Abstract
This paper describes an integrated microfluidic chip that is capable of rapidly and quantitatively measuring the concentration of a bladder cancer biomarker, apolipoprotein A1, in urine samples. All of the microfluidic components, including the fluid transport system, the micro-valve, and the micro-mixer, were driven by negative pressure, which simplifies the use of the chip and facilitates commercialization. Magnetic beads were used as a solid support for the primary antibody, which captured apolipoprotein A1 in patients' urine. Because of the three-dimensional structure of the magnetic beads, the concentration range of the target that could be detected was as high as 2000 ng ml−1. Because this concentration is 100 times higher than that quantifiable using a 96-well plate with the same enzyme-linked immunosorbent assay (ELISA) kit, the dilution of the patient's urine can be avoided or greatly reduced. The limit of detection was determined to be approximately 10 ng ml−1, which is lower than the cutoff value for diagnosing bladder cancer (11.16 ng ml−1). When the values measured using the microfluidic chip were compared with those measured using conventional ELISA using a 96-well plate for five patients, the deviations were 0.9%, 6.8%, 9.4%, 1.8%, and 5.8%. The entire measurement time is 6-fold faster than that of conventional ELISA. This microfluidic device shows significant potential for point-of-care applications.
INTRODUCTION
Bladder cancer is a type of common urinary tract carcinoma that has a high recurrence rate and a poor prognosis.1, 2 If the abnormal tissue or tumor is identified early, treatment and recovery may be easier. The standard method for the clinical detection of bladder cancer is cytology, which shows low sensitivity for low-grade bladder cancers.1, 2, 3 Cystoscopy is frequently used to examine and monitor patients for the recurrence or progression of this disease. However, this detection method is invasive and expensive.4, 5 There have been many attempts to develop an efficient, reliable, accurate, and noninvasive diagnostic procedure that can identify bladder carcinoma patients. The quantitative measurement of urinary tumor biomarkers represents a practical method for the initial detection of tumors and for the monitoring of patients for recurrence because urine is in direct contact with tumor cells for this type of cancer and is accessible for clinical analysis. Recently, apolipoprotein A1 (APOA1) has been identified as a potential biomarker that can be used for the early diagnosis of bladder cancer.1, 6, 7
Enzyme-linked immunosorbent assay (ELISA), a high-sensitivity technique, is the current standard method for the quantitative analysis of a target protein in biological samples. The amounts of certain proteins in urine samples have been suggested to be reliable and quantitative indicators of bladder cancer.8 ELISAs have been used extensively in medical research, clinical diagnostics, drug discovery, environmental monitoring, food safety, and biodefense.9 Nevertheless, the conventional immunoassay conducted with a 96-well plate requires substantial labor, the consumption of expensive reagents, and precise technical performance, making this type of assay inconvenient and impractical for point-of-care diagnosis.10, 11 The principle of ELISA is to immobilize and detect an antigen–antibody complex. In conventional ELISA, the antibodies are immobilized on the surfaces of the wells in 96-well plates. During the assay process, the sample and reagent are dispensed by manual pipetting or pipetting with a machine. Each incubation step is followed by repeated washing steps to remove unbound antibodies and nonspecific antigens. If the efficiency of the washing process is improved, the time required to complete the entire assay can be greatly shortened. In addition, the assay requires several dilution procedures to reduce the high concentration of the target antigen in urine samples. As a result, the assay requires a few hours or days for the liquid-handling, washing, and incubation procedures. Furthermore, well-trained personnel are required to conduct the entire protocol precisely.
Since its introduction, the lab-on-a-chip (LOC) system, also known as the micro total analysis system (μTAS), has been rapidly adopted to miniaturize several analytical assay systems that require large amounts of sample or reagents and bulky experimental devices.12, 13 This technique offers many advantages over conventional techniques, such as short reaction times, less consumption of expensive chemical reagents, higher sensitivity, greater portability, and automatic operation.14, 15 LOC systems are widely employed in analytical assays and clinical diagnosis. For example, electrophoresis was one of the earliest successes with microfluidics technology, and microfluidic electrophoresis devices have proven to be useful. A microfluidic electrophoresis chip with a polymerase chain reaction (PCR) DNA amplification chamber was developed in 2000.16 In addition, the combination of microfluidics with flow cytometry provides a volume-efficient and higher-precision method for determining the characteristics of suspended sample populations (e.g., cells, viruses, bacteria, yeast, aerosol particles, and microbeads).17 This combination provides higher speeds, smaller sizes, and lower costs than the conventional method. Moreover, the use of a microfluidic-based electrospray chip for mass spectrum analysis has also been demonstrated. An enrichment column, a reversed-phase separation channel, and a nanoelectrospray emitter were integrated into a single chip.18 This chip enhances the sample loading and selectivity of the mass spectrum system. Immunoassays can also be performed in microfluidic systems. For example, a miniaturized mosaic based on using a microfluidic network to pattern lines of antigens onto a chip surface has been presented.19, 20 The binding of target antibodies with their immobilized antigens on the surface results in a mosaic pattern with fluorescence labeling. This chip can be used to perform dense, parallel, and self-consistent immunoassays with nanoliter quantities of reagents and incubation times of seconds to minutes. Furthermore, microfluidic technology can provide a microenvironment for 3-dimensional cell cultures, which may better reflect actual responses than 2D monolayer cultures can. This technique has been demonstrated for the empirical testing of tumor sensitivity to drugs, which may provide a more reliable predictive value for clinic diagnoses.21 Although microfluidic techniques provide various approaches for biological measurement, the sample pretreatment process for biosamples such as blood, saliva, and urine is still an important issue affecting the general and practical use of these microfluidic devices.
