Abstract
The respiratory system is continuously modulated by numerous aminergic and peptidergic substances that act at all levels of integration: from the sensory level to the level of central networks and motor nuclei. The same neuronal networks receive inputs from multiple modulators released locally as well as from distal nuclei. All parameters of respiratory control are controlled by multiple neuromodulators. By partly converging onto similar G-proteins and second messenger systems, acetylcholine, norepinephrine, histamine, serotonin (5-HT), dopamine, ATP, substance P, cholecystokinin (CCK) can increase frequency, regularity and amplitude of respiratory activity. Yet, the same modulator can also exert differential effects on respiratory activity by acting on different receptors partly in the same neurons. In the pre-Bötzinger complex (pre-BötC) modulators can differentially modulate frequency and amplitude in different types of pacemaker neurons. Similarly motoneurons located in different motor nuclei receive differential amplitude modulation from different modulators. Thus, modulators are capable of orchestrating and modulating different parameters of respiratory activity by differentially targeting different cellular targets. A disturbance in modulatory control may lead to Sudden Infant Death Syndrome (SIDS) and erratic breathing.
Keywords: Respiratory rhythm, pre-BötC, neuromodulator, 5-HT, norepinephrine, Substance P
Introduction
Neuromodulators have multiple functions in controlling respiratory rhythmic activity. They differentially regulate the amplitude and frequency of respiratory activity. They act at the level of motoneurons, sensory neurons and neurons located in various CNS nuclei. They are involved in the reconfiguration of the respiratory network during different forms of breathing. Thus, understanding how neuromodulators control the respiratory system will provide not only important insights into principles of neuromodulation, but also into how respiratory rhythmic activity is generated and regulated.
There is increasing evidence that the respiratory rhythm is generated by neuronal networks located within the ventral respiratory column (VRC) and the parafacial respiratory group (pFRG) (Alheid et al., 2002; Feldman and Del Negro, 2006). Although, still uncertain how these regions interact to generate the breathing rhythm, it is well established that a particular area within the VRC, the so called pre-Boetzinger complex (pre-BötC) is critical for generating inspiratory activity (Gray et al., 2001). Multiple studies have demonstrated that lesioning of the pre-BötC leads to the cessation of breathing (Wenninger et al., 2004; McKay et al., 2005). Isolated in medullary slices, the pre-BötC continues to generate three distinct types of activity patterns that appear to provide the basic rhythmic drive for the generation of three distinct forms of respiratory activity patterns: normal respiratory activity (sometimes referred to as eupnea), sighs and gasps (Lieske et al., 2000). The fact that the pre-BötC can be isolated in vitro has facilitated our understanding of the role of neuromodulators in regulating respiratory rhythm generation. This neuronal network exhibits an unprecedented degree of plasticity. Synaptic and intrinsic membrane properties are under the continuous control of neuromodulators that can differently regulate amplitude and frequency of respiratory activity, as well transitions between the different types of respiratory activities.
Although we are far from understanding all aspects of neuromodulation, it is obvious that neuromodulation is an integral part of the rhythm and pattern generating process itself. This review will highlight insights gained from experiments performed in transverse medullary slices. We will focus on the discussion of only a few modulators that best exemplify certain aspects of neuromodulatory control. Hence, it must be emphasized that neuromodulation is an even more complex process and that it will play a more versatile role in governing and orchestrating respiratory network activity in the intact animal. Indeed the locally modulated pre-BötC is connected with many other neuronal networks that are themselves under continuous modulatory control and in turn modulate the pre-BötC as will be discussed in the first paragraph.
Neuromodulators affect respiratory activity from numerous areas distributed throughout the nervous system
Respiratory rhythm generating areas, such as the pre-BötC receive multiple modulatory inputs from many areas outside (Fig.1A) and within the vicinity of the pre-BötC. The pre-BötC projects to various respiratory-related areas that contain neuromodulators and are in turn modulated by multiple other areas. Most areas contain multiple neuromodulators that are partly co-released from the same neurons. Thus, neuromodulation occurs at all levels of integration, and it can be assumed that all respiratory functions are controlled by multiple neuromodulators originating from multiple regions. This paragraph reviews the anatomical origin of some of the neuromodulators known to play key roles in the neuronal control of breathing (Fig. 1A). But, it is beyond the scope of this review to cover all known modulators. Moreover it is very likely that the list of known modulators that modulate respiratory activity is far from complete.
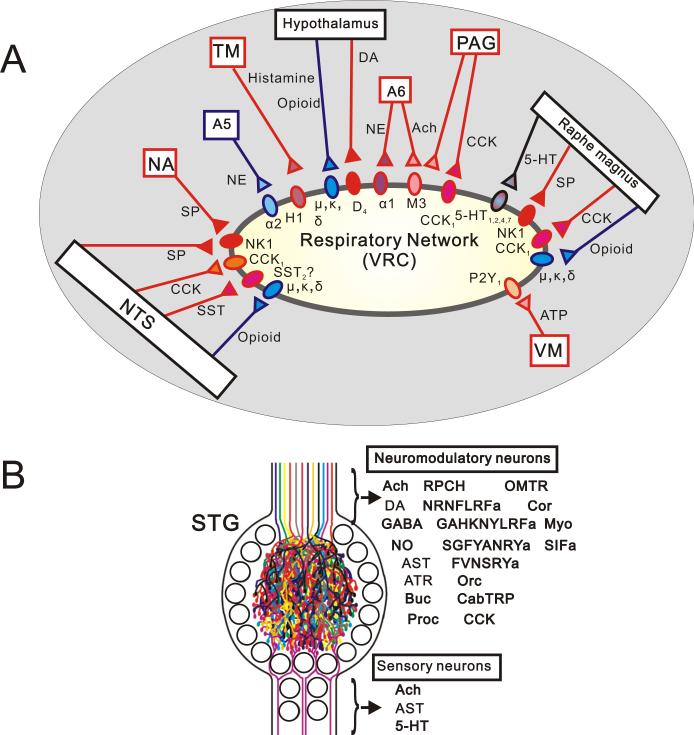
Figure 1. Neuromodulation of network activity.
(A) The respiratory network receives excitatory and inhibitory input from multiple sources that are integrated to generate breathing behavior. Abbreviations, see below. NA; nucleus of ambiguus, A5; ventrolateral pons, TM; tuberomammillary nucleus, A6; locus ceruleus, SP; substance P, NE; norepinephrine, (B) Summary of the neuroactive mediators in the somatogastoric ganglion (STG). The situation in the respiratory network is not too different from the stomatogastric ganglion in crustaceans in which modulatory inputs from multiple sources converge onto a rhythm generating network (Modified from Marder and Buchner, 2007). The stomatogastric ganglion illustrates that it shouldn't be surprising if there are many more peptides in the respiratory system that are still waiting to be discovered. For abbreviations see below. AST; allatostatin, ATR; allatotropin, Buc; buccalin, Proc; proctolin, RPCH; red pigment-concentrating hormone, FRNRNFLRFa and GAHKNYLRFa; RF amide family, SGFYANRYa and FVNSRYa; RY amide family, Orc; orcomyotropin, CabTRP; cancer borealis tachykinin-related peptide, CCK; cholecystokinin, OMTR; N/A, Cor; corazolin, Myo; myomodulin, SIFa; SIF amide.
Regions that are well known for their important modulatory roles are raphe magnus and obscurus. These regions contain a variety of neurotransmitters and neuromodulators including GABA, serotonin (5-HT), Substance P and thyrotropin releasing hormone (TRH) (Lalley, 1986; Kachidian et al., 1991). The same neurons of rape magnus contain 5-HT and substance P (Kachidian et al., 1991), but substance P is also found in neurons of the nucleus of solitary tract, the nucleus ambiguus, the dorsal motor nucleus of the vagus and the hypogrossal nucleus (Ribeiro-da-Silva and Hokfelt, 2000). All these areas either receive input from or project to the pre-BötC (Bianchi et al., 1995). Within the pre-BötC, Substance P is primarily colocalized with glutamate, but to lesser degree also with GABA (Liu et al., 2004). The pons (A5, (ventrolateral pons), A6 (locus ceruleus)) and medulla (A1, A2 regions) contain noradrenergic neurons. Some noradrenergic neurons located in the locus ceruleus possess also acetylcholine (VanderHorst and Ulfhake, 2006). Acetylcholine is present in the ventral periaquedutal gray (vPAG), the dorsal motor nucleus of vagus and others (VanderHorst and Ulfhake, 2006). The paraventricular nucleus of hypothalamus (PVN) is an important source of dopamine (Millan, 2002), while histamine is released from the tuberomammillary nucleus, a cluster of magnocellular cells in the posterior hypothalamus (Huston et al., 1997).
