Abstract
Decision-making requires stimulus categorization and localization to guide accurate responses that can be produced through multiple effectors. The success of actions is monitored so that performance can be adjusted to achieve goals. This review will survey recent empirical and theoretical developments very selectively with an emphasis on neurophysiological data from nonhuman primates that provide the clearest information about neural mechanisms.
What is a decision?
Decision-making requires multiple processes1. A choice is required when an organism is confronted with alternatives for which an action is required to achieve a goal. Choices are evaluated as good or bad according to whether goals are achieved and consequences are as expected. The term decision is used casually and technically in several non-interchangeable senses. In particular, we can refer to a decision as a deliberation process that results in the overt act of choosing. Decision as a process has two logically and mechanistically distinct meanings – “decide to”, which is a selection between alternative actions, and “decide that”, which is a selection between alternative categories of an stimulus or concept. The logical distinction is easy to see. Whereas you can “decide that” falsely, it is not intelligible to “decide to” falsely. “Decide to”, like choosing, is judged just as good or bad. However, important distinctions can be recognized between “choose” and “decide to”. Whereas choice refers most clearly to the final commitment to one among alternative actions, decision refers most clearly to the deliberation preceding the action. The polarity between deciding and choosing is highlighted further by appreciating that although choices can be predicted, decisions cannot be predicted. If you can say what you will decide, then you will have decided.
The semantic and logic differences between “decide that” and “decide to” are paralleled by their embodiment in different neural circuits. Neurons in sensory structures that encode the features of stimuli provide the input to circuits in association structures that accomplish the categorization and localization that constitutes the evidence for a decision. Neurons in motor structures that control the innervation of the muscles accomplish the actions guided, for better or worse, by the sequence of decision processes. Sensorimotor association structures, exemplified by the frontal eye field (FEF), consist of a diversity of types of neurons with different patterns of modulation derived from different inputs to different cortical layers. The distinction between “decide that” and “decide to” also corresponds to a very long history of experimental and theoretical psychology that describes response times as the outcome of successive stages of processing2. Of course, decisions have consequences. Detecting and adapting to consequences is distinct from the actual decision process. Figure 1 summarizes this framework.
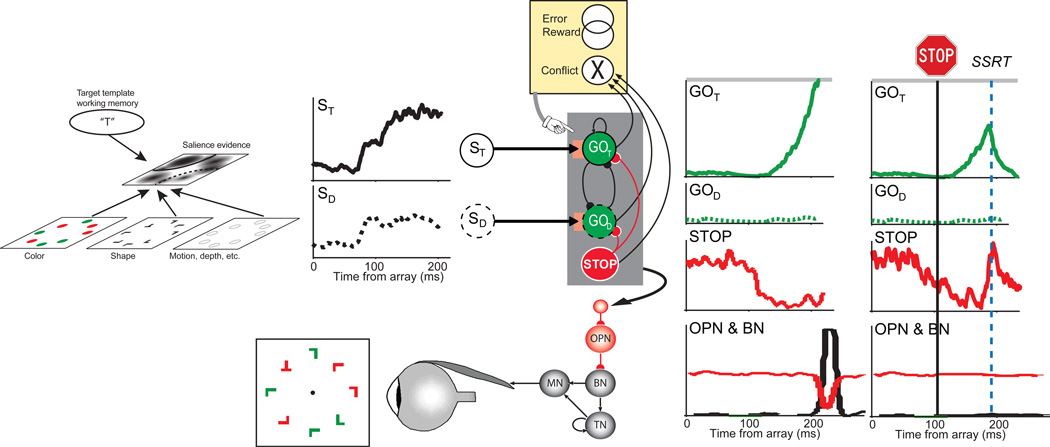
Figure 1.
