Abstract
Side effect of radiation therapy (RT) remains the most challenging issue for pancreatic cancer treatment. In this report we determined whether and how cerium oxide nanoparticles (CONPs) sensitize pancreatic cancer cells to RT. CONP pretreatment enhanced radiation-induced reactive oxygen species (ROS) production preferentially in acidic cell-free solutions as well as acidic human pancreatic cancer cells. In acidic environments, CONPs favor the scavenging of superoxide radical over the hydroxyl peroxide resulting in accumulation of the latter whereas in neutral pH CONPs scavenge both. CONP treatment prior to RT markedly potentiated the cancer cell apoptosis both in culture and in tumors and the inhibition of the pancreatic tumor growth without harming the normal tissues or host mice. Taken together, these results identify CONPs as a potentially novel RT-sensitizer as well as protectant for improving pancreatic cancer treatment.
Keywords: Cerium oxide nanoparticles, radiation, sensitizer, pancreatic cancer, ROS
Introduction
Pancreatic cancer carries an extremely poor prognosis, with 90% of pancreatic cancers being malignant and the 5-year survival rate after diagnosis hovering at 5%. Less than 20% of patients are candidates for surgical resection; therefore, chemotherapy and radiation therapy (RT) remain the only other treatment options. Ionizing radiation used in RT induces the radiolysis of water which generates reactive oxygen species (ROS), such as superoxide and hydroxyl radicals. These molecules play an important role in the subsequent cellular events such as DNA damage potentially leading to apoptosis. Unfortunately, RT induces side effects, including skin irritation, loss of appetite, fatigue, and nausea, as well as the pain associated with these conditions.
Research to reduce the unwanted side effects of RT has yielded two categories of compounds: radiation protectants and radiation sensitizers. Radiation protectants selectively protect normal tissue from the harmful impact of ionizing radiation, while radiation sensitizers, such as histone deacetylase (HDAC) inhibitors, selectively increase the damage ionizing radiation induces in cancer cells. Side effects associated with currently available protectants such as Amifostine include nausea, vomiting, and hypotension, as well as high costs, increasing the cost of the overall treatment. Suberoylanilidehydroxamic acid (SAHA), an HDAC inhibitor currently in the late stage clinical trials as a radiation sensitizer, has been associated with fatigue, dehydration, nausea, and vomiting. Therefore, creation and identification of novel compounds that improve the efficacy and therapeutic index of RT would directly improve cancer treatment. Yet, as evidenced by the disparity between for the number of patients receiving RT and how few radiation protection/sensitization compounds exist, identifying viable adjuvants has proved elusive.
Nanoparticle based therapies for cancer treatment is a rapidly growing field, with recent publications highlighting the ability of Ag microspheres and folic acid-conjugated silica-modified gold nanorodsto act as radiosensitizers. Cerium oxide nanoparticles (CONPs) have been used as an adjuvant to improve RT in pre-clinical trials. Wide range CONP applications stem from the surface chemistry of the nanoparticles. The valence state and oxygen defects allow CONPs to act as auto-regenerative redox status modulators. Recently, CONPs have been shown to be capable of entering mammalian cells and have implications in biological systems.
The antioxidant behavior of CONPs has been employed to treat diseases of the central nervous system, repair spinal cord injuries, and extend the life of neurons in vitro. The antioxidant properties of CONPs allow them to decrease the accumulation of ROS and prevent subsequent ROS-induced apoptosis in normal cells. Biochemically, CONPs have been shown to act as either a superoxide dismutase (SOD) mimetic, converting superoxide to hydrogen peroxide (H2O2), or a catalase mimetic, converting H2O2 to water. It is through these mechanisms that previous work suggests CONPs are able to protect normal tissue from radiation-induced damage in the lungs, breast, and gastrointestinal tract. Most recently, CONPs were shown to scavenge hydroxyl radicals and possess intrinsic oxidase activity, as well as cytotoxic and anti-invasive properties in melanoma cells. Due to the array of radical interactions (both pro- and antioxidant) now established, CONPs can no longer be strictly characterized as free radical scavengers and must be viewed as free radical modulators.
This study examines the ability of CONPs to drive ROS accumulation, as well as the subsequent impact on pancreatic cancer cell survival in vitro and in vivo. Our data demonstrate that the pro-oxidant activity of CONPs drives radiation-induced radical production selectively in pancreatic cancer cells resulting in radiation sensitization to apoptotic death and growth inhibition. These results identify CONPs as a potentially novel radiation sensitizer for the treatment of human pancreatic cancer.
Methods
Cell Culture and Reagents
The normal pancreatic cells (hTERT-HPNE) were obtained from American Type Culture Collection (ATCC) and maintained in 3:1 glucose free DMEM:M3 Base medium. The human pancreatic cancer cell line L3.6pl was cultured in DMEM. Both cell mediums were supplemented with 10% fetal bovine serum and 100 μg/mL penicillin-streptomycin mixture (GIBCO) and maintained at 37°C and 5% CO2. CONPs were purchased from NanoScale Corporation (Manhattan, KS) or synthesized as previously described. Hydrogen peroxide (H2O2) was purchased from Sigma Aldrich (St. Louis, MO).
Transmission Electron Microscopy of CONPs
The sizes and shapes of the nanoparticles (purchased from NanoScale Corporation, Manhattan, KS) were determined by high-resolution transmission electron microscopy (TEM) as previously described.
ROS Imaging
L3.6pl and hTERT-HPNE cells were plated in 6-well plates (5×104/well) and 24 hours later treated with 10 μM CONPs in fresh media for 24 hours. Subsequently, cells were exposed to 5 Gy RT using a 160-kV cell culture and small animal irradiator (KimtronInc, Woodbury, Connecticut). ROS production was determined 0.5 and 24 hours post RT by Image-iT LIVE Green ROS Detection Kit (Invitrogen) according to manufacturer’s protocol. The kit provides carboxy-H2DCFDA. The carboxy-H2DCFDA permeates live cells and is cleaved by cellular esterases in to carboxy-DCFH. In the presence of cellular ROS, the carboxy-DCFH is then oxidized to produce carboxy-DCF, resulting in the emission of a bright green fluorescence. Alternatively, cells were cultured in 6 well plates for 24 hours followed by exposure to RT. 24 hours post RT, the media was replaced with fresh media containing CONPs (10 μM). ROS production was determined 3 and 24 hours post addition of CONPs. Photographs are representative images from triplicate experiments, which were quantified using NIH ImageJ software to determine the number of fluorescent cells per field of view.
