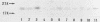Abstract
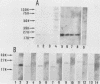
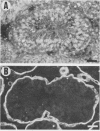
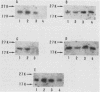
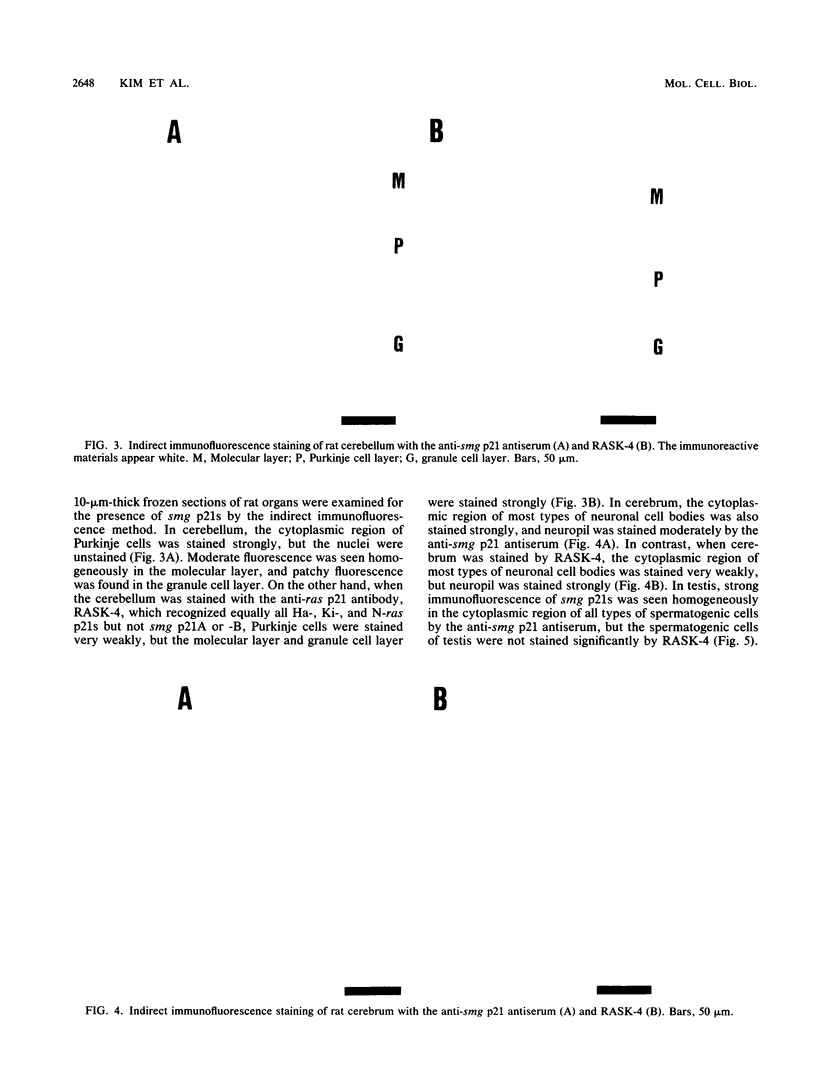
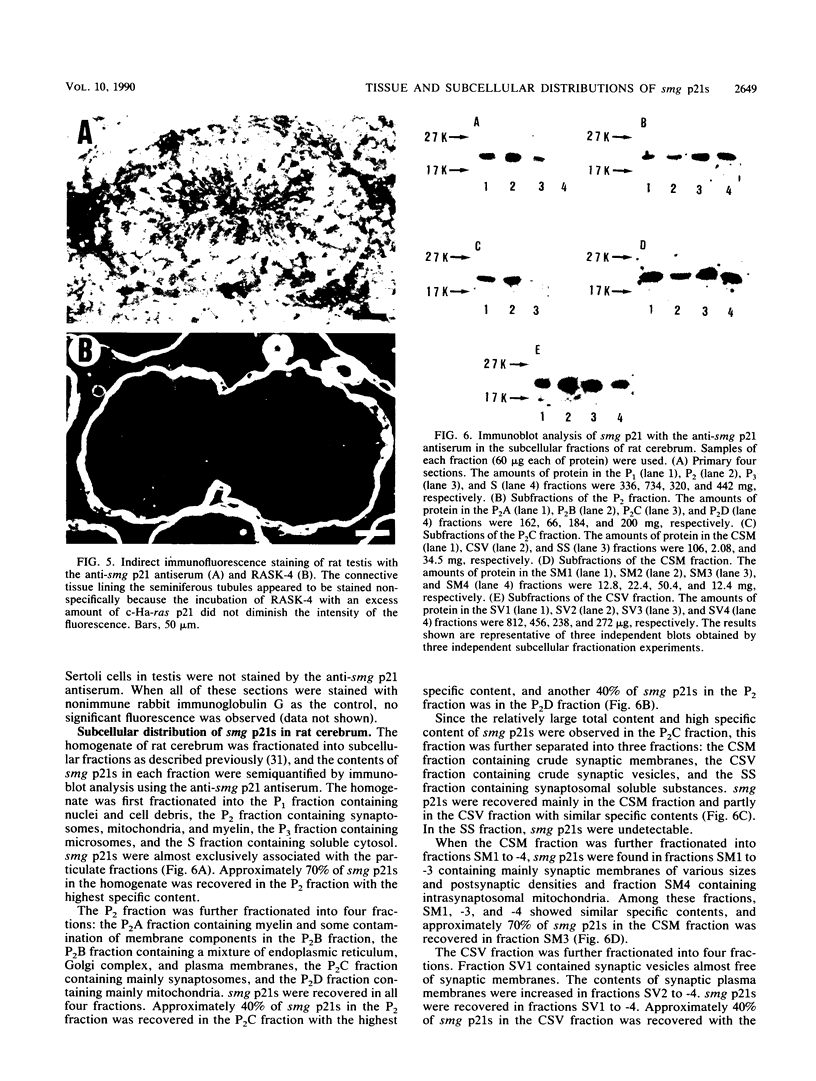
We have made a specific antiserum recognizing both smg p21A (the rap1A/Krev-1 protein) and -B (the rap1B protein), ras p21-like GTP-binding proteins having the same putative effector domain as ras p21s and have used this antiserum to study the tissue and subcellular distributions of smg p21s by immunoblot and immunocytochemical analyses. By immunoblot analysis, smg p21s were detected in various rat tissues and at the highest level in brain. By light microscopic immunocytochemical analysis, smg p21s were also detected in various rat tissues. Particularly, smg p21s in brain were found abundantly in the cytoplasmic region of most types of neuronal cell bodies and moderately in neuropil, whereas c-ras p21s were found more abundantly in neuropil than in the cytoplasmic region of most types of neuronal cell bodies. smg p21s in testis were found in spermatogenic cells, in which c-ras p21s were not significantly detected. By subcellular fractionation analysis of cerebrum, smg p21s were detected in all of the particulate fractions but not in the cytosol fraction. Among the particulate fractions, approximately 70% of smg p21s was recovered with the highest specific content in the fraction containing mainly synaptosomes, mitochondria, and myelin. In further fractionation of this fraction, approximately 40% of smg p21s was recovered in each of the synaptosome fraction and the mitochondrial fraction. This subcellular distribution of smg p21s in cerebrum was partly distinct from that of c-ras p21s, which were mainly recovered in the synaptosome and microsome fractions but present at very low levels in the mitochondrial fraction. These tissue and subcellular distributions of smg p 21s together with the fact that smg p21s have the same putative effector domain as ras p21s exert their own specific actions in addition to the actions similar or antagonistic to those of c-ras p21s.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Katada T., Northup J. K., Ui M., Gilman A. G. Purification and properties of the inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. J Biol Chem. 1984 Mar 25;259(6):3560–3567. [PubMed] [Google Scholar]
