Abstract
G3139, an antisense oligodeoxyribonucleotide (ODN) against Bcl-2, contains 2 CpG dinucleotides and has shown immunostimulatory activities in preclinical studies. It has been suggested that immunoactivation, rather than antisense activity, is primarily responsible for the therapeutic efficacy of G3139. Nanoparticle formulation naturally target phagocytic antigen presenting cells, therefore, might enhance the immunological effects of G3139. In this study, a novel formulation of lipid nanoparticles (LNPs) encapsulating G3139 was synthesized and evaluated in mice bearing L1210 subcutaneous tumors. Intravenous injection of G3139-LNPs into mice led to increased serum levels of IL-6 and IFN-γ, promoted proliferation of natural killer (NK) cells and dendritic cells (DCs), and triggered a strong anti-tumor immune response in mice. The observed effects were much greater than those induced by free G3139. Correspondingly, the G3139-LNPs more effectively inhibited tumor growth and induced complete tumor regression in some mice. In contrast, free G3139 was ineffective in tumor growth inhibition and did not prolong survival of the tumor bearing mice. These results suggest that G3139-LNPs are a potential immunomodulatory agent and may have applications in cancer therapy.
Keywords: CpG motif, G3139, oligodeoxyribonucleotide, lipid nanoparticles, immunotherapy, cancer
Introduction
Immunotherapy is a promising strategy for treatment of cancer. DNA containing unmethylated CpG dinucleotide motifs have been shown to activate innate immune system cells such as natural killer (NK) cells, macrophages and dendritic cells (DC)1, 2 through toll-like receptor-9 (TLR-9), which resides in the endosomal compartment.3–5 Synthetic oligodeoxyribnucleotides containing CpG motifs (CpG ODNs) have been investigated as antitumor agents and as vaccine adjuvants.3 They have been shown to induce Th1-like immunoresponses through stimulating NK cells proliferation and IFN-γ production.3 The type-I immune response is beneficial for elimination of cancer cells.6–8 However, immunotherapy with CpG ODNs in the clinic is likely to require further improvement in efficacy, which can potentially be accomplished through improving the formulation.
Lipid nanoparticles (LNPs) will be investigated as ODN carriers in this study. Encapsulating CpG-ODNs into lipid formulations, such as stabilized lipid particles (SALP), have been shown to enhance immune system activation both in vitro and in vivo.9, 10 However, there have been relatively few studies on antitumor activities of CpG ODN loaded nanoparticles.9–11
In this study, a novel lipid nanoparticle (LNP) formulation of G3139 was developed and investigated for immunostimulatory and antitumor activities in the murine L1210 syngeneic tumor model. G3139 (Genasense™, oblimersen sodium) is an 18-mer phosphorothioate antisense ODN developed by Genta Inc. and is designed for the catalytic cleavage of human mRNA of the antiapoptotic protein Bcl-2.12, 13 Since Bcl-2 is known to be implicated in chemoresistance by cancer, its down regulation should may lead to sensitization of these cells to chemotherapy. G3139 combined with chemotherapeutic agents has shown significant activity in preclinical models, although responses from recent clinical trials have been relatively modest. Due to rapid systemic clearance of ODNs, G3139 is currently administered by prolonged continuous infusion.12, 13 G3139 has shown significant immunostimulatory activities, probably due to its two CpG motifs.3 Our studies demonstrate that LNPs prolonged plasma half-life and tumor accumulation of G3139 and that intravenously injected G3139-LNPs rather than free G3139 can effectively activate innate immune system cells, resulting in a potent anti-tumor immune response and tumor growth inhibition.
Materials and Methods
Materials
3β-[N,N-(Dimethylaminoethane)carbamoyl] cholesterol (DC-Chol), egg yolk phosphatidylcholine (PC), and distearoylphosphatidylethanolamine-N-[methoxy(polyethylene glycol)-2000] (m-PEG2000-DSPE) were purchased from Avanti Polar Lipids (Alabaster, AL). Protamine sulfate was purchased from Sigma Chemical Co. (St. Louis, MO). 5-Bromo-2′deoxyuridine (BrdU) Flow Cytometry Assay kit was obtained from BD Pharmingen (San Diego, CA).
Oligonucleotides G3139 (5′-TCT CCC AGC GTG CGC CAT-3′), G4243 (FAM-G3139, with a 5′-fluorescein label), and control ODNs G4126 (5′-TCT CCC AGC ATG TGC CAT-3′) (2 nucleotides different from G3139 and containing no CpG motifs) were kindly provided by Genta Inc (Berkeley Heights, NJ).
Phycoerythrin (PE)-, fluorescein isothiocyanate (FITC)-, Allophycocyanin (APC)-, and (PE-Cy7)-conjugated monoclonal antibodies (mAbs), including PE-Cy5.5-CD4, APC-CD8, APC-NK-DX5, PE-CD3e, PE-INF-γ were purchased from BD Pharmingen (San Diego, CA). Anti-CD112 and anti-CD40 MAbs were purchased from BioExpress (West Lebanon, NH).
Cell culture
Human KB cell line, which has been identified as a subline of human cervical cancer HeLa cell line, was obtained as a gift from Dr. Philip Low (Purdue University, West Lafayette, IN). L1210, a murine lymphocytic leukemia cell line, were kindly provided by Dr. Manohar Ratnam (University of Toledo, Toledo, OH). Cells were cultured in RPMI 1640 medium supplemented with 100 units/mL penicillin, 100 μg/mL streptomycin, and 10% FBS in a humidified atmosphere containing 5% CO2 at 37 °C.
Preparation of ODN-Lipid nanoparticles (ODN-LNPs)
LNPs, composed of DC-Chol/egg PC/mPEG2000-DSPE (35: 60: 5, mole/mole), protamine and ODN, were prepared by EtOH dilution followed by tangential flow diafiltration (Figure 1A). The lipids were dissolved in EtOH and mixed with protamine sulfate in citrate buffer (20 mM Na citrate, pH 4.0) to achieve a lipid: protamine weight ratio of 25 and a final EtOH concentration of 66.6% (v/v). ODN in citrate buffer (20 mM, pH 4.0) was then added to the lipids/protamine solution to spontaneously form pre-LNPs at an EtOH concentration of 40% (v/v). The pre-LNPs were then diluted with 20 mM citrate buffer (pH 4.0) to further lower the EtOH concentration, and then were subjected to diafiltration in a Millipore lab-scale tangential flow filtration (TFF) unit (Billerica, MA) to remove excess EtOH and unencapsulated ODN. Finally, the resulting LNPs were buffer-exchanged into HBS (150 mM NaCl, 20 mM HEPES, pH 7.5). Empty LNPs of the same lipid composition but containing no ODN were also prepared by the same procedure.