Magnetic beads have frequently been used in microfluidic immunoassays for several reasons. First, these beads offer greater surface area-to-volume ratios than the traditional 96-well plate, thus facilitating interactions between antigens and antibodies in small volumes. In addition, magnetic beads can be easily delivered using a flowing fluid and can be separated from the medium using a magnetic field. Furthermore, multiple antigen-antibody targets can be included on a single chip if the magnetic beads are conjugated with different fluorophores. Magnetic beads allow sample pretreatment in microfluidic systems. For example, cell separation in microfluidic chips using a magnetophoresis approach has been proposed.22 Suspended cells labeled with magnetic beads in a microchannel are deflected by means of a magnetic field. Therefore, cells with different sizes or that are decorated with different numbers of magnetic beads are separated accordingly. The extraction of DNA or RNA from a cell or virus is an important technique for molecular diagnosis. The use of magnetic beads facilitates the isolation of DNA or RNA from a cell or virus in a microfluidic device. Such a device had been developed to detect viral RNA with the aid of magnetic beads.23, 24 In addition, target viruses or bacteria from a complex biosample can also be extracted directly using antibody-conjugated magnetic beads. Lien et al. purified and enriched dengue virus using magnetic beads. Next, nucleic acid amplification using a micro RT-PCR system was performed on the same chip.25 In addition, magnetic beads with ion-exchange properties were used to simulate cells with peptides bound to the cell surface by electrostatic interactions. By sequential treatment with different buffer conditions using acoustophoresis-based microfluidic chips, the medium surrounding the beads can be removed or replaced by another buffer medium, which is important for the understanding of human biology and the treatment of disease.26 Using magnetic beads has benefits in biosample pretreatment when using LOC systems. However, the microfluidic devices proposed to date have not been used for the diagnosis of bladder cancer in urine samples with a bead-based immunoassay using automatic fluid handling.
In this study, we developed a negative-pressure-driven microfluidic chip that can be used to rapidly detect a bladder cancer biomarker with the aid of magnetic bead-based ELISA. The use of only vacuum forces as the driving force for fluid transport, the vibration of the micromixer, and the opening and closing of the microvalves simplifies the corresponding components of the chip. In addition, by integrating a syringe filter into the sample inlet, the centrifugation step in the pretreatment of urine samples is no longer necessary. Five clinical urine samples were used to evaluate the use of the chip. The proposed chip is a feasible sample-in and answer-out device that can quantitate a potential bladder cancer biomarker.
MATERIALS AND METHODS
Chip design and experimental setup
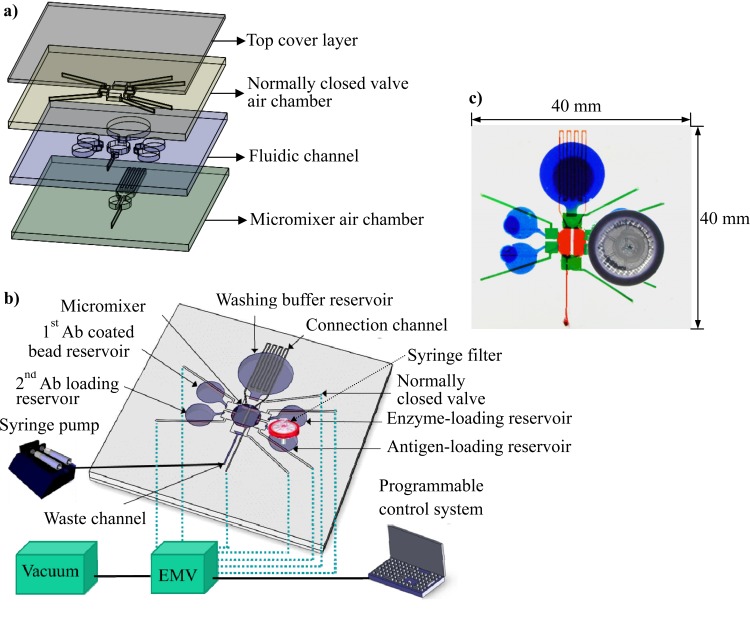
Fig. 1a is an exploded view of the chip. The chip consists of four poly(dimethylsiloxane) (PDMS) layers: the top cover layer, the air chamber layer of the normally closed valve, the fluid channel layer, and the micromixer air chamber layer at the bottom. The channel depths of the normally closed valve air chamber layer and the fluidic channel layer were both 500 μm. The bottom micromixer air chamber layer was constructed using a thick PDMS layer with a channel depth of 1.5 mm. There were five reservoirs that contained primary antibody-coated beads, secondary antibodies, antigens, enzymes, or the washing buffer, as shown in Fig. 1b. Each reservoir was connected to the central chamber through a normally closed valve. The central chamber was equipped with a micromixer and served as a reaction chamber with a volume of 14.5 μl. Note that all the driving forces—including those powering fluid transport, the micromixer, and the normally closed valves—were based on negative pressure to simplify the experimental setup. Other types of negative-pressure-driven microfluidic components can be found in the literature.27, 28, 29 The normally closed valve and micromixer were controlled and driven using a vacuum pump connected to an electromagnetic valve (EMV), and fluid transport was achieved using a syringe pump (Legato 180, KD Scientific Inc., Holliston, MA) connected to the waste outlet channel that was driven with a withdrawing force by opening the inlet to atmospheric pressure. Using a personal computer, control of the fluid in the microchannel can be performed automatically. For example, when the washing process was conducted, the valves between the washing buffer reservoir and the reaction chamber and between the reaction chamber and the waste channel were opened, and the syringe pump withdrew the remaining washing buffer to complete the washing process. This system enabled five reagents to be delivered sequentially into the reaction chamber via negative-pressure-driven fluid transport and normally closed valves. The micromixer was composed of two air chambers with thin PDMS membranes, and the two air chambers were connected with a serpentine-shaped air channel. The serpentine-shaped connecting air channel was designed to delay the airflow from one micromixer air chamber to the other. Therefore, the two thin PDMS membranes above the air chamber were deflected sequentially, leading to the swirling of the fluid in the reaction chamber after negative air pressure was applied to the air inlet (see details in Fig. 4a). Thus, the reagents and sample can be incubated with enhanced mixing efficiency. Fig. 1c shows a photograph of the integrated chip in which the normally closed valves are presented in green, the fluidic chamber is in blue, and the micromixer is in red. A commercially available syringe filter with a pore size of 0.22 μm was connected to the antigen loading chamber to filter out unwanted debris in the urine sample. The dimensions of the chip were 40 mm in length by 40 mm in width.
