Abstract
Cyclo-oxygenase (COX) enzymes are responsible for the formation from arachidonic acid of prostaglandins, among other metabolites. Prior studies have suggested that inhibition of the COX pathway attenuates the responses of sympathetic nerve activity and blood pressure during static muscle contraction. Static muscle contraction activates the exercise pressor reflex, which in turn increases sympathetic nerve activity and blood pressure. Also, COX products contribute to exaggeration of the exercise pressor reflex in heart failure (HF). This dysfunction of the exercise pressor reflex has previously been shown to be mediated primarily by muscle mechanoreflex overactivity. It is well known that COX-1 and COX-2 are two isoforms of the enzyme that lead to formation of these important biological mediators involved in the muscle reflex. Thus, in the present study, we determined whether the COX-1 and/or COX-2 pathway contribute(s) to the augmented mechanoreflex activity in HF. First, Western blot analysis was employed to examine protein expression of COX-1 and COX-2 in skeletal muscle tissue of control rats and rats with HF induced by myocardial infarction. Our data show that there is no significant difference in COX-1 expression in both experimental groups. However, COX-2 displays significant overexpression in rats with HF compared with control rats (optical density 1.06 ± 0.05 in control and 1.6 ± 0.05 in HF, P < 0.05 versus control). Second, the mechanoreflex was evoked by passive tendon stretch, and the reflex sympathetic and pressor responses to muscle stretch were examined after COX-1 and COX-2 inhibitors (FR-122047 and SC-236) were individually injected into the arterial blood supply of the hindlimb muscles. The results demonstrate that the stretch-evoked reflex responses in rats with HF were significantly attenuated by administration of SC-236, but not by FR-122047, i.e. renal sympathetic nerve activity and mean arterial pressure responses evoked by 0.5 kg of muscle tension were 52.3 ± 8.9% and 19 ± 1.4 mmHg, respectively, in control conditions and 26.4 ± 5.6% and 5.7 ± 1.6 mmHg (P < 0.05 versus control group) after 0.25 mg kg−1 of SC-236. Muscle stretch-evoked renal sympathetic nerve activity and mean arterial pressure responses were 51.8 ± 8.2% and 18.7 ± 1.2 mmHg, respectively, in control conditions and 48.3 ± 5.3% and 17.5 ± 1.9 mmHg (P > 0.05 versus control group) after 1.0 mg kg−1 of FR-122047. Accordingly, the results obtained from this study support our hypothesis that heightened COX-2 expression within the hindlimb muscles contributes to the exaggerated muscle mechanoreflex in congestive HF.
Two neural mechanisms are suggested to evoke sympathetic nerve and cardiovascular responses during exercise. The first, referred to as the ‘exercise pressor reflex’, is evoked by mechanical and metabolic stimuli that activate thin-fibre muscle afferents in the working muscle (McCloskey & Mitchell, 1972; Mitchell et al. 1983; Kaufman & Forster, 1996). Thus, the exercise pressor reflex has two functional components, namely the muscle mechanoreflex and metaboreflex. Specifically, most myelinated group III afferent nerves are stimulated by a mechanical deformation of the muscle afferent receptive field; and most unmyelinated group IV afferent nerves are activated by muscle byproducts (Kaufman et al. 1983, 1984a,b, Kaufman & Forster, 1996). Consequently, the brainstem nuclei that regulate cardiovascular activities are stimulated, leading to an increase in sympathetic nerve activity (SNA) and arterial blood pressure (BP; Mitchell et al. 1983; Kaufman & Forster, 1996). The second neural mechanism, termed ‘central command’, originates in the higher brain and is involved with motor and cardiovascular regulation through autonomic control during exercise (Goodwin et al. 1972; Waldrop et al. 1996). Additionally, the sympathetic and cardiovascular responses to exercise are modulated by the arterial baroreflex (Potts & Li, 1998; Fadel et al. 2001).
Cyclo-oxygenase (COX) is the enzyme responsible for the formation of prostaglandins from arachidonic acid (Smith et al. 2000). Prior studies demonstrated that COX pathways play an important role in regulating the exercise pressor reflex in human and animal models (Stebbins et al. 1986; Rotto et al. 1990a; Davy et al. 1993; Fontana et al. 1995; Scott et al. 2002; Middlekauff & Chiu, 2004; Hayes et al. 2006; Cui et al. 2007, 2008; Middlekauff et al. 2008). For example, static exercise increases production of arachidonic acid and prostaglandins in active muscles (Rotto et al. 1989; Symons et al. 1991). An inhibition of COX activities attenuates SNA and cardiovascular responses to static exercise in humans and cats (Stebbins et al. 1986; Davy et al. 1993; Hayes et al. 2006; Cui et al. 2008). Notably, an important work demonstrated that blocking COX pathways attenuates the discharge of group III and group IV muscle afferents during dynamic exercise in cats (Hayes et al. 2006). In this regard, arachidonic acid and the COX product, prostaglandins, are thought to sensitize muscle afferents in modulating the exercise pressor reflex (Rotto et al. 1990b; Middlekauff & Chiu, 2004; Middlekauff et al. 2008).
Congestive heart failure (HF) is a chronic condition characterized by the inadequate function of the heart to deliver an oxygen-rich blood supply to metabolizing tissues. Prior studies have shown that SNA during activation of the muscle pressor reflex is augmented in human and animal models with HF (Scott et al. 2002; Smith et al. 2006; Koba et al. 2008b; Middlekauff et al. 2008). This reflex dysfunction has been previously shown to be mediated primarily by muscle mechanoreflex overactivity (Li et al. 2004; Sinoway & Li, 2005; Smith et al. 2006). Although the specific role of the COX product, prostaglandins, in sensitizing group III and group IV muscle afferents needs to be determined in HF, the levels of prostaglandin E2 (PGE2) in active skeletal muscle have been studied in chronic HF patients (Scott et al. 2002, 2004). The results of these studies show that during rhythmic hand-grip exercise, the intramuscular concentration of PGE2 is greater in HF patients than in healthy subjects (Scott et al. 2002). Also, there is a close relationship between PGE2 and the ventilatory response to static exercise (Scott et al. 2002). Another study further demonstrates that blocking the production of COX products using indomethacin markedly attenuates the increase in SNA directed to skeletal muscles during light-intensity, rhythmic hand-grip exercise in healthy subjects and HF patients (Middlekauff et al. 2008). Light-intensity, rhythmic hand grip used in this previous study particularly activates muscle mechanoreceptors. In addition, our recent study shows that COX blockade attenuates muscle mechanoreflex-increased renal SNA to a greater degree in rats with HF than in control rats (Koba et al. 2008a). Taken together, these published data suggest that COX products contribute to the exaggerated SNA when the muscle mechanoreceptor component of the exercise pressor reflex is activated in HF.
