Abstract
Background
No consensus exists on screening to detect the estimated 2 million Americans unaware of their chronic hepatitis C infections. Advisory groups differ, recommending birth-cohort screening for baby boomers, screening only high-risk individuals, or no screening. We assessed one-time risk assessment and screening to identify previously undiagnosed 40–74 year-olds given newly available hepatitis C treatments.
Methods and Findings
A Markov model evaluated alternative risk-factor guided and birth-cohort screening and treatment strategies. Risk factors included drug use history, blood transfusion before 1992, and multiple sexual partners. Analyses of the National Health and Nutrition Examination Survey provided sex-, race-, age-, and risk-factor-specific hepatitis C prevalence and mortality rates. Nine strategies combined screening (no screening, risk-factor guided screening, or birth-cohort screening) and treatment (standard therapy–peginterferon alfa and ribavirin, Interleukin-28B-guided (IL28B) triple-therapy–standard therapy plus a protease inhibitor, or universal triple therapy). Response-guided treatment depended on HCV genotype. Outcomes include discounted lifetime costs (2010 dollars) and quality adjusted life-years (QALYs).
Compared to no screening, risk-factor guided and birth-cohort screening for 50 year-olds gained 0.7 to 3.5 quality adjusted life-days and cost $168 to $568 per person. Birth-cohort screening provided more benefit per dollar than risk-factor guided screening and cost $65,749 per QALY if followed by universal triple therapy compared to screening followed by IL28B-guided triple therapy. If only 10% of screen-detected, eligible patients initiate treatment at each opportunity, birth-cohort screening with universal triple therapy costs $241,100 per QALY. Assuming treatment with triple therapy, screening all individuals aged 40–64 years costs less than $100,000 per QALY.
Conclusions
The cost-effectiveness of one-time birth-cohort hepatitis C screening for 40–64 year olds is comparable to other screening programs, provided that the healthcare system has sufficient capacity to deliver prompt treatment and appropriate follow-on care to many newly screen-detected individuals.
Introduction
An estimated 2 million Americans are unaware that they are infected with hepatitis C (HCV) [1]. Without diagnosis and treatment, they are at risk for liver fibrosis, cirrhosis, and hepatocellular-carcinoma (HCC). HCV-caused end-stage liver disease is the leading cause of liver transplantation {Ly, #366}. The prevalence of HCV antibodies is approximately 5% in individuals born between 1950 and 1960, over twice the general adult prevalence [3]. Lifestyle factors (e.g., history of injection drug use, blood transfusion before 1992, and risky sexual behaviors) are predictive of HCV infection, though not everyone is willing to divulge their true risk status to their clinicians. Screening – whether risk-based or birth-cohort-based – could potentially prevent substantial HCV-related losses of health and life provided those identified via screening receive appropriate treatment. Given the large number of screen-eligible individuals, it is important to determine which screening strategy is most cost-effective.
The CDC recently recommended one-time screening for all individuals born between 1945 and 1965 [4]. Previously, the National Institutes of Health Consensus Panel [5] and the American Liver Foundation [6] recommend screening only high-risk individuals. In 2004, the U.S. Preventive Services Task Force (USPSTF) recommended against birth-cohort HCV screening and found that the evidence supporting screening high-risk individuals was insufficient [7]. In 2012, the USPSTF produced a draft recommendation on screening for HCV infection in high-risk adults including those with any history of intravenous drug use or blood transfusions prior to 1992, which it is currently updating [8].
HCV screening guidelines require reconsideration in light of new, more effective, treatments [9], [10], potentially combined with patient genotyping (Interleukin (IL)–28B) to personalize treatment selection. The effectiveness and cost-effectiveness of HCV screening policies will depend on screening-related factors which determine how many additional people could be identified (e.g., prevalence of undiagnosed HCV infections; the predictive power of HCV risk factors) and treatment-related factors which determine how much benefit can be delivered and at what cost for each person identified (e.g., access to and choice of treatment).
Prior studies have evaluated the cost-effectiveness of birth-cohort screening compared to risk-based screening, though none have simultaneously included a number of important clinical and epidemiological considerations. A 2001 study did not support universal HCV screening among asymptomatic, average-risk American adults [11]. More recent studies found that birth-cohort screening costs between $5,400 and $37,700 per QALY gained [12], [13], [14]. No study has simultaneously compared risk-factor guided screening to birth-cohort screening, explicitly modeling risk assessment to identify high-risk individuals; included mortality differences between risk groups, whose exclusion may bias towards the cost-effectiveness of screening; and considered how the cost-effectiveness of screening depends on the quality of follow-up care, treatment uptake and adherence for the many screen-detected individuals.
We assessed the cost-effectiveness of one-time screening of 40–74 year-olds at a routine medical visit, addressing two questions: 1) Can costs and benefits of screening be improved by using risk assessment to identify asymptomatic individuals who are more likely HCV infected? 2) How is the cost-effectiveness of HCV screening affected by subsequent disease management, HCV treatment uptake, and treatment type?
Methods
Cohorts
The decision-analytic model applies screening and treatment strategies to asymptomatic 40–74 year-old (base case age 50) U.S. adults who are unaware of their HCV infection status, with attention focused on how treatment uptake and ongoing HCV care affect outcomes. Cohorts are stratified by age, sex, race, risk history, HCV infection status, HCV genotype, treatment eligibility, IL-28B genotype, and initial liver fibrosis stage. Model inputs are presented in Table 1.
Table 1. Model Parameter Values and Ranges.
| Variable | Base Case (Range) | Reference |
| Model assumptions | ||
| Discount rate (annual) | 0.03 (0.00–0.05) | [41] |
| Time horizon | Lifetime | |
| Perspective | Societal | |
| Cohort characteristics | ||
| Cohort age, years | 50 (40–74) | |
| Stage of fibrosis distribution in HCV+ population | [17] | |
| No fibrosis (F0) | 0.13 | |
| Portal fibrosis (F1) | 0.51 | |
| Periportal fibrosis (F2) | 0.13 | |
| Bridging fibrosis (F3) | 0.10 | |
| Compensated fibrosis (F4) | 0.13 | |
| Proportion with HCV genotype 1 | 0.8 (0.7–0.9) | [5] |
| Proportion with IL-28B genotype, CC-type polymorphism (vs. non–CC type) | ||
| White | 0.37 (0.28–0.46) | [46] |
| Black | 0.14 (0.11–0.18) | |
| Risk Status (by sex, race) * | NHANES 2001–2008 | |
| Percent of high risk individuals (%) | ||
| White male | 26 (24–28) | |
| White female | 15 (13–18) | |
| Black male | 31 (28–35) | |
| Black female | 19 (17–22) | |
| Prevalence of HCV+ among high-risk individuals (%) | ||
| White male | 13 (10–16) | |
| White female | 11 (8–15) | |
| Black male | 17 (13–21) | |
| Black female | 15 (11–19) | |
| Prevalence of HCV+ among low-risk individuals (%) | ||
| White male | 2 (1–3) | |
| White female | 2 (1–2) | |
| Black male | 3 (2–4) | |
| Black female | 2 (1–3) | |
| Awareness of HCV status (%) | NHANES 2001–2008 | |
| Percent aware among HCV+ high-risk individuals | 50 (5–60) | |
| Percent aware among HCV+ low-risk individuals | 50 (0–60) | |
| Percent aware among HCV- high-risk individuals | 50 (0–60) | Assumed |
| Percent aware among HCV- low-risk individuals | 5 (0–10) | Assumed |
| Annual probability of chance identification of HCV+ | 0.037 (0.010–0.050) | [11] |
| Screening characteristics | ||
| Risk Identification | ||
| Probability of identified as “high-risk” among true high-risk individuals (sensitivity) | [24] | |