Figure 1.
(a) Exploded view of the proposed microfluidic chip. Four layers of PDMS were used to construct the negative-pressure-driven microfluidic chip. The bottom layer was an air chamber layer for the actuation of a pneumatic micromixer, the third layer was a fluidic channel layer, the second layer was another air chamber layer for the actuation of the normally closed valves, and the top layer was a flat PDMS layer that sealed the air chamber. (b) The chip was equipped with a micromixer incorporated into the reaction chamber at the center of the chip and with five reservoirs (four for sample loading and one for a wash buffer). There were six normally closed valves located between the reservoirs and the reaction chamber. A syringe filter was integrated into the antigen-loading chamber to filter out any debris in the urine samples. The driving force for fluid movement relied on a suction force provided from the waste outlet. All of the liquid handling can be performed with the aid of the integrated microfluidic components. (c) Photograph of the assembled microfluidic chip with dimensions of 40 mm × 40 mm.
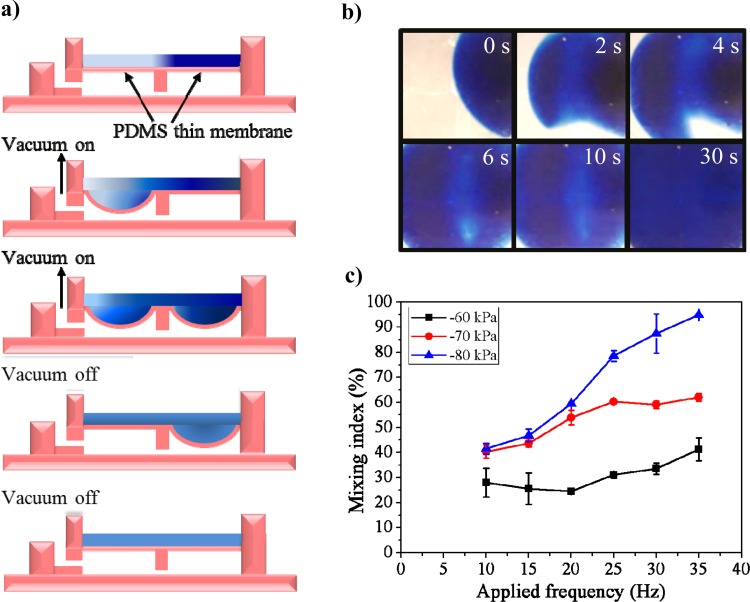
Figure 4.
Design and characterization of the suction force-driven micromixer. (a) Cross-sectional view of the micromixer. The mixer has two air chambers connected by a narrow air channel to delay the movement of air from one chamber to the other. When pulses of suction force were applied, the fluid above the PDMS membrane vibrated, thus enhancing the mixing efficiency. (b) Series of photographs of the mixer actuated for a period of 30 s. The ink and DI water were mixed together gradually. (c) Characterization of the mixing index of the micromixer with different negative air pressures and driving frequencies.
Experimental procedure
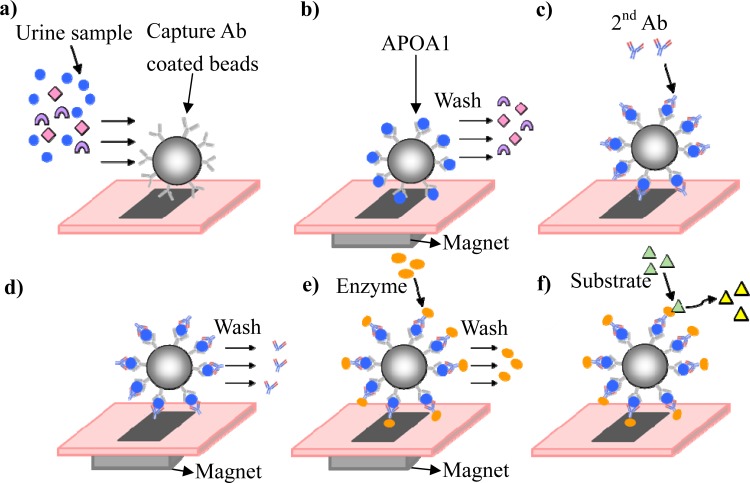
In this study, the detection of a protein, APOA1, was used to demonstrate the feasibility of using the proposed chip for cancer detection. This protein is a highly relevant protein associated with bladder cancer in urine samples. The concentrations of APOA1 were determined using a bead-based approach with a sandwich ELISA kit (Mabtech, Nacka Strand, Sweden). Fig. 2 shows a schematic illustrating the principle upon which the bead-based ELISA in the microfluidic chip is based. First, the primary antibody-coated magnetic beads and the antigen were incubated together in the reaction chamber to allow the beads to specifically capture the antigen, APOA1. The protocol for conjugating the antibody on the magnetic beads is described in the supplemental data section.30 After the antigen and beads were co-incubated, the beads were held in the chamber using an external magnet, and the nonspecific antigens were washed out of the reaction chamber (Figs. 2a, 2b). Serially diluted, purified APOA1 was used as the calibration standard. The APOA1 signals for the clinical urine samples were measured, and the standard curve was used to calculate the APOA1 concentration. Biotinylated secondary antibodies were allowed to interact with the antigens, and then unbound antibodies were washed away (Figs. 2c, 2d). Next, streptavidin-enzyme complexes were allowed to interact with the secondary antibody (Fig. 2e). Finally, the substrate was added to the reaction chamber and allowed to react with the enzyme. Then, the reagents were suspended to measure the optical density at 405 nm (Fig. 2f).