The two isoforms COX-1 and COX-2 are responsible for the formation of the important biological mediators, prostaglandins (Vane et al. 1998). These isoforms of COX are likely to play differential roles in regulating pathological mechanisms in the conditions of HF (Wong et al. 1998; Abassi et al. 2001; Ohashi et al. 2009). Accordingly, the purpose of this study was to determine whether the COX-1 and/or COX-2 pathway contribute(s) to the exaggerated muscle mechanoreflex in HF. Heart failure was induced by myocardial infarction (MI) following ligation of the coronary artery. We examined protein expression of COX-1 and COX-2 in the hindlimb muscle tissue of rats with HF (‘HF rats’) versus control rats. In addition, we examined sympathetic and cardiovascular responses to stimulation of mechanically sensitive muscle afferent nerves evoked by passive tendon stretch in HF rats and in control rats while COX-1 and COX-2 pathways were inhibited using their respective antagonists.
Methods
All procedures outlined in this study were performed in compliance with the rules and regulations described in the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 1996). These procedures were approved by the Institutional Animal Care and Use Committee of Pennsylvania State University College of Medicine. Male Sprague–Dawley rats were housed in standard rat cages and regulated on a 12 h–12 h light–dark schedule, with food and water available ad libitum. At the end of each experiment, animals were humanely killed by intravenous injection of sodium pentobarbital (120 mg kg−1), followed by 2 ml of a saturated solution of KCl. Note that the animals had been decerebrated before the KCl was given.
Coronary artery ligation
Twenty-four rats (150–200 g) were anaesthetized by inhalation of an isoflurane–oxygen mixture (2–5% isoflurane in 100% oxygen), intubated and artificially ventilated. A left thoracotomy between the fourth and fifth ribs was performed, exposing the left ventricular wall. Then, the left coronary artery was ligated. Experiments were performed 8–10 weeks after the coronary ligation. Rats matched for age and body weight served as control animals.
Transthoracic echocardiography was performed 1–2 weeks before the experiments. The rats were anaesthetized by inhalation of an isoflurane–oxygen mixture. The transducer was positioned on the left anterior chest, and left ventricular dimensions were measured. The left ventricular fractional shortening (FS) was determined by echocardiographic measurements. On the basis of FS data, the animals with FS < 30% were considered HF rats. Note that rats with FS < 30% show increases in heart weight and left ventricular end-diastolic pressure, and MI size >35% of the left ventricular area (Li et al. 2004; Gao et al. 2007; Xing et al. 2007; Koba et al. 2008b). In all control animals, FS was >40% in this study.
Immunofluorescence
Three control rats and three HF rats were anaesthetized with an isoflurane–oxygen mixture and then the white portion of gastrocnemius muscle was removed. The tissues were fixed in a 1:1 acetone and methanol solution, and permeabilized in PBS with 0.5% Triton X-100 for 10 min at room temperature. A cryostat was used to obtain muscle sections (10 μm thick).
The sections were incubated with the rabbit polyclonal anti-COX-1/COX-2 antibody (1:200 dilution; Cayman Chemical, Ann Arbor, MI, USA) overnight at 4°C. After being washed in PBS, the sections were incubated with the goat anti-rabbit Alexa Fluor 488-labelled secondary antibody (1:200 dilution; Invitrogen, Carlsbad, CA, USA) for 2 h at room temperature. Thereafter, the sections were washed in PBS and coverslipped. Fluorescence-labelled muscle tissues were examined using a Nikon Eclipse 80i microscope with appropriate filters, and the images were stored digitally on a computer.
Western blot preparation and analysis
In three control rats and three HF rats, the white portion of the gastrocnemius muscle was obtained for Western blot analysis. The muscle tissues were homogenized in ice-cold buffer containing 20 mM Hepes, 1.5 mM MgCl2, 0.2 mM EDTA, 0.1 M NaCl and 0.2 mM DTT, supplemented with protease inhibitors (Sigma-Aldrich, St Louis, MO, USA), followed by addition of NaCl to a final concentration of 0.45 M. The supernatant was collected after being centrifuged at 20,000g for 15 min at 4°C, and glycerol was added to a final concentration of 20%. The protein concentration was determined using a BCA protein assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA) and bovine serum albumin as standard. Equal amounts of protein (100 μg) were subjected to NuPAGE Bis-Tris (4–20%) gel electrophoresis (Invitrogen) and electrotransferred to a hydrophobic polyvinylidene difluoride membrane (GE Water & Process Technologies, Gloucester, MA, USA). After blocking with 10% non-fat milk in PBS containing 0.1% Tween 20 for 1 h at room temperature, the membrane was incubated overnight at 4°C with mouse polyclonal anti-COX-1 (murine)/anti-COX-2 (murine; Cayman Chemical) and glyceraldehyde 3-phosphate dehydrogenase (GADPH; Sigma-Aldrich) as loading control. After washing twice with PBS containing 0.1% Tween 20, the membrane was incubated at room temperature for 3 h with secondary anti-mouse IgG horse-radish peroxidase-conjugated antibodies (GE Healthcare, Piscataway, NJ, USA). Antigen–antibody complexes were visualized by SuperSignal® West Pico Chemiluminescent Substrate (Pierce Biotechnology, Inc.). The densities of COX-1, COX-2 and GADPH bands were determined using the NIH Scion image software (Frederick, MD, USA).
Experimental preparation for the muscle reflex
The rats were anaesthetized by inhalation of an isoflurane–oxygen mixture (2–5% isoflurane in 100% oxygen). An endotracheal tube was inserted and attached to a ventilator (Model AWS; Hallowell EMC, Pittsfield, MA, USA). Polyethylene (PE-50) catheters were inserted into an external jugular vein and the common carotid arteries for the purposes of drug administration and measurement of arterial blood pressure, respectively. A continuous infusion of physiological saline (0.1 ml h−1) into the venous line was established by using a syringe pump (Medical Industries, Subiaco, West Australia). This maintained fluid balance and basal blood pressure. The femoral artery was carefully isolated in one hindlimb. An incision was made in the artery. A PE-10 catheter was inserted into the femoral artery so that drugs could be injected into the arterial blood supply of the hindlimb muscles of the leg. As there is collateral flow to maintain limb perfusion, a catheter inserted into the femoral artery is unlikely to occlude the circulation of the limb being tested. The skin covering the triceps surae muscle and femoral region was surgically separated from the muscle below to eliminate inputs from cutaneous afferents in the hindlimb.
The animals were artificially ventilated, and tidal CO2 was monitored with a respiratory gas monitor (Model 5250; Datex-Ohmeda, Madison, WI, USA) and maintained within the normal range, as previously described (Li et al. 2004; Gao et al. 2007; Xing et al. 2007; Koba et al. 2008b). Body temperature was monitored using a rectal thermometer (ML295, AD Instrument), and was carefully maintained at 37.5–38.5°C by a heating pad and external heating lamps. Arterial blood pressure was measured by connecting the carotid arterial catheter to a pressure transducer (model 12C; Grass Instruments, West Warwick, RI, USA). Mean arterial pressure (MAP) was obtained by integrating the arterial signal with a time constant of 4 s. Heart rate (HR) was determined from the arterial pressure pulse. The renal sympathetic nerve activity (RSNA) was recorded as previously described (Gao et al. 2008; Koba et al. 2008b). Briefly, a bundle of the renal nerves was carefully dissected from other connective tissues. A piece of laboratory film was placed under the isolated nerves, and two tips of a bipolar electrode to record neural activity were placed between the nerves and the film. These were embedded in a silicone gel. Once the gel had hardened, the silicone rubber was fixed to the surrounding tissue. The RSNA signal was amplified with an amplifier (P511; Grass Instruments) with a bandpass filter of 300 Hz as the low-cut frequency and 3 kHz as the high-cut frequency and made audible.
