and dose-dependently reduced TF activation by ATG (Figure 5E-F). Importantly, RL90 did not inhibit cellular TF PCA after plasma exposure (Figure 5F), demonstrating that the PDI antibody specifically interfered with the TF activation process. Furthermore, pretreatment of THP1 cells with RL90 had no effect on ATG-induced PS externalization (Figure 5G), indicating that inhibition of cellular PCA was not caused by decreased complement activation or modulation of PDI-dependent regulation of PS asymmetry.34
Despite similar binding to various cell lines (supplemental Figure 5B), ATG only activated TF on monocytic U937 and myelomonocytic HL60 cells, but not on colorectal cancer HT29, myeloma IM9, glioma G44, or breast cancer MDA-MB231 cells (supplemental Figure 5C), pointing to a cell type–specific effect of ATG. PDI antigen was detected on HL60 cells (Figure 5H), and both surface-associated PDI activity and ATG-mediated TF activation in this cell line were inhibited by anti-PDI RL90, rutin, and bacitracin (Figure 5I), demonstrating that functional PDI was required for the TF activation process. Furthermore, following overnight culture, freshly isolated monocytes from healthy donors showed increased TF expression (supplemental Figure 5D). ATG enhanced cellular PCA on monocytes fourfold, and this effect was abolished by C5 complement inhibition using eculizumab (Figure 5J). Moreover, preincubation with 100 µM rutin specifically inhibited ATG-dependent TF activation by 66%, demonstrating that this activation mechanism is not restricted to transformed myelomonocytic cell lines.
Complement activation changes the redox state of cell surface PDI
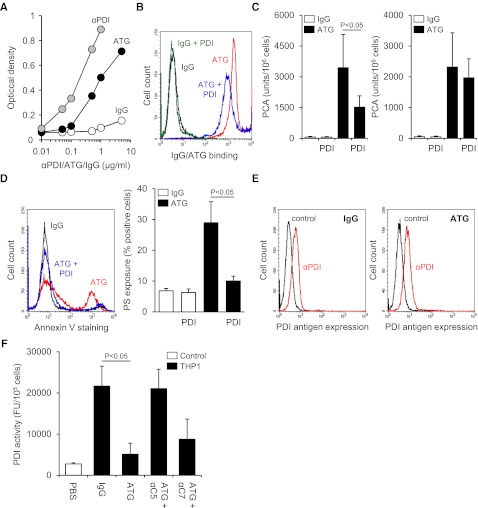
We next asked whether ATG was reactive with PDI and thereby localized to areas of the cell surface where PDI-dependent modulation of TF occurred. In a solid-phase binding assay, ATG showed specific reactivity with immobilized PDI at 0.1-5 µg/mL (Figure 6A), demonstrating that immunization with Jurkat cells led to a significant immune response toward PDI. We then neutralized the PDI-reactive fraction of ATG by preincubation with recombinant human PDI, which significantly reduced ATG binding to the surface of THP1 cells (Figure 6B), inhibited TF activation by 56% (Figure 6C), and largely abolished PS externalization (Figure 6D). Competitive inhibition of ATG-mediated TF activation by recombinant PDI was not dependent on reactive PDI free thiols (supplemental Figure 6), and PDI did not block TF activation after ATG loading (Figure 6C).
Figure 6.
Complement C5 activation induces oxidation of cell surface PDI. (A) Binding of ATG to immobilized recombinant human PDI in comparison with rabbit IgG and polyclonal rabbit anti-PDI (αPDI). (B) Rabbit IgG and ATG were preincubated with recombinant human PDI in a 1:3 molar ratio and subsequently loaded on THP1 cells for 15 minutes at RT (100 µg/mL final concentration). Following washes, cellular binding of IgG and ATG was analyzed by indirect flow cytometry. A representative experiment is shown. (C) (Left) Rabbit IgG and ATG were preincubated with recombinant human PDI in a 1:3 molar ratio before PCA of IgG- and ATG-loaded THP1 cells was measured as described above (mean ± SD, n = 5). (Right) As a control experiment, recombinant PDI was added to THP1 cells after IgG or ATG loading (mean ± SD, n = 3). (D) IgG and ATG were preincubated with recombinant PDI as described above. Following loading on THP1 cells and exposure to NHP, annexin V-FITC binding was analyzed by flow cytometry. A (left) representative experiment and (right) summary statistics are shown (mean ± SD, n = 3). (E) (Left) IgG- or (right) ATG-loaded THP1 cells were exposed to NHP and subsequently analyzed for PDI antigen expression by single-color flow cytometry using monoclonal antibody 1D3 (αPDI) in comparison with an isotype-matched control. A representative experiment is shown. (F) ATG-loaded THP1 cells were exposed to NHP in the absence or presence of 50 µg/mL eculizumab (αC5) or blocking C7 monoclonal antibody (αC7). Following washes, cell-associated PDI activity was measured as described above (mean ± SD, n = 3).