Figure 2.
Illustration of the working principles behind the bead-based ELISA using the microfluidic chip. (a) A urine sample containing the target protein and antibody-coated magnetic beads was introduced into the chip. (b) After the incubation process, the target protein was captured, and the unwanted protein was washed out. (c) and (d) The secondary antibody was bound to the antigen, and the excess antibody was washed out. (e) and (f) The enzyme was linked to the secondary antibody, and after the excess enzyme was washed out, a substrate was used to quantitatively measure the target protein.
The chip design enabled the reagents to be sequentially and automatically introduced into the reaction chamber. The detailed operation procedure for the measurement of APOA1 concentrations using the proposed chip is as follows. As shown in Fig. 1b, initially, the valves that connect the bead-loading reservoir to the reaction chamber and the reaction chamber to the waste channel were opened, and 20 μl of antibody-coated magnetic beads (109 beads ml−1) was loaded into the reaction chamber using the suction force from the outlet channel. Note that before the magnetic beads were introduced, an external magnet (approximately 300 Gauss) was placed under the reaction chamber to fix the beads in the reaction chamber. The solution in which the beads had been suspended was then suctioned out through the outlet channel. Then, 20 μl of standard APOA1 solution or a urine sample was loaded into the reaction chamber using the same control procedure. Note that the volume of the used reagent was determined by the size of the reaction chamber, i.e., 14.5 μl. After the normally closed valves were closed, the excess reagent either left via the microchannel or was suctioned out. Next, the sample and magnetic beads were mixed for 5 min using a two-membrane-type micromixer. After the incubation period, the beads were again held in place using the magnet, and the fluid was withdrawn from the waste channel using a syringe pump. During the washing process, the valves that connect the washing buffer reservoir to the reaction chamber and the reaction chamber to the waste channel were opened. Thus, the washing buffer flowed into the reaction chamber for 30 s. Next, the valves were closed, and the washing buffer was agitated for 1 min using the micromixer to remove the unbound antibody. This procedure was repeated three times for a single washing step. Other reagents, including the secondary antibody and the enzyme, were transferred into the reaction chamber and mixed sequentially. Similarly, each incubation step lasted 5 min, and the washing step was repeated three times for 1.5 min. Finally, the substrate was loaded into the reaction chamber from the bead-loading reservoir and was reacted with the enzyme for 10 min. Then, the reagents were suspended for optical measurement.
Chip fabrication
The chip was fabricated using standard PDMS photolithography, micromachining, and replication techniques. Poly(methyl methacrylate) (PMMA) master molds for the microstructures were first formed using a CNC machine (EGX-400, Roland Inc., Japan) equipped with a 0.5 mm drill bit. A silicone elastomer and an elastomer curing agent (Sylgard 184A and 184B, Sil-More Industrial Ltd., USA) were mixed in a 10:1 ratio and poured onto the PMMA mold. To form a 100-μm-thick PDMS membrane to drive the microvalve and the micromixer, the microvalve air chamber layer and the fluidic channel layer were fabricated by spin coating with 3.6 g and 4 g of PDMS, respectively, at 300 rpm for 15 s. A 600-μm-thick PDMS layer was formed with a 100-μm-thick PDMS membrane located on the microstructure of the master mold. After curing at 70 °C for 1 h, the solidified PDMS was peeled off of the master mold, and the holes for injecting air or fluid were drilled with a syringe needle. Subsequently, the four-layer PDMS structure was bonded using oxygen plasma treatment (HARRICK PLASMA, Ithaca, NY, USA).
Immunoassay reagents and urine sample preparation
Phosphate-buffered saline (PBS), bovine serum albumin (BSA), and Tween-20 surfactant were purchased from Sigma-Aldrich Co. Superparamagnetic beads (M-270 Epoxy, Dynabeads, Invitrogen) were coated with a surface epoxy group. These beads were used as a solid support to immobilize specific antibodies. In this study, all urine samples were collected at Chang Gung Memorial Hospital, Taoyuan, Taiwan (a hospital affiliated with Chang Gung University). First, morning urine samples were collected and treated with 1 mM sodium azide (Fluka, Switzerland) as a bacteriostatic reagent. A hernia patient (n = 1) was used as a control. The collected urine samples were stored at −20 °C for subsequent processing.