Neural networks for the guidance and control of visually guided saccades. Consider visual search for a red “T” among randomly oriented red and green “L”s. The color and shape of the objects are specified in feature maps that could also represent motion, depth and other visual features. These feature maps converge on a map that represents the evidence for salience at each location. This salience map is also informed by a target template in working memory. The timecourse of the salience evidence representation at the target location (ST, solid line) and a distractor location (SD, dotted line) is plotted. According to the gated accumulator model, this evidence is integrated by a network of mutually-inhibitory units that will produce a saccade to the target (GOT, solid line) or to a distractor (GOD, dotted line). A gate (orange box) prevents integration of noise by requiring the salience evidence to be of sufficient magnitude. A saccade is produced when the activation of a GO unit reaches a threshold (gray horizontal line) at which point inhibition is imposed on omnipause (OPN) neurons (red line) that releases inhibition of burst neurons (BN) that innervate motor neurons (MN) to produce a pulse of force to rotate the eye rapidly. The eye velocity signal from the BNs are integrated by a network of tonic neurons (TN) that also innervate the MN to establish a step of force necessary to maintain eccentric fixation of the target. The activation of the GO units is also influenced by gaze-holding STOP units that release inhibition on the GO units while saccade preparation transpires. If a stop signal of some kind occurs, then the STOP units potently interrupt the GO unit activation from reaching the threshold; this interruption occurs within the theoretical interval known as stop signal reaction time (SSRT) (rightmost columns). An executive control network (yellow) comprised of neurons sensitive to errors, reward and the conflict arising from co-activation of mutually incompatible response processes signals the consequences and conditions of an action. This executive control network may influence the level of the gate that systematically changes the beginning of the accumulation process to emphasize either speed or accuracy in task performance.
Decide that – Categorization and stimulus selection
If objects in the environment are not discriminated correctly, decisions cannot be effective. Neural circuits responsible for object categorization extend from the primary sensory structures that encode basic stimulus features to association areas in parietal and frontal lobes3. One well-known line of research has investigated perceptual categorization by requiring subjects to discriminate the direction of motion of a stochastic dot display4. This work has revealed much about the encoding of stimulus motion by area MT and the evolution of activity of neurons in area LIP to arrive at a categorization of the motion direction5. More recent work has demonstrated the necessity of mastering the stimulus-response association6 and elaborated the differential contribution of neurons in the caudate7 and frontal eye field (FEF)8. Other work also highlights the contribution of the frontal lobe to stimulus categorization9,10,11.
The world presents us with many stimuli, most of which must be ignored in the guidance of action. Visual search, selection of a target from among non-target objects, has also been used to study perceptual decision-making. The investigation of the neural basis of visual search has been framed by the discovery that neurons in frontal, parietal, temporal and occipital cortical areas as well as the superior colliculus and thalamus respond to target and distractor stimuli initially equivalently but then over time the activity representing the target remains elevated or increases while the activity representing distractors is attenuated12.
Several recent studies have measured this target selection process across brain regions and measurement levels. When a target is selected, does it happen more or less simultaneously across the network or in some sequence? These studies have focused on the FEF and areas in the back of the brain. The relevance of FEF for processes occurring in the back of the brain has been demonstrated vividly by the finding in monkeys and humans that stimulation of FEF influences the allocation of visual spatial attention13,14 perhaps through modulation of neural activity in extrastriate visual areas15,16. The various studies provide much more information than is summarized here, and they differ in a number of crucial details that frame their interpretation17. Every study has found that when the target is more difficult to locate, neurons in the FEF signal its location before neurons in occipital, parietal and temporal areas18,19,20,21,22. However, findings differ when the target is located easily (pop-out search). One study reports that the parietal cortex locates the target before the frontal cortex18, but two others find that parietal cortex signals do not precede frontal target selection23,24. This pattern of results was obtained with intracranial recordings of spikes and LFP and also with an event-related potential component known as the N2pc25 (or posterior contralateral negativity26) that is believed to originate from visual and association areas in the parietal and temporal lobes27,28.
The association between FEF and V4 was mediated primarily by visual and not visuomovement or movement neurons in FEF29. The evidence that visual instead of movement neurons in FEF influence V4 is consistent with recent anatomical data showing that whereas only neurons in layer 5 of FEF project to brainstem saccade structures such as the superior colliculus, the major source of input to extrastriate visual cortical areas like V4, MT and LIP arises from layer 2–3 of FEF30. Moreover, V4 and MT (and probably LIP) are innervated by different neurons in FEF that themselves have qualitatively different afferents31. Thus, the “top-down” signal from FEF to the back of the brain is not a single mechanism; each area is under some as-yet-to-be-determined different quality of influence. Additional indirect evidence that the influence of FEF on V4 is mediated by supragranular more than infragranular neurons is provided by a recent study showing that blocking D1 but not D2 receptors in FEF influenced V4 activity based on the lower density of D2 receptors in supragranular FEF32.