| Male | 0.58 (0.00–1.00) | |
| Female | 0.69 (0.00–1.00) | |
| Probability of identified as “low-risk” among true low-risk individuals (specificity) | 1 | Assumed |
| HCV screening test (ELISA) | ||
| Probability of test+among HCV+ (sensitivity) | 0.970 (0.950–0.999) | [47] |
| Probability of test - among HCV- (specificity) | 0.9996 (0.9900–1.000) | [48] |
| HCV natural history | ||
| Proportion of patients with no fibrosis (F0) who do not progress | 0.24 (0.20–0.33) | [16] |
| Annual probability of spontaneous remission from no fibrosis (F0) health state | 0.012 (0.007–0.017) | [16], [30] |
| Fibrosis progression (annual probability) | [16], [30] | |
| Males | ||
| Age 40–49 y | 0.05 (0.03–0.09) | |
| Age 50–59 y | 0.12 (0.07–0.14) | |
| Age 60–69 y | 0.20 (0.12–0.30) | |
| Age ≥70 y | 0.26 (0.14–0.38) | |
| Females | ||
| Age 40–49 y | 0.03 (0.01–0.06) | |
| Age 50–59 y | 0.06 (0.03–0.11) | |
| Age 60–69 y | 0.11 (0.04–0.21) | |
| Age 70–79 y | 0.14 (0.08–0.24) | |
| Age ≥80 y | 0.20 (0.08–0.30) | |
| Cirrhosis to decompensated cirrhosis | 0.04 (0.03–0.05) | |
| Cirrhosis (both F4 and decompensated cirrhosis) to HCC | 0.02 (0.017–0.03) | |
| Liver transplant (annual probability) | [49] | |
| Decompensated cirrhosis to liver transplant | 0.05 (0.00–0.40) | |
| HCC to liver transplant | 0.15 (0.05–0.40) | |
| Chronic HCV conversion factor | NHANES 2001–2008 | |
| Male | 0.72 (0.58–0.89) | |
| Female | 0.65 (0.60–0.70) | |
| Hazard ratio for sex-, race-, risk-, HCV-, and age-specific mortality from non-liver causes in patients with chronic HCV infection (< age 70) | Appendix S1 Table S1 | NHANES III |
| Reduction factor on background mortality after successful treatment& | 0.7 (0.3–1.0) | [22] |
| Liver-related mortality (annual probability) | ||
| Liver transplant | 0.140 (0.134–0.150) | [50] |
| After liver transplant | 0.050 (0.049–0.051) | [50] |
| Decompensated cirrhosis | 0.26 (0.12–0.33) | [16] |
| HCC | [51] | |
| First year | 0.72 (0.58–0.80) | |
| Subsequent year | 0.25 (0.16–0.30) | |
| Treatment-related mortality | 0.0050 (0.0005–0.0110) | [52] |
| Liver biopsy-related mortality | 0.0003 (0.0000–0.0033) | [53] |
| Probability of FibroTest showing F2+ for patients in F0–F1 Fibrosis | 0.13 (0.06–0.15) | [27] |
| Treatment characteristics | ||
| Percent of treatment eligible among diagnosed HCV+ | 0.86 (0.75–0.95) | [25] |
| Percentage of people accepting treatment when offered (%) | [25], [26] | |
| Genotype 1, F0–F1 fibrosis | 30 (10–90) | |
| Genotype 1, F2–F4 fibrosis | 39 (10–90) | |
| Genotype 2&3, F0–F1 fibrosis | 30 (10–90) | |
| Genotype 2&3, F2–F4 fibrosis | 39 (10–90) | |
| Effectiveness of treatment in genotype 1 patients | Details in Appendix S1 Table S2 | [15] |
| Standard therapy (PEG-INF+Rb) | [46], [54], [55] | |
| Mild fibrosis (F0/F1/F2), white | ||
| Overall probability of SVR | 0.46 (0.42–0.49) | |
| Mild fibrosis (F0/F1/F2), black | ||
| Overall probability of SVR | 0.19 (0.13–0.24) | |
| Triple therapy (PEG-INF+Rb+PI) ** | [9], [56], [57], [58], [59], [60] | |
| Adherence to triple therapy | 0.70 (0.50–0.70) | |
| Mild fibrosis (F0/F1/F2), white | ||
| Overall probability of SVR | 0.68 (0.60–0.72) | |
| Mild fibrosis (F0/F1/F2), black | ||
| Overall probability of SVR | 0.42 (0.24–0.47) | |
| Effectiveness of treatment in genotype 2&3 patients | 0.80 (0.60–0.90) | [1], [55] |
| Reduction in SVR for advanced fibrosis stage (F3 and F4) | 0.80 (0.70–1.00) | |
| Quality of life*** | ||
| Age-specific QALY weights | [28], [29] | |
| HCV-specific weights | [30], [31], [32], [33], [34] | |
| HCV mild fibrosis (F0, F1) | 0.980 (0.700–1.000) | |
| SVR after mild fibrosis | 1.000 (0.740–1.000) | |
| HCV moderate fibrosis (F2, F3) | 0.850 (0.660–1.000) | |
| SVR after moderate fibrosis | 0.933 (0.710–1.000) | |
| Compensated cirrhosis (F4) | 0.790 (0.460–1.000) | |
| SVR after cirrhosis | 0.933 (0.600–1.000) | |
| Decompensated cirrhosis | 0.720 (0.257–0.913) | |
| HCC | 0.720 (0.150–0.950) | |
| Liver transplant (during or after) | 0.825 (0.636–1.000) | |
| Standard therapy annualized decrement† | −0.110 (−0.200–0.000) | |
| Triple therapy annualized decrement† | −0.165 (−0.400–0.000) | |
| Liver transplant annualized decrement† | −0.200 (−0.364–0.000) | |
| Liver biopsy decrement ˆ | −0.055 (−0.200–0.000) | |
| HCV awareness annualized decrement | −0.020 (−0.050–0.000) | [11] |
| Cost (2010 U.S. dollars), $ | ||
| Age-specific baseline health care costs | [36] | |
| Screening | CMS | |
| HCV anti-body screening (ELISA) | 20 (10–31) | CPT 86803 |
| Risk identification (HCV+) | 36 (18–54) | CPT 99401 |
| Diagnosis (2 confirmatory ELISA, RIBA, and RNA test) | 210 (105–315) | 2 × (CPT 86803)+CPT 86804+ CPT 87522 |
| Reporting to the patient the results of a negative test | 8 (0–11) | [61] |
| HCV genotyping | 369 (184–553) | CPT 87902 |
| IL-28B genotyping | 371 (186–557) | [15] |
| Liver biopsy | 1,340 (990–1,650) | CPT 47000 |
| FibroTest | 240 (102–300) | [27] |
| Treatment (drug and medical care) | ||
| PEG-INF+Rb (F0 to F3, 24 wk) | 16,346 (6,001–24,730) | [26], [62] |
| PEG-INF+Rb (F0 to F3, 24 wk) | 17,907 (7,562–26,291) | [26], [62] |
| PEG-INF+Rb (F0 to F3, 48 wk) | 32,692 (12,002–49,460) | [26], [62] |
| PEG-INF+Rb (F4, 48 wk) | 35,814 (15,123–52,582) | [26], [62] |
| PIs (per week)† | 1,100 (781–1,430) | [63], [64] |
| AEs, standard therapy | 1,920 (1344–2,496) | [65] |
| AEs, standard therapy, PI | 2,586 (1810–3,361) | [65] |
| Annual care|| | [26], [30], [37], [66], [67] | |
| Aware of HCV status | ||
| HCV mild fibrosis (F0, F1) | 1,404 (152–4,194) | |
| HCV portal fibrosis (F2) | 1,404 (152–4,194) | |
| HCV bridging fibrosis (F3) | 1,404 (152–4,194) | |
| Compensated cirrhosis (F4) | 4,194 (152–4,194) | |
| Unaware of HCV status | ||
| HCV mild fibrosis (F0, F1) | 811 (0–1,404) | |
| HCV portal fibrosis (F2) | 811 (0–1,404) | |
| HCV bridging fibrosis (F3) | 811 (0–1,404) | |
| Compensated cirrhosis (F4) | 1,622 (0–4,194) | |
| Decompensated cirrhosis | 11,109 (5,560–16,669) | |
| HCC | 44,224 (22,117–66,341) | |
| Liver transplant, first year | 145,640 (72,825–218,455) | |
| Liver transplant, subsequent | 25,430 (12,715–38,156) | |
| Recovered states from F0 to F3 | 406 (0–702) | Assumed¶ |
| Recovered states from F4 | 811 (0–2,097) | Assumed¶ |
HCC = hepatocellular carcinoma; HCV = hepatitis C virus; IL-28B = interleukin-28B; NHANES III = Third National Health and Nutrition Examination Survey; PEG-IFN = pegylated interferon; PI = protease inhibitor; Rb = ribavirin; SVR = sustained virologic response; AE = adverse event; QALY = quality-adjusted life-year; CMS = Center for Medicare & Medicaid Services. For further details on parameter generation and the uncertainty distribution of parameters see Appendix S1 I; Appendix S1 I Table S2; Appendix S1 Table S3.
A high-risk individual is someone having a history of injection drug use, transfusion prior to 1992, or greater than 20 lifetime sex partners. The reported prevalence is estimated for the 1952–1961 birth cohort and include individuals both aware and unaware of their HCV infection status. We adjusted the prevalence to only include individuals unaware of their infection status in the cost-effectiveness analyses.
& The mortality rates for people who recovered from HCV are adjusted by a linear combination of their mortality rates with HCV and mortality rates without HCV using a factor of 0.7.
The reported triple therapy effectiveness in the base-case is similar to boceprevir.
The total quality-of-life weight for a given age and HCV disease state is computed as the product of the mean age-specific quality weight obtained from published data [28], [29] and the utility associated with the HCV disease state minus any utility decrements for events that occurred during the cycle such as receiving treatment or a liver transplant.
Unlike other utilities in this table, these utility decrements are for short-term states (that is, receiving HCV treatment or a liver transplant). The QALY decrement for receiving HCV treatment involves multiplying the annual utility decrement by the time on treatment, which can vary given the response-guided therapy rules of each strategy. ˆOne time disutility applied in a 12 weeks period.