Cell Viability Assays
L3.6pl or hTERT-HPNE cells were plated (2×103/well) and grown in 96-well plates for 24 hours and then treated with CONPs (10 μM) for an additional 24 hours, followed by exposure to 5 GyRT. Cell viability was determined 96 hours post RT by the Cell Titer-Glo Luminescent Cell Viability Assay from Promega (Madison, Wisconsin) and an Optima Fluor Star Luminometer (BMG Lab Tech, Durham, NC) following the manufacturers’ protocols.
Clonogenic Assays
L3.6pl cells were plated (1×106/10-cm dish), grown for 24 hours and then treated with CONPs (10 μM) for an additional 24 hours. Immediately after exposed to 5 Gy RT, the cells were trypsinized and re-seeded (100 cells/well) into 6 well dishes. One week later, cells were stained with 6.0% gluteraldehyde (vol/vol), 0.5% crystal violet (wt/vol) in water and photographed colonies of greater than 50 cells were counted.
Hydrogen Peroxide Assays
H2O2 production by ionizing radiation (0–30 Gy), CONPs (0–200 μM) or the combinations of both was determined in 50 μL of water or phosphate-buffered saline (PBS) at various pH values (pH 7.4, pH 5, and pH 3) by Amplex Red Assay (Invitrogen) following the manufacturer’s protocol. The combination treatments include two strategies: 1) irradiating 50 μL of CONP suspension and determining the time-course (0–25 h) of H2O2 production, and 2) irradiating 48 μL of water or PBS first, waiting for 1 or 24 hours and then adding 2 μL of CONP prior to determining the H2O2 production at a desired time point.
Intracellular Acidity Assay
L3.6pl or hTERT-HPNE cells were seeded at 500,000 cells per 6-cm dish and grown overnight. The medium was then removed and cells were incubated in 500 μL HBSS containing 1 μM BCECF-AM (Invitrogen) for 45 minutes. Cells were then trypsinized, washed twice with fresh media, and re-suspended in 1 mL HBSS. Flow cytometry was used to determine cellular fluorescence (as defined by the ratio of FITC/APC). Decreased fluorescence indicates increased intracellular acidity.
Superoxide Radical and Hydrogen Peroxide Scavenging Analysis
SOD mimetic activity of CONPs with different surface valance state to scavenge superoxide radical at neutral (pH 7) and acidic pH (pH 3) was determined using a SOD assay kit (Sigma-Aldrich, Kit #19160-1KTF) according to the manufacturer’s instructions. Catalase mimetic activity of CONPs to scavenge hydrogen peroxide under the same conditions was determined using an Amplex Red hydrogen peroxide assay kit (Life Technology, Cat # A22188) according to the manufacturer’s instruction.
Orthotopic Injection of Pancreatic Cancer Cells in Athymic Nude Mice
Female athymic nude mice (NCI-nu) were purchased from the Animal Production Area of the National Cancer Institute Frederick Cancer Research and Development Center. The mice were housed and maintained in specific pathogen-free conditions in facilities approved by the American Association for Accreditation of Laboratory Animal Care and in accordance with current regulations and standards of the United States Department of Agriculture, United States Department of Health and Human Services, and the National Institute of Health. The mice of 8 to 12 weeks of age were used in accordance under institutional guidelines with approved IACUC protocol. Human care of the mice was thoroughly considered. To develop tumors, L3.6pl cells were harvested from culture dishes and injected as previously described.
Therapy of Established Human Pancreatic Carcinoma Tumors Growing in the Pancreas of Nude Mice
Immediately following injection of cancer cells (1 × 106) into the pancreas, the mice were randomized into two groups (n = 20) as follows: (a) twice weekly intraperitoneal (i.p.) injections of saline in control groups; (b) twice weekly (Tuesday and Thursday) i.p injections of CONPs (15 μM; 0.01 mg/kg). Two weeks later each group was randomized into 2 sub-groups (n = 10) as follows: (c) continued with twice weekly i.p injections of saline (for control group) or twice weekly i.p. injections of CONPs; (d) twice weekly i.p. injections of saline or CONPs and thrice-weekly administration of 5 Gy radiation for 2 weeks (30 Gy total). All treatment groups continued to receive twice weekly i.p. injections of saline (control) or CONPs for 2 additional weeks (for a total of 6 week treatment). Mice were sacrificed at 8 weeks (day 56) and subjected to necropsy. Primary tumors in the pancreas were excised and weighed. Tumor volumes were determined by liquid displacement analysis.
Histologic Analysis of Tumors
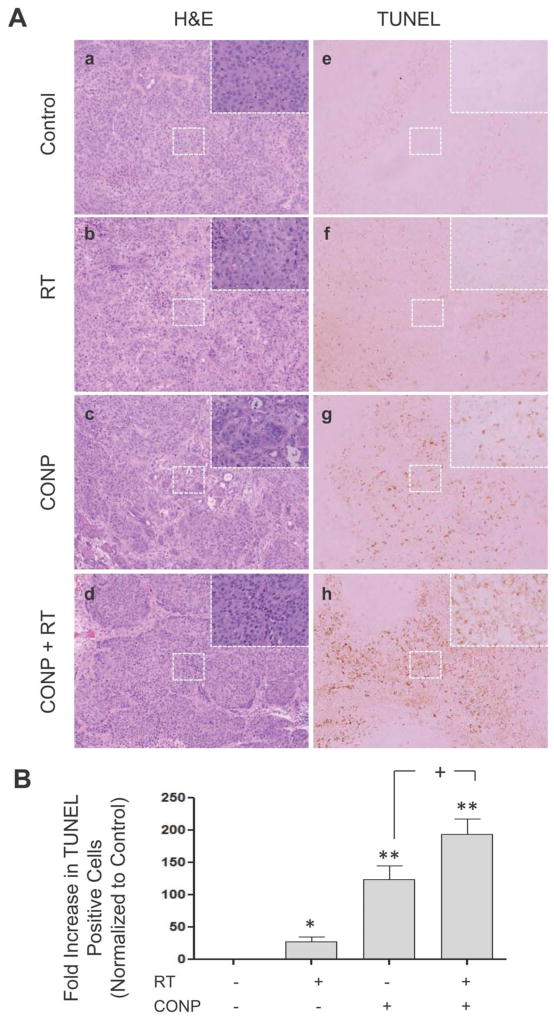
For hematoxylin and eosin (H&E) staining, tissues were fixed in formalin, embedded in paraffin, and serially sectioned at 200 μm. H & E staining was performed based on standard protocol. Paraffin-embedded tissues were used for TUNEL staining. TUNEL-positive cells were detected using the DeadEnd Colorimetric TUNEL System (Promega, Madison, WI). Immunohistological microscopy was performed using 10x and 40xobjectives on a Nikon E400 microscope (Nikon Instruments, Melville, NY). Routine procedures were used to capture images. Immunopositive cells for TUNEL staining were observed over 10 individual slides for each condition and quantified using the NIH ImageJ software to determine the number of TUNEL positive cells per field of view. Independent review by the Pathology Department at Orlando Health/MD Anderson Cancer Center Orlando confirmed the results.