RESULTS AND DISCUSSION
Characterization of the microfluidic components
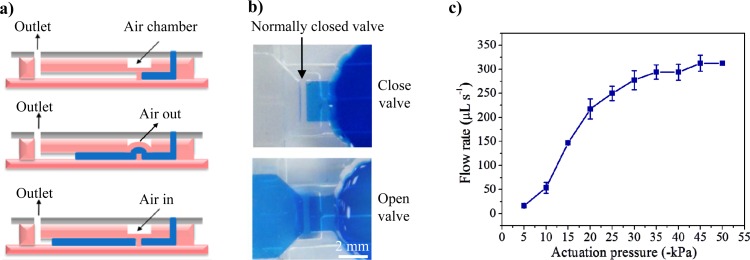
Fig. 3a illustrates the working principles of the normally closed valve. As shown in the lateral view, the normally closed valve was composed of an air chamber, a thin PDMS membrane layer, and a pillar structure in the microchannel. The PDMS membrane and the pillar structure were not bonded together. When negative air pressure from a vacuum was applied to the air chamber, the PDMS membrane was deformed and actuated, allowing the fluid to flow through the microvalve. After the vacuum was removed, the PDMS membrane recovered, and thus, the fluid flow was blocked by the valve. Fig. 3b shows a valve in the open and the closed position. The normally closed valve was characterized using a constant gravity-driven flow of deionized (DI) water (14 ml with a height of 10.5 cm). The time required for the first 5 ml of DI water to flow through the chip at different applied vacuum pressures was recorded. The flow rate was calculated using these data. By adjusting the vacuum pressure, the flow rate of the fluid that passed through the valve was varied. No fluid passed through the valve when the applied pressure was less than −4.2 kPa, i.e., the threshold vacuum pressure was −4.2 kPa for valve actuation. The flow rate increased from 16.4 μl s−1 to 312.5 μl s−1 when the actuation pressure was changed from −5 kPa to −45 kPa (as shown in Fig. 3c). When the applied pressure was greater than −45 kPa, the normally closed valve was almost fully open.
Figure 3.
Design and characterization of the normally closed valve. (a) Working principle of the valve. When the air in the chamber was suctioned out, the PDMS membrane was deflected, and the fluid could pass through the valve. (b) Photograph of the valve in the open and closed positions. (c) The DI water started flowing through the normally closed valve when the actuation pressure surpassed −4.2 kPa. The normally closed valve was almost fully open when the applied pressure was greater than −45 kPa.
The two-membrane-type micromixer was designed to mix the urine sample and reagents during incubation. Fig. 4a shows the cross-sectional view of the micromixer. The two membranes were connected by a serpentine-shaped connecting channel, and the air inlet was linked to a vacuum force. By setting the driving frequency of an EMV and using a programmable computer, the two thin membranes were vibrated at a specific frequency and in a specific sequence to enhance the mixing efficiency during incubation. To optimize the mixing efficiency of the two-membrane-type micromixer, various air pressure and applied frequency conditions were investigated. To determine the mixing efficiency of the micromixer, 2 μl of blue ink and 12.5 μl of DI water were loaded into the reaction chamber to evaluate the concentration distribution along a cross section of the reaction chamber. A mixing index was defined to quantify the mixing profile:31
| (1) |
where ρ(A) is the mixing index of the normalized concentration (C+) distributed within the sample mixing unit (A), is the initial condition in the unmixed state, and is the completely mixed state of the normalized concentration (=0.5). Fig. 4b shows the concentration distribution of the fluid in the mixing chamber for mixing periods of 0, 2, 4, 6, 10, and 30 s. After 30 s, the mixing index increased from 15 to 95% at a driving frequency of 35 Hz and an air pressure of −80 kPa. The initial mixing index of 15% was the result of the molecular diffusivity in the fluid. The time required to achieve a 100% mixing index was calculated to be approximately 60 s. However, this length of time was not sufficient for sample incubation. In practice, each incubation step lasted 5 min.
Fig. 4c shows the mixing index of the micromixer under applied air pressures of −60, −70, and −80 kPa and driving frequencies of 10, 15, 20, 25, 30, and 35 Hz after 30 s. A higher mixing index was achieved with higher applied negative air pressures and higher applied frequencies. For an air pressure of −80 kPa, the mixing index was 95% at an applied frequency of 35 Hz for 30 s. At the applied air pressures of −60 and −70 kPa, the 100-μm PDMS membrane could not be deformed completely. Therefore, the mixing index could not reach values as high as that for an applied pressure of −80 kPa. The suction-force-driven pneumatic micromixer greatly enhanced the mixing efficiency and shortened the incubation time of the bead-based ELISA for measurement of the APOA1 concentration. However, fatigue is a problem for PDMS membranes during on-off cycling, although the literature indicates that a PDMS membrane can perform more than 4 × 106 actuations without fatigue failure.32 Therefore, we used each chip three to five times to finish one sample measurement, and then used another chip for the next sample measurement to make sure that the procedure was performed by a mechanically stable PDMS membrane.
The maximum mixing efficiency was observed at a driving frequency of 35 Hz with a suction force of −80 kPa.