Figure 1. Synthesis and pharmacokinetic properties of LNPs.
(A) Flowchart of ODN-LNP preparation by EtOH dilution/diafiltration method. (B), Particle size distribution of ODN-LNPs after each step in a typical EtOH dilution/diafiltration process. (C), Plasma concentration-time profile of G4243-LNPs and free G4243 (G4243 is a fluorescein-labeled G3139) following tail vein i.v. bolus administration of 5 mg/kg of G4243-LNPs or free G4243 in DBA/2 mice (n=3). (D) Tumor accumulation profile of G4243-LNPs and free G4243 following tail vein i.v. bolus administration of 5 mg/kg of G4243-LNPs or free G4243 in DBA/2 mice (n=3). Each point represents Mean ± SD of three mice.
The particle size of the LNPs was determined by dynamic light scattering via Nicomp model 370 Submicron Particle Sizer (Particle Sizing Systems, Santa Barbara, CA). The zeta potential (ξ) of the LNPs was measured on a Brookhaven 90 plus Particle Analyzer (Holtsville, NY).
To evaluate ODN encapsulation, FITC labeled G3139 (G4243) was used instead of G3139 to enable fluorometric measurements of ODN concentration. To determined ODN content, LNPs were lysed by 1% SDS at 95 °C for 5 min and were centrifuged at 12,000 × g for 5 min. The ODN concentration in the LNPs was determined by measuring fluorescence value obtained from supernatant of LNP lysate with a spectrofluorometer (Perkin-Elmer) at excitation and emission wavelengths of 495 and 520 nm, respectively, based on a pre-established standard curve. Encapsulation efficiency was calculated based on ODN concentration in the lysate divided by ODN concentration added.
Western blot for Bcl-2
The Bcl-2 downregulatory effect of G3139-LNPs was evaluated in KB and L1210 cells. Cells were treated by lysis buffer 72 hr after treatment. From the lysate 100 μg proteins was loaded on a 15% SDS-PAGE gel (Bio-Rad, Hercules, CA) and run for 2 hr at 100 V, followed by transferring to a nitrocellulose membrane overnight. After blocking with 5% non-fat milk in Tris-buffered saline/Tween-20 (TBST) for 2 hr, the membranes were incubated with murine anti-human Bcl-2 antibody (Dako, Carpinteria, CA) for studies on KB cells or hamster anti-mouse Bcl-2 antibody (BD Pharmingen, San Diego, CA) for studies on murine L1210 cells, respectively. After 2 hr of incubation at room temperature, membranes were the treated with horseradish peroxidase-conjugated sheep anti-mouse IgG antibody (GE Health, Piscataway, NJ) for KB cell or murine anti-hamster IgG antibody (BD Pharmingen, San Diego, CA) for L1210 cell for 1 hr at room temperature. Membranes were then developed with Pierce SuperSignal West Dura Extended Duration Substrate (Pierce, Rockford, IL) and imaged with Kodak X-OMAT film (Kodak, Rochester, NY). Bcl2 protein expression levels were quantified by ImageJ software (NIH Image, Bethesda, MD) and normalized to the β-actin levels from the same samples.
In vivo assay for plasma clearance and tumor accumulation of ODN-LNP
Female DBA/2 mice (H-2d), 8 wks old, were purchased from Harlan (Indianapolis, IN). To evaluate in vivo plasma clearance and tumor accumulation of ODN-LNPs, G4243 (fluorescein labeled G3139) or G4243-LNPs were administered at 5 mg/kg ODN dose by tail vein injection. At appropriate time points, mice were anesthetized and blood was collected via the tail vein and into heparinized tubes. Plasma was separated from red blood cells via immediate centrifugation at 1000 × g for 5 min. Mice were sacrificed by carbon dioxide asphyxiation. Tumors were harvested at various time points and homogenized in microtubes containing 500 μL distilled water. Samples were then treated with 1% SDS, and heated at 95 °C for 5 min, followed by centrifugation at 12,000 × g for 5 min. The fluorescence of supernatant was determined by spectrofluorometry to determine sample ODN concentration, as described above. WinNonlin Version 3.2 (Pharsight Co., CA) was used to determine pharmacokinetic parameters, including area under the curve (AUC), total body clearance (CL) and plasma half-life.
Cytokine production and cell proliferation
To determine serum INF-γ and IL-6 levels, blood was collected from the tail vein of mice at various time points after i.v. injection of G3139-LNPs, free G3139, empty LNPs, or non-CpG containing G4126-LNPs. Three mice were used in each treatment group. The blood samples were kept at room temperature for 30 min and then centrifuged at 12000 × g to harvest serum. The levels of cytokines were determined by ELISAs using commercial kits (BD Pharmingen, San Diego, CA).
In vivo immune cell proliferation was evaluated by BrdU incorporation assay 14. BrdU (10 mg/mL) was injected i.p. into mice at 1 or 6 days after treatment. Three mice were used in each group. Splenocytes were harvested from the mice 24 hr after the BrdU administration, and were surface-stained using fluorescence-labeled mAbs to CD4, CD8, CD3 and/or CD49b (DX5), followed by intracellular staining with mAb to BrdU as instructed by the manufacturer (BD Biosciences). Then the cells were washed twice in perm/wash solution and were resuspended in 300 μL of FACS buffer for flow cytometry analysis. Data were acquired on a Becton Dickinson FACSCalibur (Becton Dickinson) and analyzed using the FlowJo software (Tree Star, Ashland, OR). In a typical assay, 100,000 events were acquired for analysis.
Histopathological and immunohistochemical (IHC) analyses
For pathological analysis, tumor samples were fixed in 10% phosphate buffered formalin solution. The tissue sections were stained with hematoxylin and eosin (H&E). For IHC analysis, tumor samples were frozen and prepared as described previously.14 Briefly, samples were fixed and washed with ice-cold PBS (pH 7.4) and stained with rat mAbs against CD4, CD8, or CD122, (2 μg/mL in PBS for 1 hr at 4 °C) followed by staining with horseradish peroxidase-conjugated rabbit anti-rat IgG..