The PI cost is added to the standard therapy cost while receiving triple therapy.
|| The total costs for a given age and HCV disease state is computed as the sum of the mean age-specific health care costs [36] and the HCV-specific health state plus any costs of testing, treatment, or liver transplant that occurred in the cycle.
We assumed costs in the recovered states are 50% of the hepatitis C–related care costs in the year before diagnosis of the corresponding unaware states [37].
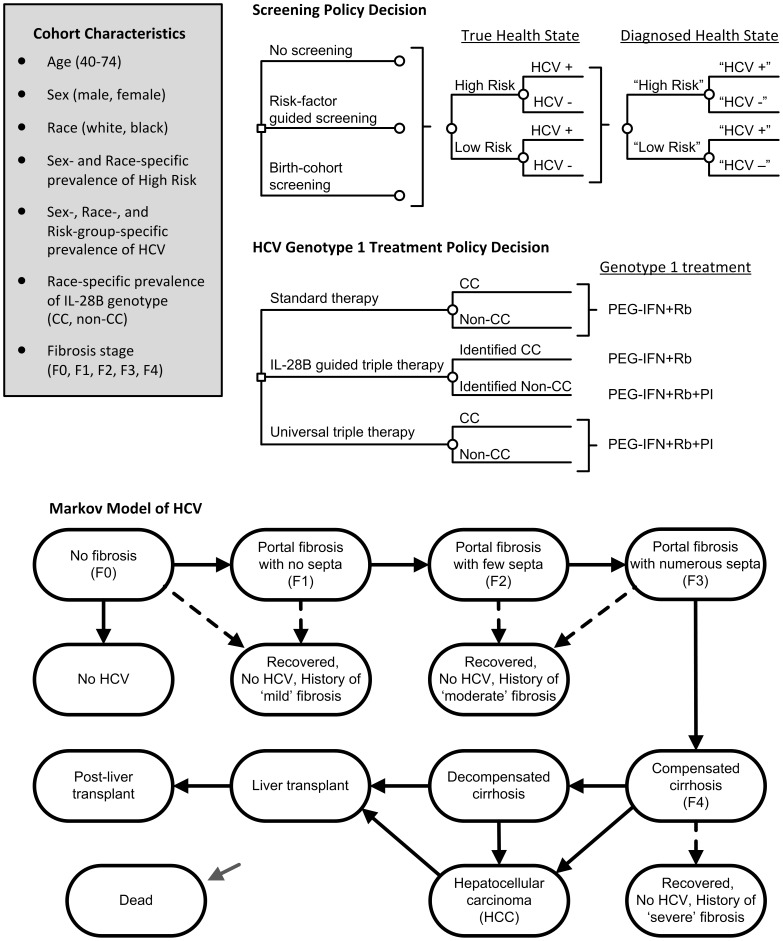
Screening and Treatment
One-time screening is performed at a routine medical visit at the outset of the analysis. We assessed screening strategies in combination with treatment strategies (Figure 1). Screening strategies included: 1) No screening: no systematic screening but HCV infected individuals may receive treatment after chance identification; 2) Risk-factor guided screening: HCV screening is only offered to individuals classified as “high risk” through an imperfect assessment of their risk history; and 3) Birth-cohort screening: all individuals are offered HCV screening. A diagnosis of “HCV-positive” occurs after a positive result on the initial enzyme immunoassay (ELISA) test, confirmed by two ELISAs, a recombinant immunoblot assay (RIBA), and a HCV RNA test to verify chronic infection. Treatment-eligible, chronically-infected individuals have their HCV infection genotyped. Three response-guided treatment strategies for HCV genotype 1 infected patients [15] were: 1) Standard therapy: patients receive pegylated interferon with ribavirin; 2) Universal triple therapy: patients receive pegylated interferon with ribavirin and a protease inhibitor; or 3) IL-28B-guided triple therapy: using IL-28B genotyping, non-CC type patients receive triple therapy and CC type patients receive standard therapy. Standard and triple therapy treatments employ specific response-guided protocols (Table S2 in Appendix S1) [15]. In all cases, patients diagnosed with genotype 2 and 3 receive 24 weeks of standard therapy.
Figure 1. Model schematics.
Small squares represent decisions. For the screening policy decision we considered the alternatives of implementing a policy of no screening, risk-factor guided screening, and birth-cohort screening. For the HCV genotype 1 treatment policy decision we considered the alternatives of standard therapy, in which patients receive pegylated interferon with ribavirin; IL-28B-guided triple therapy, in which after IL-28B genotyping patients with non-CC types receive triple therapy and patients with CC types receive standard therapy; and universal triple therapy, in which patients receive pegylated interferon with ribavirin and a protease inhibitor. In all strategies patients diagnosed with genotypes 2 and 3 receive 24 weeks of standard therapy. We considered all possible combinations of the screening policy decision and the genotype 1 treatment policy decision for a total of 9 policy alternatives. Small circles indicate chance events. Upon entering the model the cohort is stratified by true health state of risk-factor status (high risk or low risk), HCV-status (positive or negative), among HCV-positive individuals by HCV genotype (genotype 1 or other), and among HCV-positive genotype 1 individuals by IL-28B genotype (CC or non-CC type). Depending on the screening strategy, individuals may be imperfectly identified as “high-risk” or “low-risk”, may be screened for HCV, and may be imperfectly identified as “HCV+” and “HCV–”. Once individuals are classified with a diagnosis they enter one of two Markov models based on their true health state. The Markov model of HCV is shown. The Markov model of individuals who do not have HCV has only two health states, No HCV and Dead. We assume no HCV incidence in the model. HCC = hepatocellular carcinoma; HCV = hepatitis C virus; IL-28B = interleukin-28B; PEG-IFN = pegylated interferon; PI = protease inhibitor; Rb = ribavirin.
HCV Natural History
The HCV natural history follows a previously-published empirically-calibrated model [15], [16]. Chronically infected individuals start with an initial distribution of liver fibrosis stages (Metavir scores of F0, F1, F2, F3, or F4) and progress toward advanced liver disease (Figure 1) [17]. Disease progression rates depend on age and sex with possible transitions occurring every 12 weeks. We note that disease progression rates from a meta-analysis by Thein et al. [18] are within range to the values in our model. Successful treatment arrests further progression.
Risk Factors and HCV Prevalence
“High-risk” was defined as having a history of injection drug use, transfusion prior to 1992, or greater than 20 lifetime sex partners [3]. We estimated the prevalence of risk factors and of HCV among high- and low-risk individuals stratified by age, sex, and race using the National Health and Nutrition Examination Survey (NHANES) (2001–2008) (Section I in Appendix S1).
Mortality
Mortality rates by age, sex, race, risk status, and HCV infection were calculated using hazard ratios estimated from the NHANES III linked mortality data and U.S. life-tables [19] (Table S1 in Appendix S1). Successful treatment prevents long-term consequences of HCV and reduces non-liver-related mortality, which still remains higher than for individuals with no history of HCV [20], [21], [22], [23]. As age-specific mortality rates of individuals who have recovered from HCV are unknown, we reduced their pre-treatment rates by 0.80 and explored this assumption in sensitivity analyses.
Risk Assessment
Risk-based screening depends on the ability of healthcare workers to accurately identify high-risk patients. We assumed that assessment of risk-factor status had 100% specificity but a sensitivity of 60% (men) and 70% (woman) [24], varying these widely in sensitivity analyses.
Treatment Eligibility
Medical contraindications (e.g., medical and psychiatric co-morbidities, active substance abuse, and alcoholism) leave 14% of HCV patients ineligible for treatment [25].
Treatment Uptake and Ongoing Monitoring
We assumed 30% and 39% of eligible individuals with biopsy-established fibrosis stage F0–F1 and F2–F4, respectively, would initiate treatment immediately [25], [26]. For those who do not, progression surveillance occurs every 3 years using non-invasive fibrosis assessment [27]. We assume that progression to F2 leads 39% of patients with F0–F1 fibrosis at diagnosis to initiate treatment and that 39% of treatment-eligible patients with F2–F4 fibrosis will initiate treatment every three years.
Health-Related Quality of Life
Age-specific quality-of-life weights were derived from the Medical Expenditure Panel Survey [28], [29]. Quality-of-life reductions associated with chronic HCV infection were estimated by combining several studies [30], [31], [32], [33], [34]. We assumed an annual utility decrement from being aware of HCV infection [11], [35] and for being on treatment, varying these in sensitivity analyses [15], [31].
Costs
Age-specific baseline healthcare costs included out-of-pocket expenses [36]. We included additional fibrosis stage-specific costs attributable to chronic HCV infection for patients unaware and aware of their infection status [37] which we reduced by 50% for patients who achieved sustained virologic response (SVR) [26], [37]. We estimated screening-related costs using the 2010 Medicare fee schedule. We included patients’ time costs during HCV diagnosis, liver biopsy, and IL-28B genotyping by multiplying time lost by the mean 2010 hourly wage [38]. Treatment costs include drugs and medical care for the duration of treatment, which depend on a patient’s virologic response to therapy [15]. Costs were inflation-adjusted to 2010 U.S. dollars using the Consumer Price Index [39].