Statistical Analysis
All the experiments were completed in three triplicates and the data are presented as mean ± standard deviation (SD). The data presented in Table 1 are presented as mean and range. Statistical analysis was performed using Student’s t-test, and P value was calculated based on two-tailed test. P< 0.05 was considered statistically significant. GraphPad Prism software (GraphPad Software, Inc, La Jolla, CA) was used for all statistical analyses.
Table 1.
CONPs Increase Sensitivity to Radiation-Induced Inhibition of Human Pancreatic Tumor Growth
| Treatment Group | Tumor
|
Body Weight (g)
|
|||||
|---|---|---|---|---|---|---|---|
| Incidence | Volume (mm3)
|
Weight (g)
|
Mean | Range | |||
| Mean | Range | Mean | Range | ||||
| Control | 15/15 | 2100 | 1750–3000 | 1.31 | 0.48–2.63 | 28 | 22–32 |
| RT | 15/15 | 1643 | 1000–2000 | 1.38 | 1.04–2.26 | 26 | 20–31 |
| CONP | 15/15 | 1306† | 1000–2500 | 1.39 | 0.71–2.40 | 26 | 20–37 |
| CONP + RT | 15/15 | 1045*† | 250–2000 | 0.97*† | 0.23–1.66 | 27 | 21–31 |
L3.6pl cells (1 × 106) were injected into the pancreas of nude mice. Groups of mice were treated with i.p injections of saline (control) or CONP (15 μM or 0.01 mg/kg) twice weekly for 6 weeks. Sub-groups of mice also received fractionated RT (5 Gy) three times per week (total 30 Gy) during weeks 3 and 4. All mice were sacrificed on day 56. Tumor weight and volume analysis were performed as described in methods.
P< 0.01 compared to RT.
P < 0.01 compared to control.
Results
CONPs facilitate RT-inducedH2O2 production favoring lower than neutral pH
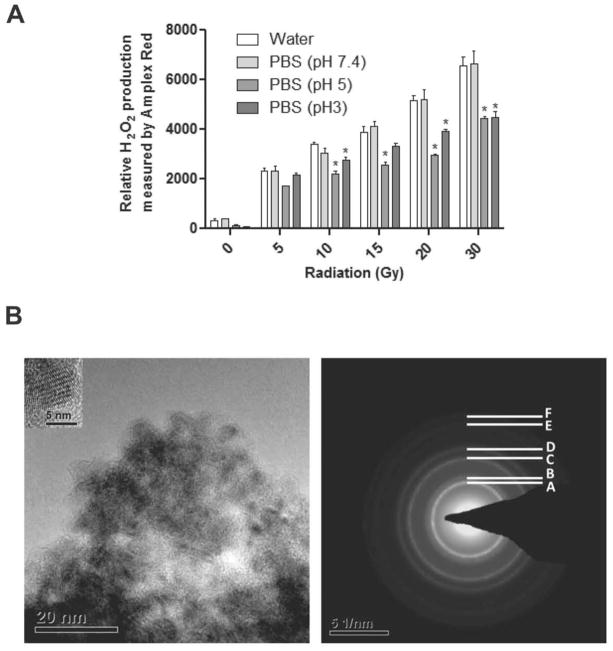
To characterize H2O2 induction in vitro by RT, water or PBS of varying pH (pH 7.4, pH 5 and pH 3) was exposed to serial doses of ionizing radiation (5–30 Gy) and relative H2O2 production was determined (Figure 1A). The results show a RT dose-dependent increase in H2O2 production immediately following RT exposure in all conditions to a similar degree. The basal as well as post-RT levels of H2O2 in acidic buffer were considerably lower than those at neutral pH, suggesting that the autodismutation activity was lower at acidic pH. In acidic pH CONPs produced H2O2 while scavenging superoxide radicals with an equation of O22−. + CeO2-x (Ce3+) +2H+→ H2O2+ CeO2 (Ce4+), indicating that in acidic pH, CONPs produce H2O2 while scavenging superoxide radical. The size and shape of the CONPs were also determined by TEM (Figure 1B).
Figure 1.
Acidic pH is relatively resistant to H2O2 production compared to neutral pH. Water (pH 7.4) and PBS at indicated pHs were irradiated at indicated doses and H2O2 production was examined by Amplex Red Assay. *P<0.05 compared to water exposed to that RT dose. B, TEM analysis of CONPs. Left panel shows TEM image of the CONPs of size between 5–8 nm (inset, high magnification images). Right panel shows selected area of electron diffraction pattern of the CONPs where A (111), B (200), C (220), D (311), E (222) and F (400) are the different crystal planes of fluorite crystal structure.