Decerebration was performed as previously described (Smith et al. 2001; Li et al. 2004; Gao et al. 2008). A transverse section was made anterior to the superior colliculus, extending ventrally to the mamillary bodies. The brain rostral to the section was then removed. This approach afforded the opportunity to examine the effect of reflex sympathetic and blood pressure responses to muscle stretch without considering the confounding effects of anaesthesia. Once the decerebration was completed, the anaesthetic agent was removed from the inhaled mixture. A recovery period of 60 min following decerebration was employed to allow sufficient time for elimination of the effects of the anaesthetic gas from the preparation.
Experimental protocols
It has been reported that COX products enhance the discharge rate of the mechanosensitive group III muscle afferents responding to static contraction and sensitize muscle afferents which modulate the exercise pressor reflex (Rotto et al. 1990b). This mechanism contributes to the exaggerated SNA in HF during activation of the muscle mechanoreflex (Cui et al. 2008; Koba et al. 2008a; Middlekauff et al. 2008). In the present experiments, therefore, we examined the specific effects of COX-1 and/or COX-2 pathways on RSNA and BP responses to stimulation of muscle mechanoreceptors evoked by passive tendon stretch. Muscle stretch (0.5 kg tension) was produced manually over ~5 s by using a rack and pinion attached to the Achilles’ tendon. Each bout of muscle stretch was maintained for 30 s after 0.5 kg of tension was achieved. In this experiment, we attempted to stimulate muscle mechanoreceptors and minimize engagement of other receptors (i.e. pain). Thus, 0.5 kg of tension was used, and this intervention was previously shown to generate sufficient RSNA and BP responses in rats (Gao et al. 2008). Muscle stretch was performed 5–10 min after arterial injection of 0.1, 0.5 and 1.0 mg (kg body weight)−1 of FR-122047 (Sigma Co.; COX-1 inhibitor; Wibberley et al. 2006) in eight control rats and in nine HF rats. Likewsie, 0.0625, 0.125 and 0.25 mg (kg body weight)−1 of SC-236 (Sigma Co.; COX-2 inhibitor; Francischi et al. 2002; You et al. 2003) was given to examine its effect on the reflex in nine control rats and in nine HF rats. It must be noted that it is possible that the drugs being injected accumulated, because they were given as a series of doses. Nevertheless, to examine this possibility, two saline injections were given as control and recovery, respectively. As the RSNA and BP responses returned to the control levels during recovery, the additive effects of the drugs were likely to be minimal. The injected volume was 0.1–0.15 ml and the duration of injections was 1 min. There was a 30 min resting period between bouts of muscle stretch.
Experimental data analysis
All measured variables of the muscle reflex were continuously recorded and stored on a computer. The responses of MAP and HR to muscle stretch were determined by the peak change from the baseline control value. The RSNA signals were transformed into absolute values, integrated over 1 s intervals, and subtracted from the 1 s integrated background noise. To quantify the RSNA response to experimental interventions, basal values were obtained by taking the mean value for the 30 s immediately before each intervention and by ascribing the mean value of 100%, and relative changes from baseline during and after the intervention were then evaluated.
All experimental data are presented as means ± SEM. Comparisons of variables for COX optical density were performed using Student’s paired t test, and comparisons of variables for MAP, HR and RSNA responses were performed using a two-way repeated-measures ANOVA, followed by Tukey’s post hoc test as appropriate. A value of P < 0.05 was considered significant. All statistic analysis was performed using SPSS for Windows 15.0 (SPSS Inc., Chicago, IL, USA).
Results
All echocardiographic measurements are shown in Table 1. Note that FS was >40% in all control animals (n = 23) and < 30% in all HF animals (n = 24) used in this study.
Table 1.
Echocardiographic measurements
| Control rats (n = 23) | HF rats (n = 24) | |
|---|---|---|
| LVDD (cm) | 0.85 ± 0.01 | 1.09 ± 0.03* |
| LVSD (cm) | 0.36 ± 0.02 | 0.82 ± 0.02* |
| Anterior wall thickness (cm) | 0.15 ± 0.01 | 0.09 ± 0.01* |
| Posterior wall thickness (cm) | 0.16 ± 0.01 | 0.15 ± 0.01 |
| FS (%) | 58 ± 3 | 25 ± 2* |
Values are means ± SEM.
Abbreviations: FS, left ventricular fractional shortening; HF rats, rats with heart failure; LVDD, left ventricular diastolic dimension; LVSD, left ventricular systolic dimension; and n, number of rats.
P < 0.05 versus control group.
Expression of COX-1 versus COX-2 in the white portion of the gastrocnemius muscle of control rats and HF rats
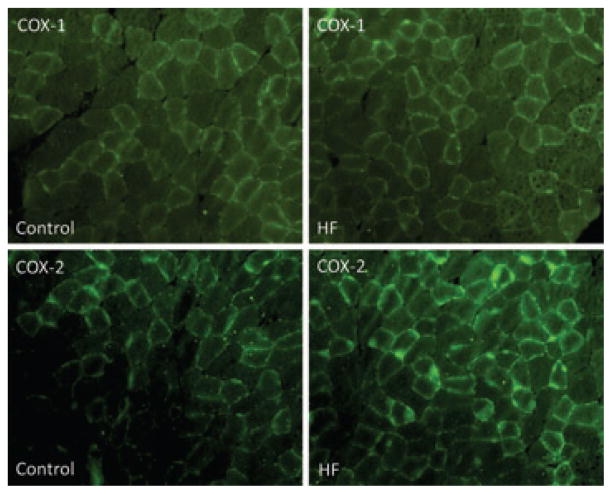
First, the protein expression of COX-1 and COX-2 in the white portion of the gastrocnemius muscle of control rats (n = 3) and HF rats (n = 3) was analysed using fluorescence immunocytochemistry methods. Figure 1 illustrates immunofluorescence images showing that COX-1 (top panels) and COX-2 (bottom panels) were localized in the muscles. Figure 1 also shows that similar COX-1 staining was observed in control rats and HF rats; however, COX-2 fluorescence staining was greater in HF rats than in control rats.
Figure 1. Immunostaining of COX-1 and COX-2.
Immunofluorescence methods were used to examine expression of cyclo-oxygenase (COX) isoforms COX-1 and COX-2 within the hindlimb muscles of control rats and rats with heart failure (HF rats). Top panels are typical images showing that COX-1 appears in the hindlimb muscles; the similar fluorescence staining is observed in control and HF rats. Bottom panels are typical immunofluorescence images showing that COX-2 is localized in the hindlimb muscles and that greater fluorescence staining is observed in HF than in control rats.