Cost-Effectiveness
Main outcomes were lifetime costs and quality-adjusted life-years (QALYs). Results are presented as population-weighted averages over race and sex [40]. We adopted a societal perspective, considered costs and benefits over a lifetime horizon, and discounted future costs and benefits at 3% annually [41]. We performed deterministic sensitivity analyses for all variables as well as probabilistic sensitivity analyses (Table S3 in Appendix S1).
Results
Main Analysis
The health benefits of both birth-cohort and risk-factor guided HCV screening compared to no screening are substantial but depend strongly on ensuring adequate treatment uptake and adherence.
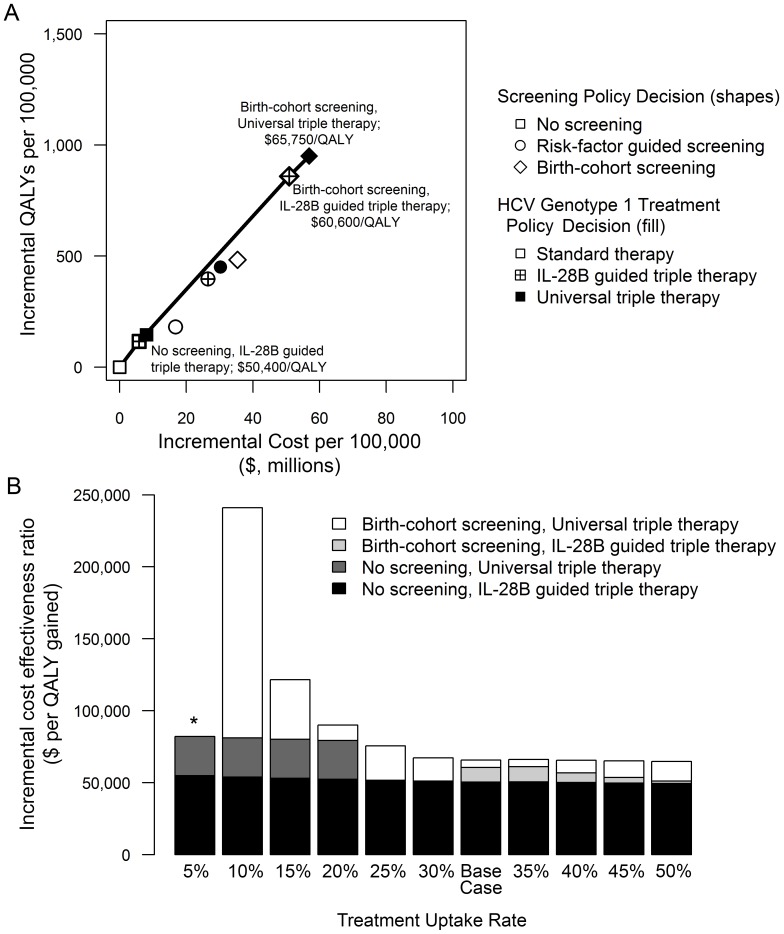
In the base case, risk-factor guided and birth-cohort screening of individuals who are currently 50 years of age, respectively, averted 4–7 and 10–15 liver transplants, 13–27 and 35–56 liver cancers, and gained 181–450 and 483–950 QALYs per 100,000 people compared to no screening, depending on the HCV treatment strategy used and assuming 30–40% treatment uptake and 70% treatment adherence (Table 2). Risk-factor guided and birth-cohort screening, respectively, increased costs by $17–30 million and $35–57 million per 100,000 people compared to no screening. Birth-cohort screening yielded greater health benefits per dollar spent than risk-factor guided screening in all cases largely because risk factors for HCV are too common and not sufficiently predictive. Compared to no screening, birth-cohort screening of individuals who are currently 50 years of age followed by IL-28B-guided triple-therapy costs $60,590 per QALY gained. Birth-cohort screening followed by universal triple therapy costs $65,749 per QALY gained (Figure 2a). Costs and benefits of each screening and treatment strategy for cohorts of 40, 50, 60, and 70 year-olds are shown in Table 3. Birth-cohort screening followed by universal triple therapy costs less than $100,000 per QALY for ages 40–64 years compared to the next best strategy (Table 3). Section II in Appendix S1 presents sex and race-stratified results as well as results for settings in which IL-28B genotyping or triple therapy is not available (Table S4, Table S5 and Figure S2 in Appendix S1).
Table 2. Base case lifetime costs, health benefits (per 100,000), and incremental costs effectiveness ratio of combined screening and treatment strategies for a cohort of individuals who are currently 50 years of age.
| COMBINED STRATEGIES | Per 100,000* | |||||
| Screening | Treatment | Liver Cancers Averted | Liver Transplants Averted | IncrementalCost ($) | IncrementalQALY | ICER($/QALY) |
| No Screening | Standard therapy | Reference | Reference | Reference | Reference | – |
| No Screening | IL-28B guided triple therapy | 7 | 1 | 5,833,793 | 116 | $50,417 |
| No Screening | Universal triple therapy | 9 | 2 | 8,076,805 | 145 | Dominated |
| Risk-Based | Standard therapy | 13 | 4 | 16,795,805 | 181 | Dominated |
| Risk-Based | IL-28B guided triple therapy | 24 | 6 | 26,537,268 | 397 | Dominated |
| Risk-Based | Universal triple therapy | 27 | 7 | 30,282,373 | 450 | Dominated |
| Birth-cohort | Standard therapy | 35 | 10 | 35,369,580 | 483 | Dominated |
| Birth-cohort | IL-28B guided triple therapy | 53 | 14 | 50,876,459 | 859 | $60,590 |
| Birth-cohort | Universal triple therapy | 56 | 15 | 56,843,606 | 950 | $65,749 |
Population weighted average (white male 44%, white female 45%, black male 5%, black female 6%) for fibrosis distribution: F0 13%, F1 51%, F2 13%, F3 10%, and F4 13%. All incremental cost and QALY are compared to the reference.
ICER = incremental cost-effectiveness ratio; IL-28B = interleukin-28B; QALY = quality-adjusted life-year.
“Dominated” indicates that the strategy costs more and provides fewer benefits than another strategy or a combination of two strategies.
Figure 2. Cost-effectiveness analysis.
(A) The graph plots the incremental discounted QALYs (y-axis) and incremental discounted lifetime costs (x-axis) for each combined screening and treatment strategy. The solid line represents the cost-effectiveness frontier, those strategies that are potentially economically efficient depending on one’s willingness-to-pay per unit of health benefit gained. (B) The bar graph shows the incremental cost-effectiveness ratios of each combined screening and treatment strategy at different levels of treatment uptake at each opportunity (varied over the range 0–50%). The asterisk denotes that, at 5% uptake, birth-cohort screening followed by universal triple therapy for screen-detected, treatment-eligible individuals is dominated. For both panels, IL-28B = interleukin-28B; QALY = quality-adjusted life-year.
Table 3. Lifetime costs, health benefits (per 100,000), and incremental costs effectiveness ratio of combined screening and treatment strategies for various patient ages.
| COMBINED STRATEGIES | Per 100,000* | ||||
| Age | Screening | Treatment | IncrementalCost ($) | IncrementalQALY | ICER($/QALY) |
| 40 | No Screening | Standard therapy | Reference | Reference | – |
| No Screening | IL-28B guided triple therapy | 3,001,814 | 68 | 44,228 | |
| No Screening | Universal triple therapy | 4,151,745 | 84 | Dominated | |
| Risk-Based | Standard therapy | 8,740,935 | 66 | Dominated | |
| Risk-Based | IL-28B guided triple therapy | 13,124,293 | 176 | Dominated | |
| Risk-Based | Universal triple therapy | 14,811,803 | 202 | Dominated | |
| Birth-cohort | Standard therapy | 15,875,395 | 184 | Dominated | |
| Birth-cohort | IL-28B guided triple therapy | 22,457,464 | 366 | Dominated | |
| Birth-cohort | Universal triple therapy | 25,006,361 | 408 | 64,719 | |
| 50 | No Screening | Standard therapy | Reference | Reference | – |
| No Screening | IL-28B guided triple therapy | 5,833,793 | 116 | 50,417 | |
| No Screening | Universal triple therapy | 8,076,805 | 145 | Dominated | |
| Risk-Based | Standard therapy | 16,795,805 | 181 | Dominated | |
| Risk-Based | IL-28B guided triple therapy | 26,537,268 | 397 | Dominated | |
| Risk-Based | Universal triple therapy | 30,282,373 | 450 | Dominated | |
| Birth-cohort | Standard therapy | 35,369,580 | 483 | Dominated | |
| Birth-cohort | IL-28B guided triple therapy | 50,876,459 | 859 | 60,590 | |
| Birth-cohort | Universal triple therapy | 56,843,606 | 950 | 65,749 | |
| 60 | No Screening | Standard therapy | Reference | Reference | – |
| No Screening | IL-28B guided triple therapy | 4,454,805 | 62 | 71,897 | |
| No Screening | Universal triple therapy | 6,181,389 | 79 | Dominated | |
| Risk-Based | Standard therapy | 16,590,388 | 115 | Dominated | |
| Risk-Based | IL-28B guided triple therapy | 25,235,234 | 250 | Dominated | |
| Risk-Based | Universal triple therapy | 28,555,220 | 285 | Dominated | |
| Birth-cohort | Standard therapy | 35,040,741 | 317 | Dominated | |
| Birth-cohort | IL-28B guided triple therapy | 49,907,383 | 571 | Dominated | |
| Birth-cohort | Universal triple therapy | 55,601,271 | 636 | 89,074 | |
| 70 | No Screening | Standard therapy | Reference | Reference | – |
| No Screening | IL-28B guided triple therapy | 2,909,047 | 25 | 116,963 | |
| No Screening | Universal triple therapy | 4,058,590 | 32 | 153,204 | |
| Risk-Based | Standard therapy | 15,037,415 | 37 | Dominated | |
| Risk-Based | IL-28B guided triple therapy | 22,001,319 | 102 | Dominated | |
| Risk-Based | Universal triple therapy | 24,700,957 | 121 | Dominated | |
| Birth-cohort | Standard therapy | 31,250,082 | 112 | Dominated | |
| Birth-cohort | IL-28B guided triple therapy | 44,286,780 | 247 | Dominated | |
| Birth-cohort | Universal triple therapy | 49,318,701 | 285 | 179,186 | |
Population weighted average (white male 44%, white female 45%, black male 5%, black female 6%) for fibrosis distribution: F0 13%, F1 51%, F2 13%, F3 10%, and F4 13%. All incremental cost and QALY are compared to the reference.