The mammalian forebrain and hypothalamic areas as well as the lower brainstem contain the cyclic polypeptide somatostatin (SST). In the brainstem, SST can be detected in somata and terminals of several nuclei related to the central respiratory groups, including the parabrachial nucleus and the nucleus of solitary tract (NTS). SST immunoreactive fibers were also seen in locus ceruleus, the NTS, and the nucleus ambiguus (Chigr et al., 1989). The NTS and the VRC contains also immunoreactive cholecystokinin (CCK) 8-like neurons (Ellenberger and Smith, 1999). The pre-BötC expresses also the trkB receptor which is activated by brain derived neurotropic factor (BDNF).
The areas and neuromodulators depicted in Figure 1 and many other modulators not shown influence respiratory activity. Thus, a major challenge for future research is to explore the metabolic conditions and behavioral states under which different neuromodulator-containing nuclei are activated (see for example Table 1B), and the extent to which different activity levels lead to the differential release of different neuromodulators. It will also be important to explore how the respiratory network copes with the concurrent release of multiple neuromodulators, and to understand whether and why some modulators are released at different times. Currently unresolved is also the question to what extent substances are released directly into the synaptic cleft or into the extracellular space.
Table 1. Excitatory modulatory effects on the respiratory rhythm.
(A): The excitatory effects of neuromodulators under standard conditions. Ach; Acetylcholine receptors, NE; adorenoceptors, 5-HT; selotonin receptors, DA; dopamine receptors, NK; neurokinin receptors, CCK;cholecystokinin receptors, M3; muscarinic3 Ach receptors, α1; α1-adorenoceptor, 2A, 2B; 5-HT2A,2B receptors, 3; 5-HT3 receptors, 4; 5-HT4 receptors, X2; P2X2 receptors, Y1; P2Y1 receptors, 1; NK1 receptors and CCK1 receptors, PAG; periaquedutal gray, X; the dorsal motor nucleus of vagus, LC; locus ceruleus, RM; raphe magnus, PVN; paraventricular nucleus of hypothalamus, VM; ventral medulla (The origin of the ATP may come from glia which is located at superficial layer of the ventral medulla), NTS; nucleus of tractus solitarius, ambiguus; nucleus of ambiguus, q/11; Gq/11 protein, s; Gs protein, PLC; phospholipase C, PIP2 ;Phosphatidylinositol bisphosphate, IP3, inositol 1,4,5-trisphosphate, PKC; protein kinase C, AC; adenylyl cyclase, cAMP, adenosine monophosphate, Amp.; amplitude, Freq.; frequency, Gener.; respiratory generator, Motor; respiratory related activity at the level of the motor nucleus or nerve, (↑); facilitatory effect on the respiratory rhythm, (↓); inhibitory effect on the respiratory rhythm, (+); Respiratory study was performed, (−); The respiratory study was not performed yet.
(B): Excitatory effects of neuromodulators on the respiratory rhythm under pathological conditions in vitro and in situ preparation. i/o; Gi/o protein, βγ; Gβγ protein, “Pentbarbital administration” means the specific condition under which pentobarbital sodium was applied to the slice preparation) or injected into the in situ preparation. “Activation of μ-opioid receptors” means the specific condition during which the agonist of the μ-opioid receptors was applied or injected.
| A | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Receptor type | Receptor subgroup | Source of endogenous ligand | G protein | Second messengers | Excitatory modulation for respiratory rhythm in vitro |
||||
| Modulation |
Location |
References | |||||||
| Amp. | Freq. | Gener. | Motor | ||||||
| Ach | M3 | PAG?,LC?, X? | q/11 | PLC, PIP2, IP3, PKC |
|
|
+ | + | Shao XM et al. 2005 |
| NE | α1 | LC | q/11 | PLC, PIP2, IP3, PKC |
|
|
+ | + | Viemari JC et al. 2006 |
| Histamine | H1 | Tuberom ammillary | q/11 | PLC, PIP2, IP3, PKC | No effect |
|
− | + | Dutschm ann M et al. 2003 |
| 2A,2B | RM | q/11 | PLC, PIP2, IP3, PKC |
|
|
+ | + | Pena et al. 2002, Gunther et al. 2006 | |
| 5-HT | 3 | RM | − | − | No effect |
|
− | + | Johnson SM et al. 2001 |
| 4 | RM | s | AC, cAMP, PKA | ? |
|
− | + | Manzke T et al. 2003 | |
| DA | ? | PVN | s? | AC, cAMP, PKA |
|
|
− | + | Johnson R et al. 1998 |
| ATP | X2 | VM (glia?) | − | − |
|
|
− | + | Lorier et al. 2007 |
| (P2) | Y1 | VM (glia?) | q/11 | PLC, PIP2, IP3, PKC |
|
|
+ | + | Lorier et al. 2003 |
| NK | 1 | RM?,NTS,Ambiguus | q/11 | PLC, PIP2, IP3, PKC |
|
|
+ | + | Pena et al, 2004 |
| CCK | 1 | PAG,NTS,RM | q/11, s | PLC, PIP2, IP3, PKC | No effect |
|
− | + | Ellenberger HH et al. 1999 |
| TRH | 1?, 2? | RM | q/11 | PLC, PIP2, IP3, PKC | ? |
|
+ | − | Dekin et al. 1985 |
| B | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Receptor type | Receptor subgroup | Source of endogenous ligand | G protein | Condition | Excitatory modulation for respiratory rhythm in vitro |
||||
| Modulation |
Location |
References | |||||||
| Amp. | Freq. | Gener. | Motor | ||||||
| 5-HT | 1 | RM | i/o,βγ | Pentbarbital administration | ? |
|
+ | + | Lalley PM et al. 1994 |
| 2A | RM | q/11 | Hypoxia (Gasping) condition | No effect |
|
+ | − | Tryba A et al. 2006 | |
| 4 | RM | s | Activation of μ-opioid receptor | ? |
|
− | + | Manzke T et al. 2003 | |
| 7 | RM | s | Activation of μ-opioid receptor | ? |
|
− | + | Richter DW et al. 2003 | |
| ATP (P2) | X2 | VM (glia?) | − | Hypoxia condition |
|
|
− | + | Gourine AV et al. 2005 |
| Y1 | VM (glia?) | q/11 | Hypoxia condition |
|
|
+ | + | Lorier et al. 2003 | |
The finding that the respiratory network is multiply modulated is not surprising from a general network perspective. It is well established that even small neuronal networks in invertebrates receive multiple modulatory inputs (Marder and Buchner, 2007, Fig.1B). Hence, it seems to be a general attribute of neuronal networks that there is no single substance and not even a few substances that are the major source of neuromodulatory input to a rhythm generating network, rather neuromodulation is a process that involves many neuromodulators acting at all levels of integration. In other words, like the situation in a classical orchestra, very different contributors are orchestrated to arrive at a common output.
Neuromodulators, receptors, subtypes and second messenger systems
The previous paragraph described that multiple neuromodulators originate from many different regions of the CNS. At the cellular level modulators act on a variety of receptor subtypes exerting inhibitory and excitatory effects on the respiratory network via different second messenger systems. In this paragraph we will discuss these cellular mechanisms for some of the key neuromodulators including acetylcholine, 5-HT, substance P and norepinephrine, but the reader is referred to Tables 1 and 2 for further details. Within the pre-BötC, acetylycholine acts on α4β2 nicotinic (nACh), M2 and M3 muscarinic (mAch) receptors (Shao and Feldman, 2005; Shao et al., 2008). M3 mAch receptors are excitatory and positively coupled to Gq/11, phospholipase C (PLC), inositol 1,4,5-trisphosphate (IP3) receptor, protein kinase C (PKC), while M2 receptors are inhibitory and suppress adenylyl cyclase (AC). Substance P binds to three main receptor types: NK1, NK2 and NK3 receptors (Quartara and Maggi, 1997). Immunostaining of the NK1 receptor is used as a maker of the of pre-BötC region (Gray et al., 1999), and its activation exerts excitatory effects. NK1 receptors are coupled to Gq/11 protein, IP3 receptor and/or PKC (Quartara and Maggi, 1997), and modulate Na+, Ca2+ , K+ channels (Quartara and Maggi, 1997), and possibly also an unknown cation channel (Pena and Ramirez, 2004).