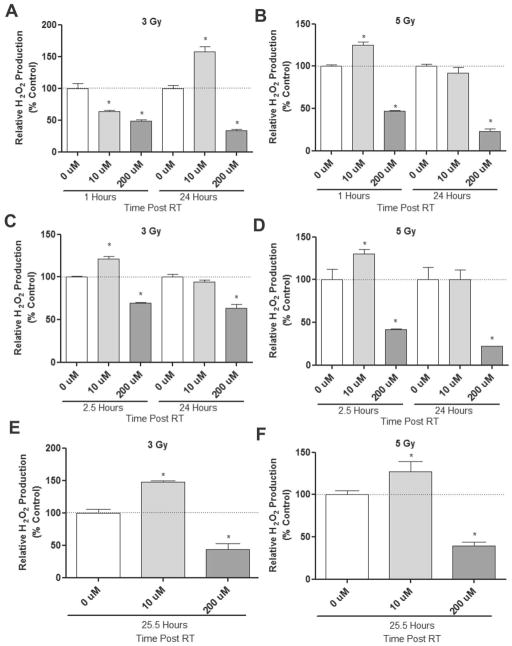
The impact of CONPs on radiation-induced radical production has been suggested but not documented. To characterize this, we first tested if prior presence of CONPs affects H2O2 induction at neutral pH by RT (Figure 2A & 2B and Figure S1A & S1B). CONPs suspended in water at serial concentrations (up to 200μM) were exposed to 3 or 5Gy RT. We noticed that CONPs alone, regardless of concentration, did not alter baseline H2O2 levels in water (pH 7.4) (data not shown). We found that the prior presence of CONPs prevented RT-induced H2O2 production in the water compared to non-CONP treated groups, although a slight increase in H2O2 levels was observed with CONPs at a concentration less than 10 μM. Similar changes were observed when CONPs were added 1 hour (Figure 2C & 2D and Figure S1C & S1D) or 24 hours (Figure 2E & 2F and Figure S1E & S1F) after RT. It should be noted that the H2O2 induction at neutral pH (7.4) is comparable between water and PBS solution (Figure S2), and that the presence of CONPs in the assay reaction or removal of CONPs from the reaction does not change the readout for H2O2 levels (Figure S3). These results suggest that the neutral pH environment seems to favor the catalase-mimetic H2O2 scavenging activity over the SOD-mimetic H2O2 producing activity of CONPs.
Figure 2.
At neutral pH CONPs generally decrease RT-induced H2O2 production. (A & B) CONP suspensions of serial concentrations up to 200 μM in water or PBS at neutral pH (Figure S2) were irradiated at indicated doses. H2O2 production was determined at indicated time points post-RT (see response to all the concentrations and time-course response in Figure S1A & S1B). (C&D) Water was irradiated at indicated doses. After 1 hour CONPs were added up to 200 μM. H2O2 production was then determined at indicated time points post-RT (see response to all the concentrations in Figure S1C & S1D). (E&F) Water was irradiated at indicated doses. After 24 hours CONPs were added up to 200 μM. H2O2 production was then determined at indicated time points post-RT (see response to all the concentrations in Figure 21E & S1F).*P<0.05 compared to 0 μM CONP at that time point.
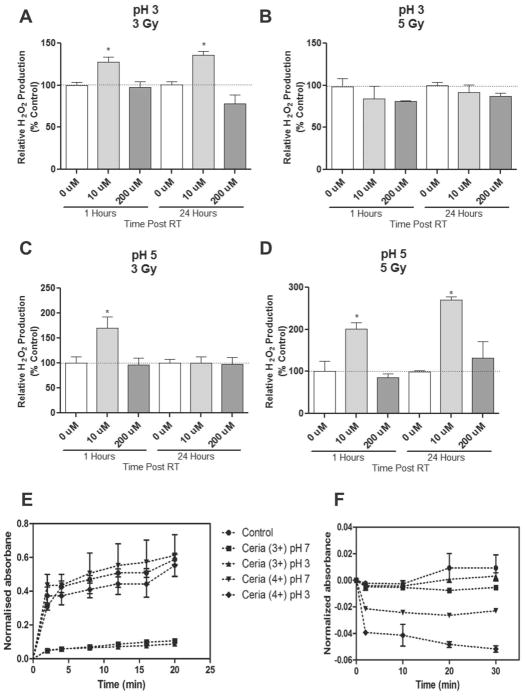
The neutral pH mimics the cellular environment of a normal cell. A cancer cell such as the L3.6pl human pancreatic cancer cells usually maintains an acidic intracellular pH (FigureS4). To assess impact of CONPs on RT-induced H2O2 production at acidic pHs, PBS-buffered solution at pH 5 or pH 3 was incubated with CONPs for 24 hours prior to RT. We found that under these acidic conditions, treatment with CONPs helped maintain the persistent high levels of H2O2 (Figure 3A–3D and Figure S5). Combined with the data obtained at the neutral pH, these results indicate that the acidic environment favors the SOD-mimetic H2O2 producing (or superoxide radical scavenging) activity over the catalase-mimetic H2O2 scavenging activity of CONPs. It has recently been suggested that the switch between the SOD-mimetic and catalase-mimetic activities of CONPs depends upon the oxidation state of CONPs with Ce3+ state favoring the H2O2 production and Ce4+ state favoring the H2O2 scavenging. To determine the potential effect of pH on the role of CONPs of either oxidation state, we added the Ce3+ or Ce4+ CONPs into water solution of pH 7, pH 5 or pH 3 and examined their superoxide radical or H2O2 scavenging activity (Figure 3E & 3F). The Ce3+ CONPs showed high activity on superoxide scavenging (Figure 3E) but little or no activity on H2O2 scavenging regardless of pH changes (Figure 3F). Conversely, the Ce4+ CONPs showed little or no activity on superoxide scavenging at both pHs (Figure 3E) but high activity on H2O2 scavenging at pH 7 which decreased dramatically at pH 3 (Figure 3F). These results imply that the acidic environment plays a major role in accumulating H2O2, presumably through increasing the Ce4+ toCe3+ switch or Ce3+/Ce4+ ratio.
Figure 4.
