Abstract
Shine and rise! GABAA–receptors are ligand-gated chloride channels that respond to the major inhibitory neurotransmitter of the mammalian central nervous system. Herein, we introduce azobenzene derivatives of propofol that increase GABA-induced currents when irradiated with light and thus function as photochromic potentiators. One of our molecules, AP2, can be employed as a light-dependent general anesthetic in translucent tadpoles.
Keywords: propofol, azobenzenes, GABA receptors, photopharmacology
GABAA receptors are pentameric ligand-gated ion channels that are activated by the major inhibitory neurotransmitter in the mammalian brain, γ-aminobutyric acid (GABA).[1] Binding of GABA results in the opening of a chloride ion-selective pore, thus hyperpolarizing the postsynaptic neuron and decreasing the likelihood of action potential firing. As such, GABAA receptors are a prominent target for anesthetic, hypnotic, and anticonvulsant drugs (Fig. 1).[2,3]
Figure 1.
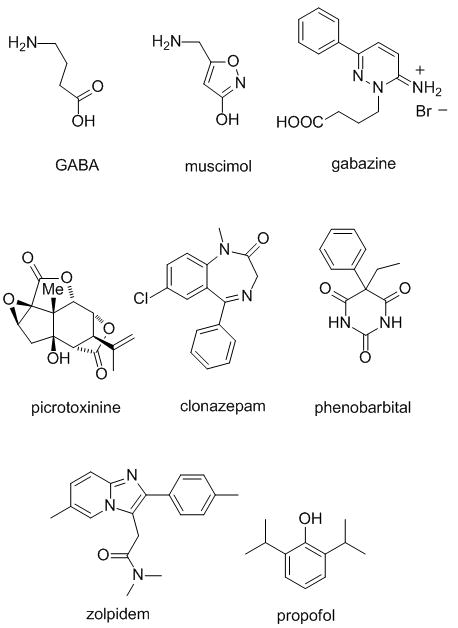
Agonists (GABA, muscimol), antagonists (gabazine) or potentiators (clonazepam, phenobarbital, zolpidem, propofol) of GABAA receptors.
While agonists, antagonists and blockers of GABAA receptors, such as muscimol, gabazine, or picrotoxinin, respectively, have proven to be valuable research tools, their impact on human medicine has been limited. Drugs that target these receptors are dominated by allosteric modulators that potentiate, i.e. increase, chloride currents elicited by the neurotransmitter. Well-established potentiators include benzodiazepines (e.g. diazepam), barbiturates (e.g. phenobarbital), the imidazopyridine zolpidem and the simple phenol propofol.[2] These bind to distinct allosteric sites on GABAA receptors increasing the mean open time or the opening frequency of the channel. However, the analysis of their exact binding sites at a molecular level has been complicated by a lack of detailed structural data.
Following its discovery in 1980, propofol has become the most widely used intravenous general anaesthetic.[4] Although its mode of action has not been fully elucidated, it is commonly accepted that the anaesthesia induced by this unusually lipophilic drug mostly results from potentitation of GABA-induced currents, as well as a direct activation of the chloride channel at low concentrations. Propofol has a rapid onset and offset of action and shows only minimal accumulation upon prolonged use. The intravenous administration of propofol is also associated with reduced postoperative nausea and vomiting.[5]
While GABAA-receptors respond to a variety of ligands, they are normally not sensitive toward light. It would be fascinating to confer light-sensitivity to these ion channels, since light is unsurpassed in terms of the temporal and spatial precision it provides. This could be indirectly achieved via ligands that act on the receptors but can be optically switched between an active and an inactive form. Reflecting the rich pharmacology of GABAA receptors, these could be photochromic agonists, antagonists or allosteric modulators. In principle, these ligands could be covalently attached as photoswitched tethered ligands (PTLs) or act as soluble photochromic ligands (PCLs).[6] Indeed, both approaches have been used to convert neuronal[7] and neuromuscular[8] nicotinic acetylcholine receptors, another type of pentameric ligand-gated ion channels, as well as ionotropic glutamate receptors[9] into artificial photoreceptors. Tethered and soluble photochromic blockers of K+, Na+ and Ca2+ have been described as well and have been used to control heart beat,[10] pain sensation[11] and visual responses[12] in a variety of animals with light.