These data suggested that the PDI-reactive fraction of ATG localized complement activation to the vicinity of PDI and TF. We next measured the redox state of PDI before and after plasma exposure of ATG-loaded THP1 cells. Whereas plasma exposure did not reduce PDI antigen expression (Figure 6E), PDI reductase activity was markedly reduced after plasma exposure dependent on complement C5 activation (Figure 6F). This finding is in accordance with the recently demonstrated consumption of cell surface reducing activity by complement activation and inhibition35 and provides a mechanism by which the complement system can promote TF oxidation in a PDI-dependent thiol-disulfide exchange pathway.
Discussion
In this study, we identify a mechanism by which immunosuppressive therapy with ATG causes systemic coagulation activation and delineate a new connection between the complement system and cell surface PDI. ATG induces cellular activation of preformed, membrane-expressed TF rather than de novo protein synthesis. Because ATG promoted markedly higher PCA relative to cell disruption, TFPI inhibition, or calcium ionophore stimulation, this mechanism represents a potent and rapid pathway of pathophysiologically relevant TF activation on myelomonocytic cells.
Although TF activation is associated with PS externalization, the magnitude of PS exposure is not correlated with TF PCA on ATG-loaded cells, and ATG-amplified PS externalization on thiol-blocked cells is insufficient to activate TF. We show that cell surface PDI is required for TF activation by ATG. The ATG used in this study is purified from rabbits that are immunized with human T-lymphoblastic Jurkat cells. However, ATG is not specific for activated T cells,36 and antigen targets ubiquitously expressed by hematopoietic cells have been identified.27 We show that ATG has reactivity with PDI but does not inhibit cell surface PDI activity. Importantly, neutralization of the PDI-reactive fraction of ATG significantly reduces ATG cell surface binding and the resulting TF activation after plasma exposure. We propose that the localization of ATG to PDI and possibly other select cell surface molecules in close proximity of TF is critically important for the Fc-mediated complement activation leading to TF decryption (Figure 7).
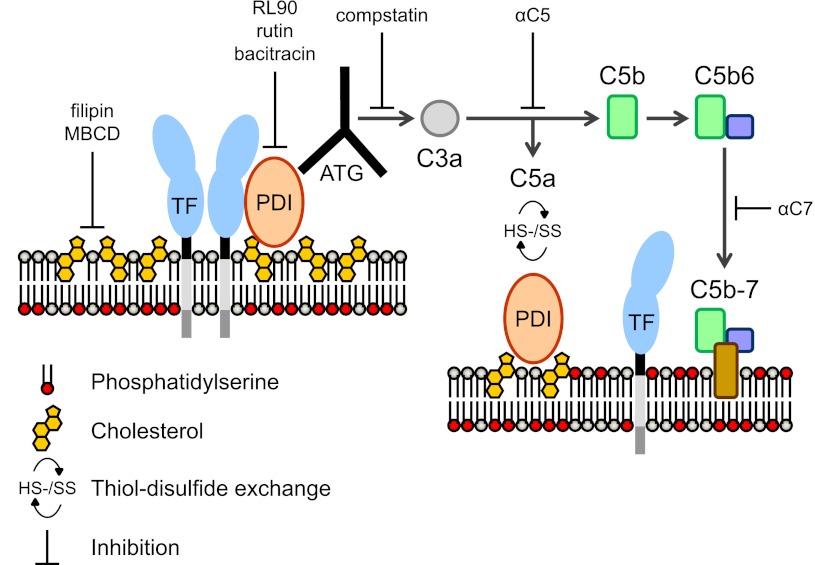
Figure 7.