ICER = incremental cost-effectiveness ratio; IL-28B = interleukin-28B; QALY = quality-adjusted life-year.
“Dominated” indicates that the strategy costs more and provides fewer benefits than another strategy or a combination of two strategies.
Lower levels of treatment uptake erode the cost-effectiveness of HCV screening (Figure 2b). If only 10% of the screened and treatment-eligible population initiate treatment at each opportunity, birth-cohort screening with triple therapy costs $241,100 per QALY compared to no screening. Birth-cohort screening costs approximately $50,000 per QALY only when treatment uptake is greater than 50%. The introduction of triple therapy into practice may improve treatment uptake due to its higher effectiveness, but its higher rates of side effects may also decrease adherence. If adherence to triple therapy is lower than standard therapy, the preferred strategy shifts from universal triple therapy to IL-28B genotype guided triple therapy (Table 4).
Table 4. Deterministic sensitivity analysis of cohort and treatment factors.
| ICER ($/QALY)* | |||||
| No Screening,IL-28Bguided tripletherapy | No Screening,Universaltriple therapy | Birth-cohortscreening,Standard therapy | Birth-cohortscreening, IL-28Bguided tripletherapy | Birth-cohortscreening,Universaltriple therapy | |
| Cohort Characteristics (age and overall HCV prevalence) | |||||
| 40 years (2.82%**) | 44,228 | Dominated | Dominated | Dominated | 64,719 |
| 50 years (2.98%) | 50,530 | Dominated | Dominated | 62,329 | 65,870 |
| 50 years (4.27%, base case) | 50,417 | Dominated | Dominated | 60,590 | 65,749 |
| 50 years (5.56%) | 50,358 | Dominated | Dominated | 59,660 | 65,684 |
| 60 years (4.27%) | 71,897 | Dominated | Dominated | Dominated | 89,074 |
| Initial Fibrosis Stage distribution | |||||
| Less severe (30% F0, 41% F1, 22% F2,3% F3, and 4% F4) | 54,222 | Dominated | Dominated | 71,337 | 73,031 |
| More severe (18% F0, 24% F1, 17% F2,13% F3, and 28% F4) | 46,939 | Dominated | Dominated | 53,746 | 59,246 |
| Quality of life reduction from awareness of HCV-positive status | |||||
| No reduction | Dominated | Dominated | Dominated | 48,863 | 70,173 |
| High reduction (-0.05) | 46,137 | 70,742 | Dominated | Dominated | 92,509 |
| Chronic HCV health care cost from awareness of HCV-positive status | |||||
| High utilization (annual cost of $4,200in F0–F3, and $8,400 in F4) | 36,944 | 66,682 | Dominated | Dominated | 100,167 |
| Same cost and utility between aware andunaware of HCV-positive status (annual costof $1,400 in F0–F3, and $4,200 in F4) | Dominated | Dominated | 28,279 | 44,092 | 70,173 |
| Treatment uptake | |||||
| Very low uptake (10%) | 53,938 | 81,115 | Dominated | Dominated | 241,066 |
| Low uptake (20%) | 52,370 | 79,357 | Dominated | Dominated | 90,129 |
| Medium High uptake (50%) | 49,447 | Dominated | Dominated | 51,165 | 64,786 |
| High uptake (70%) | Dominated | Dominated | Dominated | 45,306 | 63,602 |
| Very high uptake (90%) | Dominated | Dominated | Dominated | 42,160 | 62,866 |
| Treatment adherence & | |||||
| Low adherence to triple therapy (50%) | Dominated | Dominated | 73,265 | 79,538 | Dominated |
| Reduction of non-liver related mortality | |||||
| No reduction | 61,792 | Dominated | Dominated | Dominated | 83,980 |
| High reduction | 41,149 | Dominated | Dominated | 44,896 | 52,633 |
Population weighted average (white male 44%, white female 45%, black male 5%, black female 6%). Each strategy is compared to the next-best strategy on the efficient frontier. Risk factors were considered for all of these scenario analyses but are dominated in all cases.
Prevalence based on 1962–1971 cohort.
Adherence is defined as patients taking ≥80% of their HCV medications.
“Dominated” indicates that the strategy costs more and provides fewer benefits than another strategy or a combination of two strategies.
Population Impact
For the current screening eligible cohort of 85.3 million 40–64 year olds in the US, implementing a policy of risk-factor guided screening combined with IL-28B guided triple therapy increased 162,482 QALYs at an increased cost of $14.2 billion compared to no screening with IL-28B guided triple therapy; and combined with universal triple therapy increased 176,912 QALYs at an increased cost of $15.2 billion compared to no screening with universal triple therapy. Also compared to no screening with the same treatment strategy, implementing a policy of birth-cohort screening of 40–64 year olds combined with IL-28B guided triple therapy increased 436,394 QALYs at an increased cost of $30.1 billion; and combined with universal triple therapy increased QALYs by as much as 474,196 QALYs at an increased cost of $32.6 billion (Table 5).
Table 5. Population impact of HCV screening aged 40–64 years, total lifetime costs, health benefits, and incremental costs effectiveness ratio of combined screening and treatment strategies.
| COMBINED STRATEGIES | Incremental Cost ($) | Incremental QALY | ICER ($/QALY)* | |
| Screening | Treatment | |||
| No Screening | Standard therapy | Reference | Reference | – |
| No Screening | IL-28B guided triple therapy | 3,712,793,775 | 70,138 | 52,936 |
| No Screening | Universal triple therapy | 5,144,766,183 | 87,827 | Dominated |
| Risk-Based | Standard therapy | 11,635,472,594 | 102,088 | Dominated |
| Risk-Based | IL-28B guided triple therapy | 17,914,981,494 | 232,620 | Dominated |
| Risk-Based | Universal triple therapy | 20,332,866,318 | 264,739 | Dominated |
| Birth-cohort | Standard therapy | 23,673,786,131 | 277,782 | Dominated |
| Birth-cohort | IL-28B guided triple therapy | 33,801,361,880 | 506,532 | 68,948 |
| Birth-cohort | Universal triple therapy | 37,702,921,559 | 562,023 | 70,309 |
Population weighted average (white male 44%, white female 45%, black male 5%, black female 6%) for fibrosis distribution: F0 13%, F1 51%, F2 13%, F3 10%, and F4 13%. All incremental cost and QALY are compared to the reference. Eligible screening population in the 40–64 year-old cohort is assumed at 83.5 million.
ICER = incremental cost-effectiveness ratio; IL-28B = interleukin-28B; QALY = quality-adjusted life-year.
“Dominated” indicates that the strategy costs more and provides fewer benefits than another strategy or a combination of two strategies.
