Abstract
Background
Smoking is common in people infected with HIV but cessation support is not a routine part of clinical care. The aim was to assess whether smoking is a risk factor for pneumonia in people with HIV and whether smoking cessation ameliorates excess risk.
Methods
We performed MEDLINE and Embase database searches and included cohort or case-control studies conducted in adult patients infected with HIV extracting a hazard ratio (HR) or odds ratio (OR) that compared the incidence of bacterial pneumonia or pneumonia caused by Pneumocystis jiroveci (PCP) between two smoking categories. Studies were appraised for quality and combined using inverse variance meta-analysis.
Results
Fourteen cohort and case-control studies were included. Assessment of outcome was good, but assessment of exposure status was poor. Current smokers were at higher risk of bacterial pneumonia than former smokers: HR 1.37 (95% confidence interval (CI): 1.06, 1.78). There was no evidence that former smokers were at higher risk than never smokers: HR 1.24 (95%CI: 0.96, 1.60). Current smokers were at higher risk of bacterial pneumonia than current non-smokers: HR of 1.73 (95%CI: 1.44, 2.06). There was no evidence that smoking increased the incidence of PCP. The HR for current versus non-smokers was 0.94 (95%CI: 0.79, 1.12), but from case-control studies the OR was 1.76 (95%CI: 1.25, 2.48) with heterogeneity. Confined to higher quality studies, the OR was 0.97 (95%CI: 0.81, 1.16). Residual confounding is possible, but available data suggest this is not an adequate explanation.
Conclusions
Smoking is a risk factor for bacterial pneumonia but not PCP and smoking cessation reduces this risk.
See related article: http://www.biomedcentral.com/1741-7015/11/16
Keywords: HIV, meta-analysis, pneumonia, smoking, smoking cessation
Background
Life expectancy in patients with HIV has improved dramatically in the era of highly active antiretroviral treatments (HAART), which can prevent the decline in CD4 lymphocyte count and AIDS related morbidity and mortality [1]. However, persons with HIV still face substantially increased health risks compared with the general population. Another threat to health is that the prevalence of cigarette smoking is high amongst people with HIV at around 50% to 70% [2]. Given that many people smoke due to nicotine dependence rather than a desire to smoke and that highly cost-effective treatments for nicotine dependence are available [3], offering smoking cessation treatment to patients with HIV could be an important component of routine clinical care. Currently, smoking cessation support is not routine [2]. The case for offering cessation treatment as part of HIV care rests upon evidence that smoking cessation leads to improved outcomes specific to this population.
Smoking is a risk factor for bacterial pneumonia in the absence of HIV and without evidence of chronic obstructive pulmonary disease. However, it is unclear whether the elevated risk due to smoking is ameliorated by smoking cessation [4,5]. Many people in the general population who develop pneumonia do so without pre-existing conditions and therefore do not receive regular medical assessment and treatment. However, people with HIV undergo regular review with the prime aim of preventing opportunistic infection. Pneumonia is an important cause of morbidity and mortality in patients with HIV, and is one of the most frequent AIDS defining illnesses [6]. If smoking constitutes a risk factor for pneumonia among patients with HIV and if smoking cessation ameliorates that risk, then active management of smoking in people with HIV is supported. The best evidence of causality that smoking cessation ameliorates risk would come from randomized trials of smoking cessation support versus usual care where the outcomes included incidence of pneumonia. We identified no such trials. We therefore conducted a systematic review and meta-analysis of cohort and case-control studies to assess whether smoking cessation reduces the incidence of both bacterial pneumonia and Pneumocystis jiroveci pneumonia (PCP) in patients with HIV infection, and looked for evidence of a dose-response relationship to support causality. Furthermore, we examined whether there was evidence that any risk from smoking or benefit from cessation was modified by the use of HAART or by CD4 count.
Methods
Eligibility criteria
We included cohort or case-control studies conducted in adult patients infected with HIV virus. The studies had to have sufficient information to extract a hazard ratio (HR) or odds ratio (OR) with 95% confidence intervals (CI) that compared the risk of bacterial pneumonia or PCP incidence between two smoking exposure categories. (HRs require studies to have conducted follow-up and thus are available only from cohort studies. Case-control studies can calculate ORs, which approximate the HR when the event in question is relatively uncommon.)
Data sources and searches
We searched MEDLINE (1950 to January 2010) and Embase (1947 to January 2010) using a combination of text word and exploded medical subject headings terms related to smoking, HIV and pneumonia using the Ovid interface (Appendix 1 in Additional file 1). Two authors independently searched through the titles and abstracts to identify relevant papers for inclusion, with differences resolved through discussion. We also searched the reference lists and conducted a citation search of included studies. Many studies we included were not concerned with the association between smoking status and incidence of pneumonia but presented these data incidentally, for example as part of a multivariable adjustment for the exposure of interest. We therefore searched the full texts of all studies with relevant outcomes even if there was no mention of smoking in the title or abstract.