Second, Western blot assays were performed on the white portion of the gastrocnemius muscle from control rats (n = 3) and HF rats (n = 3). The results demonstrate that no significant differences were observed in the levels of COX-1 protein within the gastrocnemius muscle of control rats and HF rats (Fig. 2A). The optical density was 1.06 ± 0.06 in control and 1.13 ± 0.05 in HF (P > 0.05 versus control). This suggests that expression of the COX-1 pathway is probably unchanged in HF. However, there was a significant overexpression of COX-2 in the gastrocnemius muscles of HF rats compared with the control animals (Fig. 2B). The optical density was 1.06 ± 0.05 in control and 1.6 ± 0.05 in HF (P < 0.05 versus control). Thus, these data indicate that expression of COX-2 is augmented within the skeletal muscle after induction of HF.
Figure 2. Analysis of muscle COX-1 and COX-2 protein expression in control rats and HF rats.
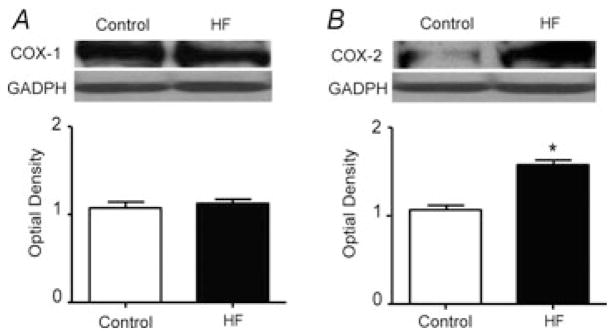
Western blot assays were performed on muscle tissues from control rats and HF rats. A, top panel shows representative bands of COX-1 expression. Bands of GADPH are used as a control for equal protein loading. The bottom panel shows average data. Results represent means ± SEM of n = 3. No significant difference was observed between control and HF groups. B, the results of Western blot assays illustrate that the optical density of COX-2 protein is higher in an HF rat than that in a control rat, shown in the top panel. The bottom panel shows average data. Significant COX-2 overexpression was seen in HF rats compared with control rats. Results represent means ± SEM of n = 3. *P < 0.05 compared with control.
Responses of RSNA and BP to muscle stretch following inhibition of COX-1 and COX-2
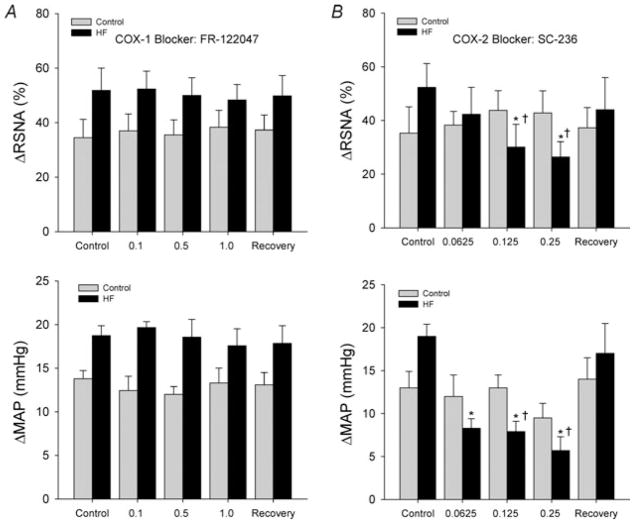
Baseline values for MAP and HR before arterial injections of FR-122047 are presented in Table 2. No significant differences were observed in baseline MAP and HR in control rats (n = 8) and HF rats (n = 9). Figure 3A shows RSNA and MAP responses to muscle stretch after inhibition of COX-1 by arterial injection of FR-122047 in control rats and HF rats. First, RSNA and MAP responses to tendon stretch were examined prior to COX-1 inhibition, showing significantly higher ΔRSNA and ΔMAP in the HF group versus the control group. Inhibition of the COX-1 isomer with FR-122047 did not elicit significant changes in RSNA and MAP responses during muscle stretch in either the control rats or the HF animals. In HF rats, RSNA and MAP responses induced by 0.5 kg of muscle tension were 51.8 ± 8.2% and 18.7 ± 1.2 mmHg, respectively in control conditions and 48.3 ± 5.3% and 17.5 ± 1.9 mmHg (P > 0.05 versus control) after 1.0 mg kg−1 of FR-122047. No significant HR responses were seen in the two groups during stretch. Original recordings shown in Fig. 4 illustrate the changes in the RSNA, BP and HR in response to tendon stretch with 0.5 kg of muscle tension in an HF rat after three interventions: saline control, arterial administrations of FR-122047 and recovery.
Table 2.
Baseline MAP and HR before arterial injection of FR-122047
| MAP (mmHg)
|
HR (beats min−1)
|
|||
|---|---|---|---|---|
| Control rats | HF rats | Control rats | HF rats | |
| Control conditions | 95 ± 6 | 97 ± 7 | 392 ± 10 | 386 ± 10 |
| FR-122047 0.1 mg kg−1 | 93 ± 10 | 89 ± 9 | 394 ± 8 | 385 ± 16 |
| FR-122047 0.5 mg kg−1 | 98 ± 9 | 96 ± 10 | 403 ± 12 | 395 ± 15 |
| FR-122047 1.0 mg kg−1 | 92 ± 8 | 97 ± 9 | 392 ± 8 | 380 ± 10 |
| Recovery | 95 ± 10 | 90 ± 6 | 392 ± 15 | 390 ± 10 |
Values are means ± SEM.
Abbreviations: HR, heart rate; and MAP, mean arterial pressure. There is no significant difference in basal values between the two groups. There were eight rats in the control group and nine in the HF group.
Figure 3. Changes of renal sympathetic nerve activity (RSNA) and mean arterial pressure (MAP) measured in response to muscle stretch after COX-1 inhibition by FR-122047 and after COX-2 inhibition by SC-236.
A, COX-1 blockade did not significantly influence sympathetic and blood pressure responses to muscle stretch in control and HF groups. Both control and HF groups displayed very little changes in overall RSNA and MAP responses after inhibition of the COX-1 isoform, maintaining steady ΔRSNA and ΔMAP after each successive dose and recovery period. The number of animals was eight in the control group and nine in the HF group. B, RSNA and MAP responses to muscle stretch were significantly attenuated in HF rats by COX-2 blockade, but not in control rats. SC-236 appears to have a dose-dependent effect. The most substantial attenuation of ΔRSNA and ΔMAP was observed in the HF group after administration of 0.25 mg kg−1 of SC-236 into the arterial blood supply of the hindlimb muscles. The responses of RSNA and MAP returned to their heightened pre-inhibitory state after a recovery period. No significant difference was observed in baseline MAP (before each injection) in control rats and HF rats. The reflex responses to muscle stretch prior to COX-2 inhibition with administration of SC-236 were higher in HF rats versus control animals. *P < 0.05 versus control. †P < 0.05, significant differences in changes in RSNA and MAP between the control group and HF group. The number of animals was nine in each group. Note that SC-236 attenuated the reflex responses in HF rats, but not in control rats.
Figure 4. Original recordings showing the changes in the RSNA, arterial blood pressure (BP), heart rate (HR) and muscle tension in control conditions, after arterial administrations of FR-122047 (1.0 mg kg−1; a COX-1 inhibitor) and during recovery in a rat with heart failure.