We now report photochromic potentiators of GABA currents that change the strength of GABA-induced currents in a light-dependent fashion. Our program was prompted by a recent report on a photoaffinity probe based on propofol, p-4-aziC5-propofol that underscored that a relatively large substituent in the para-position of the phenol would be tolerated and that the propofol pharmacophor would be compatible with photochemistry (Fig. 2a).[13] Accordingly, we designed a series of azobenzene derivatives of propofol wherein an aryldiazene unit is directly coupled to the pharmacophor. These molecules, termed Azo-Propofols 1-16 (AP1-16) are shown in Fig. 2a. Their varying substituent in the 4′-position of the azobenzene core determines their pharmacokinetic, pharmacodynamic as well as spectral properties.
Figure 2.
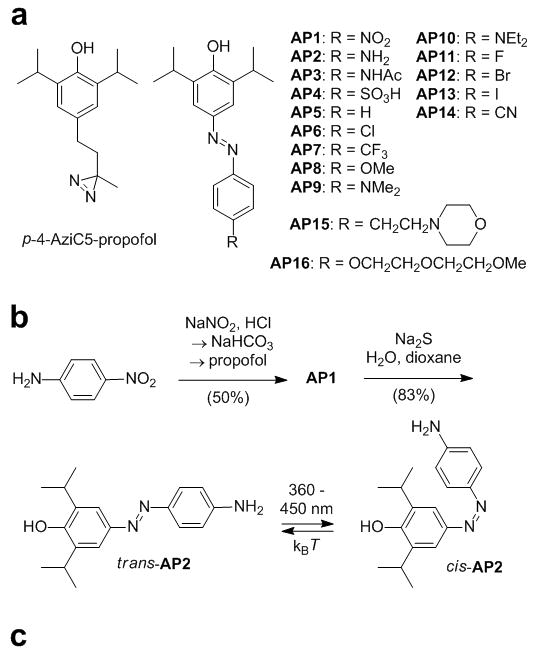
a) p-4-aziC5-propofol, a photoreactive derivative of propofol, and AP1-16, photoswitchable derivatives of propofol. b) Synthesis of AP1 and AP2, which is shown in its trans and cis configuration. c) X-ray structure of trans-AP2.
Compounds AP1-16 were synthesized using classical diazo-coupling chemistry, as shown in the representative synthesis of AP1 and AP2 (Fig. 3b, also see Supporting Information).[14] The X-ray structure of AP2 is displayed in Fig. 2c. [15] Due to crystal packing effects, the trans azobenzene is not fully planar in this solid state structure (see Supporting Information for a more detailed discussion of these effects).
Figure 3.
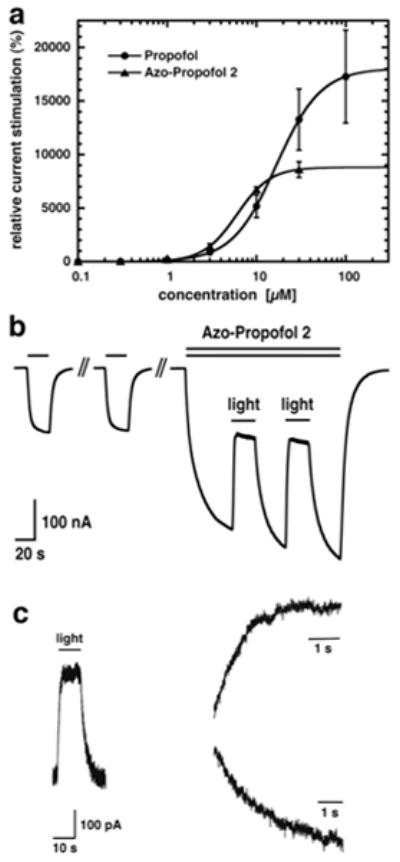
a) α1β2γ2 GABAA receptors were expressed in Xenopus oocytes. Currents were activated with a concentration of GABA eliciting 0.3 % of the maximal current amplitude (EC0.3) without or with increasing amounts of either propofol or AP2. Mean ± SEM of four experiments is shown. b) 1 μM GABA was applied repetitively until a stable current response was observed. Co-application of 1.5 μM AP2 with GABA which resulted in current potentiation. During co-application, the oocyte was exposed to a light source emanating 360–370 nm light. As a consequence, current stimulation rapidly decreased until it reached a steady level. When the light-source was turned off, the amplitude increased again. This procedure was repeated. This experiment was repeated independently six times using different oocytes. c) α1β2γ2 GABAA receptors were expressed in HEK-cells. 0.5 μM GABA was co-applied with 5 μM AP2. Subsequently, the perfusion was stopped and the cells were exposed to the light source. The inward current amplitude decreased rapidly and increased again upon turning off the light-source (trace left). Current decrease (trace top right) and increase (trace bottom right) were each fitted with a mono-exponential function with τ amounting to 1.1 ± 0.4 s (n = 7) and 2.0 ± 0.7 s (n = 6), respectively.