Data extraction and quality assessment
Two reviewers independently extracted data and resolved disagreements by recourse to the papers. We extracted data on demographic and clinical characteristics of the population (age, gender, use of HAART, CD4+ count) and on the methods of assessing exposure and outcome measures. We assessed whether the effect estimate was adjusted for the effects of potential confounders: use of intravenous street drugs, alcohol consumption, socio-economic status (such as educational attainment, income), CD4 count, viral load, and use of HAART. Each of these factors is associated with the incidence of pneumonia and could plausibly be associated with smoking.
We assessed study quality based on methods outlined by Altman [7]. We awarded points for assessment of exposure (smoking), outcome (pneumonia) and whether potential confounders were either balanced between exposure groups or controlled for in the analysis (Appendix 2 in Additional file 1). In many developed countries, 2% to 3% of smokers stop smoking annually, therefore ongoing assessment of current smoking status would be needed to ensure that people defined as current smokers were actually exposed. More importantly, almost all people who have recently stopped relapse to smoking [8], so we awarded points for baseline assessment that defined former smokers as not having smoked for at least a year. For the assessment of outcome, our ideal definition included each of the following: compatible physical symptoms, microbiological confirmation, and radiographic confirmation. As presented in some studies, we included either response to antibiotics or microbiological confirmation as alternative confirmatory signs. Additional points were awarded if outcome assessors were blinded to exposure status. The maximum point score was 16 and we classified studies as high quality when they scored six or more, which was the median score. We considered and decided against the use of sensitivity analysis based on key characteristics, namely the quality of exposure assessment and outcome assessment. Studies varied little in these two aspects and so the scoring system mainly reflected differences in the number of confounders controlled.
Data synthesis and analysis
Risk estimates were extracted as adjusted HRs or ORs with 95%CIs. If the risk estimates or 95%CIs were not presented, we calculated them from presented data using methods described by Parmar et al. [9], or using OpenEpi software [10]. Where risk estimates were presented as current or former smokers versus never smokers, we compared current smokers with former smokers using indirect comparison [11].
Adjusted ORs or HRs (where available) were combined separately from case-control and cohort studies, calculating summary ORs or HRs respectively using inverse variance fixed effects methods implemented in Review Manager 5.0.24. We assessed heterogeneity using the I2 statistic and publication bias using funnel plots (shown in Additional file 1). It is general convention to view I2 values of less than 25% as showing no appreciable heterogeneity, between 25% and 50% as moderate, and more than 50% as substantial heterogeneity. In sensitivity analysis, we confined our analysis to studies defined as high quality. We combined studies in subgroups where participants did not use HAART or where HAART was used and assessed whether use of HAART modified the association between smoking and pneumonia by the χ2 test for heterogeneity. We assessed whether the association between smoking and pneumonia incidence was modified by CD4 count. We used within-study data for this, reporting risk estimates by CD4 strata.
Results
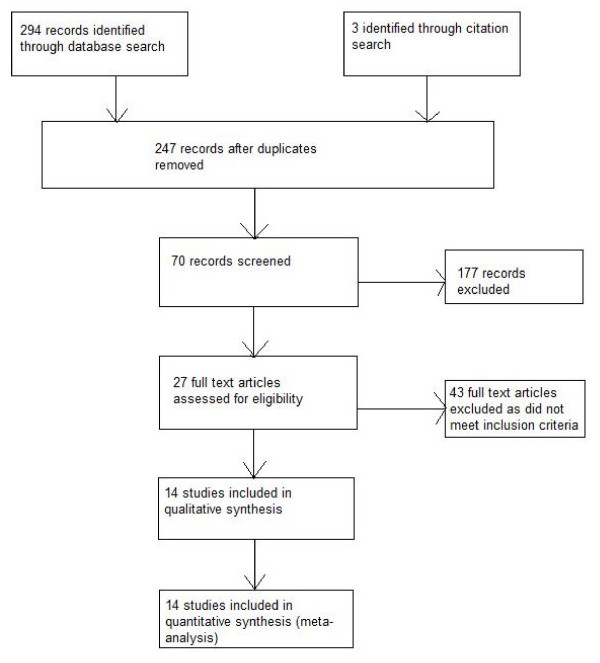
Our search identified 294 studies, of which we obtained 27 full-text articles and 14 studies were included in the review (Figure 1). Of the 14 observational studies included, 12 examined the effect of smoking status on the incidence of bacterial pneumonia, with seven (58%) including patients treated with HAART, and five examined the effect of smoking status on the incidence of PCP, with two (40%) including patients treated with HAART (Table 1). There were five case-control studies and nine cohort studies. In 10 studies the populations were based in the USA, three were based in Europe, and one in South Africa. The prevalence of smoking at cohort inception ranged from 39% to 92% with a median of 60%. Most patients were men (range 56% to 99%, median 77%) and the average age of the population was about 40 years (Table 1). Average length of follow-up in the cohort studies ranged from 1.3 to 10 years (median 4 years), a total of about 62,000 person-years of follow-up in the cohort studies. There were 1,409 participants included in the case-control studies.
Figure 1.
Flow diagram.
Table 1.