Proposed mechanism of TF activation by ATG. TF is maintained in a predominantly noncoagulant (cryptic) state by possible mechanisms of tonic inhibition by cholesterol-rich lipid rafts, homo- or hetero-dimerization, association with PDI, and/or sequestration of PS to the inner membrane leaflet (left). We propose that ATG binds to surface-located PDI in close proximity of the cryptic pools of TF and activates complement through the classical pathway. Formation of the initial membrane insertion complex C5b-7 is critical for TF activation and PS exposure (right), but full MAC assembly leading to lytic PS externalization is not required. PDI-dependent thiol-disulfide exchange reactions occur following C5 conversion through engagement of complement regulatory proteins,35 resulting in depletion of membrane reductive equivalents (ie, thioredoxin-1) with consecutive PDI and TF oxidation.38 Rearrangements of the cell membrane by C5b-7 insertion may lead to PS exposure in raft domains and thus facilitate dissociation of TF from regulatory proteins and/or TF oxidation.
Oxidative stress and extracellular thiol-disulfide exchange reactions are linked to complement attack.35 Thioredoxin (Trx)-1, a disulfide reductase controlling cellular redox potential, has recently emerged as a crucial cell surface regulator of complement activation. Trx-1 localizes to lipid rafts37 and provides reducing equivalents to support complement inhibition by C4B and factor H at the level of the C5 convertase.35 Our data show that C5 activation results in a marked decrease of cell surface PDI reductase activity requiring C5 activation. Because induction of TF PCA is furthermore dependent on intact lipid raft domains, we propose that, upon activation of the classical complement pathway by cell-bound ATG, rapid depletion of reduced Trx-1 from the cell surface causes oxidation of PDI, which in turn promotes TF activation by shifting the equilibrium from reduced to oxidized TF.38
Although ATG-mediated complement activation on human fibroblasts results in delayed TF PCA expression that strongly correlates with cytoloysis,39 our findings delineate that full MAC assembly is not required for rapid complement-dependent TF activation on monocytes. C7 does not contribute to PDI oxidation, but promotes PS externalization and TF activation. C7 binding to the last proteolytic product of complement activation, C5b6, is crucial for generating the first lipophilic intermediate—the C5b-7 complex—that stably associates with cell membranes. Although the C5b-7 complex does not form a pore that would allow transmembrane fluxes, it appears to perturb membranes sufficiently to induce cell signaling.40-42 It is thus conceivable that targeted insertion of C5b-7 complexes into lipid rafts supports TF oxidation by perturbing protein-protein interactions contributing to the suppression of TF activity, including TF dimerization7 or association with β1 integrins43 and other chaperone proteins.44 Alternatively, the exposure of PS, implicated in coordinating the lipid insertion of functionally important membrane-proximal loops of the TF extracellular domain,9,45 may aid the acquisition of a fully functional conformation during TF oxidation.
PDI is increasingly recognized as an important contributor to vascular thrombosis in animal models3,23,24 and specifically for TF-dependent thrombosis.4 However, in vitro studies that implicated PDI in TF activation through thiol exchange reactions targeting the membrane-proximal allosteric Cys186-Cys209 disulfide have been vigorously disputed.46-48 Particularly, epithelial MDA-MB231 cancer cells have been used as a model to argue against significant roles of PDI and thiol pathways in TF activation.46 Our data with various cell lines and primary monocytes demonstrate that the ATG-initiated TF activation pathway is specific for myelomonocytic cells and does not operate on tumor cell surfaces, directly resolving the existing controversies raised by studies with carcinoma cells.46 The accumulating evidence points to critical contributions of PS exposure and oxidative changes in cellular models used to study TF decryption. Our data show that activation of complement induces these changes simultaneously by the delineated effects of C5 activation favoring oxidation and C7-dependent membrane alteration.
To our knowledge, the present study provides the first identification of a pathophysiologically relevant scenario where TF activation by a PDI-dependent pathway results in clinically relevant thrombotic disease. Our study defines a novel link between complement-mediated inflammation and extrinsic coagulation pathway initiation that results in rapid activation of preformed TF on monocytes. This novel mechanism may be of broader importance for prothrombotic disorders characterized by deregulated complement activation such as the Escherichia coli–associated hemolytic uremic syndrome49 or paroxysmal nocturnal hemoglobinuria50 and for other pathophysiological scenarios in bacterial infection, organ graft rejection, and autoimmunity.