Table 2. Inhibitory modulatory effects on the respiratory rhythm.
Adenosine; adenosine receptors, Opioid; opioid receptors, SST; somatostatin receptors, α2; α2-adorenoceptor, D4; dopamine 4 receptors, A1; adenosine 1 receptors, μ, δ, κ; μ-, δ- and κ-receptors, 2?; SST 2 receptors?, Metabolism of ATP; Adenosine may result from metabolism of the ATP. PBN; parabrachial nucleus, Irregu.; irregularity of the rhythm
| Receptor type | Receptor subgroup | Source of endogenous ligand | G protein | Second messengers |
Inhibitory modulation for respiratory rhythm in vitro |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Modulation |
Location |
References | ||||||||
| Amp. | Freq. | Irregu. | Gener. | Motor | ||||||
| NE | α2 | Ventrolateral pons | i/o | AC, cAMP, PKA | No effect |
|
|
− | + | Hilaire et al. 1989 |
| DA | D4 | PVN | i/o | AC, cAMP, PKA | No effect |
|
− | + | − | Fujii M et al. 2006 |
| Adenosine | A1 | Metabolism of ATP | i/o | AC, cAMP, PKA | − |
|
|
+ | + | Herlenius E et al. 1999 |
| Opioid | μ,δ,κ | NTS, PBN, PVN, RM | i/o | AC, cAMP, PKA |
|
|
|
+ | + | Mellen NM et al. 2003 |
| SST | 2? | NTS | i/o | AC, cAMP, PKA | ? |
|
− | − | + | Llona l et al. 2004 |
5-HT exerts its respiratory effects via 5-HT1, 2, 3, 4 and 5-HT7 (Onimaru et al., 1998; Johnson et al., 2001; Pena and Ramirez, 2002; Manzke et al., 2003; Gunther et al., 2006). 5-HT1 receptors are linked to Gi/o, and inhibit AC and increase K+ conductance. 5-HT2 receptors are coupled to Gq/11, PLC, IP3 receptor, facilitate the production of PKC, mobilize intracellular Ca2+ and/or block voltage dependent K+ channel (Fink and Gothert, 2007). The 5-HT3 receptors are ionotropic and conduct cations. The 5-HT4 and 5-HT7 receptors are associated with Gs, increase AC, stimulate protein kinase A (PKA), and inactivate K+ channel or seem to activate Ih currents (Millan, 2002). 5-HT1 inhibit, while 5-HT2 and 5-HT4 receptors facilitate respiratory rhythmic activity. In addition to these traditional signaling pathways 5-HT receptor activation seems to result also in the activation of BDNF, extracellular-regulated kinase (ERK), protein kinase B (Akt), mitogen-activated protein (MAP), insulin-like growth factor-1 (IGF-1), small GTPase (Ras, Raf) phosphatidylinositol 3-kinase (PI3K) and phosphoinositide-dependent protein kinases (PDK) (Cowen, 2007). Under some circumstances the activation of 5-HT1A receptors can lead to increases in cAMP which results in increased IGF-1 levels (Cowen, 2007).
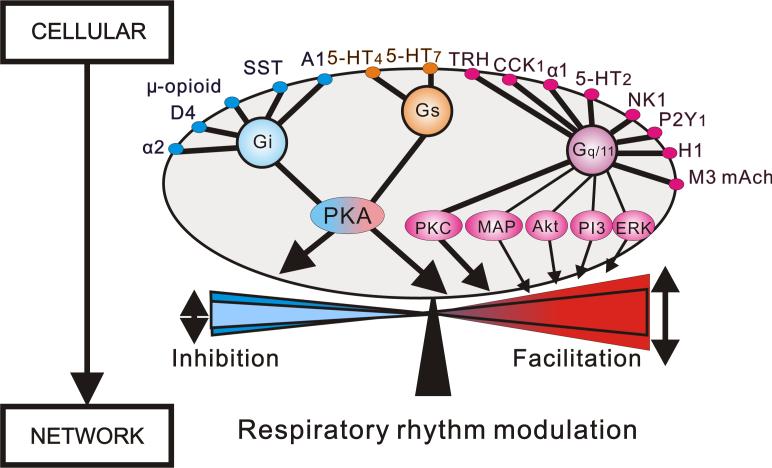
Figure 2 illustrates that many different modulators originating from different sources must converge at the receptor and second-messenger level. But, it remains unknown whether these complex modulatory influences are differentially regulated. If modulatory influences are regulated at the cellular and subcellular level it will be critical to obtain insights into the underlying rules. Specifically, the functional consequences of concurrent activation of multiple second messenger systems within the same neurons and in different areas of the respiratory system remain unknown. The insights gained in the mammalian respiratory network resemble those obtained in small neuronal networks of invertebrates (Marder and Bucher, 2007). Indeed many more cellular insights have already been obtained in these smaller networks. This clearly indicates that we are far away from a complete understanding of how respiration is modulated. But, even in absence of a complete understanding it is possible to conclude that the same neuromodulators act on different receptors, that neuromodulators affect several different voltage-gated channels within the same neuron, and that a number of different neuromodulators will converge onto the same second messenger system (Fig. 2), and that every neuron is subject to modulation by multiple substances originating from multiple areas of the respiratory system.
Figure 2. Convergence of excitatory and inhibitory neuromodulators that regulate the frequency of respiratory rhythmic activity.
Multiple neuromodulators including biogenic amines and neuropeptide converge onto respiratory neurons. Note: the activation of excitatory metabotropic receptors couple mainly to Gq/11 proteins. The Gq/11 protein links traditionally to PLC, PIP2, IP3, PKC. Additionally, it has been recently reported that Gq/11 coupled receptors, affect to mitogen-activated protein (MAP), protein kinase B (Akt), phosphatidylinositol 3-kinase (PI3) and extracellular-regulated kinase (ERK). The convergence and divergence of modulatory effects contribute to the regulation of the frequency of respiratory rhythmic activity at the network level. One of the main challenges is to identify the mechanisms that constrain these complex modulatory effects to a physiological frequency range.
Inhibitory and excitatory control of respiratory frequency
Endogenously released neuromodulators provide continuous, excitatory and inhibitory drive onto the respiratory network (Fig. 2). Some of the known modulators exert either excitatory or inhibitory effects, while others have inhibitory as well as excitatory effects on respiratory functions. Substance P is an example for a modulator with primarily excitatory effects. This peptide exerts its excitatory effects by acting on NK1 receptors within the pre-BötC, but studies obtained in the brainstem-spinal cord preparation suggests that both NK1 and NK3 receptor agonist induce a concentration-dependent increase in the respiratory frequency (Monteau et al., 1996). Another primarily excitatory neuromodulator is CCK. This peptide acts on CCK1 receptors within one or more medullary or pontine respiratory groups (Ellenberger and Smith, 1999). TRH, an important excitatory neuromodulators is known to induce bursting properties in NTS neurons, which may have important implications for respiratory control (Dekin et al., 1985). Exogenously applied histamine (H)1 and H3 receptors agonists increase the frequency of phrenic nerve activity, but only H1 receptor antagonists reduce respiratory frequency, suggesting that endogenously released histamine exerts its excitatory effects primarily by acting on H1 receptors (Dutschmann et al., 2003).
Opioids and SST (Llona and Eugenin, 2005) are primarily inhibitory modulators. Pre-BötC neurons that contain SST receptors are likely targets for the SST effect, but how SST inhibits respiratory frequency remains unknown. Much more is known about the opioid effect. By acting on different receptors and neurons they exert differential inhibitory effects on the frequency of respiratory activity. The frequency effects seem to be mediated in part by neurons within the pre-BötC since μ-opioid receptor agonists inhibit respiratory activity when injected directly into the pre-BötC (Gray et al., 1999). Here they inhibit the activity of inspiratory pre-BötC neurons. Interestingly, opioids have no effect on neurons in the parafacial nucleus. This differential opioid modulation has provided important clues into the differential control of inspiration and expiration within the respiratory network (Mellen et al., 2003). Although, delta receptor activation affects respiratory activity, these receptors seem to have no frequency effects (Lonergan et al., 2003). Kappa (κ)- and mu (μ)-opioid receptor activation depresses the frequency of respiratory activity when activated by themselves. However, the activation of κ receptors seems to oppose the μ-opioid receptor-mediated respiratory depression when activated together (Haji and Takeda, 2001). This is an important example that shows (a) that the release of different modulators must be highly regulated and coordinated by the respiratory system, and (b) that different neuromodulators with apparently similar effects may have complex modulatory consequences that are difficult to predict when concurrently released.