Evaluation of anti-tumor activity
L1210 cells (5 × 106) were subcutaneously inoculated into the flank of syngeneic DBA/2 mice. Palpable tumors developed within 4–5 days after inoculation. At 7 days post inoculation, the tumor-bearing mice were injected i.v. with PBS (pH 7.4), free ODN (G3139), empty LNPs, G3139-LNPs or non-CpG containing G4126-LNPs (1.5 mg/kg or 5 mg/kg dose of ODN) on every 4th days (Q4D). Five mice were used in each treatment group. Anti-tumor activity was determined by measuring the tumor size (width and length) using a Vernier caliper at a series of time points. Tumor volume was calculated by the formula: tumor volume = (π/6) × length (mm) × [width (mm)]2. Mice were scarified once the tumor size reached greater than 1500 mm3.
Statistical analysis
Statistical analysis was performed with Analysis of Variance (ANOVA) or Student’s t test and by JMP™ software, where appropriate. Differences in survival of mice among treatment groups were analyzed using the log-rank test. A p value of < 0.05 was considered significant.
Results
LNPs showed prolonged plasma half-life and increased G3139 accumulation in tumors
G3139-LNPs and G4243-LNPs were prepared by the EtOH dilution/diafiltration method. This method relied on particle self assembly. At high EtOH concentration, the lipids formed a self-assembled metastable bilayer structure, which enables efficient ODN loading. In the subsequent dilution and diafiltration steps, EtOH concentration was gradually decreased. This resulted in “sealing off” of the lipid bilayers. Interestingly, the particle sizes changed with EtOH concentration in each step (Figure 1B). In a typical preparation, after removal of excess EtOH, the protocol yielded small ODN-LNPs with a mean diameter of 89 ± 45.6 nm, encapsulation efficiency of > 95%, and zeta potential of 4.08 ± 0.4 mV. G3139-LNPs and G4243-LNPs had essentially identical characteristics.
Free ODNs are known to be rapidly cleared from the circulation.12 Extending circulation time should increase ODN delivery into solid tumor tissue. LNP-encapsulation is a potential means to accomplish this. The circulation time of LNP-encapsulated ODNs was evaluated by measuring plasma clearance of G4243-LNPs (G4243 is fluorescein-labeled G3139) in L1210 tumor bearing DBA/2 mice. At 24 hr after intravenous administration, ~ 25% of the injected G4243-LNPs remained in the plasma, yielding a plasma half-life of about 8 hr (Figure 1C). In contrast, only 1% of the free G4243 was detected in the plasma 24 hr after the i.v. injection, yielding a plasma half-life of about 45 min. Thus, the circulation time of G4243 was extended by > 10 times when incorporated into LNPs. Plasma concentration versus time data were analyzed by WinNonLin using non-compartmental model to determine pharmacokinetic parameters. As shown in Table 1, i.v. administration of G4243-LNPs resulted in a terminal elimination half-life (T1/2) of 0.47 h, area under the plasma concentration time curve (AUC) of 85.0 h·μg/ml, volume at steady state (Vss) of 363.6 ml/kg and clerance (CL) of 58.9 ml/kg/h. In comparison, free G4243 had a much shorter T1/2 and 10-time increased CL. These data show that the G4243-LNPs had a greatly prolonged blood circulation time and decreased elimination rate. The accumulation of G4243-LNPs in tumor tissue was also significantly enhanced. The G4243-LNPs level in tumor was at 6.9 μg ODN/g tumor tissue at 24 hr after i.v. bolus administration (Figure 1D), whereas the free G4243 in the tumor tissue was 0.75 μg ODN/g tumor tissue. These results indicated that the LNP encapsulation could extend circulation time of ODNs as well as enhance accumulation of G4243 in the tumor tissue, possibly due to enhanced permeability and retention (EPR) effect.
Table 1.
Pharmacokinetic parameters of G4243-LNPs and free G4243 after i.v. bolus administration at 5 mg/kga
| T1/2 (h) | VSS (ml/kg) | AUC (h·μg/ml) | CL (ml/kg/h) | |
|---|---|---|---|---|
| G4243-LNPs | 0.47 (7.4%) | 363.6(4.6%) | 85.0(5.0%) | 58.9 (10.3%) |
| Free G4243 | 0.08 (2.3%) | 105.0 (6.6%) | 8.7 (10.0%) | 577.4 (9.8%) |
Data generated by WinNonlin. Standard errors were shown in parenthesis as (CV%)
G3139-LNPs did not induce Bcl-2 down-regulation in murine L1210 Cells
G3139 is an antisense ODN designed for targeting the human Bcl-2. Against murine Bcl-2, G3139 has a two nucleotides mismatch. The effects of G3139 on Bcl-2 expression were evaluated in human KB and in murine L1210 cells. The cells were incubated with either G3139 or G3139-LNPs for 72 hrs and were harvested for Western-blot analysis of Bcl-2 protein expression. As shown in the Figure 2, while both free G3139 and G3139-LNPs significantly inhibited Bcl-2 expression in human KB cells (Figure 2A), they had no significant effect on Bcl-2 expression in murine L1210 cells (Figure 2B). These results suggested that Bcl-2 down-regulatory activity of G3139 is specific for human.
Figure 2. Western blot analysis of Bcl-2 protein expression.
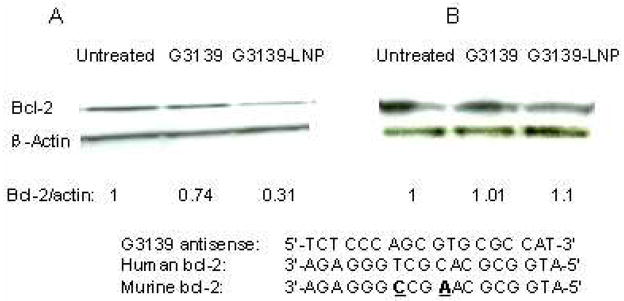
Human KB cells (A) and murine L1210 cells (B) were transfected with or without 1 μM G3139 for 72 hr, and the cells were harvested for Western-blot analysis. Ratios of Bcl-2 to β-actin were obtained by densitometry. There was a 2-nucleotide difference between the sequences of human and murine Bcl-2 mRNA.