Supplementary Material
Acknowledgments
This study was supported in part by a grant from the Eppendorfer Krebs und Leukämiehilfe e. V. (F.L.) and National Heart, Lung, and Blood Institute grant P01-HL31950 (W.R.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: F.L. designed experiments, analyzed data, and wrote the manuscript; B.S., C.F., and M.S. performed experiments and analyzed data; F.A.A., N.K., and C.B. oversaw the study, analyzed data, and critically revised the manuscript; and W.R. participated in experimental design, data interpretation, and manuscript writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Florian Langer, II. Medizinische Klinik und Poliklinik, Hubertus Wald Tumorzentrum–Universitäres Cancer Center Hamburg (UCCH), Universitätsklinikum Eppendorf, Martinistrasse 52, D-20246 Hamburg, Germany; e-mail: langer@uke.de.
References
- 1.Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007;27(8):1687–1693. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- 2.Drake TA, Ruf W, Morrissey JH, et al. Functional tissue factor is entirely cell surface expressed on lipopolysaccharide-stimulated human blood monocytes and a constitutively tissue factor-producing neoplastic cell line. J Cell Biol. 1989;109(1):389–395. doi: 10.1083/jcb.109.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reinhardt C, von Brühl ML, Manukyan D, et al. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J Clin Invest. 2008;118(3):1110–1122. doi: 10.1172/JCI32376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furlan-Freguia C, Marchese P, Gruber A, et al. P2X7 receptor signaling contributes to tissue factor-dependent thrombosis in mice. J Clin Invest. 2011;121(7):2932–2944. doi: 10.1172/JCI46129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mann KG, Krudysz-Amblo J, Butenas S. Tissue factor controversies. Thromb Res. 2012;129(suppl 2):S5–S7. doi: 10.1016/j.thromres.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietzen DJ, Page KL, Tetzloff TA. Lipid rafts are necessary for tonic inhibition of cellular tissue factor procoagulant activity. Blood. 2004;103(8):3038–3044. doi: 10.1182/blood-2003-07-2399. [DOI] [PubMed] [Google Scholar]
- 7.Bach RR, Moldow CF. Mechanism of tissue factor activation on HL-60 cells. Blood. 1997;89(9):3270–3276. [PubMed] [Google Scholar]
- 8.Bach RR. Tissue factor encryption. Arterioscler Thromb Vasc Biol. 2006;26(3):456–461. doi: 10.1161/01.ATV.0000202656.53964.04. [DOI] [PubMed] [Google Scholar]
- 9.Ohkubo YZ, Morrissey JH, Tajkhorshid E. Dynamical view of membrane binding and complex formation of human factor VIIa and tissue factor. J Thromb Haemost. 2010;8(5):1044–1053. doi: 10.1111/j.1538-7836.2010.03826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiedmer T, Esmon CT, Sims PJ. On the mechanism by which complement proteins C5b-9 increase platelet prothrombinase activity. J Biol Chem. 1986;261(31):14587–14592. [PubMed] [Google Scholar]
- 11.Saadi S, Holzknecht RA, Patte CP, et al. Complement-mediated regulation of tissue factor activity in endothelium. J Exp Med. 1995;182(6):1807–1814. doi: 10.1084/jem.182.6.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muhlfelder TW, Niemetz J, Kreutzer D, et al. C5 chemotactic fragment induces leukocyte production of tissue factor activity: a link between complement and coagulation. J Clin Invest. 1979;63(1):147–150. doi: 10.1172/JCI109269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krem MM, Di Cera E. Evolution of enzyme cascades from embryonic development to blood coagulation. Trends Biochem Sci. 2002;27(2):67–74. doi: 10.1016/s0968-0004(01)02007-2. [DOI] [PubMed] [Google Scholar]
- 14.Wolberg AS, Monroe DM, Roberts HR, et al. Tissue factor de-encryption: ionophore treatment induces changes in tissue factor activity by phosphatidylserine-dependent and -independent mechanisms. Blood Coagul Fibrinolysis. 1999;10(4):201–210. [PubMed] [Google Scholar]
- 15.Wolberg AS, Kon RH, Monroe DM, et al. Deencryption of cellular tissue factor is independent of its cytoplasmic domain. Biochem Biophys Res Commun. 2000;272(2):332–336. doi: 10.1006/bbrc.2000.2783. [DOI] [PubMed] [Google Scholar]