Decide to – Response selection and preparation
Locating and categorizing objects does not oblige any particular action. Other neural circuits are responsible for selecting and producing body movements to achieve goals. The neural dissociation between “decide that” circuits and “decide to” circuits has been demonstrated in numerous studies; for example, when no saccade is produced, the search target selection process transpires normally while presaccadic movement neurons are suppressed 29,33,34.
Research over decades has shown that movements are prepared through the progressive increase of discharge rate of neurons innervating premotor structures in the brainstem and spinal cord, and movements are initiated when the discharge rate reaches a threshold that does not vary with RT35,36,37,38. New work has suggested instead that movements are initiated when a neural population reaches some point in a high-dimensional dynamical space39. Recent findings from the FEF indicate that these alternative hypotheses may not be contradictory40.
Explaining how sensory representations lead to accurate movements is a classic problem. One approach to this problem is based on the premise that noisy evidence guiding a response is accumulated over time until a threshold is achieved at which time the response is initiated. A recent model inspired by this approach provides an explanation for how signals from neurons that represent target salience can be transformed into a saccade command41,42. The model begins with the simple assumption that the input to the neurons producing saccade responses is simply a feed-forward cascade of the output of the visual selection neurons representing the salience at the various locations in the search array. The stochastic variability in the evidence provided by the selection process is translated into variability in choice performance through the accumulation of that evidence by a network of mutually inhibitory, leaky integrators. The evidence accumulated by the network of integrators was equated with the spike trains recorded from the visual selection neurons in the FEF. Accumulated variability in the firing rates of these neurons explains the probability and timing of correct and error responses with search arrays of different set sizes if the accumulators are mutually inhibitory. Although not designed in the model, the dynamics of the stochastic accumulators quantitatively correspond to the activity of presaccadic movement neurons that initiate eye movements if gating inhibition prevents accumulation before sufficient evidence about stimulus salience has emerged. Adjustments in the level of gating inhibition can control tradeoffs in speed and accuracy that optimize visual search performance.
Although this is the only model of visual search that accounts for response time distributions43, it assumes that saccade production is guided entirely by the visual salience representation so errant saccades originate in a failure to represent evidence correctly. While this has been observed in some testing conditions44,45, in other conditions the salient target is located correctly, but the responses are incorrect46,47. If the evidence is correct, why was an error made? Obviously, the response production stage, while guided by, can operate independently of the perceptual stage. Indeed, response selection errors can be corrected before visual processing can register that the gaze shift was an error48.
Monitoring consequences
The correction of errors before sensory processing can be completed demonstrates the existence of a system that monitors performance. Research over the last 20 years has characterized the role of a circuit involving medial frontal cortex in executive control for limbs49 and eyes50. A major thread of this research began with the discovery of the error-related negativity (ERN), an event-related potential that occurs when participants produce errors51. Macaque monkeys possess the same error monitoring system as evidenced by neural spikes52,53 and local field potentials54,55 modulated after errors. In fact, monkeys also exhibit the ERN56. A diversity of other neurons signal the anticipation and delivery of feedback and reinforcement and also perhaps conflict between competing response processes57; some of these resemble signals produced by brainstem dopamine neurons58. The presence of these signals is consistent with models of executive function based on reward prediction error59,60.
Adjusting performance
Stochastic accumulator models account for adaptation of RT to minimize errors and maximize rewards most commonly through changes in the amount of accumulation necessary to trigger a response61,62,63. Recent fMRI studies have reported evidence consistent with this64,65,66,67,68. However, this conclusion may be premature. First, the areas with clearest modulation were in medial frontal cortex. Current neurophysiological evidence shows that weak electrical stimulation of SEF can elevate RT69, but individual neurons in SEF do not control directly saccade initiation70, nor do single neurons in SMA or pre-SMA control directly limb movement times71. Therefore, medial frontal areas could contribute to strategic RT adjustment necessary for SAT, but neurophysiological evidence is inconsistent with the mapping of a response threshold on activation in these areas. Also, mapping particular parameters of very simple computational models to highly-derived measures of cerebral oxygen utilization seems uncertain.