G3139-LNPs inhibited tumor growth
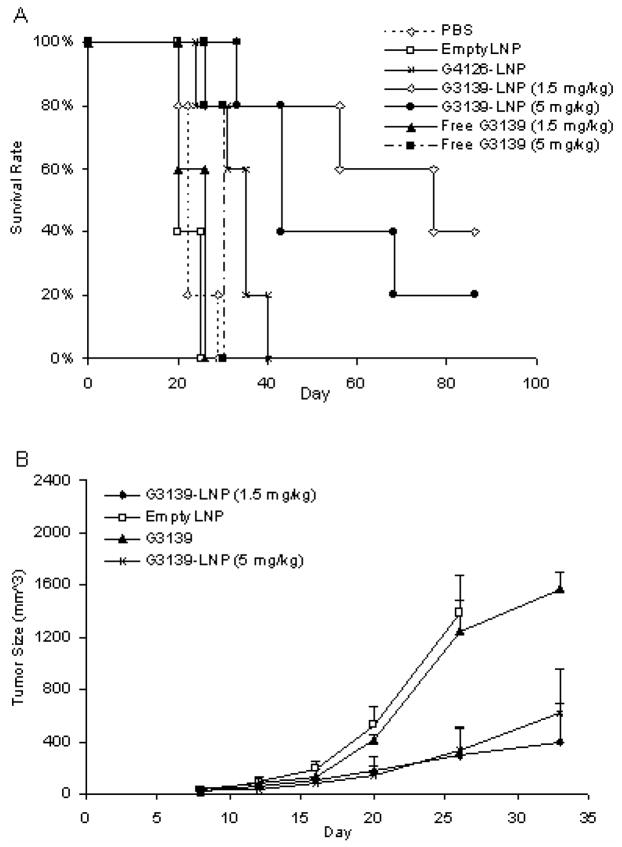
Since LNPs could extend the plasma circulation time of G3139 and enhance its accumulation in the tumor, it is possible that these effects may result in a stronger antitumor activity. To evaluate this possibility, the G3139-LNPs were studied for therapeutic efficacy in mice with established solid tumors. A tumor model was established with DBA/2 mice, which were injected subcutaneously with syngeneic L1210 tumor cells. The mice developed solid tumors of ~ 30 mm3 within 7 days, which reached sizes > 1500 mm3 within 1 month in the absence of treatment. For the therapeutic study, the mice were injected i.v. with 100 μL of G3139-LNPs every 4 days started from day 7 post inoculation. The mice of control groups were injected i.v. with the same volume of PBS (pH 7.4), empty LNPs, free G3139, or non-CpG containing G4126-LNPs. As shown in Figure 3 and Table 2, tumor growth in the mice treated with G3139-LNPs was inhibited by > 50% (p < 0.005), resulting in prolonged survival of 80% of the mice (4/5) with a median survival time (MST) of 76 days and increase-in-lifespan (ILS) value of 245% (p = 0.002) and complete rejection of tumors in 40% (2/5) of the mice after 3 injections with 1.5 mg/kg (low dose) of G3139-LNPs. In contrast, the mice treated with free G3139 (1.5 mg/kg) did not respond. For this group, the tumor size were comparable to the mice treated with PBS, empty LNPs, or G4126-LNPs (Figure 3A) and the ILS value was not significantly different from the PBS control group (p = 0.1). In fact, neither G3139 nor empty LNPs had a significant effect on tumor growth (Figure 3A & B). Moreover, the antitumor effect of G3139 was likely mediated by CpG motif, because G4126-LNPs, which lacked CpG motifs, did not inhibit tumor growth (Figure 3B).
Figure 3. Therapeutic efficacy of G3139-LNPs.
(A) DBA/2 mice were inoculated s.c. with syngeneic L1210 cells 7 days prior to treatment. The mice received i.v. injections of PBS (pH 7.4), empty LNP, G3139, G3139-LNPs, or non-CpG containing G4126-LNPs on every 4th day until the mouse had tumor size of >1500 mm3. Low dose was 1.5 mg/kg of ODN, and high dose was 5 mg/kg of ODN. There were 5 mice in each group.
(B) Comparison of antitumor effects of G3139, empty LNP, low dose G3139-LNPs (1.5 mg/kg), and high dose G3139-LNPs (5 mg/kg). Graphs show the mean tumor size (mm3), error bars indicated standard error (SE).
Table 2.
Survival of mice after treatments by LNP-G3139s and other formulations (n=5 for each treatment group)
| Formulation | Median survival time (days) | T/C (%) | Increase in lifespan (%) | Log-rank P compared to PBS group |
|---|---|---|---|---|
| PBS | 22 | 100 | 0 | |
| Empty LNP | 20 | 91 | −9 | 0.3 |
| Free G3139 (1.5 mg/kg) | 25 | 114 | 14 | 0.3 |
| Free G3139 (5 mg/kg) | 30 | 136 | 36 | 0.1 |
| G4126 LNP | 35 | 159 | 59 | 0.03 |
| LNP-G3139 (1.5 mg/kg) | 76 | 345 | 245 | 0.002 |
| LNP-G3139 (5 mg/kg) | 43 | 195 | 95 | 0.01 |
To determine whether the antitumor effect of G3139-LNPs was dose-dependent, we treated tumor-bearing mice with either 1.5 mg/kg (low dose) or 5 mg/kg (high dose) of G3139-LNPs or free G3139. Neither dosing levels of free G3139 produced antitumor activities (Figure 3A). Interestingly, as shown in Figure 3A and Table 2, high dose of G3139-LNPs (5 mg/kg) did not result in a better therapeutic effect compared to low dose (1.5 mg/kg) G3139-LNPs. The median survival was actually decreased from 76 days to 43 days, and only one mouse had complete tumor eradication. Thus, the experiments described below used only low dose of G3139-LNPs.