Detection of the bladder cancer biomarker APOA1
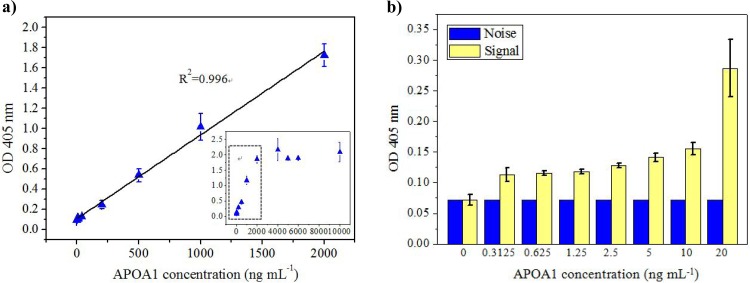
Prior to detecting the patients' samples, the incubation time for the chip was determined. Supplemental Fig. S130 shows the relationship between the detection signal and the mixing time for three concentrations of APOA1 within 20 min. Note that the mixing time represents every incubation step in each assay. It can be observed that the detection signal was increased with the increased mixing time. Notably, the signal increased rapidly before the incubation time reached 5 min. From time period of 5 min to 20 min, the signal increased 22%, 28%, and 26% with APOPA1 concentrations of 1000, 500, and 200 ng ml−1, respectively. We, therefore, chose 5 min for each incubation period, which was a trade-off between time and signal intensity. The detection limit of 5 min (10 ng ml−1) is sufficiently smaller than the cut-off valve (11.16 ng ml−1) for bladder cancer diagnosis using APOA1 as a biomarker. Therefore, each incubation step lasted 5 min. The detection range of APOA1 was measured using the proposed system. APOA1 standards with concentrations ranging from 0 to 10000 ng ml−1 were prepared using proper dilution ratios. As shown in Fig. 5a, when the concentration of APOA1 was less than 2000 ng ml−1, the optical signal increased proportionally to the APOA1 concentration with a linearity of 0.996. The calibration curve of APOA1 was calculated for repeated experiments (n = 5). When the concentration of APOA1 exceeded 2000 ng ml−1, the optical signal exhibited a nonlinear plateau. This finding revealed that the detection range for the APOA1 concentration can reach 2000 ng ml−1 when using 20 μl of magnetic beads (1 × 109 beads ml−1). In comparison, the detection range using the tradition method with a 96-well ELISA plate with the same ELISA kit was 0.2 to 20 ng ml−1. Thus, the traditional plate-based ELISA requires several repeated dilutions to measure the high concentration of APOA1 in clinical urine samples, and this requirement results in burdensome and complicated assay procedures. The maximum concentration of APOA1 in urine is approximately 9000 ng ml−1.6 Therefore, at most, one 10-fold dilution is necessary to analyze unknown samples using the proposed chip. With the greatly enhanced detection range of ELISA using the proposed method, the process of diluting samples can be omitted or reduced, which is beneficial when needing to analyze a large number of patient samples. Because the magnetic beads have a larger surface area-to-volume ratio than the 96-well plate, the beads increase the efficiency of interactions between antigens and antibodies, further enhancing the detection range of the immunoassays and shortening the measurement time. Although the cutoff value for diagnosing bladder cancer was 11.16 ng ml−1, we also tried to determine the relationship between the stage of the bladder cancer and the concentration of APOA1.6 If such a relationship can be identified, the proposed chip can be used not only for the early detection but also for stage differentiation of bladder cancer. To determine the detection limit, an APOA1 concentration range from 0 to 20 ng ml−1 was used. Figure 5b shows the background noise and the detection signal for the low concentrations of APOA1. The detection limit of APOA1 on the chip was determined to be approximately 10 ng ml−1. The cutoff value for diagnosing bladder cancer using the APOA1 protein has been reported to be 11.16 ng ml−1 (n = 126, 94.6% sensitivity and 92.0% specificity).6 Thus, the detection limit of 10 ng mL−1 is sufficient to diagnose bladder cancer with a urine sample. The chip requires only 5 min for each incubation process, which is less than the 1 h required for the 96-well plate on a shaker. Thus, the total measurement time with the chip is greatly reduced, totaling approximately 40 min, which is much shorter than the time needed to run a conventional plate-based ELISA (more than 4 h). In another bead-based microfluidic assay approach using surface tension valves for fluidic control,33 the measurement of the concentration of the biomarker in that chip was performed off-chip, but in the proposed chip, it was performed in the same chip, which eliminates the need for manual operation and facilitates automatic control of the measurement process.
Figure 5.
The detection ability of the proposed chip. (a) The detection range was up to 2000 ng ml−1 with 20 μl of magnetic beads (1 × 109 beads ml−1), and the detection linearity was 0.996 in this range. (b) The detection limit was approximately 10 ng ml−1 for APOA1 for the chip.