Characteristics of participants in included studies for bacterial pneumonia and Pneumocystis jiroveci pneumonia.
| Reference | Number. of participants | Men (%) | Mean/median age(SD) in years | Smokers (%) | Non-smokers (%) | Mean/median CD4+ count | Use of HAART |
|---|---|---|---|---|---|---|---|
| Bacterial pneumonia | |||||||
| [12] | 40 | 75 | Median 30 to 40 | NR | NR | NR | Pre HAART era |
| [21] | 867 | 99 | Mean 50 (10) | 63% | 22% former smokers,15% never smokers | NR | Post-HAART era |
| [13] | 509 | 100 | Median 30 | 80% | 20% non-smokers | 177 cells/μL | Post-HAART era |
| [23] | 5472 | 73 | Median 43 | 41% | 25% former, 34% never smokers | >350 cells/μL | Post-HAART era |
| [14] | 1130 | NR | NR | NR | NR | NR | Pre-HAART era |
| [15] | 885 | 0 (all female) | Mean 36 | 76% | 24% | NR | Pre and Post HAART era |
| [16] | 1203 | 77 | Mean 36 | 66% unknown orcurrent smokers | 34% currently not smoking | 279 cells/μL | Post-HAART era |
| [17] | 300 | 56 | Mean 37 (7) | 92% | 8% non-smokers | 327 cells/μL | Post-HAART era |
| [18] | 285 | NR | Mean 34 (8) | 55% | 45% non-smokers | 182 cells/μL | Pre-HAART era |
| Bacterial pneumonia and Pneumocystis jiroveci pneumonia | |||||||
| 19] | 3221 | 83 | Mean 37 | 57% | 19% former smokers,24%, never smoker | 327 cells/μL | Pre-HAART era |
| [20] | 232 | 100 | Median 36 to 38 | 46% | 54% | NR | Pre-HAART era |
| [25] | 521 | 58 | Mean 42 (9) | 63% | 12% former smokers,25% never smokers | 172 cells/μL | Post-HAART era |
| Pneumocystis jiroveci pneumonia only | |||||||
| [22] | 2499 | 100 | NR | 39% | 61% | >200 cells/μL | Pre-HAART era |
| [24] | 54 | 59 | Mean 40 | NR | NR | 257 cells/μL | Post-HAART era |
HAART: highly active antiretroviral treatments; NA: not applicable; NR: not reported.
Quality assessment
Seven of the 14 studies were not primarily concerned with the association between smoking and the incidence of pneumonia, and details of the definition of smoking exposure were scant [12-18]. Even in studies focused on smoking, the definitions of smoking exposure given were suboptimal and studies lost quality points as a consequence [19-25]. No studies dealt with the fact that former smokers might relapse while current smokers might become former smokers. Most studies met the quality criteria for outcome assessment. Eight of the 12 estimates for bacterial pneumonia risk were adjusted for some predefined confounders [12,14-16,19,21,23,25], with individual studies adjusting for between three and seven confounders (median four). Four of the five studies on PCP adjusted for confounding, range one to four, median 2/3 [19,22,24,25]. We classified six studies scoring six and above as high quality, of which five had outcomes of bacterial pneumonia [12,14-16,23] and one of PCP [22] (Table 2).
Table 2.
Methodological characteristics of studies.
| Author (year) | Study design | Exposure assessment | Outcome assessment | Number of confounders controlled | Study quality score | |||
|---|---|---|---|---|---|---|---|---|
| Definition of smoking | Definition of non smoking | Method of assessingsmoking status | Definition ofpneumonia | Blinding ofassessors toexposure status (cohort studies) | ||||
| Bacterial pneumonia | ||||||||
| [12] | Case control | >1 cig/day | Never smoked | Medical history | 2 | 0 | 3 | 7 |
| [21] | Cohort | NR | Former and never, otherwise undefined | Questionnaire or clinical notes | 1 | 0 | 4 | 5 |
| [13] | Nested case-control | NR | NR | NR | 2 | 1 | 0 | 3 |
| [23] | Cohort | NR | NR | Interview | 2 | 1 | 4 | 7 |
| [14] | Cohort | NR | Never smokers <100 cigarettes in lifetime | NR | 2 | 0 | 4 | 6 |
| [15] | Cohort | NR | Not current smokers | NR | 2 | 0 | 7 | 9 |
| [16] | Cohort | NR | NR | Questionnaire | 2 | 0 | 4 | 6 |
| [17] | Cohort | NR | NR | Questionnaire | 2 | 0 | 0 | 2 |
| [18] | Case control | >6 cigs/day | NR | NR | 2 | 0 | 0 | 3 |
| Bacterial pneumonia and Pneumocystis jiroveci pneumonia | ||||||||
| [19] | Cohort | NR | Self-defined | Clinical notes | 0 | 0 | 4 | 4 |
| [20] | Cohort | Cigarettes/day in multiple categories | NR | Questionnaire | 1 | 0 | 0 | 2 |
| [25] | Case control | NR | NR | Questionnaire | 2 | 0 | 3 | 5 |
| Pneumocystis jiroveci pneumonia only | ||||||||
| [22] | Cohort | Defined according to daily cigarette consumption and change in smoking status over time | Not currently smoking | Questionnaire | 2 | 0 | 2 | 6 |
| [24] | Case control | Current tobacco use | Current non-smoking | Questionnaire and clinical notes | 2 | 0 | 1 | 4 |
NR: not reported.