Although several of the Azo-Propofols shown in Fig. 2 function as photochromic potentiators, AP2 emerged early on as our most promising candidate due its favorable pharmacological and photochemical features. By virtue of its aminosubstituent, the photoswitch has a red-shifted action spectrum, which means that maximum cis-content is achieved at irradiation with 404 nm light. However, due to its broad absorption spectrum, slightly longer or shorter wavelengths could be used effectively (see Supporting Information). In addition to this, the substitution of the azobenzene core with electron-donating substituents greatly decreases the thermal stability of the cis isomer. Therefore, AP2 quickly reverts to its trans form once the light is switched off. Since the absorption spectra of the cis and trans isomers are very similar (see Supporting Information), this process cannot be accelerated by irradiation with a different wavelength. Other APs studied, have less favorable photophysical properties, show decreased potency (e.g. AP3, AP9, AP10), no activity at all (e.g. AP4),[14c] or unfavorable solubility and distribution (e.g. AP6).
The effect of AP2 on Cl− currents was investigated with electrophysiology using α1β2γ2 GABAA receptors expressed in Xenopus oocytes (Fig. 3).[16] This receptor subtype represents the most prevalent form in the human brain.[17] First, the heterologoulsy expressed GABAA receptors were exposed to GABA at a concentration eliciting 0.3 % of the maximal current amplitude in combination with increasing concentrations of propofol or AP2 in the dark to compare the relative effect of the compounds. From the resulting dose-response curves, we extracted an EC50 value of 17.1 ± 2.9 μM for propofol and of 6.1 ± 0.4 μM for AP2 (n = 4, SEM) (Fig. 3a). Thus, AP2 in its dark-adapted trans form has a significantly higher affinity than propofol itself, albeit its efficacy is reduced by about two-fold when compared with its parent.
Having established that AP2, in its dark-adapted form, has an effect on GABAA receptors, the light-dependency of the current potentiation was investigated. UV-VIS light from an Ultrafire® 1 Watt UV LED pocket lamp (YonC Trading, Zürich; emission wavelength 390–450 nm) had no effect on the GABA response or the combined GABA/propofol response (data not shown). Fig. 3b illustrates the effect of UV/Vis light on currents elicited by the combined application of GABA and AP2. Stimulation of GABA currents by 1.5 μM of this compound was 159 ± 25 % (mean ± SEM, n = 6). Exposure to light decreased the residual stimulation to 18 ± 3 % (mean ± SEM, n = 6). Similar observations were made using a UV high power LED pocket lamp, 5 Watt (Uveco GmbH, Bruckmühl, Germany) emission wavelength 355–380 nm equipped with a CHROMA bandpass filter D365/10x, to limit light emission to 360–370 nm. The variety of these light-sources reflects the broad abortion spectrum of AP2. Due to redistribution of the hydrophobic compound AP2 into egg yolk, rate of photo-switching could not be determined in Xenopus oocytes. For this purpose, we expressed α1β2γ2 GABAA receptors in HEK-cells and performed experiments using the whole-cell patch-clamp technique. GABA was co-applied with AP2. Subsequently, the perfusion was stopped to prevent arrival of new trans-AP2 during the measurement and the cells were exposed to the light. The current amplitude decreased rapidly and increased again upon turning off the light-source. Current traces were fitted with a mono-exponential function. The time constant τ amounted 1.1 ± 0.4 s (n = 7) for the trans to cis transition and 2.0 ± 0.7 s (n = 6) for the cis to trans transition, respectively.