- 16.Le DT, Rapaport SI, Rao LV. Relations between factor VIIa binding and expression of factor VIIa/tissue factor catalytic activity on cell surfaces. J Biol Chem. 1992;267(22):15447–15454. [PubMed] [Google Scholar]
- 17.Chen VM, Ahamed J, Versteeg HH, et al. Evidence for activation of tissue factor by an allosteric disulfide bond. Biochemistry. 2006;45(39):12020–12028. doi: 10.1021/bi061271a. [DOI] [PubMed] [Google Scholar]
- 18.Versteeg HH, Ruf W. Thiol pathways in the regulation of tissue factor prothrombotic activity. Curr Opin Hematol. 2011;18(5):343–348. doi: 10.1097/MOH.0b013e32834981de. [DOI] [PubMed] [Google Scholar]
- 19.Azimi I, Wong JW, Hogg PJ. Control of mature protein function by allosteric disulfide bonds. Antioxid Redox Signal. 2011;14(1):113–126. doi: 10.1089/ars.2010.3620. [DOI] [PubMed] [Google Scholar]
- 20.van den Hengel LG, Kocatürk B, Reitsma PH, et al. Complete abolishment of coagulant activity in monomeric disulfide-deficient tissue factor. Blood. 2011;118(12):3446–3448. doi: 10.1182/blood-2011-06-364612. [DOI] [PubMed] [Google Scholar]
- 21.van den Hengel LG, Osanto S, Reitsma PH, et al. Murine tissue factor coagulant activity is critically dependent on the presence of an intact allosteric disulfide. Haematologica. 2013;98(1):153–158. doi: 10.3324/haematol.2012.069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Versteeg HH, Ruf W. Tissue factor coagulant function is enhanced by protein-disulfide isomerase independent of oxidoreductase activity. J Biol Chem. 2007;282(35):25416–25424. doi: 10.1074/jbc.M702410200. [DOI] [PubMed] [Google Scholar]
- 23.Cho J, Furie BC, Coughlin SR, et al. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J Clin Invest. 2008;118(3):1123–1131. doi: 10.1172/JCI34134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jasuja R, Passam FH, Kennedy DR, et al. Protein disulfide isomerase inhibitors constitute a new class of antithrombotic agents. J Clin Invest. 2012;122(6):2104–2113. doi: 10.1172/JCI61228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang XM, Fitzgerald M, Grant CM, et al. Redox control of exofacial protein thiols/disulfides by protein disulfide isomerase. J Biol Chem. 1999;274(4):2416–2423. doi: 10.1074/jbc.274.4.2416. [DOI] [PubMed] [Google Scholar]
- 26.Ahamed J, Versteeg HH, Kerver M, et al. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc Natl Acad Sci USA. 2006;103(38):13932–13937. doi: 10.1073/pnas.0606411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia. 2007;21(7):1387–1394. doi: 10.1038/sj.leu.2404683. [DOI] [PubMed] [Google Scholar]
- 28.Weber M, Kröger N, Langer F, et al. Non-overt disseminated intravascular coagulation in patients during treatment with antithymocyte globulin for unrelated allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2003;31(9):817–822. doi: 10.1038/sj.bmt.1703921. [DOI] [PubMed] [Google Scholar]
- 29.Giese A, Hagel C, Kim EL, et al. Thromboxane synthase regulates the migratory phenotype of human glioma cells. Neuro-oncol. 1999;1(1):3–13. doi: 10.1093/neuonc/1.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahamed J, Belting M, Ruf W. Regulation of tissue factor-induced signaling by endogenous and recombinant tissue factor pathway inhibitor 1. Blood. 2005;105(6):2384–2391. doi: 10.1182/blood-2004-09-3422. [DOI] [PubMed] [Google Scholar]
- 31.Sevinsky JR, Rao LV, Ruf W. Ligand-induced protease receptor translocation into caveolae: a mechanism for regulating cell surface proteolysis of the tissue factor-dependent coagulation pathway. J Cell Biol. 1996;133(2):293–304. doi: 10.1083/jcb.133.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi J, Shi Y, Waehrens LN, et al. Lactadherin detects early phosphatidylserine exposure on immortalized leukemia cells undergoing programmed cell death. Cytometry A. 2006;69(12):1193–1201. doi: 10.1002/cyto.a.20345. [DOI] [PubMed] [Google Scholar]