Dopamine, serotonin and norepinephrine are neuromodulators that exert inhibitory as well as excitatory effects. Dopamine (D) increases the frequency of respiratory activity when bath-applied (Johnson et al., 1998), while specific activation of the D4 receptors seems to depress respiratory rhythmic activity of pre-inspiratory neurons (Fujii et al., 2004). Bath-applied 5-HT increases and subsequently decreases bursting frequency in pre-inspiratory and inspiratory neurons. This biphasic modulation of the respiratory rhythm seems to be best explained by the activation of 5-HT2A (excitatory), 5-HT2C (excitatory) and 5-HT1A (inhibitory) receptors (Onimaru et al., 1998). But, it remains largely unknown under what natural conditions these receptors are differentially activated. Indeed, a major task of future research will be to unravel the differential activation of different receptor systems in more physiological context. This must include understanding the activation pattern of the different neuromodulatory neurons and their projections. These studies may arrive at conclusions that are unexpected from in vitro experiments. This applies not only to serotonin, but to all known neuromodulators. Norepinephrine is clearly another good example for this issue. Like serotonin, norepinephrine exerts also excitatory and inhibitory by acting on different receptors. The A5 region in the pons seems to have inhibitory effects on respiratory activity, while locus coeruleus neurons from the pons activate the medullary respiratory network presumably via alpha-1 adrenergic receptors. Indeed exogenously applied alpha-1 agonists cause frequency increases in isolated transverse slices (Viemari and Ramirez, 2006b). However, antagonists of alpha-1 receptors do not alter spontaneous rhythmic activity in medullary slices, suggesting that alpha-1 receptors are endogenously activated only by noradrenergic neurons located outside the medulla. For further details on the role of excitatory as well as inhibitory noradrenergic descending drive onto the respiratory network the reader is referred to the accompanying review by JC Viemari.
Medullary control of respiratory frequency
Insights gained into locally released neuromodulators were gained by manipulating endogenous, excitatory drive in spontaneously active medullary slices that contain the pre-BötC. Unilateral microinjection of the acetylcholinesterase inhibitor physostigmine into the pre-BötC increases the frequency of respiratory rhythmic activity, an effect that is partially blocked by either M3 mACh receptor antagonists or α4β2 nACh receptor antagonists (Shao and Feldman, 2005). The frequency of respiratory activity is also increased by applying 5-HT uptake blockers in rhythmic medullary slices. This frequency effect is mediated by 5-HT2A receptors, since blockade of endogenously activated 5-HT2A receptors with three different antagonists decreases the frequency of respiratory population activity (Pena and Ramirez, 2002). The frequency of respiratory rhythmic activity is also reduced following pre-treatment with spantide, a NK1 receptor antagonist, suggesting that endogenously released substance P provides also local excitatory drive within the transverse slice preparation (Telgkamp et al., 2002).
Taken together, these findings indicate that the respiratory network is continuously and simultaneously driven by endogenously and locally released Ach, 5-HT and substance P acting on muscarinic M3, nicotinic α4β2 receptors, 5-HT2A and NK1 receptors. It appears that these neuromodulators have similar effects on respiratory frequency because they partly converge onto the same second messenger pathways. But, only very few studies have really investigated the functional significance of simultaneously activating the same second messenger pathways by different neuromodulators. The observation that the blockade of each receptor type (5-HT2A, NK1, mACh and nACh receptors) results in a frequency decline, suggests that the simultaneous receptor activation will have cumulative consequences, and that a single neuromodulators is not sufficient to maximally activate a given second messenger system. However, at this point this is speculation and further experiments will be necessary to specifically address the role of converging modulatory pathways (Figure 2).
Indeed many questions associated with the convergence of neuromodulators remain unresolved: In particular, why is the frequency controlled by so many endogenously and locally released neuromodulators? Is the frequency control by multiple neuromodulators just a matter of redundancy? Is there an advantage of having multiple neuromodulators concurrently providing excitatory drive? Or alternatively is the multiple frequency control just an unavoidable consequence of the convergence of different modulators at the level of second messenger pathways? For example, if two “excitatory neuromodulators” are released within the pre-BötC for different reasons, they both will also increase the frequency if both of them activate PKC. Unfortunately, at this point we do not even know whether individual neurons receive excitatory inputs from all these neuromodulators or whether different subsets of respiratory neurons are modulated by subsets of different neuromodulators. Another important question is whether different neuromodulators can compensate for each other? Specifically, is the existence of multiple “frequency” modulators an important safety mechanism? Could this explain for example why genetic lesions of excitatory neuromodulators have surprisingly small effects on frequency regulation? Mice in which substance P production (Telgkamp et al., 2002) was genetically blocked show baseline respiratory frequencies that are on average not different from control mice. The same is true for NK1 receptor knock-out mice (Hilaire et al., 2003). These reports suggest mechanisms compensating for the loss of substance P production and NK1 receptors. However, such potential mechanisms may not always be sufficient to compensate for the loss of neuromodulators and their receptors. An interesting example is the hypercapnic ventilatory response of Lmx1b knock-out mice, which in the absence of central 5-HT neurons was decreased by 50% compared to wild-type mice (Hodges et al., 2008). This result underlines the important role of 5-HT receptor in CO2 sensitivity, and the limited ability of the nervous system to fully compensate for the loss of serotonergic modulation.
Future studies need to address the combinatory effects of different neuromodulators. For example by simultaneously blocking muscarinic, nicotinic, 5-HT2 and NK1 receptors and comparing the magnitude of the combined blockade versus the blockade of individual neuromodulators it should be possible to learn whether the contribution of each neuromodulator is cumulative and whether the drive of all neuromodulators is necessary for maintaining baseline frequency. If multiple modulators regulate frequency, what are the constraints that limit the frequency modulation to a given frequency range that is physiologically meaningful? What are the mechanisms that maintain respiratory stability despite the multiplicity of modulatory control? All these questions are critical for understanding the role of neuromodulators in respiratory control.
Neuromodulatory control of regularity of rhythmic activity
From existing experiments there seems to be a tight correlation between modulatory control of frequency and regularity of respiratory rhythmic activity. Blockade of endogenous, excitatory drive decreases, while increasing endogenous excitatory drive increases respiratory frequency as well as regularity of the rhythm. The regularity of respiratory activity in transverse rhythmic slices is for example increased by 5-HT uptake blockers that mimic the “regularizing” effect of exogenously applied 5-HT2A agonists (Pena and Ramirez, 2002). Conversely, blockade of endogenous excitatory drive with 5-HT2A receptors antagonist results in irregular respiratory rhythmic activity. Similarly, pre-treatment of spantide, a NK1 receptor antagonist, increases the irregularity score, suggesting that the endogenous substance P and 5-HT release regulate regularity of respiratory activity (Telgkamp et al., 2002).
The tight correlation between modulatory control of frequency and regularity of respiratory rhythmic activity may indicate that both of these parameters depend on the same or at least highly intertwined cellular/network mechanism. Recordings from neurons that give rise to the generation of respiratory rhythmic activity could yield important insights into the relationship between regularity and frequency of respiratory activity (Ramirez et al., 2004).
Cadmium-insensitive pacemakers and the control of frequency and regulatory of rhythmic activity
Inspiratory neurons located within the pre-BötC exhibit two types of pacemaker properties (Thoby-Brisson and Ramirez, 2001). Pacemaker bursting in one population of inspiratory neurons seems to depend on the calcium-activated non-selective cation (CAN) current (Pena et al., 2004). These neurons are referred to as “cadmium-sensitive’ pacemaker neurons, because bursting in these neurons is abolished following blockade of calcium influx with extracellularly applied cadmium. Pacemaker bursting in the other population of inspiratory neurons seems to depend on the persistent sodium current (Pena et al., 2004). These neurons are referred to as “cadmium-insensitive” pacemaker neurons, because bursting in these neurons continues even following blockade of calcium currents with extracellularly applied cadmium. Neuromodulators that cause a frequency and regularity increase in respiratory network activity evoke a frequency increase specifically in the endogenous bursting of isolated cadmium-insensitive pacemakers. Indeed substance P, norepinephrine and 5-HT increase the frequency of intrinsic bursting of these pacemakers upon pharmacological isolation (Pena and Ramirez, 2002, 2004; Viemari and Ramirez, 2006a). Although an intriguing correlation, the similarity in the modulatory frequency effect at the level of single pacemaker neurons and the respiratory network does not necessarily imply a causal relationship. However, this correlation between single cell and network response is consistent with a hypothesis proposed by Ramirez et al. 2004. According to this hypothesis the population of cadmium-insensitive pacemaker neurons may be involved in kindling inspiratory network activity (Ramirez et al., 2004). Specifically, it was hypothesized that closely timed bursts in several pacemaker neurons might trigger or “kindle” an inspiratory population burst that is associated with the synaptic recruitment of other pacemaker and non-pacemaker neurons, including not only excitatory but also inhibitory neurons. In the respiratory network, the intrinsic inactivation properties of pacemaker currents will reduce the probability of bursting directly after an inspiration, thereby preventing a premature kindling of the next inspiration. Conversely, an increased burst frequency of these neurons could result in an increased probability of kindling an inspiratory network burst resulting in an increased frequency in network activity. An increased probability of kindling a network burst could in turn also result in an increased regularity of bursting. For further details regarding this “kindling hypothesis”, the reader is referred to the review by Ramirez et al. 2004.