G3139-LNPs potently activated innate immune system Cells
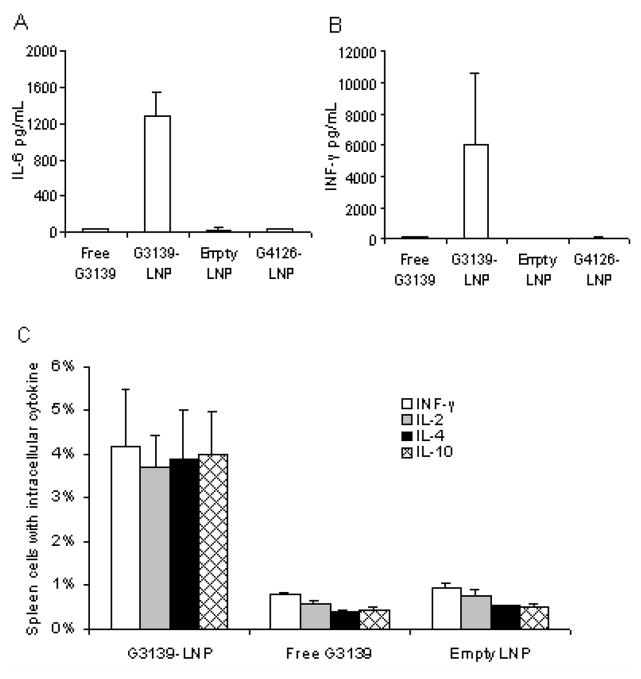
CpG-ODNs are known to stimulate innate immune responses.1, 2 Thus we examined cytokine production and innate immune cell proliferation in mice treated with G3139-LNPs. The levels of IL-6 and IFN-γ were evaluated in the peripheral blood because these are important for the induction of Th17 and Th1 responses, respectively16, 17. DBA/2 mice were injected i.v. with 1.5 mg/kg of G3139, G3139-LNPs, non-CpG containing G4126-LNPs or empty LNPs. The serum levels of IL-6 and IFN-γ were determined by ELISA after 4 and 8 hour of injection, respectively (Figure 4A, B). The highest level of IL-6 was observed at 4 hr following intravenous injection of G3139-LNP, whereas the highest level of INF-γ was detected at 8 hr after injection. Only low levels of IL-6 or INF-γ were detected in the sera of mice treated with free G3139, non-CpG containing G4126-LNPs, or empty LNPs. Meanwhile, the splenocytes from the mice treated with G3139-LNPs produced more cytokines, including IFN-γ, IL-2, IL-4 and IL-10, than those treated with free G3139 or empty LNPs, as shown by immunohistochemical staining of spleen (Figure 5A). These results suggested that the antitumor activity of G3139-LNPs might be associated with their high pontecy in cytokine induction.
Figure 4. G3139-LNPs activated serum cytokine expression in mice.
For serum cytokine detection, eight-week-old DBA/2 mice were injected i.v. with 1.5 mg/kg of G3139, G3139-LNPs, empty LNPs, or non-CpG containing G4126-LNPs. (A) IL-6 was measured at 4hr, and (B) INF-γ was measure at 8 hr by ELISA. Three mice were used in each group.
Figure 5. G3139-LNPs enhanced intracellular cytokine expression in spleen cells and enlarged the spleen size.
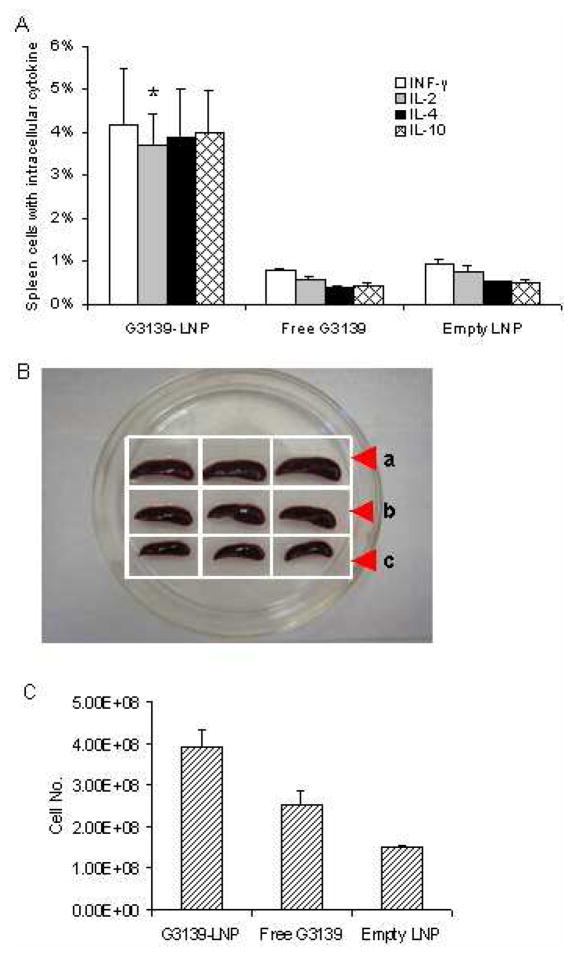
(A) For intracellular cytokines expression in spleen cells, eight-week-old DBA/2 mice were injected i.v. with 1.5 mg/kg of G3139, G3139-LNPs, and empty LNPs. There were 3 mice in each group. Spleen cells were harvested from mice 2 days after treatment, stained with fluorescence labeled MAbs, and measured by FACS. (B) Spleens harvested 7 days after i.v. administration of (a) G3139-LNPs (1.5 mg/kg of G3139), (b) free G3139 (1.5 mg/kg), and (c) empty LNPs in DBA/2 mice. Three mice were in each group. (C) Total cell numbers of the above spleen, G3139-LNP treated group has significantly more spleen cells than free G3139 (p = 0.0017) and empty LNP treated groups (p < 0.0001). (* indicates p < 0.05, by Student’s t test)
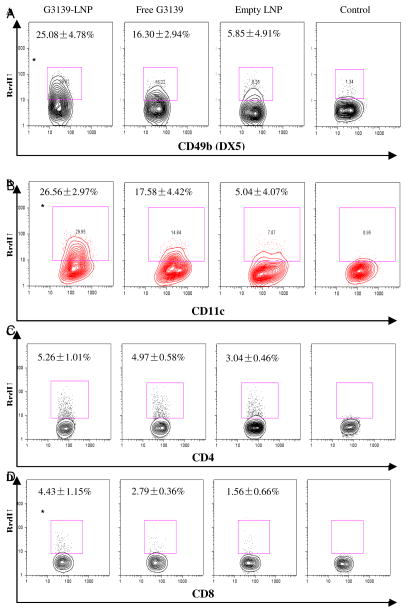
In addition to cytokine production, G3139-LNPs also promoted immune cell proliferation. LNP-treated mice showed significantly enlarged spleens and increased spleen cells at 7 days after treatment. The effect was much more pronounced compared to in mice treated with G3139 (p = 0.0017) or empty LNPs (p < 0.0001) (Figure 5B, C). To verify that the expansion of the spleen cells was associated with proliferation of innate immune cells, such as NK and dendritic cells (DCs), we examined BrdU incorporation by these cells. BrdU, an analog of thymidine, can replace thymidine during cell division, and has been widely used for quantification of cell proliferation, especially in vivo.18, 19 The mice bearing L1210 tumors were treated with G3139-LNPs, G3139 or LNPs for 2 days, and BrdU was administered i.p. The mice were then sacrificed 24 hrs later and analyzed, as described in in Materials and Methods. As shown in Figure 6, LNPs alone had little effect on NK and DC expansion, at 5.85% and 5.05%, respectively. Meanwhile, free G3139 ODN had a significant effect on NK and DC proliferation, at 16.30% and 17.58%, respectively. Interestingly, LNPs loaded with G3139 induced a much greater effect than free G3139 and empty LNPs on the expansion of NK and DCs (25.08% and 26.56%, respectively, p < 0.05) (Figure 6). The studies were repeated twice, and produced similar results. These results indicated that G3139-LNPs not only elicited innate immune cells to produce cytokines, but also promoted their proliferation.