- 33.Roberts PA, Morgan BP, Campbell AK. 2-Chloroadenosine inhibits complement-induced reactive oxygen metabolite production and recovery of human polymorphonuclear leucocytes attacked by complement. Biochem Biophys Res Commun. 1985;126(2):692–697. doi: 10.1016/0006-291x(85)90240-2. [DOI] [PubMed] [Google Scholar]
- 34.Popescu NI, Lupu C, Lupu F. Extracellular protein disulfide isomerase regulates coagulation on endothelial cells through modulation of phosphatidylserine exposure. Blood. 2010;116(6):993–1001. doi: 10.1182/blood-2009-10-249607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.King BC, Nowakowska J, Karsten CM, et al. Truncated and full-length thioredoxin-1 have opposing activating and inhibitory properties for human complement with relevance to endothelial surfaces. J Immunol. 2012;188(8):4103–4112. doi: 10.4049/jimmunol.1101295. [DOI] [PubMed] [Google Scholar]
- 36.Greco B, Bielory L, Stephany D, et al. Antithymocyte globulin reacts with many normal human cell types. Blood. 1983;62(5):1047–1054. [PubMed] [Google Scholar]
- 37.Hara T, Kondo N, Nakamura H, et al. Cell-surface thioredoxin-1: possible involvement in thiol-mediated leukocyte-endothelial cell interaction through lipid rafts. Antioxid Redox Signal. 2007;9(9):1427–1437. doi: 10.1089/ars.2007.1661. [DOI] [PubMed] [Google Scholar]
- 38.Schwaller M, Wilkinson B, Gilbert HF. Reduction-reoxidation cycles contribute to catalysis of disulfide isomerization by protein-disulfide isomerase. J Biol Chem. 2003;278(9):7154–7159. doi: 10.1074/jbc.M211036200. [DOI] [PubMed] [Google Scholar]
- 39.Carson SD, Johnson DR. Consecutive enzyme cascades: complement activation at the cell surface triggers increased tissue factor activity. Blood. 1990;76(2):361–367. [PubMed] [Google Scholar]
- 40.Nicholson-Weller A, Halperin JA. Membrane signaling by complement C5b-9, the membrane attack complex. Immunol Res. 1993;12(3):244–257. doi: 10.1007/BF02918256. [DOI] [PubMed] [Google Scholar]
- 41.Carney DF, Lang TJ, Shin ML. Multiple signal messengers generated by terminal complement complexes and their role in terminal complement complex elimination. J Immunol. 1990;145(2):623–629. [PubMed] [Google Scholar]
- 42.Niculescu F, Rus H, Shin S, et al. Generation of diacylglycerol and ceramide during homologous complement activation. J Immunol. 1993;150(1):214–224. [PubMed] [Google Scholar]
- 43.Dorfleutner A, Hintermann E, Tarui T, et al. Cross-talk of integrin alpha3beta1 and tissue factor in cell migration. Mol Biol Cell. 2004;15(10):4416–4425. doi: 10.1091/mbc.E03-09-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhattacharjee G, Ahamed J, Pedersen B, et al. Regulation of tissue factor—mediated initiation of the coagulation cascade by cell surface grp78. Arterioscler Thromb Vasc Biol. 2005;25(8):1737–1743. doi: 10.1161/01.ATV.0000173419.31242.56. [DOI] [PubMed] [Google Scholar]
- 45.Ruf W, Miles DJ, Rehemtulla A, et al. Tissue factor residues 157-167 are required for efficient proteolytic activation of factor X and factor VII. J Biol Chem. 1992;267(31):22206–22210. [PubMed] [Google Scholar]
- 46.Pendurthi UR, Ghosh S, Mandal SK, et al. Tissue factor activation: is disulfide bond switching a regulatory mechanism? Blood. 2007;110(12):3900–3908. doi: 10.1182/blood-2007-07-101469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang HP, Hogg PJ. Critical importance of the cell system when studying tissue factor de-encryption. Blood. 2008;112(3):912–913, author reply 913. doi: 10.1182/blood-2008-05-158766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pendurthi UR, Rao VM. Tissue factor de-encryption: the cell model system. Blood. 2008;112(3):913. [Google Scholar]
- 49.Orth D, Würzner R. Complement in typical hemolytic uremic syndrome. Semin Thromb Hemost. 2010;36(6):620–624. doi: 10.1055/s-0030-1262883. [DOI] [PubMed] [Google Scholar]
- 50.Ziakas PD, Poulou LS, Pomoni A. Thrombosis in paroxysmal nocturnal hemoglobinuria at a glance: a clinical review. Curr Vasc Pharmacol. 2008;6(4):347–353. doi: 10.2174/157016108785909742. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.