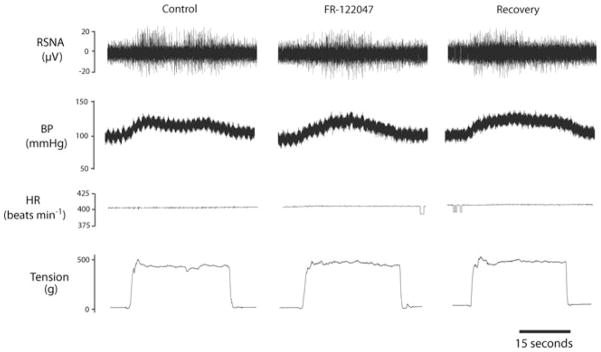
FR-122047 failed to attenuate the increases in RSNA, AP and HR elicited by muscle stretch. Note that the same level of tension was loaded in three interventions.
Table 3 shows baseline values for MAP and HR before arterial injections of SC-236. There were no significant differences in basal MAP and HR before injections in the control and HF groups (n = 9 in each group). Figure 3B illustrates RSNA and MAP responses to muscle stretch after inhibition of COX-2 by arterial injection of SC-236 in control rats and HF rats. The RSNA and MAP in response to muscle stretch were examined prior to COX-2 inhibition with SC-236, showing higher ΔRSNA and ΔMAP in HF rats versus control rats. Then, increasing doses of SC-236 were administered. Attenuation of RSNA and MAP responses to muscle stretch was observed in the HF group after successive doses of SC-236. The RSNA and MAP responses during muscle stretch were significantly attenuated in HF rats after 0.125 and 0.25 mg kg−1 of SC-236 were injected. The RSNA and MAP responses evoked by 0.5 kg of muscle tension were 52.3 ± 8.9% and 19 ± 1.4 mmHg, respectively, in control conditions, and 26.4 ± 5.6% and 5.7 ± 1.6 mmHg (P < 0.05 versus control group) after 0.25 mg kg−1 of SC-236. The RSNA and MAP responses of HF rats returned to their control levels after the recovery period. Note that control rats displayed no significant changes in RSNA and MAP responses after inhibition of the COX-2 isoform. There were no significant HR changes during tendon stretch in both groups. Original recordings shown in Fig. 5 demonstrate the reflex responses of RSNA, BP and HR evoked by 0.5 kg of muscle tension in an HF rat after the three interventions (saline control, arterial administrations of SC-236 and recovery) were conducted.
Table 3.
Baseline MAP and HR before arterial injection of SC-236
| MAP (mmHg)
|
HR (beats min−1)
|
|||
|---|---|---|---|---|
| Control rats | HF rats | Control rats | HF rats | |
| Control conditions | 105 ± 10 | 99 ± 7 | 405 ± 10 | 396 ± 15 |
| SC-236 0.0625 mg kg−1 | 103 ± 12 | 103 ± 9 | 404 ± 12 | 395 ± 12 |
| SC-236 0.125 mg kg−1 | 93 ± 9 | 96 ± 7 | 402 ± 18 | 405 ± 10 |
| SC-236 0.25 mg kg−1 | 95 ± 5 | 97 ± 10 | 389 ± 10 | 400 ± 15 |
| Recovery | 95 ± 15 | 107 ± 17 | 392 ± 9 | 388 ± 10 |
Values are means ± SEM. There is no significant difference in basal values between the two groups. There were nine rats in each group.
Figure 5. Original recordings showing that RSNA, BP and HR responses to muscle stretch were attenuated by the prior administration of SC-236 (0.25 mg kg−1) to inhibit activity of the COX-2 pathway.
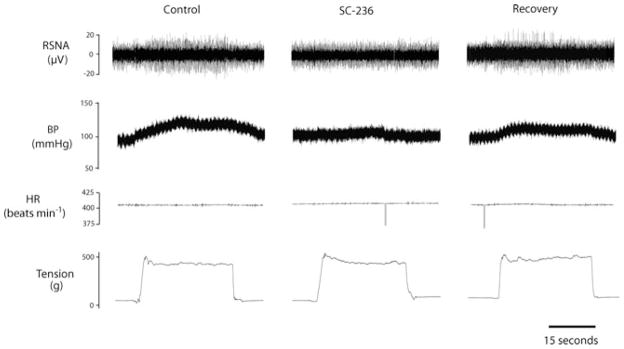
The data were obtained from a rat with heart failure. The reflex RSNA and AP responses were recovered to the levels of control 30 min after the end of the SC-236 intervention.
Discussion
In our previous study, we demonstrated that indomethacin, a blocker of COX, significantly attenuated the sympathetic responses to intermittent muscle contraction (an intervention is considered to stimulate mainly muscle mechanoreceptors) to a greater degree in HF rats compared with control rats (Koba et al. 2008a). This indicates that COX products contribute to the exaggerated SNA in HF during activation of the muscle mechanoreceptors. However, the isoforms of COX involved in the greater sympathetic responses evoked by the muscle mechanoreflex in HF were not specifically identified. Thus, the purpose of the present report was to determine the role played by the COX-1 and/or COX-2 pathways in abnormal SNA when the muscle mechanoreflex is activated in HF.
Expression of both COX-1 and COX-2 was localized in the white portion of the gastrocnemius muscle (Fig. 1). The effect of fibre-type composition on the cardiovascular response evoked by static muscle contraction has been studied previously (Wilson et al. 1995) and indicates that the reflex response evoked by static contraction of oxidative muscle (red portion) is smaller compared with the changes elicited by contraction of glycolytic muscle (white portion). In addition, data from our earlier study (Xing et al. 2008) further suggest that dorsal root ganglion neurons with nerve endings in the white muscle develop greater inward current responses to metabolic stimulation, such as capsaicin and acid. Thus, in this present study we examined the white portion of the gastrocnemius muscle, which contains mainly glycolytic muscle fibres.
Additionally, Western blot analysis demonstrated that there was no significant difference observed in the levels of COX-1 protein within the gastrocnemius muscles of control rats and HF rats (Fig. 2A). This result suggests that expression of the COX-1 pathway is probably unchanged following HF. In contrast, there was a significant overexpression of COX-2 in the gastrocnemius muscle of HF rats compared with control animals (Fig. 2B). Thus, these data indicate that expression of COX-2 is augmented within the muscles after induction of HF. In agreement with these findings, the results of our experiments examining the muscle mechanoreflex further demonstrated that COX-1 inhibition had no significant effects on RSNA and MAP responses to muscle stretch in both control and HF groups (Fig. 3A). Nevertheless, blocking COX-2 had a greater effect on RSNA and MAP responses to muscle stretch compared with blocking COX-1 (Fig. 3B). In particular, the effects were significantly increased in the HF group, with no significant effect noted in the control animals. The explanation for this result may be that in HF rats the muscle mechanoreflex is sensitized by overexpression of COX-2, to a greater degree. Thus, a larger attenuation in the reflex RSNA and BP responses seen after SC-236 administration in the HF rats is likely to be due to a greater reduction in the sensitizing effect of COX-2 products on activation of mechanosensitive afferents, compared with the control rats. The exaggerated exercise pressor reflex has been previously shown to be mediated primarily by muscle mechanoreflex overactivity in HF (Li et al. 2004; Sinoway & Li, 2005; Smith et al. 2006). Taken together, results obtained from the present report suggest that expression of COX-2 within the gastrocnemius muscles is heightened in congestive HF, thereby increasing mechanoreceptor sensitivity. This may make an important contribution to mechanoreceptor-mediated exaggerations in the exercise pressor reflex in HF.