Moreover, two recent neurophysiological studies provide compelling evidence that the accumulator models do not account for all of the adjustments that mediate speed-accuracy adaptation. One study showed that the adaptive slowing of RT in the stop signal task is accomplished not by a change of threshold, baseline, or accumulation rate, but instead through a change in the time when presaccadic movement activity first begins to accumulate72. Another study trained macaque monkeys to trade speed for accuracy on cue during visual search73. This speed-accuracy tradeoff was accomplished through several distinct neural adjustments. When accuracy was cued, baseline discharge rate was reduced before visual search arrays appeared, visual response magnitude was attenuated, neural target selection time was delayed, and movement-related activity accumulated more slowly to a lower level before saccades. This surprising pattern of modulation demonstrates that the popular stochastic accumulator models do not provide an accurate or complete description of how the brain adjusts performance.
Summary
Decision-making is accomplished by a diversity of neural circuits that influence one another is ways that remain poorly understood. Computational models of decision-making are simplifications that embody particular assumptions based on intuitions about simplicity and optimality. These models have proven very effective at describing performance on decision tasks. The evident parallels between the form of activity of some neurons and the form of the processes in these models has invited and encouraged rather direct mapping of model process onto particular neurons. However, new data indicate that correct formulation of such linking propositions will require an unexpected degree of subtlety and nuance. For example, models that require discrete stage completion times are inconsistent with the diversity of times when stimulus categorization and localization is accomplished in different structures. Also, models that explain all of the variation of error and correct responses in a single evidence-accumulating categorization stage are inconsistent with the production of errors by the response selection circuit. How to translate between different levels of analysis and description remains a major challenge. The heterogeneity and anatomical specificity of neural circuits, timings and processes (that cannot be resolved by measures like ERP and fMRI) must be appreciated to evaluate how models of decision-making actually map onto neural mechanisms.
Highlights.
Categorization and stimulus selection are accomplished by sensory and association circuits
Response preparation is accomplished by association and motor circuits
Monitoring and adjustment involve medial frontal areas
Speed-accuracy adjustments involve perceptual and response stages
Acknowledgements
The author’s research has been supported by National Eye Institute, National Institute of Mental Health, National Science Foundation, McKnight Endowment Fund for Neuroscience, Air Force Office of Scientific Research and by Robin and Richard Patton through the E. Bronson Ingram Chair in Neuroscience.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schall JD. Decision making. Curr Biol. 2005;15:R9–R11. doi: 10.1016/j.cub.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 2. Sternberg S. Modular processes in mind and brain. Cogn Neuropsychol. 2011;28:156–208. doi: 10.1080/02643294.2011.557231. This paper describes a powerful approach, termed separate modifiability, to identifying cognitive modules and associated neural processes. Specific examples of reliable and unreliable approaches are reviewed.
- 3.Romo R, Lemus L, de Lafuente V. Sense, memory, and decision-making in the somatosensory cortical network. Curr Opin Neurobiol. 2012 Aug 28; doi: 10.1016/j.conb.2012.08.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- 5.Churchland AK, Ditterich J. New advances in understanding decisions among multiple alternatives. Curr Opin Neurobiol. 2012 May 1; doi: 10.1016/j.conb.2012.04.009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Law CT, Gold JI. Neural correlates of perceptual learning in a sensory-motor, but not a sensory, cortical area. Nat Neurosci. 2008;11:505–513. doi: 10.1038/nn2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding L, Gold JI. Caudate encodes multiple computations for perceptual decisions. J Neurosci. 2010;30:15747–15759. doi: 10.1523/JNEUROSCI.2894-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ding L, Gold JI. Neural correlates of perceptual decision making before, during, and after decision commitment in monkey frontal eye field. Cereb Cortex. 2012;22:1052–1067. doi: 10.1093/cercor/bhr178. This paper with [7] describes the diverse patterns of activity of neurons in the caudate nucleus and FEF in the well-known stochastic motion direction discrimination task.