Association between smoking cessation and incidence of bacterial pneumonia
One cohort study provided an HR for the difference in incidence between current and former smokers [19]. We used indirect methods to calculate HRs for this comparison from data in three studies [14,21,23]. In comparison to former smokers, current smoking was associated with a 37% increased risk in developing bacterial pneumonia (HR 1.37; 95%CI: 1.06, 1.78; P = 0.02) (Figure 2). Effect estimates were similar in all four studies with an I2 value of 0%, with no evidence of difference in the pre- to post-HAART eras (P = 0.70). After excluding studies of lower quality, the summary estimate did not change greatly, but was no longer significant (HR 1.28; 95%CI: 0.93, 1.76).
Figure 2.
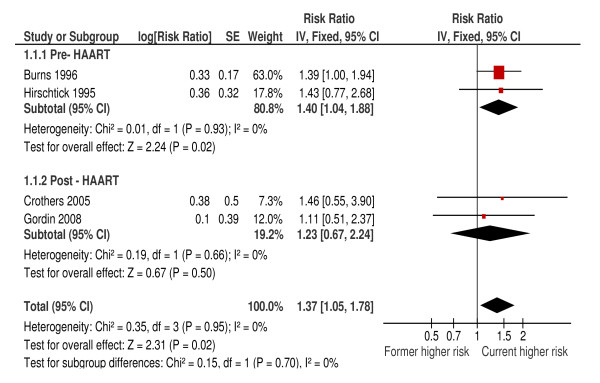
Comparison of risk of bacterial pneumonia between HIV seropositive current smokers and former smokers.
Former smokers versus never smokers and incidence of bacterial pneumonia
There was no evidence that former smokers were at greater risk of bacterial pneumonia than never smokers (HR 1.24; 95%CI: 0.96, 1.60; P = 0.10; I2 = 22%) (Figure 3), which remained the case after exclusion of lower quality studies (HR 1.29; 95%CI: 0.79, 2.10). Subgroup analysis for treatment era showed some evidence that former smokers on HAART were at higher relative risk from their past smoking than those not on HAART although the difference between groups was not significant (P = 0.07).
Figure 3.
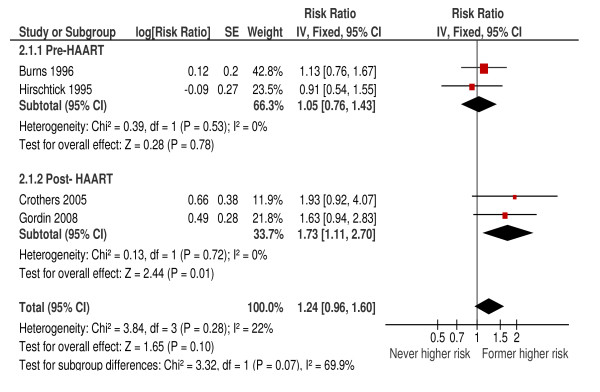
Risk of bacterial pneumonia in former versus never smokers (cohort studies).
Current smokers versus current non-smokers and incidence of bacterial pneumonia
These results, which give direct evidence on the possible effect of cessation, were supported by more extensive indirectly relevant data. We pooled eight cohort studies where the incidence of pneumonia in current smokers was compared with a combined group of never and former smokers at study entry (hereafter referred to as non-smokers for simplicity). This produced a summary HR of 1.73 (95%CI: 1.44, 2.06; P <0.001; I2 = 21%) (Figure 4). Confined to four higher quality cohort studies, the HR was 1.64 (95%CI: 1.28, 2.10; I2 = 30%). There were four case-control studies comparing risk of pneumonia in current and non-smokers, which gave a pooled OR of 2.12 (95%CI: 1.63, 2.75; P <0.001) but with more substantial heterogeneity (I2 = 46%) (Figure 5). No case-control studies were rated high quality. In both these meta-analyses there was weak evidence that the association between smoking and bacterial pneumonia was modified by the use of HAART. For current versus non-smokers, the p value for subgroup differences for cohort studies was 0.17 (Figure 4) and for case-control studies it was 0.11 (Figure 5). However the direction of possible effect modification differed, with participants using HAART who smoked being at higher risk from smoking in cohort studies and at lower risk in case-control studies.
Figure 4.
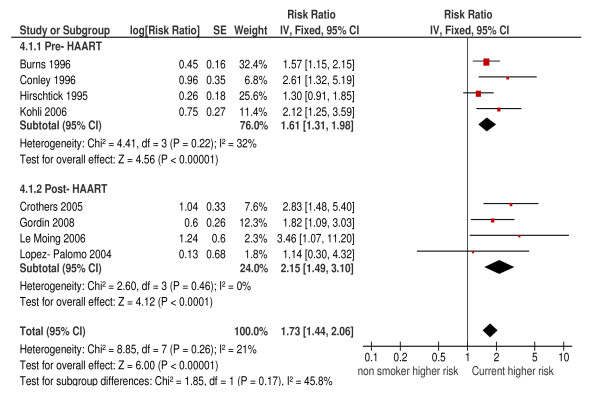
Risk of bacterial pneumonia in current versus non-smokers (cohort studies).