- Cotman C. W., Taylor D. Isolation and structural studies on synaptic complexes from rat brain. J Cell Biol. 1972 Dec;55(3):696–711. doi: 10.1083/jcb.55.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubruck H., Disela C., Wagner P., Gallwitz D. The ras-related ypt protein is an ubiquitous eukaryotic protein: isolation and sequence analysis of mouse cDNA clones highly homologous to the yeast YPT1 gene. EMBO J. 1987 Dec 20;6(13):4049–4053. doi: 10.1002/j.1460-2075.1987.tb02750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshijima M., Kikuchi A., Kawata M., Ohmori T., Hashimoto E., Yamamura H., Takai Y. Phosphorylation by cyclic AMP-dependent protein kinase of a human platelet Mr 22,000 GTP-binding protein (smg p21) having the same putative effector domain as the ras gene products. Biochem Biophys Res Commun. 1988 Dec 30;157(3):851–860. doi: 10.1016/s0006-291x(88)80953-7. [DOI] [PubMed] [Google Scholar]
- Hoshijima M., Kondo J., Kikuchi A., Yamamoto K., Takai Y. Purification and characterization from bovine brain membranes of a GTP-binding protein with a Mr of 21,000, ADP-ribosylated by an ADP-ribosyltransferase contaminated in botulinum toxin type C1--identification as the rhoA gene product. Brain Res Mol Brain Res. 1990 Jan;7(1):9–16. doi: 10.1016/0169-328x(90)90067-n. [DOI] [PubMed] [Google Scholar]
- Jahn R., Schiebler W., Ouimet C., Greengard P. A 38,000-dalton membrane protein (p38) present in synaptic vesicles. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4137–4141. doi: 10.1073/pnas.82.12.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawata M., Kawahara Y., Araki S., Sunako M., Tsuda T., Fukuzaki H., Mizoguchi A., Takai Y. Identification of a major GTP-binding protein in bovine aortic smooth muscle membranes as smg p21, a GTP-binding protein having the same effector domain as ras p21s. Biochem Biophys Res Commun. 1989 Sep 29;163(3):1418–1427. doi: 10.1016/0006-291x(89)91137-6. [DOI] [PubMed] [Google Scholar]
- Kawata M., Kikuchi A., Hoshijima M., Yamamoto K., Hashimoto E., Yamamura H., Takai Y. Phosphorylation of smg p21, a ras p21-like GTP-binding protein, by cyclic AMP-dependent protein kinase in a cell-free system and in response to prostaglandin E1 in intact human platelets. J Biol Chem. 1989 Sep 15;264(26):15688–15695. [PubMed] [Google Scholar]
- Kawata M., Matsui Y., Kondo J., Hishida T., Teranishi Y., Takai Y. A novel small molecular weight GTP-binding protein with the same putative effector domain as the ras proteins in bovine brain membranes. Purification, determination of primary structure, and characterization. J Biol Chem. 1988 Dec 15;263(35):18965–18971. [PubMed] [Google Scholar]