Screening in Different Birth Cohorts
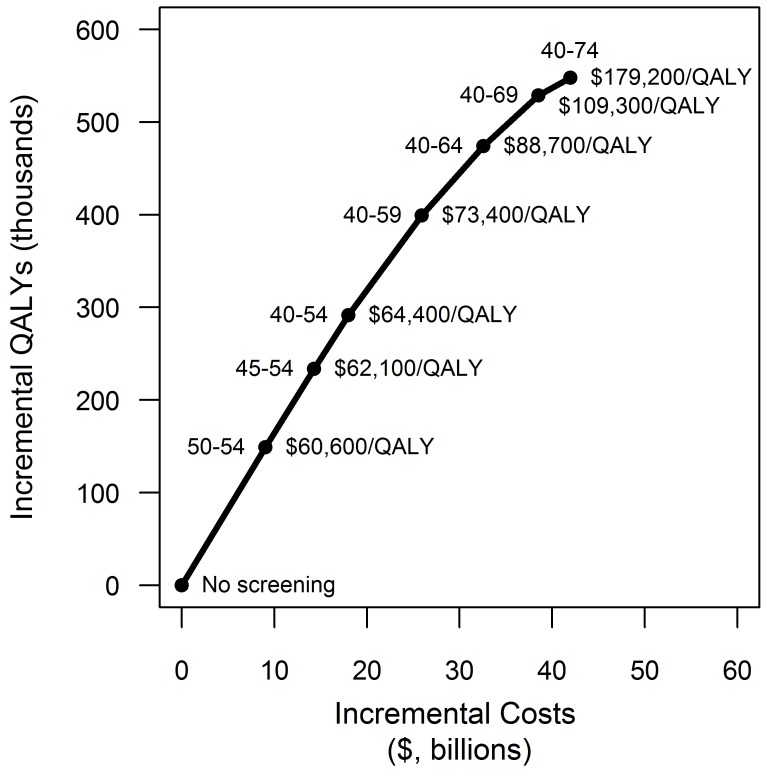
Holding the treatment decision constant, we evaluated various possible birth-cohorts to include in an efficient screening program. In this analysis we are essentially evaluating the new CDC recommendation of one-time birth-cohort screening and answering the question: if it is not possible to screen all birth cohorts, which age groups should be screened to most effectively address chronic HCV infections in the US? If standard therapy continues to be the widely used treatment choice, screening individuals aged 40–59 costs $90,090 per QALY gained. Expanding screening to include the next older cohort, individuals aged 60–64 increases the cost to $110,576 per QALY gained. If IL-28B guided triple therapy or universal triple therapy became the widely adopted treatment actions, screening individuals aged 40–64 costs approximately $89,000 per QALY gained (Figure 3). Expanding screening to include the next older cohort, individuals aged 65–69, increases the cost to approximately $110,000 per QALY gained. Screening individuals aged 70–74 costs approximately $180,000 per QALY gained if either IL-28B guided or universal triple therapy are the treatment action and costs $277,800 per QALY gained if HCV-infected patients are treated with standard two-drug therapy.
Figure 3. Cost-effectiveness of birth-cohort screening by age group.
The graph plots the incremental discounted QALYs and incremental discounted lifetime costs for screening various birth cohorts. The analysis shown in the graph assumes that the treatment strategy used is universal triple therapy. For clarity, the graph shows only those strategies on the cost-effectiveness frontier (i.e., those that are not dominated) although all combinations of birth-cohort groups (40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74 years of age) were considered in the analysis.
Sensitivity Analyses
Deterministic sensitivity analyses
The impact of varying cohort age and HCV prevalence, screening-related factors, and treatment-related factors on the findings are presented in Table 4 (with additional information provided in Section II, Table S6 in Appendix S1).
Our main analysis considered individuals aged 50 in 2011. Birth-cohort screening followed by universal triple therapy cost $64,700, $65,700, $89,100, and $179,200 per QALY gained for 40 year-olds, 50 year-olds, 60 year-olds, and 70 year-olds respectively. In the oldest cohorts, high HCV prevalence is offset by the shorter horizon over which to accrue benefits from averted serious liver diseases.
Screening factors such as the ability to elicit a true risk history from patients, the cost of risk assessment, the test characteristics, and costs of HCV screening and diagnostic tests did not alter the main results.
The fibrosis-stage distribution of individuals diagnosed through screening strongly influences cost-effectiveness (Figure S1 in Appendix S1). If more HCV-infected individuals detected via screening have mild liver fibrosis, screening is less cost-effective. If the fibrosis distribution were 71% F0–F1 and 29% F2–F4 [12], birth-cohort screening with triple therapy costs $73,000 per QALY whereas if the distribution were 42% F0–F1 and 58% F2–F4 [42], it costs $59,200 per QALY.
People newly diagnosed with HCV may increase their use of health care services both to manage their illness and because they become concerned, anxious, or worried, also leading to a lower quality-of-life. If this occurs, birth-cohort screening 50 year olds costs over $100,200 per QALY (Table 4). Proper counseling may help to ensure that patients do not experience decreased quality-of-life or worry-related increases in heath-resource consumption.
While chronic HCV treatment may also reduce non-liver related mortality [22], some studies suggest that this is because patients selected for treatment are relatively healthier. If non-liver related mortality does not change with successful HCV treatment, birth-cohort screening followed by triple therapy costs $84,000 per QALY.
Interferon-free direct-acting antiviral drugs that are currently in phase I and phase II trials show potential for high efficacy and reduced side-effects. As of yet, these therapies have not established efficacy and safety profiles in large phase III trials nor have they been FDA approved for use in routine care. We included a scenario analysis in which treatment is more effective (95% SVR for HCV genotype 1 patients with IL-28B genotype of CC and 80% SVR for HCV genotype 1 patients with non-CC IL-28B genotypes), with a better side-effect profile (QALY decrements of 0.025 instead of 0.055), and cost 70% of triple therapy (due to lower drug costs and/or shorter therapy duration). Result showed birth-cohort screening followed by universal triple therapy was preferred for birth cohorts of ages 40–64 years with a cost between $30,000 and $50,000 per QALY gained.
Probabilistic sensitivity analyses
At a willingness-to-pay threshold of $50,000 per QALY, screening was optimal 25% of the time. However, at a willingness-to-pay of $60,000 and $100,000 per QALY, birth-cohort screening was optimal 60% and 98% of the time, respectively. If treatment uptake were only 20%, even at $100,000 per QALY, birth-cohort screening was optimal only 67% of the time. Risk-factor guided screening was never preferred to birth-cohort screening (Figure S3 in Appendix S1).
Discussion
An estimated 2 million Americans are unaware of their chronic HCV infections. The health and mortality burdens experienced by this group are expected to rise as many were infected more than 30 years ago and have experienced long-term, asymptomatic liver fibrosis progression. Because newer treatments are more effective, early disease identification and treatment via birth-cohort screening of individuals who are currently 40 to 64 years old could improve health and increase life expectancy. A dramatic expansion of the current screening and treatment programs would also be costly. If high treatment uptake rates can be maintained for screen-detected individuals, compared to no systematic screening, birth-cohort screening costs between $60,590 and $65,749 per QALY gained depending on the recommended treatment regimen. However, if treatment uptake were lower, birth-cohort screening could cost over $200,000 per QALY gained.
Our study expands upon results from prior cost-effectiveness analyses of U.S. HCV screening policies [12], [13], [14]: explicitly examining risk-factor guided screening via detailed modeling of risk assessment; incorporating mortality hazard rates stratified by risk group and HCV status which permit more accurate estimates of the potential benefits of HCV screening and treatment; considering the role of IL-28B-guided triple therapy; and considering the cost-effectiveness of screening in age groups of 40–74 years of age. Like other recent studies, we find that birth-cohort screening is more cost-effective than risk-based screening for 40–64 year-olds. However, unlike these studies, the cost per QALY gained that we estimate is substantially higher, especially in scenarios in which treatment uptake is lower, the costs of downstream chronic HCV care are higher, or the quality of chronic HCV care worsens due to capacity limitations with large numbers of new screen-detected individuals.
We closely examined risk-factor guided HCV screening and found that it is not preferred to birth-cohort screening for several reasons. First, in the case of HCV, NHANES-derived prevalence estimates show that a relatively large fraction of infected individuals (28–47%) have no known risk factors. Second, risk factors such as history of injection drug use, blood transfusion before 1992, and greater than 20 lifetime sex partners are also relatively common among uninfected individuals and, therefore, are not sufficiently predictive. Third, chronically infected individuals without risk factors are often better treatment candidates than individuals with risk factors due to lower overall mortality from comorbidities. Finally, identifying patients with stigmatized risk factors, such as a history of injection drug, use may prove difficult. Despite these challenges, risk-based screening may still be important given tightening healthcare budgets. Implementing birth-cohort screening of individuals currently age 40–64 years costs between $12.0 and $17.4 billion more than implementing risk-based screening.
Maintaining high levels of treatment uptake and adherence is crucial for ensuring the cost-effectiveness of HCV screening. It is possible that treatment initiation rates will increase with the introduction of more effective triple therapy or direct-acting antiviral-only regimens when available, but these drugs’ side effects may also decrease adherence. In parts of the U.S., large numbers of newly screen-detected treatment candidates may exceed existing capacity, effectively lowering uptake rates.
The importance of providing appropriate counseling to reduce HCV related co-morbidities and minimize quality-of-life loss from awareness of HCV-positive status as part of HCV screening and treatment cannot be overstated. A driver of the relatively high cost per QALY gained from HCV screening is that knowing one’s HCV status reduces quality-of-life given anxiety surrounding the daunting possibility of intense treatment along with potential side effects and social stigma [11], [35]. If proper counseling can mitigate these effects and reduce associated increases in health resource consumption, birth-cohort screening of 50-year olds with IL-28B guided triple therapy costs $44,100 per QALY compared to no screening.