Figure 5.
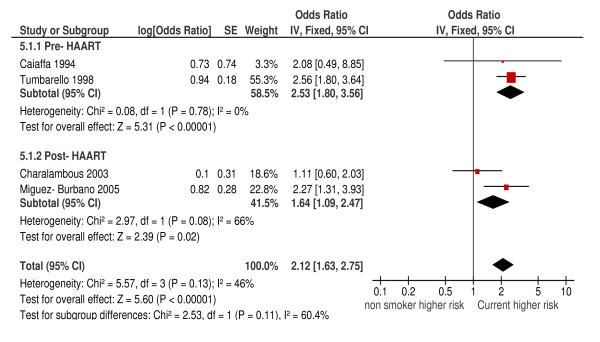
Risk of bacterial pneumonia in current versus non-smokers (case-control studies).
The association between smoking cessation and PCP
Three of five studies containing data on PCP were cohort studies [19,20,22]. One study reported data that allowed us to calculate the risk of PCP for current compared with former smokers [19]. There was no evidence that the risk differed (relative risk (RR) 0.81; 95%CI: 0.55, 1.20).
The overall pooled estimate for current versus non-smokers from the three cohort studies gave an HR of 0.94 (95%CI: 0.79, 1.12; P = 0.31; I2 = 15%), and from the two case-control studies gave an OR of 1.76 (95%CI: 1.25, 2.48; P = 0.001) but with substantial heterogeneity (I2 = 65%) (Figures 6 and 7). The three cohort studies were all conducted in the pre-HAART era and the two case-control studies in the post-HAART era. For the two higher quality cohort studies, the summary HR was 0.97 (95%CI: 0.81, 1.16) with no heterogeneity (I2 = 0%). There were no case-control studies with a quality score six or above.
Figure 6.
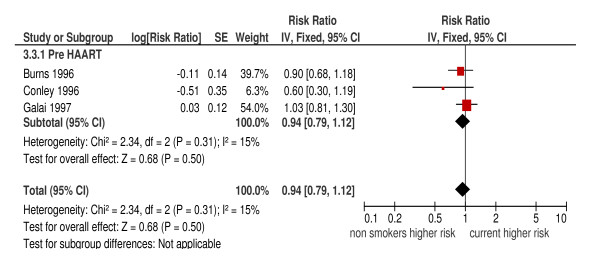
Risk of Pneumocystis jiroveci pneumonia in current smokers compared to non-smokers (cohort studies).
Figure 7.
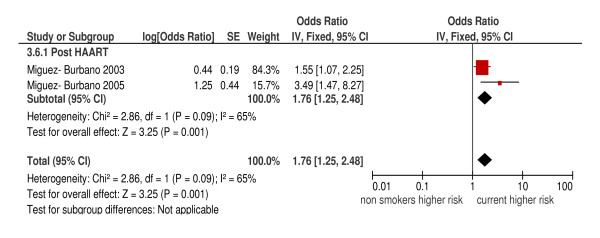
Risk of Pneumocystis jiroveci pneumonia in current smokers compared to non-smokers (case-control studies).
Assessing the effect of confounding factors
Four studies reported data on both unadjusted and adjusted HRs in bacterial pneumonia. Hirschtick et al. reported that adjustment reduced the HR between current and never smokers but direct numerical comparisons were not possible [14]. In Gordin et al., adjustment for confounding by intravenous drug use, use of HAART, CD4 count and viral load reduced the HR slightly, from 1.80 to 1.64 (former versus never) and 2.19 to 1.82 (current versus never) [23]. In Caiaffa et al., adjustment for CD4 count, age and illicit drug use increased the OR slightly from 2.00 to 2.08 [12]. In Miguez-Burbano et al., adjustment for CD4 count, viral load and use of HAART changed the OR from 1.80 to 2.28 [25].
There was only one study that allowed comparison between unadjusted and adjusted estimates of the risk of PCP. Miguez-Burbano et al. reported that the OR for current versus non-smokers decreased on adjustment from approximately 16 to 1.56 [24].
Dose-response relationships between smoking and outcomes
No studies examined the effect of years since cessation on the incidence of pneumonia or even reported on years of cessation achieved in former smokers. Conley et al. reported a significant linear trend for risk of bacterial pneumonia with heaviness of consumption in current smokers, but no trend for PCP [20]. Miguez-Burbano et al. reported a significant dose-response relationship, with the odds of bacterial pneumonia increasing by 3% per cigarette per day in a linear trend analysis [25]. Galai et al. reported no association between number of cigarettes smoked and increasing risk of PCP [22].
Is the association between smoking and bacterial pneumonia or PCP modified by CD4 count?
Three studies reported data for bacterial pneumonia, but none allowed a quantitative summary of the effect estimates of smoking on pneumonia by CD4 count category. Hirschtick et al. reported that a low CD4 count (<200 lymphocytes per microliter) was associated with an increased relative hazard from current smoking [14], Miguez-Burbano et al. reported a lower relative hazard [25], and Burns reported no difference in relative hazard by CD4 count [19]. Galai et al. reported that CD4 count did not significantly modify the effect of smoking on incidence of PCP [22].