Neuromodulation and the neuronal control of the amplitude of inspiratory activity
Exogenously applied agonists for alpha-1 adrenergic receptors, 5-HT2A receptors and NK1 receptors as well as 5-HT uptake blockers increase not only the frequency, but also the amplitude of respiratory activity at the level of the pre-BötC (Pena and Ramirez, 2002, 2004; Viemari and Ramirez, 2006a). Interestingly, these excitatory neuromodulators caused predominantly an increase in the amplitude of the depolarizing drive potential in cadmium-sensitive pacemaker neurons. An increased depolarizing drive potential gives rise to an increased frequency of action potentials that when integrated will result in an increased amplitude in the integrated inspiratory population burst. Blockade of the CAN current with flufenamic acid abolishes bursting in cadmium-sensitive pacemaker neurons and also the amplitude modulation at the network level. This finding is consistent with the hypothesis that the CAN current plays a critical role in amplitude modulation as proposed by Viemari and Ramirez, 2006a. The hypothesis that intrinsic bursting properties can cause an amplification in the network amplitude by amplifying excitatory synaptic drive potentials has first been demonstrating by injecting hyperpolarizing current pulses that caused linear drops in the burst amplitude (Ramirez and Richter, 1996). The role of intrinsic membrane currents in amplifying network activity has more recently also been described by Del Negro et al. (Del Negro et al., 2005).
It must be emphasized that the amplitude modulation of both population activity and single cell burst at the level of the pre-BötC does not necessarily result in a corresponding amplitude modulation at the level of the motor output. This is the case because motor neurons have their own intrinsic membrane properties, and synaptic inputs that are under modulatory control and that can be regulated independently from the modulatory effects on the rhythm generating network. An example for the independent amplitude control of rhythm generating network and motor output is seen in the response to hypoxia. The respiratory network in the pre-BötC shows primarily a frequency modulation. By contrast hypoglossal motor neurons exhibit a massive depolarization and amplitude modulation that is not reflected in a corresponding amplitude modulation of the pre-BötC (Telgkamp and Ramirez, 1999). This independent amplitude control presumably allows the respiratory system to differentially regulate different motor outputs. This seems critical since the requirements for diaphragmatic control may be strikingly different from those of upper airway or thoracic muscles. For an in depth review of the modulatory control of motoneurons the reader is referred to a publication by Feldman et al (Feldman et al., 2003).
The modulatory control of gasps and sighs, and the association with Sudden Infant Death Syndrome
In vitro preparations containing the pre-BötC show a distinct activity pattern during severe hypoxia. This activity pattern has been referred to as “fictive gasping” (Lieske et al., 2000). Like in vivo gasping this activity pattern is characterized by a faster rise time, shorter burst duration and lower frequency under hypoxic conditions (Ramirez and Garcia, 2007). The transition from normal respiratory activity into gasping activity is associated with a reconfiguration in network activity. Under normal conditions two types of pacemaker neurons (cadmium-sensitive and cadmium-insensitive) are active, and excitatory and inhibitory synaptic mechanisms are critical for rhythm generation. Under hypoxic conditions, the activity of cadmium-sensitive pacemakers and the majority of non-pacemaker neurons ceases, while cadmium-insensitive pacemakers continue to burst. Blockade of the persistent sodium current with riluzole abolished selectively bursting in cadmium-insensitive pacemaker neurons and also gasping (Pena et al., 2004). Although these pharmacological experiments are associated with significant caveats as also discussed by Pena et al. 2004, the data obtained are consistent with the hypothesis that cadmium-insensitive pacemakers become the main drivers of gasping under hypoxic conditions (Pena et al., 2004). The hypothesis proposed by Pena et al. 2004, i.e. that (a) respiratory rhythm generation during gasping depends on persistent sodium current and bursting in cadmium-insensitive pacemakers and (b) that the respiratory network reconfigures during hypoxia was later confirmed by (Paton et al., 2006). Additional support for the hypothesis by Pena et al. 2004, came from in vivo experiments. The administration of persistent sodium channel blocker, riluzole, in cisterna cerebellomedullaris drastically reduced gasping generation (Pena and Aguileta, 2007). Experiments in the in situ perfused preparation also suggest that both 5-HT2 and alpha-1 adrenergic receptors are important to sustain gasping after hypoxia-induced depression (St-John et al., 2007). A recent publication by Tryba et al. 2008 suggests that cadmium-insensitive pacemakers are also important for the generation of sighs (Tryba et al., 2008), and the study by Pena et al. 2004 suggested that riluzole blocks not only fictive gasping but also sighs.
The hypotheses put forward by Pena et al. 2004, may have important implications for Sudden Infant Death Syndrome (SIDS). Increasing evidence suggests that SIDS is associated with disturbed sighs, gasps and arousal mechanisms (Hunt, 1992; Poets et al., 1999; Harper et al., 2000; Poets, 2004; Fewell, 2005; Thach, 2005). As described by Lijowska et al (1997) the infants’ arousal response consists of four highly stereotyped behaviors: sighs, startles, thrashing limb movements, and full arousal (eyes open, cry). According to Lijowska et al (1997) these behaviors occurred always in the same sequence: first a sigh coupled with a startle, then thrashing, then full arousal (Lijowska et al., 1997). Orem and Trotter described that sighs usually occurred just before, or coincided with, awakening and other sleep state transitions in adult cats (Orem and Trotter, 1993). These authors also suggested that the neural mechanisms regulating both sighs and arousals are closely linked. A similar association between sighs and arousals has been described by Phillipson et al. for dogs (Phillipson et al., 1978; Lijowska et al., 1997; McNamara et al., 1998; Gerard et al., 2002; McNamara et al., 2002). In SIDS the number of sighs and the number of gasps are decreased (Kahn et al., 1988; Poets et al., 1999), and often gasps are not associated with autoresuscitation or appropriate heart rate responses (Poets, 2004). Together with the observation that riluzole, blocks not only selectively bursting in cadmium-insensitive pacemaker neurons but also gasps and sighs makes this an intriguing finding (Pena et al., 2004).
The hypothesis that cadmium-insensitive pacemakers are not only the critical drivers of gasping, but are also dependent on serotonin is also interesting in this context (Pena et al., 2004; Tryba et al., 2006). An increased risk of SIDS has been associated with mutations in the promoter of the 5-HT transporter protein gene (Weese-Mayer et al., 2003), and abnormalities in 5-HT receptor expression within the medulla (Kinney, 2005; Paterson et al., 2006; Sahni et al., 2007). Interesting, is also the association between 5-HT, thermoregulation and CO2 sensitivity, a disturbance of these mechanisms could also contribute to SIDS (Hodges et al., 2008). SIDS has not only been associated with serotonin, but also with a disturbance in noradrenergic systems (Hilaire, 2006). Through their widespread projections, the locus coeruleus and the other noradrenergic nuclei supply norepinephrine throughout the CNS, which functions as the brain's major arousal and attentional system (Coull et al., 2001; Berridge and Waterhouse, 2003). Interestingly, a recent report suggests a relationship between sudden unexpected fetal death and an altered NK1 immunopositivity and developmental alterations of pre-BötC neurons (Lavezzi and Matturri, 2008).