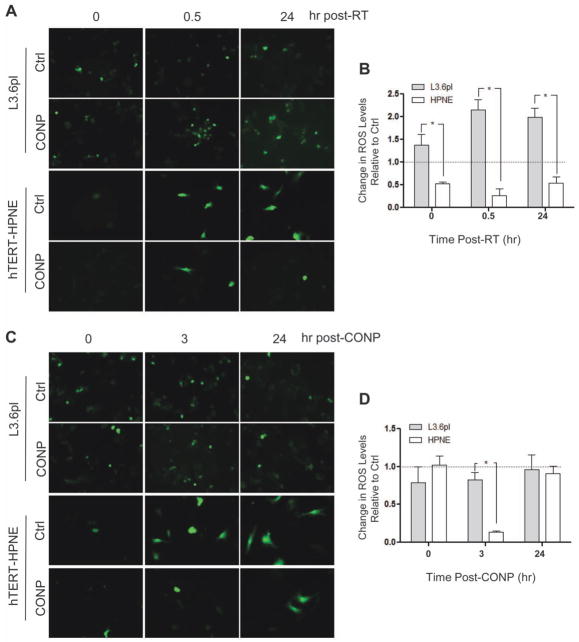
CONP treatment prior to, but not post, RT increase RT-induced ROS levels in acidic pancreatic cancer cells and decrease RT-induced ROS levels in neutral pancreatic normal cells. (A&B) The cancer (L3.6pl) and normal hTERT-HPNE cells were treated with (CONP) or without (Ctrl) CONPs for 24 hours followed by 5 Gy RT. ROS levels were then determined and compared between the cells at indicated times. Relative fold changes were normalized to the control groups. (C&D) The cells were treated with 5 Gy RT for 24 hours prior to CONP treatment. ROS levels were then determined and compared between the cells at indicated times. Relative fold changes are normalized to the control groups. *P < 0.001. The acidic cancer cellular environment relative to the neutral normal cellular environment was confirmed (see Figure S5)
Figure 3.
Under acidic conditions CONPs enhances H2O2 production and lose H2O2 scavenging activity. (A–D) CONPs of serial concentrations (up to 200 μM) were included in acidic PBS solutions at indicated pHs for 24 hours followed by RT at indicated doses. H2O2 production was determined at indicated time points post-RT (see response to all the concentrations and time-course response in Figure S4A–D). *P<0.01 compared to 0 μM at the same time point.
(E&F) CONPs with predominant Ce3+ and Ce4+ on the surface were included in water at indicated pHs for indicated periods of time before concentration of superoxide radical (E) or H2O2 (F) was determined using a SOD assay kit and Amplex Red assay kit, respectively as described in Methods.
CONPs increase radiation-induced ROS levels in pancreatic cancer cells
Next, we investigated whether and to what extent CONPs might affect radiation-induced ROS production in pancreatic cancer cells and normal pancreatic cells. Our results show a persisting increase (> 2-fold, at 0.5 hour through 24 hours post RT) in radiation-induced ROS production in L3.6pl cells pretreated with CONPs (10 μM) for 24 hours (sufficing for CONP uptake by cells before 5 GyRT exposure, as compared to cells exposed to radiation alone (Figure 4A& 4B; P=0.006). In sharp contrast, the same treatment led to a constant decrease (> 50%) in radiation-induced ROS production in hTERT-HPNE cells (Figure 4A& 4B P=0.006). The difference was even greater if the ROS levels were compared between the cancer and normal cells (Figure 4B; P < 0.001). Consistent with the buffer only based experiments shown in Figure 3, these results suggest that radiation action on pre-existing CONPs is critical for ROS production selectively in the cancer cells, presumably by increasing the Ce3+/Ce4+ ratio in the acidic cellular environment. This was further supported by the little effect of post-RT treatment of the cells with CONPs (Figure 4C & 4D). Notably, CONP treatment alone (Figure 4B, 0 hour post-RT) could also increase the ROS levels in the cancer cells but decrease it in the normal cells, suggesting an interesting possibility that the basal oxidation state or Ce3+/Ce4+ ratio of CONPs depends upon the acidity of the cellular environment. While the ROS data show CONPs do act as an antioxidant under the condition of normal cells, the radicals generated by RT seem to eventually overwhelm the antioxidant ability of the CONPs. This is evidenced by the ROS levels 24 hours versus 3 hours post RT, further supporting the potentially differential impact of RT on the Ce3+/Ce4+ ratio in the acidic cancerous versus neutral normal cellular environment. Nevertheless, the CONP-then-RT treatment seems to be the favorable strategy to enhance ROS levels selectively in the cancer cells.
CONPs enhance radiation-induced cell death in pancreatic cancer cells
L3.6pl and hTERT-HPNE cells were incubated with CONPs concentrations (10μM) for 24 hours followed by 5Gy RT and subject to cell viability assays (Figure 5A). CONPs alone induced cancer cell death (12.5%, P=0.0055) compared to control (no treatment) and sensitized L3.6pl cells to 5 GyRT resulting in an additional 12.9% (P=0.0196) increase in cell death compared to cells exposed to radiation alone. Excitingly, CONP treatment caused neither statistically significant increase in cell death nor sensitization to RT in the normal cells. Consistent results were obtained by another independent, clonogenic assays (Figure 5B & 5C). These results indicate that CONPs alone can induce cell toxicity and enhance sensitization to RT selectively in acidic cancer cells.
Figure 5.
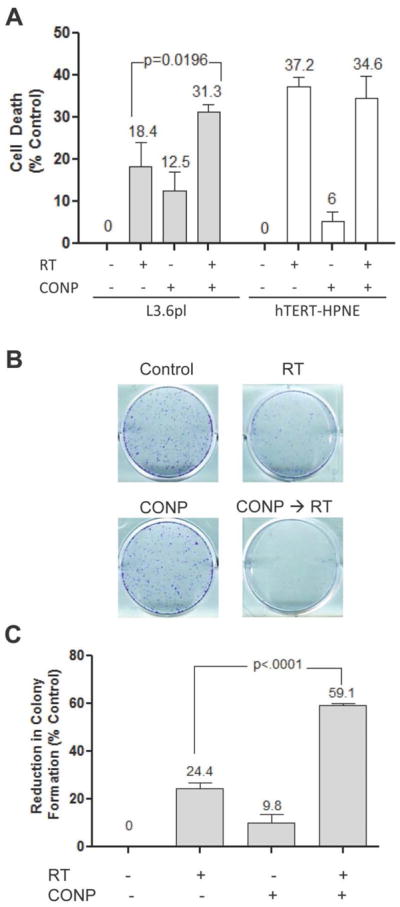
CONP pretreatment selectively sensitizes pancreatic cancer cells to RT-induced cell death in culture. (A) Indicated cells were pre-treated with 10 μM CONPs for 24 hours followed by RT at 5 Gy. Cell viability was determined 96 hours post-RT. Cell death was normalized to untreated group. (B & C) L3.6pl cells were treated similarly as in A. Immediately after the treatment, cells were detached, replated and grown for 7 days before colonies were counted.