Discussion
We found strong statistical evidence that, in patients with HIV, current smoking was associated with an approximate 70% to 100% increased risk of bacterial pneumonia compared with non-smokers, and moderate evidence that stopping smoking decreased this by about 27%. There was some evidence that former smokers were at slightly increased risk compared with never smokers, which was not significant, however the estimate was insufficiently precise to exclude a substantial increased risk. There was no good evidence that the risk of smoking was modified either by the use of HAART or by CD4 count. There was mixed evidence that smoking increased the risk of PCP, but higher quality evidence indicated no substantial increase in risk of PCP from current smoking. The evidence from all studies was somewhat clouded by poor definition of exposure status (smoking) and failure to control for a full range of confounders. However, studies that reported the effects of adjustment suggested that adjustment for several confounders had an insubstantial effect on these estimates.
The strengths of this study relate to the comprehensive search of studies that had relevant outcomes even though the titles or abstracts included no mention of smoking. We found seven studies this way that did not have an investigation of smoking as a risk factor for pneumonia as their prime aim. It is plausible that several cohort studies investigating smoking as a risk factor failed to find a significant association, and therefore did not publish data. Incorporating studies where smoking was not the main focus offers reassurance that the association is not due to publication bias and funnel plots also suggested no evidence of bias.
There are some limitations with the review and the data we reviewed. We might have searched a larger number of databases and we did not search the grey literature. We included observational data, and there were inherent limitations. First, the measurement of smoking was suboptimal. Over time, people who were smokers at baseline would have stopped smoking, while some ex-smokers might have relapsed; this will mix exposure assignment and generally underestimate any true risk of smoking on pneumonia [26]. Another limitation is that few studies adjusted for a full range of confounders. People who smoke are more likely than people who do not to have other factors associated with an increased risk of pneumonia, for example, intravenous drug use or failure to adhere to HAART. Our ability to assess the importance of this explanation was compromised by failure to report unadjusted and adjusted estimates, but those that were presented suggested a small effect of adjustment and this is unlikely to explain the associations observed.
Taken together, we believe this systematic review gives reasonable evidence that the association between smoking and bacterial pneumonia is causal in HIV patients. There are no strong reasons to doubt the validity of the association and there was a dose-response relationship between smoking and risk of pneumonia. There are supporting data that suggest biologically plausible mechanisms, such as reduced local defenses in the lung. Smokers with HIV have lower concentrations of CD4 and CD8 lymphocytes in lung tissue and lower concentrations of cytokines IL-1β and TNF-α [27]. There is also evidence that smoking reduces the phagocytic function of alveolar macrophages in individuals infected with HIV [28]. Another possible explanation is that free radicals in tobacco smoke increase oxidative stress within cells and therefore enhance the likelihood of infection [29].
This review produced inconclusive evidence on whether there is an association between current smoking and risk of PCP in patients with HIV. There was no evidence of a dose-response relationship and, given the weight of evidence, we conclude that smoking is not a risk factor for development of PCP. HAART prevents the occurrence of PCP and antibiotic prophylaxis against PCP can be stopped in people with a CD4 count greater than 100 cells/μL [30]. It is clear then that CD4 count is critical and this reinforces our conclusion that smoking is not an important risk factor for this pneumonia.
In this meta-analysis, the relative risk of bacterial pneumonia in current smokers compared to non-smokers was 26% higher than the relative risk of current smokers compared to non-smokers. In western countries, where nearly all of these studies were completed, most non-smokers aged around 40 years (the average age of participants in included studies) are never smokers rather than former smokers [31]. The risk of former smokers in comparison to never smokers was imprecisely estimated and we could not therefore exclude a continued risk in former smokers. These data are clouded further by the definitions of smoking status used in these studies. We cannot therefore be clear that the risk of pneumonia for former smokers is the same as never smokers, but it is clear that it is lower than among current smokers.
To our knowledge, no other systematic reviews have been published on this topic, but narrative reviews have addressed it [32-34]. This is the first review to explicitly compare current smokers with former smokers. All but one study published data comparing the risk of current smoking to never smokers, but a person who smokes cannot become a never smoker. Using indirect comparisons allowed us to estimate the association that is most clinically relevant. We were, however, unable to estimate how long the risk of current smoking remains elevated after stopping before the benefits of cessation are manifest. There are numerous cohort studies of people with HIV and they are likely to have recorded data on smoking repeatedly during the follow-up of the cohorts. It would therefore be useful for these cohorts to examine the difference in risk by time since cessation and including these data would allay concerns that smoking status is likely to have changed over time. There is evidence that smoking predisposes to tuberculosis, but rather less data on the effect of smoking cessation on tuberculosis [35]. Such associations could also be investigated in these studies.