Our study has several limitations. First, we analyzed NHANES to estimate the prevalence of HCV stratified by sex, race, and risk-factor status depending on relatively small sample sizes. Reassuringly, varying these parameters across the range of uncertainty does not alter our main findings. Second, we did not include people with HIV or hepatitis B co-infections due to complexities of the two diseases and clinical challenges of safely and effectively treating both diseases [43], [44]. Third, observational studies that provide long-term follow-up data on differential mortality rates among people in various risk groups and HCV infection statuses are limited. We used the NHANES III linked mortality data, but there remains uncertainty about this effect. Fourth, because the protease inhibitors used in triple therapy were only recently approved, the feasibility of implementing response-guided therapy in routine practice, treatment effectiveness, and adherence are unknown. Finally, we did not include reductions in HCV transmission due to screening and successful treatment and, thus, may underestimate benefits associated with screening. Transmission effects are likely limited because our targeted screening group, older American adults, is responsible for a low percentage of HCV transmission [45].
One-time, birth-cohort HCV screening at a routine medical visit for asymptomatic adults currently aged 40–64 years followed by IL28B-guided or universal triple therapy for HCV infected patients provides substantial benefits and is likely cost-effective provided a sufficiently high treatment uptake rate and quality of follow-on care are ensured. Along with providing birth-cohort screening, HCV policies should focus on ensuring that screen-detected individuals receive prompt treatment and high-quality HCV care.
Supporting Information
Contains appendices with supporting information.
(DOCX)
Acknowledgments
The authors thank Kim Babiarz for suggestions on statistical analyses, Douglas Owens for helpful comments on the manuscript, and Paul Barnett and Steven Asch for helpful suggestions on previous, related HCV work.
Funding Statement
SL is supported by a Stanford Graduate Fellowship. LEC is supported by a doctoral scholarship from the Social Sciences and Humanities Research Council of Canada and by the Seth Bonder Scholarship for Applied Operations Research in Health Services from the Institute for Operations Research and the Management Sciences. MH is supported by the Department of Veterans Affairs and supported in part by R01 DA15612-016. JDGF is supported in part by a National Institutes of Health National Institute on Aging Career Development Award (K01 AG037593-01A1; principal investigator, JDGF). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ghany MG, Strader DB, Thomas DL, Seeff LB (2009) Diagnosis, Management, and Treatment of Hepatitis C: An Update. Hepatology 49: 1335–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, et al. (2012) The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med 156: 271–278. [DOI] [PubMed] [Google Scholar]
- 3. Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, et al. (2006) The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 144: 705–714. [DOI] [PubMed] [Google Scholar]
- 4. Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, et al. (2012) Hepatitis C virus testing of persons born during 1945–1965: recommendations from the Centers for Disease Control and Prevention. Ann of Intern Med 157: 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NIH Consensus Development Program website. National Institutes of Health Consensus Statement on Management of hepatitis C: 2002. June 10–12; 19(3) 1–46. Available: http://consensus.nih.gov/2002/2002HepatitisC2002116main.htm. Accessed 2009 October 1.
- 6.ALF website. American Liver Foundation Position. Available: http://www.liverfoundation.org/about/advocacy/hcvscreening/#AASLD. Accessed 2011 November 1.
- 7. Chou R, Clark EC, Helfand M (2004) Screening for hepatitis C virus infection: A review of the evidence for the US Preventive Services Task Force. Ann of Intern Med 140: 465–479. [DOI] [PubMed] [Google Scholar]
- 8.USPSTF website. USPSTF Screening for Hepatitis C Virus Infection in Adults: U.S. Preventive Services Task Force Recommendation Statement DRAFT. Available: http://www.uspreventiveservicestaskforce.org/uspstf12/hepc/hepcdraftrec.htm. Accessed 2013 February 26.
- 9. Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, et al. (2011) Boceprevir for Untreated Chronic HCV Genotype 1 Infection. N Engl J Med 364: 1195–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, et al. (2011) Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 364: 2405–2416. [DOI] [PubMed] [Google Scholar]
- 11. Singer ME, Younossi ZM (2001) Cost effectiveness of screening for hepatitis C virus in asymptomatic, average-risk adults. American Journal of Medicine 111: 614–621. [DOI] [PubMed] [Google Scholar]
- 12. Rein DB, Smith BD, Wittenborn JS, Lesesne SB, Wagner LD, et al. (2011) The cost-effectiveness of birth-cohort screening for hepatitis C antibody in U.S. primary care settings. Ann Intern Med 156: 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coffin PO, Scott JD, Golden MR, Sullivan SD (2012) Cost-effectiveness and population outcomes of general population screening for hepatitis C. Clin Infect Dis. 54: 1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McGarry LJ, Pawar VS, Panchmatia HR, Rubin JL, Davis GL, et al. (2011) Economic model of a birth cohort screening program for hepatitis C virus. Hepatology 55: 1344–1355. [DOI] [PubMed] [Google Scholar]
- 15. Liu S, Cipriano LE, Holodniy M, Owens DK, Goldhaber-Fiebert JD (2012) New protease inhibitors for the treatment of chronic hepatitis C: a cost-effectiveness analysis. Ann Intern Med 156: 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ (2002) Empirically calibrated model of hepatitis C virus infection in the United States. American Journal of Epidemiology 156: 761–773. [DOI] [PubMed] [Google Scholar]
- 17. Mallette C, Flynn MA, Promrat K (2008) Outcome of screening for hepatitis C virus infection based on risk factors. Am J Gastroenterol 103: 131–137. [DOI] [PubMed] [Google Scholar]
- 18. Thein HH, Yi QL, Dore GJ, Krahn MD (2008) Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: A meta-analysis and meta-regression. Hepatology 48: 418–431. [DOI] [PubMed] [Google Scholar]
- 19.Arias E National Vital Statistics Reports, United States Life Tables, 2006. Center for Disease Control, Division of Vital Statistics. [PubMed]
- 20. Walter SR, Thein HH, Amin J, Gidding HF, Ward K, et al. (2011) Trends in mortality after diagnosis of hepatitis B or C infection: 1992–2006. J Hepatol 54: 879–886. [DOI] [PubMed] [Google Scholar]
- 21. El-Kamary SS, Jhaveri R, Shardell MD (2011) All-Cause, Liver-Related, and Non-Liver-Related Mortality Among HCV-Infected Individuals in the General US Population. Clinical Infectious Diseases 53: 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Di Martino V, Crouzet J, Hillon P, Thevenot T, Minello A, et al. (2011) Long-term outcome of chronic hepatitis C in a population-based cohort and impact of antiviral therapy: a propensity-adjusted analysis. J Viral Hepat 18: 493–505. [DOI] [PubMed] [Google Scholar]
- 23.Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, et al.. (2011) A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol 9: 509–516 e501. [DOI] [PubMed]
- 24. Fischer LR, Tope DH, Conboy KS, Hedblom BD, Ronberg E, et al. (2000) Screening for hepatitis C virus in a health maintenance organization. Archives of Internal Medicine 160: 1665–1673. [DOI] [PubMed] [Google Scholar]
- 25. Narasimhan G, Sargios TN, Kalakuntla R, Homel P, Clain DJ, et al. (2006) Treatment rates in patients with chronic hepatitis C after liver biopsy. Journal of Viral Hepatitis 13: 783–786. [DOI] [PubMed] [Google Scholar]
- 26. Mitra D, Davis KL, Beam C, Medjedovic J, Rustgi V (2010) Treatment Patterns and Adherence among Patients with Chronic Hepatitis C Virus in a US Managed Care Population. Value in Health 13: 479–486. [DOI] [PubMed] [Google Scholar]
- 27. Liu S, Schwarzinger M, Carrat F, Goldhaber-Fiebert JD (2011) Cost effectiveness of fibrosis assessment prior to treatment for chronic hepatitis C patients. PLoS One 6: e26783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nyman JA, Barleen NA, Dowd BE, Russell DW, Coons SJ, et al. (2007) Quality-of-life weights for the US population - Self-reported health status and priority health conditions, by demographic characteristics. Medical Care 45: 618–628. [DOI] [PubMed] [Google Scholar]
- 29. Sullivan PW, Ghushchyan V (2006) Preference-based EQ-5D index scores for chronic conditions in the United States. Medical Decision Making 26: 410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ (2003) Cost-effectiveness of treatment for chronic hepatitis C infection in an evolving patient population. Jama-Journal of the American Medical Association 290: 228–237. [DOI] [PubMed] [Google Scholar]
- 31. Grieve R, Roberts J, Wright M, Sweeting M, DeAngelis D, et al. (2006) Cost effectiveness of interferon or peginterferon with ribavirin for histologically mild chronic hepatitis C. Gut. 55: 1332–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sherman KE, Sherman SN, Chenier T, Tsevat J (2004) Health values of patients with chronic hepatitis C infection. Archives of Internal Medicine 164: 2377–2382. [DOI] [PubMed] [Google Scholar]
- 33. Chong CAKY, Gulamhussein A, Heathcote EJ, Lilly L, Sherman M, et al. (2003) Health-state utilities and quality of life in hepatitis C patients. American Journal of Gastroenterology 98: 630–638. [DOI] [PubMed] [Google Scholar]
- 34. McLernon DJ, Dillon J, Donnan PT (2008) Health-state utilities in liver disease: A systematic review. Medical Decision Making 28: 582–592. [DOI] [PubMed] [Google Scholar]
- 35. Rodger AJ, Jolley D, Thompson SC, Lanigan A, Crofts N (1999) The impact of diagnosis of hepatitis C virus on quality of life. Hepatology 30: 1299–1301. [DOI] [PubMed] [Google Scholar]
- 36. Meara E, White C, Cutler DM (2004) Trends in medical spending by age, 1963–2000. Health Affairs 23: 176–183. [DOI] [PubMed] [Google Scholar]
- 37. Poret AW, Ozminkowski R, Goetzel R, Pew J, Balent J (2002) Cost Burden of Illness for Hepatitis C Patients with Employer-Sponsored Health Insurance. Disease Management 5: 95–107. [Google Scholar]
- 38.Bureau of Labor Statistics website. May 2010 National Occupational Employment and Wage Estimates United States. Available: http://www.bls.gov/oes/2010/may/oes_nat.htm. Accessed 2011 January 1.