Detection of APOA1 in clinical urine samples
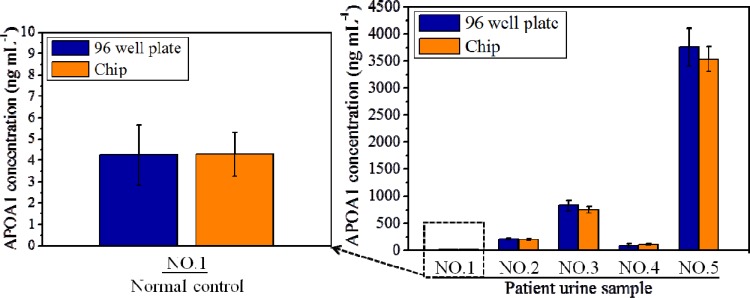
To evaluate the feasibility of using the proposed chip for the analysis of clinical urine samples, patient urine samples collected at the Chang Gung Memorial Hospital were used. The experimental results were then compared with the results of conventional ELISA conducted in a 96-well plate. One normal control urine sample from a hernia patient and four samples from bladder cancer patients were tested. The concentrations of APOA1 in the urine samples (1, 2, 3, 4, and 5) were 4.24, 207.3, 826.3, 1038.7, and 3754.7 ng ml−1, respectively. With the exception of sample 5, the samples were measured in their native undiluted form. The concentration of APOA1 in sample number 5 was diluted 10-fold prior to analysis. Each sample was assessed three times (n = 3), and the mean value was calculated. Because clinical urine samples may contain debris, a syringe filter was integrated into the inlet of the chip to eliminate unwanted debris from the urine sample. Fig. 6 shows the experimental results for the quantitative detection of APOA1 using the proposed chip and the traditional ELISA method. The variations between the two sets of results were found to be 0.9%, 6.8%, 9.4%, 1.8%, and 5.8%. Note that the values represented by the error bars are the maximum/minimum values of the experimental data. The variation between the two sets of results may be the result of several factors, such as the absorption of protein to the PDMS surface or stiction between the magnetic beads and the PDMS surface, which can cause bead loss during each incubation process. The experimental data (Fig. 6) show that most measurement results for the APOA1 concentration that were obtained with the proposed chip were a little lower than the results from the ELISA plate. In addition, the absorption of protein on PDMS surfaces is a well-known issue when PDMS is used in microfluidic chips.34, 35 Therefore, we infer that the absorption of protein on the PDMS surface is one of the factors that caused the difference in detection. Furthermore, observations made during the experimental procedure indicated that there were some magnetic beads attached to the microchannel surface that could not participate in the reaction. This might be the other reason why the detected concentration in the chip was lower than in the ELISA plate. However, in general, the results confirm that the detection performance of ELISA using the proposed device is broadly comparable to that of traditional ELISA but that approximately 6-fold less time is required for the chip-based assay.
Figure 6.
Comparison of the results for five patient samples obtained using the proposed chip and using traditional 96-well ELISA. The differences in the results were 0.9%, 6.8%, 9.4%, 1.8%, and 5.8% for samples 1, 2, 3, 4, and 5, respectively.
CONCLUSION
We evaluated the use of a microfluidic chip equipped with a negative-pressure-driven microvalve, micromixer, and fluid transport system for bead-based ELISA, and we used this form of ELISA to successfully quantitatively measure the bladder cancer biomarker APOA1 in human urine. The microvalve was determined to have an applied air pressure threshold of −4.2 kPa to open the valve, and the optimal mixing efficiency of the suction-type micromixer was found to occur at an air pressure of −80 kPa and a frequency of 35 Hz. Because magnetic beads were used as the solid support for the primary antibody, the surface area-to-volume ratio for the interaction between the reagent and sample was greatly increased compared with the ratio for the plate-based ELISA. Therefore, the concentration range for measurement was greatly enhanced, and the measurement time was also reduced. Five human urine samples were analyzed using the proposed chip, and the results were compared with those from the 96-well plate-based ELISA test. The maximum variation between the two sets of results was found to be 9.4%, for patient number 3. In general, the performance of the proposed chip was comparable to that of 96-well plate-based ELISA. The proposed microfluidic device is a promising tool for the point-of-care diagnosis of bladder cancer.
ACKNOWLEDGMENTS
This work was supported by grants to Chang Gung University from the Ministry of Education (EMRPD1A0761) of Taiwan, Republic of China; Chang Gung University (UERPD2A0051, UERPD2B0091); and the National Science Council of Taiwan, Republic of China (100-2221-E-182-021-MY3).
References
- Li H., Li C., Wu H., Zhang T., Wang J., Wang S., and Chang J., Proteome Sci. 9, 21 (2011). 10.1159/000052495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanahashi T., Matsumura Y., Ohmori H., and Tanaka T., Acta Med. Okayama 32, 139 (1978). [PubMed] [Google Scholar]
- Lotana Y. and Roehrborna C. G., Urology 61, 109 (2003). 10.1016/S0090-4295(02)02136-2 [DOI] [PubMed] [Google Scholar]