- 9.Ferrera VP, Yanike M, Cassanello C. Frontal eye field neurons signal changes in decision criteria. Nat Neurosci. 2009;12:1458–1462. doi: 10.1038/nn.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kusunoki M, Sigala N, Gaffan D, Duncan J. Detection of fixed and variable targets in the monkey prefrontal cortex. Cereb Cortex. 2009;19:2522–2534. doi: 10.1093/cercor/bhp005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cromer JA, Roy JE, Miller EK. Representation of multiple, independent categories in the primate prefrontal cortex. Neuron. 2010;66:796–807. doi: 10.1016/j.neuron.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schall JD, Cohen JY. The neural basis of saccade target selection. In: Liversedge Simon P, Gilchrist Iain P, Everling Stefan., editors. Oxford Handbook on Eye Movements. Oxford University Press; 2011. [Google Scholar]
- 13.Moore T, Fallah M. Microstimulation of the frontal eye field and its effects on covert spatial attention. J. Neurophysiol. 2004;91:152–162. doi: 10.1152/jn.00741.2002. [DOI] [PubMed] [Google Scholar]
- 14.Grosbras MH, Paus T. Transcranial magnetic stimulation of the human frontal eye field: effects on visual perception and attention. J Cogn Neurosci. 2002;14:1109–1120. doi: 10.1162/089892902320474553. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong KM, Fitzgerald JK, Moore T. Changes in visual receptive fields with microstimulation of frontal cortex. Neuron. 2006;50:791–798. doi: 10.1016/j.neuron.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Silvanto J, Lavie N, Walsh V. Stimulation of the human frontal eye fields modulates sensitivity of extrastriate visual cortex. J Neurophysiol. 2006;96:941–945. doi: 10.1152/jn.00015.2006. [DOI] [PubMed] [Google Scholar]
- 17.Schall JD, Paré M, Woodman GF. Comment on "Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices". Science. 2007;318:44. doi: 10.1126/science.1144865. author reply 44. [DOI] [PubMed] [Google Scholar]
- 18. Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. This paper describes the first direct comparison of the responses of neurons in frontal and parietal areas during a visual search task. The results obtained agree in some respects and differ in others in comparison to other studies for reasons that are considered in [17].
- 19. Cohen JY, Heitz RP, Schall JD, Woodman GF. On the origin of event-related potentials indexing covert attentional selection during visual search. J Neurophysiol. 2009;102:2375–2386. doi: 10.1152/jn.00680.2009. This paper and [24] report the first neurophysiological data on the intracranial origin of the N2pc and demonstrate the contribution of the FEF to this ERP that is supposed to arise from current sources in parietal and temporal cortex.
- 20. Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324:1207–1210. doi: 10.1126/science.1171402. This paper with 21 and 29 describe the first data from simultaneous recordings from FEF and V4. These papers demonstrate remarkable specificity in the interactions between FEF and V4 that arises from the specificity of the anatomical connectivity. Such findings indicate the lack of utility in treating cortical areas as unitary entities in the analysis of neurophysiological or neuroimaging data.
- 21.Zhou H, Desimone R. Feature-based attention in the frontal eye field and area V4 during visual search. Neuron. 2011;70:1205–1217. doi: 10.1016/j.neuron.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Monosov IE, Sheinberg DL, Thompson KG. Paired neuron recordings in the prefrontal and inferotemporal cortices reveal that spatial selection precedes object identification during visual search. Proc Natl Acad Sci U S A. 2010;107:13105–13110. doi: 10.1073/pnas.1002870107. This paper is the first to describe data from simultaneous recordings from FEF and IT.
- 23.Katsuki F, Constantinidis C. Early involvement of prefrontal cortex in visual bottom-up attention. Nat Neurosci. 2012;15:1160–1166. doi: 10.1038/nn.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purcell BA, Schall JD, Woodman GF. On the origin of event-related potentials indexing covert attentional selection during visual search: Timing of selection during pop-out search. J Neurophysiol. 2012 Oct 24; doi: 10.1152/jn.00549.2012. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luck SJ. The Oxford Handbook of Event-Related Potential Components. Oxford University Press; 2012. Electrophysiological correlates of the focusing of attention within complex visual scenes: N2pc and related ERP components; pp. 329–360. [Google Scholar]
- 26.Töllner T, Rangelov D, Müller HJ. How the speed of motor-response decisions, but not focal-attentional selection, differs as a function of task set and target prevalence. Proc Natl Acad Sci U S A. 2012;109:E1990–E1999. doi: 10.1073/pnas.1206382109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hopf JM, Luck SJ, Girelli M, Hagner T, Mangun GR, Scheich H, Heinze HJ. Neural sources of focused attention in visual search. Cereb Cortex. 2000;10:1233–1241. doi: 10.1093/cercor/10.12.1233. [DOI] [PubMed] [Google Scholar]
- 28.Boehler CN, Tsotsos JK, Schoenfeld MA, Heinze HJ, Hopf JM. Neural mechanisms of surround attenuation and distractor competition in visual search. J Neurosci. 2011;31:5213–5224. doi: 10.1523/JNEUROSCI.6406-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gregoriou GG, Gotts SJ, Desimone R. Cell-type-specific synchronization of neural activity in FEF with V4 during attention. Neuron. 2012;73:581–594. doi: 10.1016/j.neuron.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pouget P, Stepniewska I, Crowder EA, Leslie MW, Emeric EE, Nelson MJ, Schall JD. Visual and motor connectivity and the distribution of calcium-binding proteins in macaque frontal eye field: implications for saccade target selection. Front Neuroanat. 2009;3:2. doi: 10.3389/neuro.05.002.2009. This paper demonstrates that different neurons in FEF send axons to the superior colliculus and send axons to area V4. While known for other cortical areas in monkeys and in other species, this paper demonstrated this to be the case emphasized for the FEF and highlighted the implications of such wiring specificity for understanding functional capacity of the different pathways issued from FEF.