CONPs enhance growth inhibition and apoptosis induced by RT in vivo in human pancreatic tumors
To determine if CONPs can increase pancreatic cancer cell sensitivity to RT in vivo, we examined the effect of CONPs on orthotopic L3.6pl tumor growth in athymic nude mice (Table 1). The data show that, compared to RT alone, the addition of CONP treatment to RT causeda dramatic decrease in tumor weight (P=0.0112) and tumor volume (P=0.0006). Interestingly, like RT alone, CONPs alone also resulted in a striking decrease in tumor volume although the change in tumor weight was marginal. While the mean tumor volume may suggest that the synergistic effects of the co-treatment were only marginally better than the CONPs or RT alone, it is not necessarily indicative of the efficacy of the co-treatment. In the co-treated group, 50% of the mice had tumors with a volume less than 1000 mm3, whereas no other group had a single mouse with a tumor that small. Body weight was not changed among all treatment groups as compared with control mice. These results suggest that CONPs can act synergistically to enhance RT-induced inhibition of the tumor growth without causing obvious undesired toxicity to the host.
To determine the mechanisms underlying the tumor growth inhibition, effects of CONPs (alone or in combination with RT) on apoptosis were analyzed on the tumors harvested from the different treatment groups. H & E (Figure 6A, panels a–d) and TUNEL staining (Figure 6A, panels e–h) revealed that there was no apoptosis in untreated control tumors (Figure 6Aa & 6Ae). Apoptosis was increased by 25 times in RT-treated tumors (Figures 6Ab, 6Af and 6B), 120 times in CONP-treated tumors (Figures 6Ac, 6Ag and 6B), and 180 times in CONPs plus RT treated tumors (Figures 6Ad, 6Ah and 6B), with all groups being statistically significantly different from one another following TUNEL quantification (Figure 6B). The apoptosis appeared to be restricted to the tumor tissue, suggesting that CONPs selectively sensitize tumor cells to and protect normal cells from RT-induced apoptosis.
Figure 6.
CONPs enhance tumor cell apoptosis in vivo. (A–D) Histologic evaluations using hematoxylin and eosin (H&E) staining. (E–H) TUNEL staining of apoptosis cells in situ. Tumor cell implantation and treatment of mice are described in Table 1. Tumor tissues along with adjacent normal pancreatic tissues were collected at the time when mice were sacrificed. Formalin-fixed and paraffin-embedded, immediately adjacent tissue sections were used for the staining. Immunopositive cells for TUNEL staining were observed over 10 individual slides for each condition and quantified using the NIH ImageJ software to determine the number of TUNEL positive cells per field of view.
Discussion
This is the first study to examine a potential role of CONPs as an adjuvant for radiation therapy to treat pancreatic cancer. The results demonstrate that CONPs selectively sensitize human pancreatic cancer cells to RT acting as pro-oxidant and induce apoptosis due to acidic tumor cell environment.
The fact that acidic environment allows lower basal levels of H2O2 and more resistance to RT induction of H2O2 than neutral environment (see Figure 1) suggests that the autodismutation activity is lower in acidic than neutral environment. This could make a significant, if not major, contribution to resistance to RT by (acidic) tumor cells as well as RT-induced toxicity to (neutral) normal tissues. Therefore, increasing SOD activity in tumor cells and H2O2 scavenging activity in normal cells appears to be a key to sensitizing cancer cells to RT.
RT alone treatment killed the hTERT-HPNE normal cells (18.4%) more than the L3.6pl cancer cells (37.2%) (Figure 5A). This result clearly indicates that the cancer cells have developed some mechanisms such as high acidity that make them more resistant to RT than the normal cells do. The radiation resistance is often selected during the course of RT and seen in post-radiation tumor regrowth. For this reason, RT sensitization therapy is critical so that the same dose of RT can kill much more cancer cells without increasing the side effect on normal cells. The pre-treatment with CONPs has achieved exactly such a goal of sensitizing the cancer cells selectively to the subsequent RT (Figure 5 and 6) without increasing the RT toxicity to the normal cells (Figure 5A). Previous studies have shown that CONPs are capable of acting as both a SOD mimetic to scavenge superoxide radicals and a catalase mimetic to scavenge H2O2, and that acidic pH promotes SOD mimetic activity of CONPs while inhibiting catalase mimetic activity, resulting in increased accumulation of H2O2. Consistently, our results further demonstrate that the acidity-dependent differential role of Ce3+ versus Ce4+ oxidation state of CONPs is a critical mechanism in the regulation of the activity switch between SOD mimetic and catalase mimetic and subsequent H2O2 accumulation (see Figure 3). This could explain why CONPs alone was almost as toxic as RT to the cancer cells but showed little or no toxicity to the normal cells (Figure 5A). This finding points out an important role of CONPs as a stand-alone therapeutic for cancer treatment. More importantly, our results also indicate that RT further increases the SOD mimetic activity and decreases the catalase mimetic activity of CONPs in acidic environment (see Figure 2 and Figure 3), suggesting that RT may induce a switch of oxidation states from Ce4+ to Ce3+ of CONPs favorably in acidic tumor environment, which is supported by the chemical reaction equation of O22−. + CeO2-x (Ce3+) +2H+ → H2O2+ CeO2 (Ce4+). Indeed, we have observed a few percent increase in Ce3+/Ce4+ ratio at neutral pH induced by radiation (data not shown). It remains unknown and interesting how the SOD activity of Ce3+ and catalase activity of Ce4+ are differentially regulated by different pH and to what extent RT can switch the oxidation states from Ce4+ to Ce3+ at acidic pHs.