Next, we investigated anesthetic activity and photo-reversibility of both propofol and AP2 in a small animal model, albino Xenopus laevis tadpoles. Groups of animals were placed in aqueous solutions containing either propofol or AP2 and tested every five minutes for loss of righting reflexes (LORR), a standard assay for anesthesia.[18] Steady-state LORR results were observed at 10 min for propofol and 25 min for AP2. After 30 min in drug solution, each animal was exposed for 5 to 10 sec to 360–370 nm bandpass filtered UV light (details in Supporting Information) while retesting for LORR. Propofol alone produced LORR with an EC50 of 1.3 μM (Fig. 4a). Illumination induced vigorous swimming activity in un-anesthetized tadpoles (suggesting that it represents a noxious stimulus) and produced a small rightward shift in the propofol vs. LORR relationship (EC50 ≈ 2.0 μM; Fig. 4a). AP2 alone produced LORR with an EC50 similar to that of propofol (1.1 μM; Fig. 4b). However, illumination produced a large rightward shift in the AP2 EC50 to 4.6 μM (Fig. 4b). All animals recovered from anesthesia when returned to water alone. In an independent set of experiments (see video in the Supporting Information), 3 μM propofol produced LORR in all tadpoles with or without light, whereas in 3 μM AP2, all animals showed LORR without light and all spontaneously righted themselves and swam during illumination with UV light.
Figure 4.
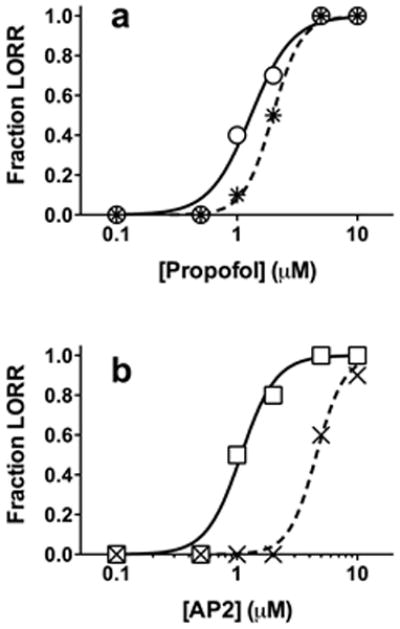
Light-dependent anesthesia in tadpoles. Loss of righting reflexes is plotted against aqueous anesthetic concentration, overlayed with logistic fits. Each point represents data from ten animals. a) 360–370 nm light, an apparently noxious stimulus in Xenopus tadpoles, produces a small rightward shift in propofol-dependent loss of righting reflexes (LORR) from 1.1 ± 0.1 μM (circles) to 2.0 ± 0.1 μM (stars). b) 360–370 nm light shifts the AP2-dependent LORR curve to the right from 1.1 ± 0.1 μM (squares) to 4.6 ± 0.2 μM (X’s). This larger shift is due to photo-isomerization of AP2.
The photo-reversibility of both AP2-induced GABAA receptor modulation and its anesthetic action in animals supports the hypothesis that anesthesia caused by AP2 and propofol is largely mediated by GABAA receptors. However, evidence also implicates other targets, including HCN1 channels,[19] in propofol’s anesthetic actions. Examination of AP2 effects and photo-reversibility on these targets might help further elucidate their roles in the pharmacology of general anesthesia.
In summary, we have developed photoswitchable versions of propofol that allow for the indirect optical control of GABAA receptors. Functionally, our compounds differ from previously introduced PCLs, as they act as photochromic potentiators rather than photochromic agonists, antagonists or channel blockers. Application of our lead compound, AP2, in the dark potentiates GABA-induced Cl− currents, which can be reversed upon irradiation with violet light. The ability of Azo-Propofols to control neural systems has been demonstrated, since AP2 functions as a light-dependent anesthetic in translucent tadpoles. Future work will address the usefulness of Azo-Propofols in other systems, such as brain slices and retinas lacking innate photoreceptors, wherein photochromic potentiators could restore visual responses through their action on interneurons expressing GABAA receptors.