These results have direct clinical implications. The prevalence of smoking was high in these cohorts, much higher than the population in general, in keeping with the higher prevalence in gay men and intravenous drug users [36,37]. Two studies reported incidence rates for bacterial pneumonia, the weighted mean of which was 7.8 per 100 person-years in smokers [14,15]. This means that 25% of smokers would develop pneumonia over 10 years due to their smoking that would have been prevented by smoking cessation. Of cases of pneumonia in people with HIV, 22% are due to current smoking if the median prevalence of smoking in these studies (60%) applies generally. Thus it seems imperative that physicians caring for people with HIV become proficient in smoking cessation treatment, regularly offer treatment, and support their patients to stop smoking. Brief advice to stop smoking is insufficient. The large majority of people will fail to stop with advice alone [38]. Many smokers who are gay or current or former intravenous drug users want to stop smoking but find this difficult due to nicotine addiction; effective treatment programs exist that can ameliorate this [3]. The decision on whether such programs become routine practice in HIV medicine depends upon showing that they are cost-effective in this population. Smoking cessation programs have been called 'among the most cost-effective of all healthcare interventions' (p7 of [39]) in the general population, so it seems likely that they would be similarly cost-effective in people with HIV. There are several small scale trials published of treatment for smoking cessation in people with HIV [40-43], though there is no reason to imagine that treatment regimens in this subgroup need be very different from standard smoking cessation treatment. However, it was outside the scope of this review to examine evidence of efficacy in supporting cessation or its cost-effectiveness.
Conclusions
There is reasonable evidence that smoking cessation is casually associated with a reduced risk of bacterial pneumonia but not PCP in people with HIV. Smoking cessation programs should be incorporated into HIV treatment programs.
Abbreviations
CI: confidence interval; HAART: highly active antiretroviral treatments; HIV: human immunodeficiency virus; HR: hazard ratio; IL: interleukin; OR: odds ratio; PCP: Pneumocystis jiroveci pneumonia; TNF: tumor necrosis factor.
Competing interests
PA has done research and consultancy for manufacturers of smoking cessation medication. All other authors declare that they have no competing interests.
Authors' contributions
AF conceived the study and all authors contributed to developing the protocol. All authors searched the literature. PD and PA extracted and analyzed the data. PD and PA wrote the first draft and all authors edited it. All authors have read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Search strategy, quality criteria, and funnel plots. A line-by-line search strategy, the criteria used to assign quality points to studies, and the funnel plots of each of Figures 2 to 7.
Contributor Information
Preeti De, Email: p.de@ucl.ac.uk.
Amanda Farley, Email: a.c.farley@bham.ac.uk.
Nicola Lindson, Email: n.l.lindson@bham.ac.uk.
Paul Aveyard, Email: paul.aveyard@phc.ox.ac.uk.
Acknowledgements
The authors are grateful to Alice May and Charlotte Griffin for their help in the literature search. The study received no special funding. AP, NL and PA are members of UK Centre for Tobacco Control Studies, a UK Clinical Research Collaboration Public Health Research: Centre of Excellence. Funding from British Heart Foundation, Cancer Research UK, Economic and Social Research Council, Medical Research Council and the National Institute for Health Research, under the auspices of the UK Clinical Research Collaboration, is gratefully acknowledged. PA was funded by the National Institute of Health Research.
References
- Crum NF, Riffenburgh RH, Wegner S, Agan BK, Tasker SA, Spooner KM, Armstrong AW, Fraser S, Wallace MR. on Behalf of the Triservice AIDS Clinical Consortium. Comparisons of causes of death and mortality rates among HIV-Infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr. 2006;41:194–200. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- Reynolds NR. Cigarette smoking and HIV: more evidence for action. AIDS Educ Prev. 2009;21:106–121. doi: 10.1521/aeap.2009.21.3_supp.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aveyard P, West R. Managing smoking cessation. BMJ. 2007;335:37–41. doi: 10.1136/bmj.39252.591806.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almirall J, Bolibar I, Balanzo X, Gonzalez CA. Risk factors for community-acquired pneumonia in adults: a population-based case-control study. Eur Respir J. 1999;13:349–355. doi: 10.1183/09031936.99.13234999. [DOI] [PubMed] [Google Scholar]
- Nuorti JP, Butler JC, Farley MM, Harrison LH, McGeer A, Kolczack MS, Breiman RF. Cigarette smoking and invasive pneumococcal disease. New Engl J Med. 2010;342:681–689. doi: 10.1056/NEJM200003093421002. [DOI] [PubMed] [Google Scholar]
- Madeddu G, Fiori ML, Mura MS. Bacterial community-acquired pneumonia in HIV-infected patients. Curr Opin Pulm Med. 2010;16:201–207. doi: 10.1097/MCP.0b013e3283375825. [DOI] [PubMed] [Google Scholar]