- 31. Ninomiya T, Sawamura H, Inoue K, Takada M. Segregated pathways carrying frontally derived top-down signals to visual areas MT and V4 in macaques. J Neurosci. 2012;32:6851–6858. doi: 10.1523/JNEUROSCI.6295-11.2012. This anatomical tracing study has profound cortical computation implications. Using trans-synaptic retrograde tracers, the authors found that area V4, like area MT, gets first-order input from FEF and LIP and second-order input from ventral area 46. In contrast area MT also gets second-order input from the supplementary eye field. Using simultaneous retrograde tracers in V4 and MT, the authors found no neurons in FEF or LIP projecting to both areas, consistent with effectively all other cortical mapping studies.
- 32.Noudoost B, Moore T. Control of visual cortical signals by prefrontal dopamine. Nature. 2011;474:372–375. doi: 10.1038/nature09995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bennur S, Gold JI. Distinct representations of a perceptual decision and the associated oculomotor plan in the monkey lateral intraparietal area. J Neurosci. 2011;31:913–921. doi: 10.1523/JNEUROSCI.4417-10.2011. By introducing flexible stimulus-response mapping, this paper presents results indicating that perceptual decision-making and action selection are different brain processes that only appear to be inseparable under particular behavioral contexts.
- 34.Thompson KG, Biscoe KL, Sato TR. Neuronal basis of covert spatial attention in the frontal eye field. J Neurosci. 2005;25:9479–9487. doi: 10.1523/JNEUROSCI.0741-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanes DP, Patterson WF, 2nd, Schall JD. Role of frontal eye fields in countermanding saccades: visual, movement, and fixation activity. J Neurophysiol. 1998;79:817–834. doi: 10.1152/jn.1998.79.2.817. [DOI] [PubMed] [Google Scholar]
- 36.Cisek P, Kalaska JF. Neural mechanisms for interacting with a world full of action choices. Annu Rev Neurosci. 2010;33:269–298. doi: 10.1146/annurev.neuro.051508.135409. [DOI] [PubMed] [Google Scholar]
- 37.Lecas JC, Requin J, Anger C, Vitton N. Changes in neuronal activity of the monkey precentral cortex during preparation for movement. J Neurophysiol. 1986;56:1680–1702. doi: 10.1152/jn.1986.56.6.1680. [DOI] [PubMed] [Google Scholar]
- 38.Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science. 1996;274:427–430. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- 39.Shenoy KV, Kaufman MT, Sahani M, Churchland MM. A dynamical systems view of motor preparation: implications for neural prosthetic system design. Prog Brain Res. 2011;192:33–58. doi: 10.1016/B978-0-444-53355-5.00003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Purcell BA, Heitz RP, Cohen JY, Schall JD. Response variability of frontal eye field neurons modulates with sensory input and saccade preparation but not visual search salience. J Neurophysiol. 2012 Sep 5; doi: 10.1152/jn.00613.2012. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Purcell BA, Heitz RP, Cohen JY, Schall JD, Logan GD, Palmeri TJ. Neurally constrained modeling of perceptual decision making. Psychol Rev. 2010;117:1113–1143. doi: 10.1037/a0020311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Purcell BA, Schall JD, Logan GD, Palmeri TJ. From salience to saccades: multiple-alternative gated stochastic accumulator model of visual search. J Neurosci. 2012;32:3433–3446. doi: 10.1523/JNEUROSCI.4622-11.2012. This paper builds on 41 to develop the first neurally-based model of multi-alternative decision-making.