Acetylcholine is another modulator that is associated with the etiology of SIDS. Exposure to tobacco has been identified as a key risk factor, and polymorphisms in both the glutathione S-transferase theta 1 (GSTT1) and cytochrome P450 1A1 (CYP1A1) genes have been reported to impact the metabolic detoxification process for cigarette smoke and have been associated with low birth weight (Rand et al., 2006). Acetylcholine has also been associated with the neuronal control of sighs (Tryba et al., 2008). Muscarinic agonists promote the generation of sighs and large amplitude bursts in a subpopulation of cadmium-insensitive pacemaker neurons. The muscarinic facilitation of sighs is blocked by pretreatment with M3 mAch antagonists (Tryba et al., 2008). This is interesting, since M3 mAch activates the same second messenger system as the other Gq/11 coupling receptors: 5-HT2A, alpha-1adrenergic and NK1 receptors.
Neuromodulation and Respiratory Disorders
Because of the important role of neuromodulators in regulating frequency, regularity and amplitude of respiratory activity it shouldn't surprise that a disturbance in any aspect of modulatory control may also lead to breathing disorders. A neurological disorder that has been linked to a disturbance in modulatory control is Rett syndrome. These patients have X-linked mutations in the methyl-CpG binding protein 2 gene (Mecp2), and typically suffer from severe breathing abnormalities that include hyperventilation, breath-holding and respiratory dysrhythmia (Elian and Rudolf, 1991; Kerr, 1992; Woodyatt and Murdoch, 1996; Morton et al., 1997; Cooper et al., 1998; Kerr and Julu, 1999; Weese-Mayer et al., 2007). These breathing disturbances are associated with deficiencies in SP (Matsuishi et al., 1997; Deguchi et al., 2000), serotonin, noradrenaline and dopamine (Lekman et al., 1989; Zoghbi et al., 1989; Segawa, 1997). Adult mice deficient for the Mecp2 gene have erratic breathing reminiscent of those of human patients (Viemari et al., 2005). In this animal model highly variable respiratory rhythm and frequent apneas are associated with reduced norepinephrine and serotonin content in the medulla, and drastic reductions in medullary tyrosine-hydroxylase expressing neurons in the A2/C2 group (Viemari et al., 2005). The severe respiratory disturbances are also evident in the pre-BötC isolated from the medulla of Mecp2 deficient mice (Viemari et al., 2005). The finding that disturbed respiratory network activity in vitro can be stabilized with norepinephrine has led to pharmacological experiments in whole animals. Interestingly, noradrenaline and serotonin-uptake inhibitors were capable of not only stabilizing breathing, but possibly also extending the life span in this mouse model (Roux et al., 2007), an intriguing finding with important clinical implications.
Another disorder associated with a disturbance in aminergic neuromodulation is the Prader-Willi syndrome. These patients have mutations in the necdin gene which is associated with severe breathing irregularities and disturbances in the 5-HT metabolism (Zanella et al., 2008a). Given the complexity and multiplicity of modulatory control it is likely that also other modulators are altered in these patients. Indeed, opiates used as analgesics have exaggerated depressive effects on breathing in Prader-Willi Syndrome patients (Zanella et al., 2008b).
Concluding remarks
The examples discussed in this review illustrate that neuromodulators play critical roles in the neuronal control of breathing regularity, frequency and amplitude. Disturbances in the modulatory milieu seem to have severe clinical consequences that are possibly amenable to therapeutic treatments aimed at reestablishing normal levels of modulatory control. However, given the complexity of modulatory control as described in the first part of this review, it is probably difficult to predict how a given neuromodulator acting at many different levels of the respiratory system will affect breathing. This prediction will be particularly difficult, because changes in one modulator could potentially lead to various different compensatory responses. Indeed, given that so many modulators could potentially compensate for the defect in one modulatory system a disturbance in one or a few modulators should have only limited functional consequences. Hence, understanding how neuromodulators can compensate for each other will be an important task that is probably relevant to all neuronal networks, i.e. not only to the respiratory network. Importantly this is an issue that needs to be resolved if we want to enter the next phase in which basic scientific insights are potentially translated into the clinic.
Acknowledgement
This manuscript was supported by NIH 68860.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alheid GF, Gray PA, Jiang MC, Feldman JL, McCrimmon DR. Parvalbumin in respiratory neurons of the ventrolateral medulla of the adult rat. J Neurocytol. 2002;31:693–717. doi: 10.1023/a:1025799830302. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Chigr F, Najimi M, Leduque P, Charnay Y, Jordan D, Chayvialle JA, Tohyama M, Kopp N. Anatomical distribution of somatostatin immunoreactivity in the infant brainstem. Neuroscience. 1989;29:615–628. doi: 10.1016/0306-4522(89)90135-8. [DOI] [PubMed] [Google Scholar]
- Cooper RA, Kerr AM, Amos PM. Rett syndrome: critical examination of clinical features, serial EEG and video-monitoring in understanding and management. Eur J Paediatr Neurol. 1998;2:127–135. doi: 10.1016/s1090-3798(98)80028-7. [DOI] [PubMed] [Google Scholar]
- Coull JT, Nobre AC, Frith CD. The noradrenergic alpha2 agonist clonidine modulates behavioural and neuroanatomical correlates of human attentional orienting and alerting. Cereb Cortex. 2001;11:73–84. doi: 10.1093/cercor/11.1.73. [DOI] [PubMed] [Google Scholar]
- Cowen DS. Serotonin and neuronal growth factors - a convergence of signaling pathways. J Neurochem. 2007;101:1161–1171. doi: 10.1111/j.1471-4159.2006.04420.x. [DOI] [PubMed] [Google Scholar]
- Deguchi K, Antalffy BA, Twohill LJ, Chakraborty S, Glaze DG, Armstrong DD. Substance P immunoreactivity in Rett syndrome. Pediatr Neurol. 2000;22:259–266. doi: 10.1016/s0887-8994(00)00120-x. [DOI] [PubMed] [Google Scholar]
- Dekin MS, Richerson GB, Getting PA. Thyrotropin-releasing hormone induces rhythmic bursting in neurons of the nucleus tractus solitarius. Science. 1985;229:67–69. doi: 10.1126/science.3925552. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Morgado-Valle C, Hayes JA, Mackay DD, Pace RW, Crowder EA, Feldman JL. Sodium and calcium current-mediated pacemaker neurons and respiratory rhythm generation. J Neurosci. 2005;25:446–453. doi: 10.1523/JNEUROSCI.2237-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Bischoff AM, Busselberg D, Richter DW. Histaminergic modulation of the intact respiratory network of adult mice. Pflugers Arch. 2003;445:570–576. doi: 10.1007/s00424-002-0904-z. [DOI] [PubMed] [Google Scholar]
- Elian M, Rudolf ND. EEG and respiration in Rett syndrome. Acta Neurol Scand. 1991;83:123–128. doi: 10.1111/j.1600-0404.1991.tb04660.x. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Smith FM. Sulfated cholecystokinin octapeptide in the rat: pontomedullary distribution and modulation of the respiratory pattern. Can J Physiol Pharmacol. 1999;77:490–504. [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewell JE. Protective responses of the newborn to hypoxia. Respir Physiol Neurobiol. 2005;149:243–255. doi: 10.1016/j.resp.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Fink KB, Gothert M. 5-HT receptor regulation of neurotransmitter release. Pharmacol Rev. 2007;59:360–417. doi: 10.1124/pr.107.07103. [DOI] [PubMed] [Google Scholar]
- Fujii M, Umezawa K, Arata A. Dopaminergic modulation on respiratory rhythm in rat brainstem-spinal cord preparation. Neurosci Res. 2004;50:355–359. doi: 10.1016/j.neures.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Gerard CM, Harris KA, Thach BT. Physiologic studies on swaddling: an ancient child care practice, which may promote the supine position for infant sleep. J Pediatr. 2002;141:398–403. doi: 10.1067/mpd.2002.127508. [DOI] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBotzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBotzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther S, Maroteaux L, Schwarzacher SW. Endogenous 5-HT2B receptor activation regulates neonatal respiratory activity in vitro. J Neurobiol. 2006;66:949–961. doi: 10.1002/neu.20253. [DOI] [PubMed] [Google Scholar]