The exercise pressor reflex is one of the neural mechanisms evoking the sympathetic nervous and cardiovascular responses to exercise (Kaufman & Forster, 1996; Sinoway & Li, 2005). The afferent arm of this reflex is composed of myelinated group III and unmyelinated group IV thin-fibre nerves, which respond to mechanical deformation of the muscle afferent receptive fields and metabolic stimulation (Kaufman et al. 1983, 1984a,b). Both groups of muscle afferents can be stimulated and/or sensitized by muscle metabolic byproducts, such as arachidonic acid, prostaglandins and ATP, produced by the working muscle (Rotto et al. 1990a,b; Li & Sinoway, 2002; Li et al. 2004; Hayes et al. 2006; Cui et al. 2007; Gao et al. 2007; Koba et al. 2008a). Arachidonic acid and its metabolites produced via the COX pathway have been reported to contribute to the cardiovascular reflexes evoked by stimulating thin-fibre muscle afferents during muscle contraction (Stebbins et al. 1986; Rotto et al. 1990a,b; Davy et al. 1993; Hayes et al. 2006). For example, Rotto et al. (1990a)) demonstrated that group IV muscle afferents were stimulated by injection of arachidonic acid into the arterial blood supply of the hindlimb of cats, an effect that could be greatly attenuated by blocking the activity of COX. Indomethacin, a COX inhibitor, has been reported to attenuate the exercise pressor reflex when it is administered into the hindlimb muscles of cats (Hayes et al. 2006). 20-Hydroxyeicosatetraenoic acid (20-HETE), a primarily metabolized product of arachidonic acid, has been shown to be converted to 20-OH-PGE2 by a COX-dependent mechanism (Roman, 2002). Gao et al. (2008) have demonstrated that arterial injections of 20-HETE can sensitize sympathetic and blood pressure responses evoked by stimulation of mechanically sensitive afferents during muscle stretch. Furthermore, this study demonstrated that indomethacin, a COX inhibitor, can significantly attenuate the sensitized sympathetic response evoked by injection of 20-HETE into the working muscle (Gao et al. 2008). Overall, these findings suggest that COX pathways mediate the reflex sympathetic and pressor responses to stimulation of muscle mechanoreceptors during exercise.
In HF, reflex sympathetic nerve and pressor responses to activation of the mechanoreceptor component of the exercise pressor reflex are enhanced (Li et al. 2004; Sinoway & Li, 2005; Smith et al. 2006; Koba et al. 2008b). In this disease, abnormal metabolic changes mediated by expression of the COX pathway are likely to play a role in stimulating and/or sensitizing certain pools of muscle afferents (Scott et al. 2002, 2004; Koba et al. 2008a; Middlekauff et al. 2008). COX-1 and COX-2 are two isoforms of the enzyme responsible for the formation of biologically important mediators (such as PGEs) that are demonstrated to invoke sympathetic nerve activity during exercise. Previous studies have found COX-2 protein in relatively large amounts in the tissues of HF subjects (Wong et al. 1998; Abassi et al. 2001), suggesting that COX-2 activity is generally greater in the disease. Also, COX-2 has a possible role in the pathology of HF, such as enhanced sympathetic outflows, heart inflammation and scar formation (Wong et al. 1998; Frans, 2007). Winaver and colleagues have demonstrated that the COX-2 isoform, rather than COX-1, is upregulated in the kidney of rats with experimental HF, in proportion to the severity of the disease (Abassi et al. 2001).
The results of the present study have demonstrated that protein expression of COX-2 is amplified within the gastrocnemius muscles of diseased animals compared with control rats. However, it was found that there is no significant difference in COX-1 expression in both experimental groups. These data indicate that there is a linkage between pathological processes in HF and the COX-2 overexpression observed within skeletal muscle tissues, in addition to the overexpression shown previously heart, kidney etc. (Wong et al. 1998; Abassi et al. 2001).
A recent study reported the effects of COX blockade with indomethacin on the muscle mechanoreceptor-induced sympathetic nervous response in a rat model of HF versus a healthy control group (Koba et al. 2008a). In that study, arterial injection of indomethacin attenuated the sympathetic nervous responses induced by the muscle mechanoreflex to a larger degree in HF animals than in the healthy control group. In the present study, the attenuation of the sympathetic and pressor responses to muscle stretch appeared to be greater in HF rats than in control animals when the COX-2 isoform specifically was inhibited. On the contrary, specific inhibition of the COX-1 isoform did not significantly affect reflex responses in either experimental group. These findings suggest a relationship between COX-2 and the exaggerated sympathetic and cardiovascular activities observed in HF rats during activation of the muscle mechanoreflex. Also, these data are congruent with the COX-2 overexpression found within the gastrocnemius muscles of the diseased animals in this report.
Exercise tolerance declines significantly in patients with HF, in part due to increased sympathetic nerve activity and thereby heightened vasoconstriction that forces the heart to overwork to compensate for the elevated oxygen demands of the metabolically active muscle (Minotti et al. 1991). Experimental evidence indicates that an increase in COX products in contracting muscles stimulates muscle afferent nerves and sensitizes afferents responding to contraction, thereby contributing to cardiorespiratory responses to exercise (Scott et al. 2002, 2004; Koba et al. 2008a; Middlekauff et al. 2008). The muscle reflex activated by contraction comprises the muscle mechanoreflex and metaboreflex (Kaufman & Forster, 1996; Sinoway & Li, 2005). Prior studies demonstrated attenuation of the muscle metaboreflex and hyperactivity of the mechanoreflex in rats with MI-induced chronic HF (Sinoway & Li, 2005; Smith et al. 2006). Additional support for this observation that muscle mechanoreceptor sensitivity is increased in HF patients was demonstrated by others (Middlekauff et al. 2008). The heightened muscle mechanoreflex responses observed in the diseased group can be attenuated, as demonstrated in our present study, by administering a specific COX-2 blocker. Taken together with these findings, the significance of our present investigation is that the COX-2 isoform plays a role in the exaggerated muscle mechanoreflex that leads to the augmented sympathetic nerve activity seen during exercise in congestive HF.