- 43.Wolfe JM, Palmer EM, Horowitz TS. Reaction time distributions constrain models of visual search. Vision Res. 2010;50:1304–1311. doi: 10.1016/j.visres.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson KG, Bichot NP, Sato TR. Frontal eye field activity before visual search errors reveals the integration of bottom-up and top-down salience. J Neurophysiol. 2005;93:337–351. doi: 10.1152/jn.00330.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heitz RP, Cohen JY, Woodman GF, Schall JD. Neural correlates of correct and errant attentional selection revealed through N2pc and frontal eye field activity. J Neurophysiol. 2010;104:2433–2441. doi: 10.1152/jn.00604.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murthy A, Ray S, Shorter SM, Schall JD, Thompson KG. Neural control of visual search by frontal eye field: effects of unexpected target displacement on visual selection and saccade preparation. J Neurophysiol. 2009;101:2485–2506. doi: 10.1152/jn.90824.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trageser JC, Monosov IE, Zhou Y, Thompson KG. A perceptual representation in the frontal eye field during covert visual search that is more reliable than the behavioral report. Eur J Neurosci. 2008;28:2542–2549. doi: 10.1111/j.1460-9568.2008.06530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murthy A, Ray S, Shorter SM, Priddy EG, Schall JD, Thompson KG. Frontal eye field contributions to rapid corrective saccades. J Neurophysiol. 2007;97:1457–1469. doi: 10.1152/jn.00433.2006. [DOI] [PubMed] [Google Scholar]
- 49.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 50.Schall JD, Boucher L. Executive control of gaze by the frontal lobes. Cogn Affect Behav Neurosci. 2007;7:396–412. doi: 10.3758/cabn.7.4.396. [DOI] [PubMed] [Google Scholar]
- 51.Gehring WJ, Liu Y, Orr JM, Carp J. The error-related negativity (ERN/Ne) In: Luck SJ, Kappenman E, editors. Oxford handbook of event-related potential components. New York: Oxford University Press; 2011. pp. 231–291. [Google Scholar]
- 52.Stuphorn V, Taylor TL, Schall JD. Performance monitoring by the supplementary eye field. Nature. 2000;408:857–860. doi: 10.1038/35048576. [DOI] [PubMed] [Google Scholar]
- 53.Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- 54.Emeric EE, Leslie M, Pouget P, Schall JD. Performance monitoring local field potentials in the medial frontal cortex of primates: supplementary eye field. J Neurophysiol. 2010;104:1523–1537. doi: 10.1152/jn.01001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Emeric EE, Brown JW, Leslie M, Pouget P, Stuphorn V, Schall JD. Performance monitoring local field potentials in the medial frontal cortex of primates: anterior cingulate cortex. J Neurophysiol. 2008;99:759–772. doi: 10.1152/jn.00896.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Godlove DC, Emeric EE, Segovis CM, Young MS, Schall JD, Woodman GF. Event-related potentials elicited by errors during the stop-signal task. I. Macaque monkeys. J Neurosci. 2011;31:15640–15649. doi: 10.1523/JNEUROSCI.3349-11.2011. This paper is the first demonstration of the ERN in nonhuman primates, forming a keystone in the bridge linking human and nonhuman primate studies on the neural basis of performance monitoring.