Figure 6. G3139-LNPs activated proliferation of innate immune cells.
DBA/2 mice were treated with G3139-LNP, free G3139 or empty LNPs, and then injected i.p. with BrdU. Three mice were in each group. Twenty four hours after treatment, spleen cells were harvested, and the activation status of DX5+ NK cells (A), CD11c+ DCs (B), CD4+ T cells (C), and CD8+ T cells (D) were evaluated by BrdU incorporation rate. Results represent the average ± SD of three independent experiments. (* indicates p < 0.05, by Student’s t test)
The effect of G3139-LNPs on adaptive anti-tumor immunity
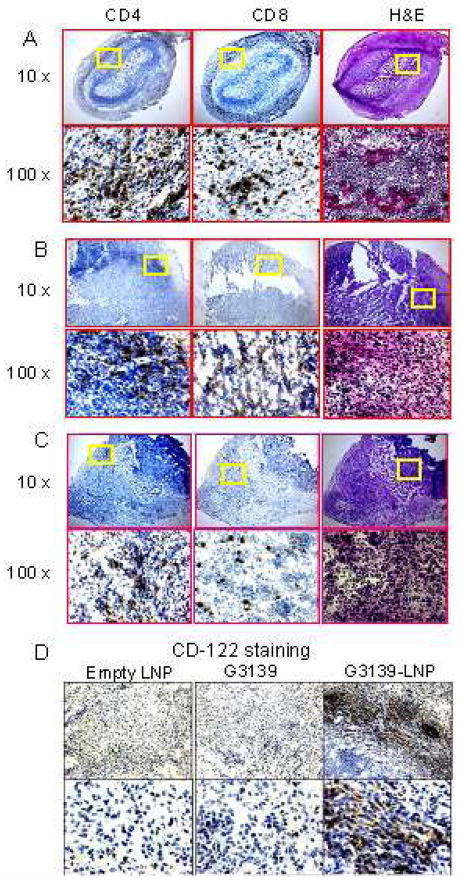
Since activation of innate immune cells can induce adaptive immunity, we further characterized the adaptive immunity in the tumor-bearing mice treated with G3139-LNPs. Since the IFN-γ-mediated adaptive immune response is important for anti-tumor immunity, we examined IFN-γ production by CD4+ and CD8+ T cells in the spleen of the mice at day 2 and 7 after treatment. At day 2 post-treatment, IFN-γ-producing cells among CD4+ and CD8+ T cells were scarce in the tumor-bearing mice regardless of the agents used for treatment (up to about 5%). On the day 7 of treatment, IFN-γ-producing cells were significantly increased among CD8+, but not CD4+ T cells. Importantly, G3139-LNPs were much more potent in inducing IFN-γ production by CD8+ T cells (26.84%), compared to G3139 (19.42%) and empty LNPs (10.38 %) (Figure 7). There was no significant change of the INF-γ expression in CD4+ cells on either day 2 (3.16%) or day 7 (5.73%) after treatment with G3139-LNPs. These findings suggest that G3139-LNPs can induce an adaptive immune response that shifts to type 1 with an increase in INF-γ-producing CD8+ cytotoxic T cells (CTLs). This was further supported by identification of a large number of CD4+ and CD8+ T cells in the tumors. Since tumor regression was observed in the mice treated with G3139-LNPs started from day 4 to 7 post treatment, the frozen tumor sections from the mice treated with G3139-LNPs, G3139, or LNPs for 7 days were analyzed by immunohistochemistry (IHC) for the infiltrated CD4+, CD8+ and CD122+ cells. As shown in Figure 8, CD4+ and CD8+ cells were found ubiquitously infiltrating the tumor tissue except for the necrotic areas in tumors from the mice treated with G3139-LNPs, but not those that were treated with G3139 or LNPs (Figure 8). In addition, more CD122+ cells were detected in the tissue sections of tumors from the mice treated with G3139-LNPs than those from the mice treated with G3139 or LNPs, although the number of infiltrating CD122+ cells was much lower than those of CD4+ and CD8+ cells in the same group (Figure 8D). These results suggested that adaptive immunity may have played a critical role in rejection of established tumors and that G3139-LNPs can induce a strong adaptive anti-tumor immunity.
Figure 7. G3139-LNPs induced IFN-γ production and activated innate and acquired immunity.
INF-γ expression was determined in CD4 (A) and CD8 (B) cells 2 days or 7 days after treatment. Three mice were used in each group. Spleen cells were isolated and stained with INF-γ, CD4, and CD8-specific mAbs as described in Materials and Methods. Data showed the percentage of INF-γ expressing cells identified by FACS. Results represent the average ± SD of three independent experiments. (* indicates p < 0.05, by Student’s t test)
Figure 8. Immunohistochemistry (IHC) Staining of L1210 tumors.
Frozen sections were prepared from tumors 7 days after treatment with G3139-LNPs (A), G3139 alone (B) or empty LNPs (C), and stained with anti-CD4, or anti-CD8 antibodies, or with hematoxylin & eosin (H&E). D, Tumor frozen sections from A, B, and C groups were stained with anti-CD122.
Discussion
In the present study, it was demonstrated that ODN can be efficiently loaded into LNPs by EtOH dilution/diafiltration method, and encapsulating G3139 into LNPs dramatically changed its plasma clearance profile and enhanced its immunostimulatory effects.