Some study limitations should be acknowledged here. First, the differences in expression of COX-1 and COX-2 in the white portion of the gastrocnemius muscle using immunocytochemistry were not quantified. Western blot analysis was used to examine the protein levels of muscle COX-1 and COX-2. No significant differences were observed in protein expression of COX-1 in the gastrocnemius muscle. Three animals were used in each group to analyse expression of COX-1 in this experiment, which could account for the lack of difference seen in COX-1 expression. Second, as the venous outflow from the limb was not occluded during injection of drugs in this study, the drugs delivered could have had a systemic effect and affected the reflex responses. Third, a prior study has examined the discharge rate of group III afferents during tendon stretch and static contraction (Hayes et al. 2005) and demonstrated that whilst there is some overlap, tendon stretch and static contraction stimulate somewhat different populations of group III mechanoreceptors. However, the discharge of the group III mechanoreceptors that responded to both tendon stretch and to static contraction was similar. Thus, tendon stretch used to evoke the mechanical component of the exercise pressor reflex in the present study represents activity of only part of the available mechanoreceptive afferent pool that would be available in exercise. Fourth, the results of the present study were obtained from a rat model of HF. The potential problems in translating this relatively ‘acute’ heart failure model to the human ‘chronic’ heart failure situation should be noted.
In summary, the data of this report support the hypothesis that the COX-2 isoform, but not COX-1, is overexpressed within the hindlimb muscles following induction of HF. Furthermore, specific inhibition of the COX-2 isoform significantly attenuates the augmented sympathetic nervous and blood pressure responses to muscle stretch in rats with MI-induced HF compared with control animals. In addition, the data demonstrate that inhibition of the COX-1 isoform did not significantly affect the muscle stretch-induced sympathetic and pressor responses in either experimental group, suggesting that COX-1 is unlikely to alter the muscle mechanoreflex directly. Notably, the role of COX-1 and COX-2 isoforms in sensitizing muscle mechanoreceptive afferents in a rat model of HF is congruent with their expression within the hindlimb muscles. Therefore, the present study has identified inducible COX-2 as a primary contributor to the over-reactive muscle mechanoreflex observed in HF.
Acknowledgments
The authors thank Chunying Yang for performing experiments that involved the recording of sympathetic nerve activity. Ariel Morales is from the School of Science, Technology and Engineering Management, St Thomas University, Miami, FL, USA and sponsored by the Penn State Hershey Summer Undergraduate Research Internship Program (SURIP). This study was supported by NIH R01 HL090720 and American Heart Association Established Investigator Award 0840130N.
References
- Abassi Z, Brodsky S, Gealekman O, Rubinstein I, Hoffman A, Winaver J. Intrarenal expression and distribution of cyclooxygenase isoforms in rats with experimental heart failure. Am J Physiol Renal Physiol. 2001;280:F43–F53. doi: 10.1152/ajprenal.2001.280.1.F43. [DOI] [PubMed] [Google Scholar]
- Cui J, McQuillan P, Momen A, Blaha C, Moradkhan R, Mascarenhas V, Hogeman C, Krishnan A, Sinoway LI. The role of the cyclooxygenase products in evoking sympathetic activation in exercise. Am J Physiol Heart Circ Physiol. 2007;293:H1861–H1868. doi: 10.1152/ajpheart.00258.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Moradkhan R, Mascarenhas V, Momen A, Sinoway L. Cyclooxygenase inhibition attenuates sympathetic responses to muscle stretch in humans. Am J Physiol Heart Circ Physiol. 2008;294:H2693–H2700. doi: 10.1152/ajpheart.91505.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy KP, Herbert WG, Williams JH. Effect of indomethacin on the pressor responses to sustained isometric contraction in humans. J Appl Physiol. 1993;75:273–278. doi: 10.1152/jappl.1993.75.1.273. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Ogoh S, Watenpaugh DE, Wasmund W, Olivencia-Yurvati A, Smith ML, Raven PB. Carotid baroreflex regulation of sympathetic nerve activity during dynamic exercise in humans. Am J Physiol Heart Circ Physiol. 2001;280:H1383–1390. doi: 10.1152/ajpheart.2001.280.3.H1383. [DOI] [PubMed] [Google Scholar]
- Fontana GA, Pantaleo T, Bongianni F, Cresci F, Lavorini F, Guerra CT, Panuccio P. Prostaglandin synthesis blockade by ketoprofen attenuates respiratory and cardiovascular responses to static handgrip. J Appl Physiol. 1995;78:449–457. doi: 10.1152/jappl.1995.78.2.449. [DOI] [PubMed] [Google Scholar]
- Francischi JN, Chaves CT, Moura ACL, Lima AS, Rocha OA, Ferreira-Alves DL, Bakhle YS. Selective inhibitors of cyclo-oxygenase-2 (COX-2) induce hypoalgesia in a rat paw model of inflammation. Br J Pharmacol. 2002;137:837–844. doi: 10.1038/sj.bjp.0704937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frans HL. Brain mechanisms contributing to sympathetic hyperactivity and heart failure. Circ Res. 2007;101:221–223. doi: 10.1161/CIRCRESAHA.107.158261. [DOI] [PubMed] [Google Scholar]
- Gao Z, Koba S, Sinoway L, Li J. 20-HETE increases renal sympathetic nerve activity via activation of chemically and mechanically sensitive muscle afferents. J Physiol. 2008;586:2581–2591. doi: 10.1113/jphysiol.2008.150730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Xing J, Sinoway L, Li J. P2X receptor-mediated muscle pressor reflex in myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;292:H939–H945. doi: 10.1152/ajpheart.00911.2006. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol. 1972;226:173–190. doi: 10.1113/jphysiol.1972.sp009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SG, Kindig AE, Kaufman MP. Comparison between the effect of static contraction and tendon stretch on the discharge of group III and IV muscle afferents. J Appl Physiol. 2005;99:1891–1896. doi: 10.1152/japplphysiol.00629.2005. [DOI] [PubMed] [Google Scholar]
- Hayes SG, Kindig AE, Kaufman MP. Cyclooxygenase blockade attenuates responses of group III and IV muscle afferents to dynamic exercise in cats. Am J Physiol Heart Circ Physiol. 2006;290:H2239–H2246. doi: 10.1152/ajpheart.01274.2005. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. Chapter 10. Oxford University Press; New York: 1996. pp. 381–447. [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol. 1984a;57:644–650. doi: 10.1152/jappl.1984.57.3.644. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Waldrop TG, Rybicki KJ, Ordway GA, Mitchell JH. Effects of static and rhythmic twitch contractions on the discharge of group III and IV muscle afferents. Cardiovasc Res. 1984b;18:663–668. doi: 10.1093/cvr/18.11.663. [DOI] [PubMed] [Google Scholar]