- Karakiewicz P. I., Benayoun S., Zippe C., Lüdecke G., Boman H., Sanchez-Carbayo M., Casella R., Mian C., Friedrich M. G., Eissa S., Akaza H., Huland H., Hedelin H., Rupesh R., Miyanaga N., Sagalowsky A. I., Marberger M. J., and Shariat S. F., BJU Int. 97, 997 (2006). 10.1111/j.1464-410X.2006.06036.x [DOI] [PubMed] [Google Scholar]
- Mitropoulos D., Kiroudi-Voulgari A., Nikolopoulos P., Manousakas T., and Zervas A., J. Endourol. 19, 861 (2005). 10.1089/end.2005.19.861 [DOI] [PubMed] [Google Scholar]
- Chen Y.-T., Chen C.-L., Chen H.-W., Chung T., Wu C.-C., Chen C.-D., Hsu C.-W., Chen M.-C., Tsui K.-H., Chang P.-L., Chang Y.-S., and Yu J.-S., J. Proteome Res. 9, 5803 (2010). 10.1021/pr100576x [DOI] [PubMed] [Google Scholar]
- Chen Y.-T., Chen H.-W., Domanski D., Smith D. S., Liang K.-H., Wu C.-C., Chen C.-L., Chung T., Chen M.-C., Chang Y.-S., Parker C. E., Borchers C. H., and Yu J.-S., J. Proteomics 75, 3529 (2012). 10.1016/j.jprot.2011.12.031 [DOI] [PubMed] [Google Scholar]
- McCabe R. P., Lamm D. L., Haspel M. V., Pomato N., Smith K. O., Thompson E., and M. G.Hanna, Jr., Cancer Res. 44, 5886 (1984). [PubMed] [Google Scholar]
- Hüttenhain R., Malmström J., Picotti P., and Aebersold R., Curr. Opin. Chem. Biol. 13, 518 (2009). 10.1016/j.cbpa.2009.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C. T. and Zhang Y., Biosens. Bioelectron. 22, 1197 (2007). 10.1016/j.bios.2006.06.005 [DOI] [PubMed] [Google Scholar]
- Herr A. E., Hatch A. V., Throckmorton D. J., Tran H. M., Brennan J. S., Giannobile W. V., and Singh A. K., Proc. Natl. Acad. Sci. U. S. A. 104, 5268 (2007). 10.1073/pnas.0607254104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes D. R., Iossifidis D., Auroux P.-A., and Manz A., Anal. Chem. 74, 2623 (2002). 10.1021/ac0202435 [DOI] [PubMed] [Google Scholar]
- Auroux P.-A., Iossifidis D., Reyes D. R., and Manz A., Anal. Chem. 74, 2637 (2002). 10.1021/ac020239t [DOI] [PubMed] [Google Scholar]
- Bange A., Halsall H. B., and Heineman W. R., Biosens. Bioelectron. 20, 2488 (2005). 10.1016/j.bios.2004.10.016 [DOI] [PubMed] [Google Scholar]
- Hong J. W. and Quake S. R., Nat. Biotechnol. 21, 1179 (2003). 10.1038/nbt871 [DOI] [PubMed] [Google Scholar]
- Lagally E. T., Simpson P. C., and Mathies R. A., Sens. Actuators B 63, 138–146 (2000). 10.1016/S0925-4005(00)00350-6 [DOI] [Google Scholar]
- Huh D., Gu W., Kamotani Y., Grotberg J. B., and Takayama S., Physiol. Meas. 26, R73 (2005). 10.1088/0967-3334/26/3/R02 [DOI] [PubMed] [Google Scholar]
- Fortier M.-H., Bonneil E., Goodley P., and Thibault P., Anal. Chem. 77, 1631 (2005). 10.1021/ac048506d [DOI] [PubMed] [Google Scholar]
- Bernard A., Michel B., and Delamarche E., Anal. Chem. 73, 8 (2001). 10.1021/ac0008845 [DOI] [PubMed] [Google Scholar]
- Cesaro-Tadic S., Dernick G., Juncker D., Buurman G., Kropshofer H., Michel B., Fattinger C., and Delamarche E., Lab Chip 4, 563 (2004). 10.1039/b408964b [DOI] [PubMed] [Google Scholar]
- Das T., Meunier L., Barbe L., Provencher D., Guenat O., Gervais T., and Mes-Masson A., Biomicrofluidics 7, 011805 (2013). 10.1063/1.4774309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamme N. and Wilhelm C., Lab Chip 6, 974 (2006). 10.1039/b604542a [DOI] [PubMed] [Google Scholar]
- Zaytseva N. V., Montagna R. A., and Baeumner A. J., Anal. Chem. 77, 7520 (2005). 10.1021/ac0509206 [DOI] [PubMed] [Google Scholar]
- Wang C.-H., Lien K.-Y., Wang T.-Y., Chen T.-Y., and Lee G.-B., Biosens. Bioelectron. 26, 2045 (2011). 10.1016/j.bios.2010.08.083 [DOI] [PubMed] [Google Scholar]
- Lien K.-Y., Lin J.-L., Liu C.-Y., Lei H.-Y., and Lee G.-B., Lab Chip 7, 868 (2007). 10.1039/b700516d [DOI] [PubMed] [Google Scholar]
- Augustsson P., Malm J., and Ekstrom S., Biomicrofluidics 6, 034115 (2012). 10.1063/1.4749289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng C.-H., Hsieh I.-S., Hung L.-Y., Lin H.-I., Shiesh S.-C., Chen Y.-L., and Lee G.-B., Microfluid. Nanofluid. 14(3-4), 753 (2013). 10.1007/s10404-012-1095-3 [DOI] [Google Scholar]
- Chang W.-H., Yang S.-Y., Wang C.-H., Tsai M.-A., Wang P.-C., Chen T.-Y., Chen S.-C., and Lee G.-B., “Rapid isolation and detection of aquaculture pathogens in an integrated microfluidic system using loop-mediated isothermal amplification,” Sens. Actuators B (in press). 10.1016/j.snb.2011.12.054 [DOI]
- Cui J. and Pan T., J. Micromech. Microeng. 21, 065034 (2011). 10.1088/0960-1317/21/6/065034 [DOI] [Google Scholar]
- See supplementary material at http://dx.doi.org/10.1063/1.4794974 for supporting text and figure.
- Yang S.-Y., Lin J.-L., and Lee G.-B., J. Micromech. Microeng. 19, 035020 (2009). 10.1088/0960-1317/19/3/035020 [DOI] [Google Scholar]
- Unger M. A., Chou H.-P., Thorsen T., Scherer A., and Quake S. R., Science 288, 113 (2000). 10.1126/science.288.5463.113 [DOI] [PubMed] [Google Scholar]
- Adams N. M., Creecy A. E., Majors C. E., Wariso B. A., Short P. A., Wright D. W., and Haselton F. R., Biomicrofluidics 7, 014104 (2013). 10.1063/1.4788922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eteshola E. and Leckband D., Sens. Actuators B 72, 129 (2001). 10.1016/S0925-4005(00)00640-7 [DOI] [Google Scholar]
- Wu D., Zhao B., Dai Z., Qin J., and Lin B., Lab Chip 6, 942 (2006). 10.1039/b600765a [DOI] [PubMed] [Google Scholar]