- 39.U.S. Department Of Labor, Bureau of Labor Statistics Consumer Price Index. Available: ftp://ftp.bls.gov/pub/special.requests/cpi/cpiai.txt. Accessed 2010 June 1.
- 40.U.S. Census Bureau website. U.S. Census 2009 Population Estimates National Characteristics. Available: http://www.census.gov/popest/data/national/asrh/2009/index.html. Accessed 2012 March 1.
- 41.Gold MR (1996) Cost-effectiveness in health and medicine. New York: Oxford University Press. xxiii, 425 p. p.
- 42. Siddiqui FA, Ehrinpreis MN, Janisse J, Dhar R, May E, et al. (2008) Demographics of a large cohort of urban chronic hepatitis C patients. Hepatology International 2: 376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heeswijk RV, Vandevoorde A, Boogaerts G, Vangeneugden T, Paepe E, et al. (2011) Pharmacokinetic Interactions between ARV Agents and the Investigational HCV Protease Inhibitor TVR in Healthy Volunteers. 18th Conference on Retroviruses and Opportunistic Infections (CROI 2011). Boston. February 27–March 2, 2011. Abstract 119. Available: http://www.retroconference.org/2011/Abstracts/41437.htm. Accessed 2013 January 1.
- 44.Kasserra C, Hughes E, Treitel M, Gupta S, O’Mara E (2011) Clinical Pharmacology of BOC: Metabolism, Excretion, and Drug-Drug Interactions. 18th Conference on Retroviruses and Opportunistic Infections (CROI 2011). Boston. February 27–March 2, 2011. Abstract 118. Available: http://www.retroconference.org/2011/Abstracts/41140.htm. Accessed 2013 January 1.
- 45. Williams IT, Bell BP, Kuhnert W, Alter MJ (2011) Incidence and transmission patterns of acute hepatitis C in the United States, 1982–2006. Archives of Internal Medicine 171: 242–248. [DOI] [PubMed] [Google Scholar]
- 46. Thompson AJ, Muir AJ, Sulkowski MS, Ge D, Fellay J, et al. (2010) Interleukin-28B Polymorphism Improves Viral Kinetics and Is the Strongest Pretreatment Predictor of Sustained Virologic Response in Genotype 1 Hepatitis C Virus. Gastroenterology 139: 120–U178. [DOI] [PubMed] [Google Scholar]
- 47. Gretch DR (1997) Diagnostic tests for hepatitis C. Hepatology. 26: 43S–47S. [DOI] [PubMed] [Google Scholar]
- 48. Hyland CA, Kearns S, Young IF, Battistutta D, Morgan CL (1992) Predictive markers for hepatitis C antibody ELISA specificity in Australian blood donors. Transfus Med 2: 207–213. [DOI] [PubMed] [Google Scholar]
- 49. Hutton DW, Tan D, So SK, Brandeau ML (2007) Cost-effectiveness of screening and vaccinating Asian and pacific islander adults for hepatitis B. Ann of Intern Med. 147: 460–469. [DOI] [PubMed] [Google Scholar]
- 50.United Network for Organ Sharing website. Available: http://www.unos.org/. Accessed 2011 January 1.
- 51.National Cancer Institute website. SEER Cancer Stat Fact Sheets. Available: http://seer.cancer.gov/statfacts/. Accessed 2011 April 3.
- 52. Fattovich G, Giustina G, Favarato S, Ruol A, Macarri G, et al. (1996) A survey of adverse events in 11241 patients with chronic viral hepatitis treated with alfa interferon. Journal of Hepatology 24: 38–47. [DOI] [PubMed] [Google Scholar]
- 53. Poynard T, Ratziu V, Bedossa P (2000) Appropriateness of liver biopsy. Canadian Journal of Gastroenterology 14: 543–548. [DOI] [PubMed] [Google Scholar]
- 54. McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, et al. (2009) Peginterferon Alfa-2b or Alfa-2a with Ribavirin for Treatment of Hepatitis C Infection. New England Journal of Medicine 361: 1027–1027. [DOI] [PubMed] [Google Scholar]
- 55. Davis GL, Wong JB, McHutchison JG, Manns MP, Harvey J, et al. (2003) Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology. 38: 645–652. [DOI] [PubMed] [Google Scholar]
- 56.Merck (2011) FDA Antiviral Drugs Advisory Committee Meeting Boceprevir Capsules (NDA 202–258) Briefing Document. Available: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AntiviralDrugsAdvisoryCommittee/UCM252343.pdf. Accessed May 15.
- 57.Vertex Pharmaceuticals (2011) Telaprevir 375-mg Film-Coated Tablet for the Treatment of Genotype 1 Chronic Hepatitis C, Antiviral Drugs Advisory Committee Briefing Document (Telaprevir NDA 201–917). Available: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AntiviralDrugsAdvisoryCommittee/UCM252562.pdf. Accessed May 15.
- 58.Birnkrant D, Team TR (2011) Advisory Committee Briefing Document for NDA 201–917 Telaprevir 375 mg tablets. Silver Spring: Department of Health & Human Services, Public Health Service, Food and Drug Administration. Available: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AntiviralDrugsAdvisoryCommittee/UCM252561.pdf. Accessed 1 May 2011.
- 59.NATAP website. Levin J. Telaprevir in Combination with Peginterferon alfa-2a and Ribavirin in Genotype 1 HCV Treatment-Naïve patients: Final results of Phase 3 ADVANCE Study 2010; Boston, MA, Hynes Convention Center. Available: http://www.natap.org/2010/AASLD/AASLD_23.htm. Accessed 1 March 2011.
- 60.Background Materials for Boceprevir Advisory Committee Division of Antiviral Products (DAVP). Available: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/drugs/AntiviralDrugsAdvisoryCommittee/ucm252341.pdf. Accessed 2011 May 15.
- 61. Farnham PG, Hutchinson AB, Sansom SL, Branson BM (2008) Comparing the costs of HIV screening strategies and technologies in health-care settings. Public Health Rep 123 Suppl 3 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thomson Corporation. (2009) Red book: pharmacy's fundamental reference. Montvale, NJ: Thomson PDR. pp. v.
- 63.The New York Times website. Pollack A (2011) Second Drug Wins Approval for Treatment of Hepatitis C. Available: http://www.nytimes.com/2011/05/24/business/24drug.html?_r=1.Accessed 2011 June 1.
- 64.United States Department of Veterans Affairs website. Federal Supply Schedule, Drug Pharmaceutical Prices 2011. Available: http://www.pbm.va.gov/DrugPharmaceuticalPrices.aspx. Accessed 2011 June 24.
- 65.Stephens CJM, Carter J, Gao X, Haider S, Rustgi VK. Adverse Event-Related Treatment Costs Associated with Protease Inhibitor-Based Combination Therapy for Hepatitis C; 2010 September 6, 2010. AASLD ePoster. Available: http://trs.scivee.tv/node/4033?destination=node%2F4033. Accessed 2012 December 1.
- 66. Armstrong EP, Charland SL (2004) Burden of illness of hepatitis C from a managed care organization perspective. Current Medical Research and Opinion 20: 671–679. [DOI] [PubMed] [Google Scholar]
- 67.Bennett WG, Inoue Y, Beck JR, Wong JB, Pauker SG, et al.. (1997) Estimates of the cost-effectiveness of a single course of interferon-alpha 2b in patients with histologically mild chronic hepatitis C. Ann of Intern Med 127: 855–&. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Contains appendices with supporting information.
(DOCX)