- 57.Amiez C, Joseph JP, Procyk E. Reward encoding in the monkey anterior cingulate cortex. Cereb Cortex. 2006;16:1040–1055. doi: 10.1093/cercor/bhj046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schultz W. Dopamine signals for reward value and risk: basic and recent data. Behav Brain Funct. 2010;23(6):24. doi: 10.1186/1744-9081-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nieuwenhuis S, Holroyd CB, Mol N, Coles MG. Reinforcement-related brain potentials from medial frontal cortex: origins and functional significance. Neurosci Biobehav Rev. 2004;28:441–448. doi: 10.1016/j.neubiorev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 60.So N, Stuphorn V. Supplementary eye field encodes reward prediction error. J Neurosci. 2012;32:2950–2963. doi: 10.1523/JNEUROSCI.4419-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bogacz R, Wagenmakers EJ, Forstmann BU, Nieuwenhuis S. The neural basis of the speed-accuracy tradeoff. Trends Neurosci. 2010;33:10–16. doi: 10.1016/j.tins.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Simen P, Contreras D, Buck C, Hu P, Holmes P, Cohen JD. Reward rate optimization in two-alternative decision making: empirical tests of theoretical predictions. J Exp Psychol Hum Percept Perform. 2009;35:1865–1897. doi: 10.1037/a0016926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ratcliff R, McKoon G. The diffusion decision model: Theory and data for two-choice decision tasks. Neural Computation. 2008;20:873–922. doi: 10.1162/neco.2008.12-06-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Forstmann BU, Dutilh G, Brown S, Neumann J, von Cramon DY, Ridderinkhof KR, Wagenmakers EJ. Striatum and pre-SMA facilitate decision-making under time pressure. Proc Natl Acad Sci U S A. 2008;105:17538–17542. doi: 10.1073/pnas.0805903105. Like [65, 66, 67, 68] this paper reports functional brain imaging data associated with adjustments of speed versus accuracy. 64 reports elevated activation in the striatum and pre-SMA when speed was stressed. 65 reports that individuals who flexibly change the response threshold of an accumulator model have stronger connections between presupplementary motor area and striatum. 66 reports that when speed was stressed, a positive correlation was measured between the amount of evidence accumulated by an accumulator model and the BOLD activation in preSMA and dorsal ACC, but when accuracy was stressed, a positive correlation was measured between total evidence and BOLD activation in another part of ACC. 67 reports that when speed was stressed, more BOLD activation is found in SMA and medial precuneus with right SMA activation correlated negatively with model response threshold, but when accuracy was stressed, more activation is found in left DLPFC with a positive correlation with the model threshold. 68 reports that when accuracy but not when speed was stressed in an orientation discrimination task, a measure of orientation tuning in primary visual cortex predicted the rate of an accumulator model and accuracy. Taken together, these studies focus on contributions of particular brain structures and attribute qualitatively different functions to different pathways and structures. Such localization of function does not correspond to the massively distributed parallel and sequential activation that is observed in neurophysiology studies.
- 65.Forstmann BU, Anwander A, Schäfer A, Neumann J, Brown S, Wagenmakers EJ, Bogacz R, Turner R. Cortico-striatal connections predict control over speed and accuracy in perceptual decision making. Proc Natl Acad Sci U S A. 2010;107:15916–15920. doi: 10.1073/pnas.1004932107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Maanen L Brown SD, Eichele T, Wagenmakers EJ, Ho T, Serences J, Forstmann BU. Neural correlates of trial-to-trial fluctuations in response caution. J Neurosci. 2011;31:17488–17495. doi: 10.1523/JNEUROSCI.2924-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wenzlaff H, Bauer M, Maess B, Heekeren HR. Neural characterization of the speed-accuracy tradeoff in a perceptual decision-making task. J Neurosci. 2011;31:1254–1266. doi: 10.1523/JNEUROSCI.4000-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ho T, Brown S, van Maanen L, Forstmann BU, Wagenmakers EJ, Serences JT. The optimality of sensory processing during the speed-accuracy tradeoff. J Neurosci. 2012;32:7992–8003. doi: 10.1523/JNEUROSCI.0340-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stuphorn V, Schall JD. Executive control of countermanding saccades by the supplementary eye field. Nat Neurosci. 2006;9:925–931. doi: 10.1038/nn1714. [DOI] [PubMed] [Google Scholar]
- 70.Stuphorn V, Brown JW, Schall JD. Role of supplementary eye field in saccade initiation: executive, not direct, control. J Neurophysiol. 2010;103:801–816. doi: 10.1152/jn.00221.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Scangos KW, Stuphorn V. Medial frontal cortex motivates but does not control movement initiation in the countermanding task. J Neurosci. 2010;30:1968–1982. doi: 10.1523/JNEUROSCI.4509-09.2010. Like [70], this paper demonstrates how the supplementary motor area complex of areas enables executive but not direct control over responses.
- 72. Pouget P, Logan GD, Palmeri TJ, Boucher L, Paré M, Schall JD. Neural basis of adaptive response time adjustment during saccade countermanding. J Neurosci. 2011;31:12604–12612. doi: 10.1523/JNEUROSCI.1868-11.2011. This paper provides new insights into the neural instantiation of stochastic accumulator models and the mechanisms through which executive control can be exerted.
- 73. Heitz RP, Schall JD. Neural mechanisms of speed-accuracy tradeoff. Neuron. 2012;76:616–628. doi: 10.1016/j.neuron.2012.08.030. This paper reveals for the first time the neurophysiological processes accomplishing the immediate tradeoff of speed and accuracy. The results are not anticipated by previous modeling or neuroimaging research.