The LNPs encapsulating ODN were produced by a EtOH dilution/diafiltration method. A similar method has been used previously for preparation of stabilized-plasmid-lipid-particles (SPLP), and yielded up to a 90% encapsulation rate.20 We have also used this method to prepare cationic lipid/ODN particles and obtained a ~ 80% loading efficiency.21, 22 Compared to liposomes, Protamines are small, arginine-rich, nuclear proteins that replace histones late in the haploid phase of spermatogenesis and promote sperm head condensation and DNA stabilization.23 Protamine sulfate was added as an additional excipient. The choice of DC-Chol as the cationic lipid and incorporation of PEG-DSPE into the LNPs were both important for long circulation and serum stability. DC-Chol has a titratable tertiary amine group with apparent pKa of 7.8. When the external pH is close to neutral pH, DC-Chol is partially deprotonated resulting in reduced surface charge, as confirmed by zeta potential analysis. PEG on the LNP surface can decrease uptake of particle by the RES, resulting in longer in vivo circulation. In addition, it served as a steric barrier that minimizes LNP aggregation and fusion during the formulation synthesis and storage. This novel LNP formulation has enabled high encapsulation efficiency for the ODN and good colloidal stability. Further studies on the role of LNP composition would be highly interesting. We plan to carry out additional studies in this area in the future.
Immune responses activated by G3139-LNPs rather than Bcl-2 downregulation ability was most likely responsible for the antitumor activity exhibited in the present study. G3139 was designed originally as a human Bcl-2 antisense ODN.12, 24 Western blot results showed that the G3139 had Bcl-2 down-regulatory activity in human KB cells, but not in murine L1210 cells (Figure 2). Moreover, upon removal of CpG motifs from G3139, the resulting non-CpG containing G4126 formulated in LNPs did not show immune stimulatory effect or antitumor activity (Figure 4).
Although free G3139 has been shown to induce immunoactivation previously, G3139-LNPs induced a much stronger cytokine response and a much greater therapeutic activity. Previous studies have also shown significantly enhanced immunostimulatory activities of CpG ODNs upon loading into liposomes, such as stabilized antisense lipid particles (SALP).9, 25, 26 Results in this study are consistent with these previous reports. The increased activity of the nanoparticles may be due to more efficient uptake of the LNPs by tumor resident macrophages and dendritic cells, resulting in greater local immunoactivation, as shown by immunohistochemical staining of the tumor sections (Figure 8). Keeping LNP particle size below 200 nm is important for efficient extravasation of the particles at the site of the tumor and maintaining long systemic circulation time.25
ODNs are large polyanions and cannot diffuse across the cell membrane. The mechanism for cellular uptake of CpG ODN is not well understood.27 CpG motifs activate immune system via toll-like-receptor-9 (TLR-9), a pattern recognition receptor (PRR) that triggers initiation of innate immune activation in response to infection.3–5, 28 Increased uptake of G3139-LNPs by phagocytic cells provides greater accessibility for CpG motifs to TLR-9 than free G3139. In this study, we observed that G3139-LNPs dramatically promoted proliferation of both DCs and NK cells based on BrdU incorporation (Figure 6). Since murine NK cells express little TLR-9 and thus may not be directly activated by CpG motif7, it is possible that G3139-LNPs-stimulated DCs and/or macrophages produce factors that indirectly stimulated NK cell proliferation.
Our data showed the G3139-LNPs were highly effective as a monotherapeutic agent. In fact, 1.5 mg/kg dose was very effective in activating immune responses and inhibit tumor growth in mice. In contrast, both low (1.5 mg/kg) and high (5 mg/kg) dose of free G3139 did not inhibit tumor growth (Figure 3). In previous reports, tumor growth inhibition was observed by administration of free G3139 in human xenograft tumor model.29 This could conceivably be partly due to its anti-Bcl-2 activity. However, the potential of anti-Bcl-2 function of G3139 was abolished in the murine tumor model used in the current study, thus excluding this mechanism. Interestingly, high dose was found to be no more effective than low dose. This was not surprising since Ballas et al. reported that free CpG ODN also demonstrated a non-linearity of immune activation in their Listeria model.7
The innate immunity can trigger a cascade of events that may further influence acquired immunity. 30 CpG containing ODNs favors strong Th-1 cytokine induction and activation of innate effector cells 25, 31, 32, which further promots adaptive immunity. As a result, CpG ODNs have been used as vaccine adjuvants.3,17 Type 1 innate and acquired immunity are both critical to overcoming immunosuppression in tumor-bearing animals.32, 33 In the present study, we observed elevated expression of INF-γ as well as high proliferation of innate effector cells, including NK cells and DCs, which play pivotal roles in acquired immunity. The experiment also showed that CD8+ cells were apparently up-regulated to express elevated levels of INF-γ at 7 days after treatment. In addition, IHC staining of tumor sections clearly demonstrated much higher levels of CD4+ and CD8+ cells infiltrating the tumor and greater tumor cell killing in G3139-LNP group than free G3139 or empty LNP treatment groups had (Figure 8). These data support the possibility that G3139-LNPs induced protective immunity by activating type 1 innate as well as acquired immunity. It should be noted that G3139 has no antitumor effect on its own (Figure 3 and 4) in the L1210 model, while it did have an effect on spleen expansion (Figure 5), NK and DCs expansion (Figure 6) and induction of INF-γ (Figure 7). This appears to be a contradiction. A possible explanation is that tumor infiltration of CD8+ T cells was more critical for antitumor activity than peripheral cytokine production (Figure 8). G3139-LPN was shown to be significantly more potent than G3139 in inducing CD8+ T cell infiltration in tumors (Figure 8). This was likely a result of the high tumor accumulation level of LNPs (Figure 1D). Further studies are warranted to investigate the therapeutic potential of G3139-LNPs as an immunotherapeutic agent, alone and in combination with other therapeutic modalities.
Acknowledgments
This work was supported in part by NSF grant EEC-0425626 (RJL), an OSU Comprehensive cancer center seed grant (XJG), and American Cancer Society grant IRG-112367 (XJG).