- Kikuchi A., Yamashita T., Kawata M., Yamamoto K., Ikeda K., Tanimoto T., Takai Y. Purification and characterization of a novel GTP-binding protein with a molecular weight of 24,000 from bovine brain membranes. J Biol Chem. 1988 Feb 25;263(6):2897–2904. [PubMed] [Google Scholar]
- Kim S., Kikuchi A., Mizoguchi A., Takai Y. Intrasynaptosomal distribution of the ras, rho and smg-25A GTP-binding proteins in bovine brain. Brain Res Mol Brain Res. 1989 Nov;6(2-3):167–176. doi: 10.1016/0169-328x(89)90051-x. [DOI] [PubMed] [Google Scholar]
- Kitayama H., Sugimoto Y., Matsuzaki T., Ikawa Y., Noda M. A ras-related gene with transformation suppressor activity. Cell. 1989 Jan 13;56(1):77–84. doi: 10.1016/0092-8674(89)90985-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsui Y., Kikuchi A., Kawata M., Kondo J., Teranishi Y., Takai Y. Molecular cloning of smg p21B and identification of smg p21 purified from bovine brain and human platelets as smg p21B. Biochem Biophys Res Commun. 1990 Jan 30;166(2):1010–1016. doi: 10.1016/0006-291x(90)90911-6. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A., Kim S., Ueda T., Takai Y. Tissue distribution of smg p25A, a ras p21-like GTP-binding protein, studied by use of a specific monoclonal antibody. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1438–1445. doi: 10.1016/0006-291x(89)90835-8. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A., Ueda T., Ikeda K., Shiku H., Mizoguti H., Takai Y. Localization and subcellular distribution of cellular ras gene products in rat brain. Brain Res Mol Brain Res. 1989 Jan;5(1):31–44. doi: 10.1016/0169-328x(89)90015-6. [DOI] [PubMed] [Google Scholar]
- Nagata K., Itoh H., Katada T., Takenaka K., Ui M., Kaziro Y., Nozawa Y. Purification, identification, and characterization of two GTP-binding proteins with molecular weights of 25,000 and 21,000 in human platelet cytosol. One is the rap1/smg21/Krev-1 protein and the other is a novel GTP-binding protein. J Biol Chem. 1989 Oct 15;264(29):17000–17005. [PubMed] [Google Scholar]
- Nagata K., Nagao S., Nozawa Y. Low Mr GTP-binding proteins in human platelets: cyclic AMP-dependent protein kinase phosphorylates m22KG(I) in membrane but not c21KG in cytosol. Biochem Biophys Res Commun. 1989 Apr 14;160(1):235–242. doi: 10.1016/0006-291x(89)91646-x. [DOI] [PubMed] [Google Scholar]
- Ohmori T., Kikuchi A., Yamamoto K., Kim S., Takai Y. Small molecular weight GTP-binding proteins in human platelet membranes. Purification and characterization of a novel GTP-binding protein with a molecular weight of 22,000. J Biol Chem. 1989 Jan 25;264(3):1877–1881. [PubMed] [Google Scholar]