Supplementary Material
Acknowledgments
We thank Dr. Peter Mayer (LMU Munich) for the determination of the X-ray structure. M. S. is grateful to the Fonds der Chemischen Industrie for a Ph.D. fellowship. This work was supported by the Swiss National Science Foundation grants 31003A-132806/1 (E.S.) and the European Science Foundation (ERC grant No 268795 to D.T.) and the National Institutes of Health (GM089745 to S.A.F and GM087316 to D.E.R.).
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
Contributor Information
Marco Stein, Department of Chemistry, Ludwig-Maximilians-Universität München and Center of Integrated Protein Science, D-81377 Munich, Germany, Fax: (+49) 89 2180-77972.
Simon J. Middendorp, Institute for Biochemistry and Molecular Medicine, University of Bern, Bühlstr. 28, CH-3010 Bern.
Valentina Carta, Institute for Biochemistry and Molecular Medicine, University of Bern, Bühlstr. 28, CH-3010 Bern.
Ervin Pejo, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, 32 Fruit Street, Boston, Massachusetts 02114, United States.
Douglas E. Raines, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, 32 Fruit Street, Boston, Massachusetts 02114, United States
Stuart A. Forman, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, 32 Fruit Street, Boston, Massachusetts 02114, United States
Erwin Sigel, Email: erwin.sigel@mci.unibe.ch, Institute for Biochemistry and Molecular Medicine, University of Bern, Bühlstr. 28, CH-3010 Bern.
Dirk Trauner, Email: dirk.trauner@lmu.de, Department of Chemistry, Ludwig-Maximilians-Universität München and Center of Integrated Protein Science, D-81377 Munich, Germany, Fax: (+49) 89 2180-77972.
References
- 1.Macdonald RL, Olsen RW. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 2.Sieghart W. Pharmacol Rev. 1995;47:181–233. [PubMed] [Google Scholar]
- 3.Sigel E, Lüscher BP. Curr Trends in Med Chem. 2011;11:241–246. doi: 10.2174/156802611794863562. [DOI] [PubMed] [Google Scholar]
- 4.Vanlersberghe C, Camu F. Handb Exp Pharmacol. 2008;182:227–252. doi: 10.1007/978-3-540-74806-9_11. [DOI] [PubMed] [Google Scholar]
- 5.Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, Zernak C, Danner K, Jokela R, Pocock SJ, Trenkler S, Kredel M, Biedler A, Sessler DI, Roewer N. N Engl J Med. 2004;350:2441–2451. doi: 10.1056/NEJMoa032196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fehrentz T, Schönberger M, Trauner D. Angew Chem Int Ed. 2011;16:12156–12182. doi: 10.1002/anie.201103236. [DOI] [PubMed] [Google Scholar]
- 7.Tochitsky I, Banghart MR, Mourot A, Zhao JZ, Gaub B, Kramer RH, Trauner D. Nat Chem. 2012;4:105–111. doi: 10.1038/nchem.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartels E, Wassermann NH, Erlanger BF. Proc Natl Acad Sci USA. 1971;68:1820–1823. doi: 10.1073/pnas.68.8.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Volgraf M, Gorostiza P, Numano R, Kramer RH, Isacoff EY, Trauner D. Nat Chem Bio. 2006;1:47–52. doi: 10.1038/nchembio756. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Volgraf M, Gorostiza P, Szobota S, Helix MR, Isacoff MREY, Trauner D. J Am Chem Soc. 2007;129:260–261. doi: 10.1021/ja067269o. [DOI] [PubMed] [Google Scholar]; c) Szobota S, Gorostiza P, Del Bene F, Wyart C, Fortin DL, Kolstad KD, Tulyathan O, Volgraf M, Numano R, Aaron HL, Scott EK, Kramer RH, Flannery J, Baier H, Trauner D, Isacoff EY. Neuron. 2007;54:535–545. doi: 10.1016/j.neuron.2007.05.010. [DOI] [PubMed] [Google Scholar]; d) Gorostiza P, Volgraf M, Numano R, Szobota S, Trauner D, Isacoff EY. Proc Natl Acad Sci. 2007;104:10865–10870. doi: 10.1073/pnas.0701274104. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Numano R, Szobota S, Laud AY, Gorostiza P, Volgraf M, Roux B, Trauner D, Isacoff EY. Proc Natl Acad Sci. 2009;106:6814–6819. doi: 10.1073/pnas.0811899106. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Janovjak H, Szobota S, Wyart C, Trauner D, Isacoff EY. Nat Neurosci. 2010;13:1027–1032. doi: 10.1038/nn.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Stawski P, Janovjak H, Trauner D. Bioorg Med Chem. 2010;18:7759–7772. doi: 10.1016/j.bmc.2010.09.012. [DOI] [PubMed] [Google Scholar]; h) Caporale N, Kolstad KD, Lee T, Tochitsky I, Dalkara D, Trauner D, Kramer RH, Dan Y, Isacoff EY, Flannery JG. Mol Ther. 2011;19:1212–1219. doi: 10.1038/mt.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fortin DL, Banghart MR, Dunn TD, Borges K, Wagnaar DA, Gaudry Q, Karakossian M, Otis TW, Kristan WB, Trauner D, Kramer RH. Nat Meth. 2008;5:331–338. doi: 10.1038/nmeth.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mourot A, Fehrentz T, Bautista D, Trauner D, Kramer RH. Nat Meth. 2012;9:396–402. doi: 10.1038/nmeth.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polosukhina A, Litt J, Tochitsky I, Nemargut J, Sychey Y, De Kouchkovsky I, Huang T, Borges K, Trauner D, Van Gelder RN, Kramer RH. Neuron. 2012;75:271–282. doi: 10.1016/j.neuron.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart DS, Savechenkov PY, Dostalova Z, Chiara DC, Ge R, Raines DE, Cohen JB, Forman SA, Bruzik KS, Miller KW. J Med Chem. 2011;54:8124–8135. doi: 10.1021/jm200943f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.a) Geidysh LS, Nikiforov GA, Ershov VV. Bio Bull Acad Sci USSR. 1969;18:2552–2555. [Google Scholar]; b) Silk JA, Summers LA. J Chem Soc. 1963:3472–3474. [Google Scholar]; c) Borah JC, Mujtaba S, Karakikes I, Zeng L, Muller M, Patel J, Moshkina N, Morohashi K, Zhang W, Gerona-Navarro G, Hajjar RJ, Zhou MM. Chem Biol. 2011;18:531–541. doi: 10.1016/j.chembiol.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.AP2: C18H23N3O, Mr = 297.395 g mol−1, red block, 0.14 × 0.25 × 0.30 mm, monoclinic, P21, a = 10.5454(3), b = 9.5435(3), c = 17.0651(4) Å, α = 90°, β = 90.0955(16)°, γ = 90°, V = 1704.28(8) Å3, Z = 4, ρ = 1.159 g cm−3, μ(MoKα) = 0.073 mm−1, MoKα radiation (λ = 0.71073 Å), T = 200 K, 2θmax 55.02°, 13750 refls., 4143 independent, 3271 with I ≥ 2σ(I), Rint = 0.042, mean σ(I)/I = 0.0392, 429 parameters, R(Fobs) = 0.0432, Rw(F2) = 0.1097, S = 1.014, min. and max. residual electron density: −0.21, 0.15 e Å−3; data collection by means of a Nonius KappaCCD diffractometer equipped with a rotating anode generator (ω-scans), structure solution by direct methods with SIR97, structure refinement with SHELXL-97, O- and N-bonded H have been refined freely, C-bound H have been added geometrically treated as riding on their parent atoms; crystallographic data (excluding structure factors) for the structure reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC-890176. Copies of the data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB21EZ (fax: (+44)1223-336-033; e-mail: deposit@ccdc.cam.ac.uk).
- 16.Sigel E, Minier F. Mol Nutr Food Res. 2005;49:228–234. doi: 10.1002/mnfr.200400104. [DOI] [PubMed] [Google Scholar]
- 17.Olsen RW, Sieghart W. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart DS, Savechenkov PY, Dostalova Z, Chiara DC, Ge R, Raines DE, Cohen JB, Forman SA, Bruzik KS, Miller KW. J Med Chem. 2011;54:8124–8135. doi: 10.1021/jm200943f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X, Shu S, Bayliss DA. J Neurosci. 2009;29:600–609. doi: 10.1523/JNEUROSCI.3481-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