- Koba S, Xing J, Sinoway LI, Li J. Cyclooxygenase products contribute to enhanced muscle reflex in heart failure. FASEB J. 2008a;22 (Program no. 952.16) [Google Scholar]
- Koba S, Xing J, Sinoway LI, Li J. Sympathetic nerve responses to muscle contraction and stretch in ischemic heart failure. Am J Physiol Heart Circ Physiol. 2008b;294:H311–H321. doi: 10.1152/ajpheart.00835.2007. [DOI] [PubMed] [Google Scholar]
- Li J, Sinoway AN, Gao Z, Maile MD, Pu M, Sinoway LI. Muscle mechanoreflex and metaboreflex responses after myocardial infarction in rats. Circulation. 2004;110:3049–3054. doi: 10.1161/01.CIR.0000147188.46287.1B. [DOI] [PubMed] [Google Scholar]
- Li J, Sinoway LI. ATP stimulates chemically sensitive and sensitizes mechanically sensitive afferents. Am J Physiol Heart Circ Physiol. 2002;283:H2636–H2643. doi: 10.1152/ajpheart.00395.2002. [DOI] [PubMed] [Google Scholar]
- McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlekauff HR, Chiu J. Cyclooxygenase products sensitize muscle mechanoreceptors in healthy humans. Am J Physiol Heart Circ Physiol. 2004;287:H1944–H1949. doi: 10.1152/ajpheart.00329.2004. [DOI] [PubMed] [Google Scholar]
- Middlekauff HR, Chiu J, Hamilton MA, Fonarow GC, Maclellan WR, Hage A, Moriguchi J, Patel J. Cyclooxygenase products sensitize muscle mechanoreceptors in humans with heart failure. Am J Physiol Heart Circ Physiol. 2008;294:H1956–H1962. doi: 10.1152/ajpheart.01304.2007. [DOI] [PubMed] [Google Scholar]
- Minotti JR, Christoph I, Oka R, Weiner MW, Wells L, Massie BM. Impaired skeletal muscle function in patients with congestive heart failure. Relationship to systemic exercise performance. J Clin Invest. 1991;88:2077–2082. doi: 10.1172/JCI115537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol. 1983;45:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Ouchi N, Sato K, Higuchi A, Ishikawa T, Herschman H, Kihara S, Walsh K. Adiponectin promotes revascularization of ischemic muscle through a cyclooxygenase 2-dependent mechanism. Mol Cell Biol. 2009;29:3487–3499. doi: 10.1128/MCB.00126-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts JT, Li J. Interaction between carotid baroreflex and exercise pressor reflex depends on baroreceptor afferent input. Am J Physiol Heart Circ Physiol. 1998;274:H1841–H1847. doi: 10.1152/ajpheart.1998.274.5.H1841. [DOI] [PubMed] [Google Scholar]
- Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- Rotto DM, Hill JM, Schultz HD, Kaufman MP. Cyclooxygenase blockade attenuates responses of group IV muscle afferents to static contraction. Am J Physiol Heart Circ Physiol. 1990a;259:H745–H750. doi: 10.1152/ajpheart.1990.259.3.H745. [DOI] [PubMed] [Google Scholar]
- Rotto DM, Massey KD, Burton KP, Kaufman MP. Static contraction increases arachidonic acid levels in gastrocnemius muscles of cats. J Appl Physiol. 1989;66:2721–2724. doi: 10.1152/jappl.1989.66.6.2721. [DOI] [PubMed] [Google Scholar]
- Rotto DM, Schultz HD, Longhurst JC, Kaufman MP. Sensitization of group III muscle afferents to static contraction by arachidonic acid. J Appl Physiol. 1990b;68:861–867. doi: 10.1152/jappl.1990.68.3.861. [DOI] [PubMed] [Google Scholar]
- Scott AC, Wensel R, Davos CH, Georgiadou P, Ceri Davies L, Coats AJ, Francis DP, Piepoli MF. Putative contribution of prostaglandin and bradykinin to muscle reflex hyperactivity in patients on Ace-inhibitor therapy for chronic heart failure. Eur Heart J. 2004;25:1806–1813. doi: 10.1016/j.ehj.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Scott AC, Wensel R, Davos CH, Kemp M, Kaczmarek A, Hooper J, Coats AJ, Piepoli MF. Chemical mediators of the muscle ergoreflex in chronic heart failure: a putative role for prostaglandins in reflex ventilatory control. Circulation. 2002;106:214–220. doi: 10.1161/01.cir.0000021603.36744.5e. [DOI] [PubMed] [Google Scholar]
- Sinoway LI, Li J. A perspective on the muscle reflex: implications for congestive heart failure. J Appl Physiol. 2005;99:5–22. doi: 10.1152/japplphysiol.01405.2004. [DOI] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol. 2001;537:961–970. doi: 10.1111/j.1469-7793.2001.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp Physiol. 2006;91:89–102. doi: 10.1113/expphysiol.2005.032367. [DOI] [PubMed] [Google Scholar]
- Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- Stebbins CL, Maruoka Y, Longhurst JC. Prostaglandins contribute to cardiovascular reflexes evoked by static muscular contraction. Circ Res. 1986;59:645–654. doi: 10.1161/01.res.59.6.645. [DOI] [PubMed] [Google Scholar]
- Symons JD, Theodossy SJ, Longhurst JC, Stebbins CL. Intramuscular accumulation of prostaglandins during static contraction of the cat triceps surae. J Appl Physiol. 1991;71:1837–1842. doi: 10.1152/jappl.1991.71.5.1837. [DOI] [PubMed] [Google Scholar]
- Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Ann Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- Waldrop TG, Eldridge FL, Iwamoto GA, Mitchell JH. Central neural control of respiration and circulation during exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. Chapter 9. Oxford University Press; New York: 1996. pp. 333–380. [Google Scholar]
- Wibberley A, McCafferty GP, Evans C, Edwards RM, Hieble JP. Dual, but not selective, COX-1 and COX-2 inhibitors, attenuate acetic acid-evoked bladder irritation in the anaesthetised female cat. Br J Pharmacol. 2006;148:154–161. doi: 10.1038/sj.bjp.0706715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LB, Dyke CK, Parsons D, Wall PT, Pawelczyk JA, Williams RS, Mitchell JH. Effect of skeletal muscle fiber type on the pressor response evoked by static contraction in rabbits. J Appl Physiol. 1995;79:1744–1752. doi: 10.1152/jappl.1995.79.5.1744. [DOI] [PubMed] [Google Scholar]
- Wong SC, Fukuchi M, Melnyk P, Rodger I, Giaid A. Induction of cyclooxygenase-2 and activation of nuclear factor-κB in myocardium of patients with congestive heart failure. Circulation. 1998;98:100–103. doi: 10.1161/01.cir.98.2.100. [DOI] [PubMed] [Google Scholar]
- Xing J, Koba S, Kehoe V, Gao Z, Rice K, King N, Sinoway L, Li J. Interstitial norepinephrine concentrations in skeletal muscle of ischemic heart failure. Am J Physiol Heart Circ Physiol. 2007;293:H1190–H1195. doi: 10.1152/ajpheart.00231.2007. [DOI] [PubMed] [Google Scholar]
- Xing J, Sinoway L, Li J. Differential responses of sensory neurons innervating glycolytic and oxidative muscle to protons and capsaicin. J Physiol. 2008;586:3245–3252. doi: 10.1113/jphysiol.2008.154450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You HJ, Morch CD, Chen J, Arendt-Nielsen L. Differential antinociceptive effects induced by a selective cyclooxygenase-2 inhibitor (sc-236) on dorsal horn neurons and spinal withdrawal reflexes in anesthetized spinal rats. Neuroscience. 2003;121:459–472. doi: 10.1016/s0306-4522(03)00296-3. [DOI] [PubMed] [Google Scholar]