It is not known whether the acidic cellular environment also determines a preferential uptake of CONPs into cancer cells. The differential role and oxidation state of CONPs in acidic versus neutral pH environment make the implication of CONP uptake into cancer versus normal cells more complicated. Assuming that as postulated CONPs act primarily as a producer of H2O2 in (acidic) cancer environment and a scavenger of H2O2 in (neutral) normal tissues, one would wish both the cancer and normal cells take up sufficient amount of CONPs during the combination therapy. This would make CONPs a ‘super sensitizer’ of RT which not only sensitizes cancer cells to the therapeutic effect of RT but also desensitizes normal cells to the toxic side-effect of RT. On the other hand, RT could kill the cancer cells and normal cells by distinct mechanisms which could interpret why CONPs reduce ROS levels in the normal cells (Figure 4A) but fails to reduce the killing effect of RT on the cells (Figure 5A). Indeed, in addition to apoptosis, mitotic cell death accounts for a significant portion of radiation induced cytotoxicity. When this death mechanism fails to kill, the survived cancer cells become more resistant to RT. This mechanism also poses a risk of aneuploidy and oncogenesis in the normal cells that have survived the undesired exposure to RT. Given that ROS pathways can induce both mitotic and apoptotic cytotoxicity, it will be interesting to determine whether or not CONP treatment also sensitizes the cancer cells to RT-induced mitotic cell death as well.
Previous work has documented the differential uptake of CONPs by lung cancer and normal cells in culture. However, it remains very challenging to assess CONP uptake in vivo or in situ by tumor versus normal tissues including its levels, distribution, oxidation states as well as subcellular versus extracellular localizations and so on. Furthermore, in addition to regulating ROS, CONPs likely play many other biological roles to be identified in the cells or tissues. Further addressing these ‘nanodynamic’ and ‘nanokinetic’ issues would enhance the interpretation of the pre-clinical therapeutic role of CONPs.
It would be interesting to test whether the H2O2 accumulation is the primary factor contributing to the tumor selective apoptosis (see Figure 6) and whether the apoptosis occurred to the cancer cells only, the tumor stromal cells only or both. One of the tumor-associated stromal cell types is vascular endothelial cells which are the building blocks of tumor angiogenesis that is in turn essential for aggressive tumor growth as well as metastasis. Since all these processes are closely favored by hypoxia-induced acidification within tumor microenvironment, both intracellular and extracellular, of the vast majority types of cancer, CONPs could also play a role selectively against these processes as well of tumor progression of many cancer types including pancreatic cancer. Experiments are in progress to address these important questions.
In summary, this work demonstrates the novel role of CONPs to enhance RT-induced ROS production and cell death selectively in human pancreatic tumor cells while protecting normal tissues from the toxic side-effect of RT depending upon the environmental acidity. These findings suggest that CONPs may be further developed as a novel tumor tissue sensitizer and normal tissue protectant to increase the therapeutic index of RT for improving treatment of pancreatic cancer patients.
Supplementary Material
Acknowledgments
Financial Support: Bankhead-Coley Cancer Research Program, Florida Department of Health to CHB (09BN-01-23081), National Health Institute to JT (CA128865) and JZ (CA132977), and National Science Foundation to SS (CBET 1007495). This is part of the Ph.D. thesis work of MW at University of Central Florida
We thank Drs. William T. Self and Annette Khaled and Ms. Rebecca Boohaker for helpful comments and technical advice. We also thank all the members of JZ laboratory particularly Dr. Heng Lu for technical assistance and critical discussions.
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sharma C, Eltawil KM, Renfrew PD, Walsh MJ, Molinari M. Advances in diagnosis, treatment and palliation of pancreatic carcinoma: 1990–2010. World J Gastroenterol. 2011;17:867–897. doi: 10.3748/wjg.v17.i7.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kouvaris JR, Kouloulias VE, Vlahos LJ. Amifostine: the first selective-target and broad-spectrum radioprotector. Oncologist. 2007;12:738–747. doi: 10.1634/theoncologist.12-6-738. [DOI] [PubMed] [Google Scholar]
- 3.Stolarska M, Mlynarski W, Zalewska-Szewczyk B, Bodalski J. Cytoprotective effect of amifostine in the treatment of childhood neoplastic diseases--a clinical study including the pharmacoeconomic analysis. Pharmacol Rep. 2006;58:30–34. [PubMed] [Google Scholar]
- 4.Krug LM, Curley T, Schwartz L, Richardson S, Marks P, Chiao J, Kelly WK. Potential role of histone deacetylase inhibitors in mesothelioma: clinical experience with suberoylanilide hydroxamic acid. Clin Lung Cancer. 2006;7:257–261. doi: 10.3816/CLC.2006.n.003. [DOI] [PubMed] [Google Scholar]
- 5.Huang P, Yang DP, Zhang C, Lin J, He M, Bao L, Cui D. Protein-directed one-pot synthesis of Ag microspheres with good biocompatibility and enhancement of radiation effects on gastric cancer cells. Nanoscale. 2011;3:3623–3626. doi: 10.1039/c1nr10586h. [DOI] [PubMed] [Google Scholar]
- 6.Huang P, Bao L, Zhang C, Lin J, Luo T, Yang D, He M, Li Z, Gao G, Gao B, Fu S, Cui D. Folic acid-conjugated silica-modified gold nanorods for X-ray/CT imaging-guided dual-mode radiation and photo-thermal therapy. Biomaterials. 2011;32:9796–9809. doi: 10.1016/j.biomaterials.2011.08.086. [DOI] [PubMed] [Google Scholar]
- 7.Gupta S, Brouwer P, Bandyopadhyay S, Patil S, Briggs R, Jain J, Seal S. TEM/AFM investigation of size and surface properties of nanocrystalline ceria. J Nanosci Nanotechnol. 2005;5:1101–1107. doi: 10.1166/jnn.2005.151. [DOI] [PubMed] [Google Scholar]