- Haji A, Takeda R. Effects of a kappa-receptor agonist U-50488 on bulbar respiratory neurons and its antagonistic action against the mu receptor-induced respiratory depression in decerebrate cats. Jpn J Pharmacol. 2001;87:333–337. doi: 10.1254/jjp.87.333. [DOI] [PubMed] [Google Scholar]
- Harper RM, Kinney HC, Fleming PJ, Thach BT. Sleep influences on homeostatic functions: implications for sudden infant death syndrome. Respir Physiol. 2000;119:123–132. doi: 10.1016/s0034-5687(99)00107-3. [DOI] [PubMed] [Google Scholar]
- Hilaire G. Endogenous noradrenaline affects the maturation and function of the respiratory network: possible implication for SIDS. Auton Neurosci. 2006;126−127:320–331. doi: 10.1016/j.autneu.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Burnet H, Ptak K, Sieweke M, Blanchi B, De Felipe C, Hunt S, Monteau R. Deletion of tachykinin NK1 receptor gene in mice does not alter respiratory network maturation but alters respiratory responses to hypoxia. Adv Exp Med Biol. 2003;536:497–504. doi: 10.1007/978-1-4419-9280-2_63. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt CE. The cardiorespiratory control hypothesis for sudden infant death syndrome. Clin Perinatol. 1992;19:757–771. [PubMed] [Google Scholar]
- Huston JP, Wagner U, Hasenohrl RU. The tuberomammillary nucleus projections in the control of learning, memory and reinforcement processes: evidence for an inhibitory role. Behav Brain Res. 1997;83:97–105. doi: 10.1016/s0166-4328(97)86052-4. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Johnson SM, Mitchell GS. Catecholaminergic modulation of respiratory rhythm in an in vitro turtle brain stem preparation. J Appl Physiol. 1998;85:105–114. doi: 10.1152/jappl.1998.85.1.105. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Wilkerson JE, Henderson DR, Wenninger MR, Mitchell GS. Serotonin elicits long-lasting enhancement of rhythmic respiratory activity in turtle brain stems in vitro. J Appl Physiol. 2001;91:2703–2712. doi: 10.1152/jappl.2001.91.6.2703. [DOI] [PubMed] [Google Scholar]
- Kachidian P, Poulat P, Marlier L, Privat A. Immunohistochemical evidence for the coexistence of substance P, thyrotropin-releasing hormone, GABA, methionine-enkephalin, and leucin-enkephalin in the serotonergic neurons of the caudal raphe nuclei: a dual labeling in the rat. J Neurosci Res. 1991;30:521–530. doi: 10.1002/jnr.490300309. [DOI] [PubMed] [Google Scholar]
- Kahn A, Blum D, Rebuffat E, Sottiaux M, Levitt J, Bochner A, Alexander M, Grosswasser J, Muller MF. Polysomnographic studies of infants who subsequently died of sudden infant death syndrome. Pediatrics. 1988;82:721–727. [PubMed] [Google Scholar]
- Kerr AM. A review of the respiratory disorder in the Rett syndrome. Brain Dev. 1992;14(Suppl):S43–45. [PubMed] [Google Scholar]
- Kerr AM, Julu PO. Recent insights into hyperventilation from the study of Rett syndrome. Arch Dis Child. 1999;80:384–387. doi: 10.1136/adc.80.4.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC. Abnormalities of the brainstem serotonergic system in the sudden infant death syndrome: a review. Pediatr Dev Pathol. 2005;8:507–524. doi: 10.1007/s10024-005-0067-y. [DOI] [PubMed] [Google Scholar]
- Lalley PM. Serotoninergic and non-serotoninergic responses of phrenic motoneurones to raphe stimulation in the cat. J Physiol. 1986;380:373–385. doi: 10.1113/jphysiol.1986.sp016291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzi AM, Matturri L. Functional neuroanatomy of the human pre-Botzinger complex with particular reference to sudden unexplained perinatal and infant death. Neuropathology. 2008;28:10–16. doi: 10.1111/j.1440-1789.2007.00824.x. [DOI] [PubMed] [Google Scholar]
- Lekman A, Witt-Engerstrom I, Gottfries J, Hagberg BA, Percy AK, Svennerholm L. Rett syndrome: biogenic amines and metabolites in postmortem brain. Pediatr Neurol. 1989;5:357–362. doi: 10.1016/0887-8994(89)90049-0. [DOI] [PubMed] [Google Scholar]
- Lieske SP, Thoby-Brisson M, Telgkamp P, Ramirez JM. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps [see comment]. Nat Neurosci. 2000;3:600–607. doi: 10.1038/75776. [DOI] [PubMed] [Google Scholar]
- Lijowska AS, Reed NW, Chiodini BA, Thach BT. Sequential arousal and airway-defensive behavior of infants in asphyxial sleep environments. J Appl Physiol. 1997;83:219–228. doi: 10.1152/jappl.1997.83.1.219. [DOI] [PubMed] [Google Scholar]
- Liu YY, Wong-Riley MT, Liu JP, Wei XY, Jia Y, Liu HL, Fujiyama F, Ju G. Substance P and enkephalinergic synapses onto neurokinin-1 receptor-immunoreactive neurons in the pre-Botzinger complex of rats. Eur J Neurosci. 2004;19:65–75. doi: 10.1111/j.1460-9568.2004.03099.x. [DOI] [PubMed] [Google Scholar]
- Llona I, Eugenin J. Central actions of somatostatin in the generation and control of breathing. Biol Res. 2005;38:347–352. doi: 10.4067/s0716-97602005000400006. [DOI] [PubMed] [Google Scholar]
- Lonergan T, Goodchild AK, Christie MJ, Pilowsky PM. Presynaptic delta opioid receptors differentially modulate rhythm and pattern generation in the ventral respiratory group of the rat. Neuroscience. 2003;121:959–973. doi: 10.1016/s0306-4522(03)00591-8. [DOI] [PubMed] [Google Scholar]
- Manzke T, Guenther U, Ponimaskin EG, Haller M, Dutschmann M, Schwarzacher S, Richter DW. 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science. 2003;301:226–229. doi: 10.1126/science.1084674. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- Matsuishi T, Nagamitsu S, Yamashita Y, Murakami Y, Kimura A, Sakai T, Shoji H, Kato H, Percy AK. Decreased cerebrospinal fluid levels of substance P in patients with Rett syndrome. Ann Neurol. 1997;42:978–981. doi: 10.1002/ana.410420621. [DOI] [PubMed] [Google Scholar]
- McKay LC, Janczewski WA, Feldman JL. Sleep-disordered breathing after targeted ablation of preBotzinger complex neurons. Nat Neurosci. 2005;8:1142–1144. doi: 10.1038/nn1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara F, Wulbrand H, Thach BT. Characteristics of the infant arousal response. J Appl Physiol. 1998;85:2314–2321. doi: 10.1152/jappl.1998.85.6.2314. [DOI] [PubMed] [Google Scholar]
- McNamara F, Lijowska AS, Thach BT. Spontaneous arousal activity in infants during NREM and REM sleep. J Physiol. 2002;538:263–269. doi: 10.1113/jphysiol.2001.012507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron. 2003;37:821–826. doi: 10.1016/s0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Monteau R, Ptak K, Broquere N, Hilaire G. Tachykinins and central respiratory activity: an in vitro study on the newborn rat. Eur J Pharmacol. 1996;314:41–50. doi: 10.1016/s0014-2999(96)00529-8. [DOI] [PubMed] [Google Scholar]
- Morton RE, Bonas R, Minford J, Tarrant SC, Ellis RE. Respiration patterns during feeding in Rett syndrome. Dev Med Child Neurol. 1997;39:607–613. doi: 10.1111/j.1469-8749.1997.tb07496.x. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Shamoto A, Homma I. Modulation of respiratory rhythm by 5-HT in the brainstem-spinal cord preparation from newborn rat. Pflugers Arch. 1998;435:485–494. doi: 10.1007/s004240050543. [DOI] [PubMed] [Google Scholar]
- Orem J, Trotter RH. Medullary respiratory neuronal activity during augmented breaths in intact unanesthetized cats. J Appl Physiol. 1993;74:761–769. doi: 10.1152/jappl.1993.74.2.761. [DOI] [PubMed] [Google Scholar]
- Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. Jama. 2006;296:2124–2132. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- Paton JF, Abdala AP, Koizumi H, Smith JC, St-John WM. Respiratory rhythm generation during gasping depends on persistent sodium current. Nat Neurosci. 2006;9:311–313. doi: 10.1038/nn1650. [DOI] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci. 2002;22:11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Substance P-mediated modulation of pacemaker properties in the mammalian respiratory network. J Neurosci. 2004;24:7549–7556. doi: 10.1523/JNEUROSCI.1871-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena F, Aguileta MA. Effects of riluzole and flufenamic acid on eupnea and gasping of neonatal mice in vivo. Neurosci Lett. 2007;415:288–293. doi: 10.1016/j.neulet.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Pena F, Parkis MA, Tryba AK, Ramirez JM. Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron. 2004;43:105–117. doi: 10.1016/j.neuron.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Phillipson EA, Sullivan CE, Read DJ, Murphy E, Kozar LF. Ventilatory and waking responses to hypoxia in sleeping dogs. J Appl Physiol. 1978;44:512–520. doi: 10.1152/jappl.1978.44.4.512. [DOI] [PubMed] [Google Scholar]
- Poets CF. Apparent life-threatening events and sudden infant death on a monitor. Paediatr Respir Rev. 2004;5(Suppl A):S383–386. doi: 10.1016/s1526-0542(04)90068-1. [DOI] [PubMed] [Google Scholar]
- Poets CF, Meny RG, Chobanian MR, Bonofiglo RE. Gasping and other cardiorespiratory patterns during sudden infant deaths. Pediatr Res. 1999;45:350–354. doi: 10.1203/00006450-199903000-00010. [DOI] [PubMed] [Google Scholar]
- Quartara L, Maggi CA. The tachykinin NK1 receptor. Part I: ligands and mechanisms of cellular activation. Neuropeptides. 1997;31:537–563. doi: 10.1016/s0143-4179(97)90001-9. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Richter DW. The neuronal mechanisms of respiratory rhythm generation. Curr Opin Neurobiol. 1996;6:817–825. doi: 10.1016/s0959-4388(96)80033-x. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Garcia A., 3rd Point: Medullary pacemaker neurons are essential for both eupnea and gasping in mammals. J Appl Physiol. 2007;103:717–718. doi: 10.1152/japplphysiol.00003.2007. discussion 722. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Tryba AK, Pena F. Pacemaker neurons and neuronal networks: an integrative view. Curr Opin Neurobiol. 2004;14:665–674. doi: 10.1016/j.conb.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Rand CM, Weese-Mayer DE, Maher BS, Zhou L, Marazita ML, Berry-Kravis EM. Nicotine metabolizing genes GSTT1 and CYP1A1 in sudden infant death syndrome. Am J Med Genet A. 2006;140:1447–1452. doi: 10.1002/ajmg.a.31306. [DOI] [PubMed] [Google Scholar]
- Ribeiro-da-Silva A, Hokfelt T. Neuroanatomical localisation of Substance P in the CNS and sensory neurons. Neuropeptides. 2000;34:256–271. doi: 10.1054/npep.2000.0834. [DOI] [PubMed] [Google Scholar]
- Roux JC, Dura E, Moncla A, Mancini J, Villard L. Treatment with desipramine improves breathing and survival in a mouse model for Rett syndrome. Eur J Neurosci. 2007;25:1915–1922. doi: 10.1111/j.1460-9568.2007.05466.x. [DOI] [PubMed] [Google Scholar]
- Sahni R, Fifer WP, Myers MM. Identifying infants at risk for sudden infant death syndrome. Curr Opin Pediatr. 2007;19:145–149. doi: 10.1097/MOP.0b013e32808373b6. [DOI] [PubMed] [Google Scholar]
- Segawa M. Pathophysiology of Rett syndrome from the standpoint of early catecholamine disturbance. Eur Child Adolesc Psychiatry. 1997;6(Suppl 1):56–60. [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Cholinergic neurotransmission in the preBotzinger Complex modulates excitability of inspiratory neurons and regulates respiratory rhythm. Neuroscience. 2005;130:1069–1081. doi: 10.1016/j.neuroscience.2004.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao XM, Tan W, Xiu J, Puskar N, Fonck C, Lester HA, Feldman JL. Alpha4* nicotinic receptors in preBotzinger complex mediate cholinergic/nicotinic modulation of respiratory rhythm. J Neurosci. 2008;28:519–528. doi: 10.1523/JNEUROSCI.3666-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-John WM, Waki H, Dutschmann M, Paton JF. Maintenance of eupnea of in situ and in vivo rats following riluzole: a blocker of persistent sodium channels. Respir Physiol Neurobiol. 2007;155:97–100. doi: 10.1016/j.resp.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Telgkamp P, Ramirez JM. Differential responses of respiratory nuclei to anoxia in rhythmic brain stem slices of mice. J Neurophysiol. 1999;82:2163–2170. doi: 10.1152/jn.1999.82.5.2163. [DOI] [PubMed] [Google Scholar]
- Telgkamp P, Cao YQ, Basbaum AI, Ramirez JM. Long-term deprivation of substance P in PPT-A mutant mice alters the anoxic response of the isolated respiratory network. J Neurophysiol. 2002;88:206–213. doi: 10.1152/jn.2002.88.1.206. [DOI] [PubMed] [Google Scholar]
- Thach BT. The role of respiratory control disorders in SIDS. Respir Physiol Neurobiol. 2005;149:343–353. doi: 10.1016/j.resp.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Ramirez JM. Identification of two types of inspiratory pacemaker neurons in the isolated respiratory neural network of mice. J Neurophysiol. 2001;86:104–112. doi: 10.1152/jn.2001.86.1.104. [DOI] [PubMed] [Google Scholar]
- Tryba AK, Pena F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci. 2006;26:2623–2634. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryba AK, Pena F, Lieske SP, Viemari JC, Thoby-Brisson M, Ramirez JM. Differential modulation of neural network and pacemaker activity underlying eupnea and sigh-breathing activities. J Neurophysiol. 2008;99:2114–2125. doi: 10.1152/jn.01192.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderHorst VG, Ulfhake B. The organization of the brainstem and spinal cord of the mouse: relationships between monoaminergic, cholinergic, and spinal projection systems. J Chem Neuroanat. 2006;31:2–36. doi: 10.1016/j.jchemneu.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Ramirez JM. Norepinephrine differentially modulates different types of respiratory pacemaker and nonpacemaker neurons. J Neurophysiol. 2006a;95:2070–2082. doi: 10.1152/jn.01308.2005. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Ramirez JM. Fictive gasping and eupneic activity in transverse slices require activity activation of noradrenergic receptors. Society of neuroscience meeting abstract. 2006b:455.16. [Google Scholar]
- Viemari JC, Roux JC, Tryba AK, Saywell V, Burnet H, Pena F, Zanella S, Bevengut M, Barthelemy-Requin M, Herzing LB, Moncla A, Mancini J, Ramirez JM, Villard L, Hilaire G. Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. J Neurosci. 2005;25:11521–11530. doi: 10.1523/JNEUROSCI.4373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weese-Mayer DE, Ackerman MJ, Marazita ML, Berry-Kravis EM. Sudden Infant Death Syndrome: review of implicated genetic factors. Am J Med Genet A. 2007;143:771–788. doi: 10.1002/ajmg.a.31722. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Zhou L, Berry-Kravis EM, Maher BS, Silvestri JM, Marazita ML. Association of the serotonin transporter gene with sudden infant death syndrome: a haplotype analysis. Am J Med Genet A. 2003;122:238–245. doi: 10.1002/ajmg.a.20427. [DOI] [PubMed] [Google Scholar]
- Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic J, Hodges MR, Feroah TR, Davis S, Forster HV. Large lesions in the pre-Botzinger complex area eliminate eupneic respiratory rhythm in awake goats. J Appl Physiol. 2004;97:1629–1636. doi: 10.1152/japplphysiol.00953.2003. [DOI] [PubMed] [Google Scholar]
- Woodyatt GC, Murdoch BE. The effect of the presentation of visual and auditory stimuli on the breathing patterns of two girls with Rett syndrome. J Intellect Disabil Res. 1996;40(Pt 3):252–259. doi: 10.1111/j.1365-2788.1996.tb00628.x. [DOI] [PubMed] [Google Scholar]
- Zanella S, Barthelemy M, Muscatelli F, Hilaire G. Necdin gene, respiratory disturbances and Prader-Willi syndrome. Adv Exp Med Biol. 2008a;605:159–164. doi: 10.1007/978-0-387-73693-8_28. [DOI] [PubMed] [Google Scholar]
- Zanella S, Watrin F, Mebarek S, Marly F, Roussel M, Gire C, Diene G, Tauber M, Muscatelli F, Hilaire G. Necdin plays a role in the serotonergic modulation of the mouse respiratory network: implication for Prader-Willi syndrome. J Neurosci. 2008b;28:1745–1755. doi: 10.1523/JNEUROSCI.4334-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghbi HY, Milstien S, Butler IJ, Smith EO, Kaufman S, Glaze DG, Percy AK. Cerebrospinal fluid biogenic amines and biopterin in Rett syndrome. Ann Neurol. 1989;25:56–60. doi: 10.1002/ana.410250109. [DOI] [PubMed] [Google Scholar]