- Pizon V., Chardin P., Lerosey I., Olofsson B., Tavitian A. Human cDNAs rap1 and rap2 homologous to the Drosophila gene Dras3 encode proteins closely related to ras in the 'effector' region. Oncogene. 1988 Aug;3(2):201–204. [PubMed] [Google Scholar]
- Pizon V., Lerosey I., Chardin P., Tavitian A. Nucleotide sequence of a human cDNA encoding a ras-related protein (rap1B). Nucleic Acids Res. 1988 Aug 11;16(15):7719–7719. doi: 10.1093/nar/16.15.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn M. T., Parkos C. A., Walker L., Orkin S. H., Dinauer M. C., Jesaitis A. J. Association of a Ras-related protein with cytochrome b of human neutrophils. Nature. 1989 Nov 9;342(6246):198–200. doi: 10.1038/342198a0. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C., Robishaw J. D. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984 Nov 25;259(22):13806–13813. [PubMed] [Google Scholar]
- Takai Y., Kaibuchi K., Tsuda T., Hoshijima M. Role of protein kinase C in transmembrane signaling. J Cell Biochem. 1985;29(2):143–155. doi: 10.1002/jcb.240290209. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Ida N., Waki C., Shimoda H., Slamon D. J., Cline M. J. Cell type-specific expressions of c-ras gene products in the normal rat. Mol Cell Biochem. 1987 May;75(1):23–32. doi: 10.1007/BF00231605. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T., Greengard P., Berzins K., Cohen R. S., Blomberg F., Grab D. J., Siekevitz P. Subcellular distribution in cerebral cortex of two proteins phosphorylated by a cAMP-dependent protein kinase. J Cell Biol. 1979 Nov;83(2 Pt 1):308–319. doi: 10.1083/jcb.83.2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker V. P., Michaelson I. A., Kirkland R. J. The separation of synaptic vesicles from nerve-ending particles ('synaptosomes'). Biochem J. 1964 Feb;90(2):293–303. doi: 10.1042/bj0900293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Kondo J., Hishida T., Teranishi Y., Takai Y. Purification and characterization of a GTP-binding protein with a molecular weight of 20,000 in bovine brain membranes. Identification as the rho gene product. J Biol Chem. 1988 Jul 15;263(20):9926–9932. [PubMed] [Google Scholar]
- Yamashita T., Yamamoto K., Kikuchi A., Kawata M., Kondo J., Hishida T., Teranishi Y., Shiku H., Takai Y. Purification and characterization of c-Ki-ras p21 from bovine brain crude membranes. J Biol Chem. 1988 Nov 15;263(32):17181–17188. [PubMed] [Google Scholar]