- 8.Patil S, Sandberg A, Heckert E, Self W, Seal S. Protein adsorption and cellular uptake of cerium oxide nanoparticles as a function of zeta potential. Biomaterials. 2007;28:4600–4607. doi: 10.1016/j.biomaterials.2007.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das M, Patil S, Bhargava N, Kang JF, Riedel LM, Seal S, Hickman JJ. Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials. 2007;28:1918–1925. doi: 10.1016/j.biomaterials.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunner TJ, Wick P, Manser P, Spohn P, Grass RN, Limbach LK, Bruinink A, Stark WJ. In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubility. Environ Sci Technol. 2006;40:4374–4381. doi: 10.1021/es052069i. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Patil S, Seal S, McGinnis JF. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat Nanotechnol. 2006;1:142–150. doi: 10.1038/nnano.2006.91. [DOI] [PubMed] [Google Scholar]
- 12.Heckert EG, Karakoti AS, Seal S, Self WT. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials. 2008;29:2705–2709. doi: 10.1016/j.biomaterials.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pirmohamed T, Dowding JM, Singh S, Wasserman B, Heckert E, Karakoti AS, King JE, Seal S, Self WT. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem Commun (Camb) 2010;46:2736–2738. doi: 10.1039/b922024k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colon J, Herrera L, Smith J, Patil S, Komanski C, Kupelian P, Seal S, Jenkins DW, Baker CH. Protection from radiation-induced pneumonitis using cerium oxide nanoparticles. Nanomedicine. 2009;5:225–231. doi: 10.1016/j.nano.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Tarnuzzer RW, Colon J, Patil S, Seal S. Vacancy engineered ceria nanostructures for protection from radiation-induced cellular damage. Nano Lett. 2005;5:2573–2577. doi: 10.1021/nl052024f. [DOI] [PubMed] [Google Scholar]
- 16.Colon J, Hsieh N, Ferguson A, Kupelian P, Seal S, Jenkins DW, Baker CH. Cerium oxide nanoparticles protect gastrointestinal epithelium from radiation-induced damage by reduction of reactive oxygen species and upregulation of superoxide dismutase 2. Nanomedicine. 2010;6:698–705. doi: 10.1016/j.nano.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Xue Y, Luan QF, Yang D, Yao X, Zhou KB. Direct Evidence for Hydroxyl Radical Scavenging Activity of Cerium Oxide Nanoparticles. Journal of Physical Chemistry C. 2011;115:4433–4438. [Google Scholar]
- 18.Asati A, Santra S, Kaittanis C, Nath S, Perez JM. Oxidase-like activity of polymer-coated cerium oxide nanoparticles. Angew Chem Int Ed Engl. 2009;48:2308–2312. doi: 10.1002/anie.200805279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alili L, Sack M, Karakoti AS, Teuber S, Puschmann K, Hirst SM, Reilly CM, Zanger K, Stahl W, Das S, Seal S, Brenneisen P. Combined cytotoxic and anti-invasive properties of redox-active nanoparticles in tumor-stroma interactions. Biomaterials. 2011;32:2918–2929. doi: 10.1016/j.biomaterials.2010.12.056. [DOI] [PubMed] [Google Scholar]
- 20.Lin W, Huang YW, Zhou XD, Ma Y. Toxicity of cerium oxide nanoparticles in human lung cancer cells. Int J Toxicol. 2006;25:451–457. doi: 10.1080/10915810600959543. [DOI] [PubMed] [Google Scholar]
- 21.Park EJ, Choi J, Park YK, Park K. Oxidative stress induced by cerium oxide nanoparticles in cultured BEAS-2B cells. Toxicology. 2008;245:90–100. doi: 10.1016/j.tox.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 22.Vincent A, Babu S, Heckert E, Dowding J, Hirst SM, Inerbaev TM, Self WT, Reilly CM, Masunov AE, Rahman TS, Seal S. Protonated nanoparticle surface governing ligand tethering and cellular targeting. ACS Nano. 2009;3:1203–1211. doi: 10.1021/nn9000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruns CJ, Harbison MT, Kuniyasu H, Eue I, Fidler IJ. In vivo selection and characterization of metastatic variants from human pancreatic adenocarcinoma by using orthotopic implantation in nude mice. Neoplasia. 1999;1:50–62. doi: 10.1038/sj.neo.7900005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karakoti AS, Kuchibhatla SVNT, Suresh Babu K, Seal S. Direct Synthesis of Nanoceria in Aqueous Polyhydroxyl Solutions. J Phys Chem C. 2007;111:17232–17240. [Google Scholar]
- 25.Deshpande S, Patil S, Kuchibhatla ST, Seal S. Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. APPLIED PHYSICS LETTERS. 2005;87 [Google Scholar]
- 26.Oleshko VP, Howe JM, Shukla S, Seal S. High-resolution and analytical TEM investigation of metastable-tetragonal phase stabilization in undoped nanocrystalline zirconia. J Nanosci Nanotechnol. 2004;4:867–875. doi: 10.1166/jnn.2004.110. [DOI] [PubMed] [Google Scholar]
- 27.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 28.Singh V, Singh S, Das S, Kumar A, Self WT, Seal S. A facile synthesis of PLGA encapsulated cerium oxide nanoparticles: release kinetics and biological activity. Nanoscale. 2012 doi: 10.1039/c2nr12131j. [DOI] [PubMed] [Google Scholar]
- 29.Baker CH, Kedar D, McCarty MF, Tsan R, Weber KL, Bucana CD, Fidler IJ. Blockade of epidermal growth factor receptor signaling on tumor cells and tumor-associated endothelial cells for therapy of human carcinomas. Am J Pathol. 2002;161:929–938. doi: 10.1016/S0002-9440(10)64253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker CH, Solorzano CC, Fidler IJ. Blockade of vascular endothelial growth factor receptor and epidermal growth factor receptor signaling for therapy of metastatic human pancreatic cancer. Cancer Res. 2002;62:1996–2003. [PubMed] [Google Scholar]
- 31.Konduri S, Colon J, Baker CH, Safe S, Abbruzzese JL, Abudayyeh A, Basha MR, Abdelrahim M. Tolfenamic acid enhances pancreatic cancer cell and tumor response to radiation therapy by inhibiting surviv in protein expression. Mol Cancer Ther. 2009;8:533–542. doi: 10.1158/1535-7163.MCT-08-0405. [DOI] [PubMed] [Google Scholar]
- 32.Singh S, Kumar A, Karakoti A, Seal S, Self WT. Unveiling the mechanism of uptake and sub-cellular distribution of cerium oxide nanoparticles. Mol Biosyst. 2010;6:1813–1820. doi: 10.1039/c0mb00014k. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.