References
- 1.Roman M, Martin-Orozco E, Goodman JS, Nguyen MD, Sato Y, Ronaghy A, Kornbluth RS, Richman DD, Carson DA, Raz E. Nat Med. 1997;3(8):849–54. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 2.Hartmann G, Weiner GJ, Krieg AM. Proc Natl Acad Sci U S A. 1999;96(16):9305–10. doi: 10.1073/pnas.96.16.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krieg AM. Annu Rev Immunol. 2002;20:709–60. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 4.Krieg AM. Nat Med. 2003;9(7):831–5. doi: 10.1038/nm0703-831. [DOI] [PubMed] [Google Scholar]
- 5.Krieg AM. Nat Rev Drug Discov. 2006;5(6):471–84. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 6.Whiteside TL, Vujanovic NL, Herberman RB. Curr Top Microbiol Immunol. 1998;230:221–44. doi: 10.1007/978-3-642-46859-9_13. [DOI] [PubMed] [Google Scholar]
- 7.Ballas ZK, Rasmussen WL, Krieg AM. J Immunol. 1996;157(5):1840–5. [PubMed] [Google Scholar]
- 8.Ballas ZK, Krieg AM, Warren T, Rasmussen W, Davis HL, Waldschmidt M, Weiner GJ. J Immunol. 2001;167(9):4878–86. doi: 10.4049/jimmunol.167.9.4878. [DOI] [PubMed] [Google Scholar]
- 9.Mui B, Raney SG, Semple SC, Hope MJ. J Pharmacol Exp Ther. 2001;298(3):1185–92. [PubMed] [Google Scholar]
- 10.Gursel I, Gursel M, Ishii KJ, Klinman DM. J Immunol. 2001;167(6):3324–8. doi: 10.4049/jimmunol.167.6.3324. [DOI] [PubMed] [Google Scholar]
- 11.Ishii KJ, Kawakami K, Gursel I, Conover J, Joshi BH, Klinman DM, Puri RK. Clin Cancer Res. 2003;9(17):6516–22. [PubMed] [Google Scholar]
- 12.Marcucci G, Byrd JC, Dai G, Klisovic MI, Kourlas PJ, Young DC, Cataland SR, Fisher DB, Lucas D, Chan KK, Porcu P, Lin ZP, Farag SF, Frankel SR, Zwiebel JA, Kraut EH, Balcerzak SP, Bloomfield CD, Grever MR, Caligiuri MA. Blood. 2003;101(2):425–32. doi: 10.1182/blood-2002-06-1899. [DOI] [PubMed] [Google Scholar]
- 13.Morris MJ, Tong WP, Cordon-Cardo C, Drobnjak M, Kelly WK, Slovin SF, Terry KL, Siedlecki K, Swanson P, Rafi M, DiPaola RS, Rosen N, Scher HI. Clin Cancer Res. 2002;8(3):679–83. [PubMed] [Google Scholar]
- 14.Gao JX, Zhang H, Bai XF, Wen J, Zheng X, Liu J, Zheng P, Liu Y. J Exp Med. 2002;195(8):959–71. doi: 10.1084/jem.20011948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semple SC, Klimuk SK, Harasym TO, Dos Santos N, Ansell SM, Wong KF, Maurer N, Stark H, Cullis PR, Hope MJ, Scherrer P. Biochim Biophys Acta. 2001;1510(1–2):152–66. doi: 10.1016/s0005-2736(00)00343-6. [DOI] [PubMed] [Google Scholar]
- 16.Iwanami K, Inoue A, Matsumoto I, Mamura M, Watanabe Y, Goto D, Ito S, Tsutsumi A, Sumida T. Clinical Immunology. 2007;123(Supplement 1):S156–S157. [Google Scholar]
- 17.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. J Exp Med. 1997;186(10):1623–31. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gratzner HG. Science. 1982;218(4571):474–5. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- 19.De Boer RJ, Mohri H, Ho DD, Perelson AS. J Immunol. 2003;170(5):2479–87. doi: 10.4049/jimmunol.170.5.2479. [DOI] [PubMed] [Google Scholar]
- 20.Jeffs LB, Palmer LR, Ambegia EG, Giesbrecht C, Ewanick S, MacLachlan I. Pharm Res. 2005;22(3):362–72. doi: 10.1007/s11095-004-1873-z. [DOI] [PubMed] [Google Scholar]
- 21.Chiu SJ, Liu S, Perrotti D, Marcucci G, Lee RJ. J Control Release. 2006;112(2):199–207. doi: 10.1016/j.jconrel.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Chiu SJ, Marcucci G, Lee RJ. Anticancer Res. 2006;26(2A):1049–56. [PubMed] [Google Scholar]
- 23.Cui Z, Han SJ, Vangasseri DP, Huang L. Mol Pharm. 2005;2(1):22–8. doi: 10.1021/mp049907k. [DOI] [PubMed] [Google Scholar]
- 24.Jansen B, Wacheck V, Heere-Ress E, Schlagbauer-Wadl H, Hoeller C, Lucas T, Hoermann M, Hollenstein U, Wolff K, Pehamberger H. Lancet. 2000;356(9243):1728–33. doi: 10.1016/S0140-6736(00)03207-4. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki Y, Wakita D, Chamoto K, Narita Y, Tsuji T, Takeshima T, Gyobu H, Kawarada Y, Kondo S, Akira S, Katoh H, Ikeda H, Nishimura T. Cancer Res. 2004;64(23):8754–60. doi: 10.1158/0008-5472.CAN-04-1691. [DOI] [PubMed] [Google Scholar]
- 26.Li WM, Bally MB, Schutze-Redelmeier MP. Vaccine. 2001;20(1–2):148–57. doi: 10.1016/s0264-410x(01)00277-8. [DOI] [PubMed] [Google Scholar]
- 27.Akhtar S, Hughes MD, Khan A, Bibby M, Hussain M, Nawaz Q, Double J, Sayyed P. Adv Drug Deliv Rev. 2000;44(1):3–21. doi: 10.1016/s0169-409x(00)00080-6. [DOI] [PubMed] [Google Scholar]
- 28.Iwasaki A, Medzhitov R. Nat Immunol. 2004;5(10):987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 29.Gekeler V, Gimmnich P, Hofmann HP, Grebe C, Rommele M, Leja A, Baudler M, Benimetskaya L, Gonser B, Pieles U, Maier T, Wagner T, Sanders K, Beck JF, Hanauer G, Stein CA. Oligonucleotides. 2006;16(1):83–93. doi: 10.1089/oli.2006.16.83. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Science. 1999;284(5418):1313–8. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 31.Steinman RM, Dhodapkar M. Int J Cancer. 2001;94(4):459–73. doi: 10.1002/ijc.1503. [DOI] [PubMed] [Google Scholar]
- 32.Ikeda H, Chamoto K, Tsuji T, Suzuki Y, Wakita D, Takeshima T, Nishimura T. Cancer Sci. 2004;95(9):697–703. doi: 10.1111/j.1349-7006.2004.tb03248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trinchieri G. Nat Rev Immunol. 2003;3(2):133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]