- Altman DG. Systematic reviews of evaluations of prognostic variables. BMJ. 2001;323:224–228. doi: 10.1136/bmj.323.7306.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- Parmar MKP, Torri V, Stewart L. Extracting summary statistics to perform meta-analysis of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Dean AG, Sullivan KM, Soe MM. Open Source Epidemiologic Statistics for Public Health, Version 2.3.1. 2009. http://www.OpenEpi.com
- Song F, Glenny AM, Altman DG. Indirect comparison in evaluating relative efficacy illustrated by antimicrobial prophylaxis in colorectal surgery. Control Clin Trials. 2000;21:488–497. doi: 10.1016/S0197-2456(00)00055-6. [DOI] [PubMed] [Google Scholar]
- Caiaffa WT, Vlahov D, Graham NMH, Astemborski J, Solomon L, Nelson KE, Munoz A. Drug smoking, Pneumocystis carinii pneumonia, and immunosuppression increase risk of bacterial pneumonia in human immunodeficiency virus- seropositive injection drug users. Am J Respir Crit Care Med. 1994;150:1493–1498. doi: 10.1164/ajrccm.150.6.7952605. [DOI] [PubMed] [Google Scholar]
- Charalambous S, Day JH, Fielding K, De Cock KM, Churchyard GJ, Corbett EL. HIV infection and chronic chest disease as risk factors for bacterial pneumonia: a case-control study. AIDS. 2003;17:1531–1537. doi: 10.1097/00002030-200307040-00014. [DOI] [PubMed] [Google Scholar]
- Hirschtick RE, Glassroth J, Jordan MC, Wilcosky TC, Wallace JM, Kvale PA, Markowitz N, Rosen MJ, Mangura BT, Hopewell PC. Bacterial pneumonia in persons infected with the human immunodeficiency virus. New Engl J Med. 1995;333:845–851. doi: 10.1056/NEJM199509283331305. [DOI] [PubMed] [Google Scholar]
- Kohli R, Lo Y, Homel P, Flanigan TP, Gardner LI, Howard AA, Rompalo AM, Moskaleva G, Schuman P, Schoenbaum EE. Bacterial pneumonia, HIV therapy, and disease progression among HIV-infected women in the HIV epidemiologic research (HER) study. Clin Infect Dis. 2006;43:90–98. doi: 10.1086/504871. [DOI] [PubMed] [Google Scholar]
- Le Moing V, Rabaud C, Journot V, Duval X, Cuzin L, Cassuto JP, Al Kaied F, Dellamonica P, Chêne G, Raffi F. APROCO Study Group. Incidence and risk factors of bacterial pneumonia requiring hospitalization in HIV-infected patients started on a protease inhibitor-containing regimen. HIV Med. 2006;7:261–267. doi: 10.1111/j.1468-1293.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Palomo C, Martin-Zamorano M, Benitez E, Fernandez-Gutierrez C, Guerrero F, Rodriguez-Iglesias M. Pneumonia in HIV-infected patients in the HAART era: incidence, risk, and impact of the pneumococcal vaccination. J Med Virol. 2004;72:517–524. doi: 10.1002/jmv.20045. [DOI] [PubMed] [Google Scholar]
- Tumbarello M, Tacconelli E, De GK, Ardito F, Pirronti T, Cauda R, Ortona L. Bacterial pneumonia in HIV-infected patients: analysis of risk factors and prognostic indicators. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18:39–45. doi: 10.1097/00042560-199805010-00006. [DOI] [PubMed] [Google Scholar]
- Burns DN, Hillman D, Neaton JD, Sherer R, Mitchell T, Capps L, Vallier WG, Thurnherr MD, Gordin FM. Cigarette smoking, bacterial pneumonia, and other clinical outcomes in HIV-1 infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13:374–383. doi: 10.1097/00042560-199612010-00012. [DOI] [PubMed] [Google Scholar]
- Conley LJ, Bush TJ, Buchbinder SP, Penley KA, Judson FN, Holmberg SD. The association between cigarette smoking and selected HIV-related medical conditions. AIDS. 1996;10:1121–1126. [PubMed] [Google Scholar]
- Crothers K, Griffith TA, McGinnis KA, Rodriguez-Barradas MC, Leaf DA, Weissman S, Gibert CL, Butt AA, Justice AC. The impact of cigarette smoking on mortality, quality of life, and comorbid illness among HIV-positive veterans. J Gen Intern Med. 2005;20:1142–1145. doi: 10.1111/j.1525-1497.2005.0255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galai N, Park LP, Wesch J, Visscher B, Riddler S, Margolick JB. Effect of smoking on the clinical progression of HIV-1 infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:451–458. doi: 10.1097/00042560-199704150-00009. [DOI] [PubMed] [Google Scholar]
- Gordin FM, Roediger MP, Girard P-M, Lundgren JD, Miro JM, Palfreeman A, Rodriguez-Barradas MC, Wolff MJ, Easterbrook PJ, Clezy K, Slater LN. Pneumonia in HIV-infected persons: increased risk with cigarette smoking and treatment interruption. Am J Respir Crit Care Med. 2008;178:630–636. doi: 10.1164/rccm.200804-617OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguez-Burbano MJ, Burbano X, Ashkin D, Pitchenik A, Allan R, Pineda L, Rodriguez N, Shor-Posner G. Impact of tobacco use on the development of opportunistic respiratory infections in HIV seropositive patients on antiretroviral therapy. Addiction Biol. 2003;8:39–43. doi: 10.1080/1355621031000069864. [DOI] [PubMed] [Google Scholar]
- Miguez-Burbano MJ, Ashkin D, Rodriguez A, Duncan R, Pitchenik A, Quintero N, Flores M, Shor-Posner G. Increased risk of Pneumocystis carinii and community-acquired pneumonia with tobacco use in HIV disease. Int J Infect Dis. 2005;9:208–217. doi: 10.1016/j.ijid.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Sorahan T, Gilthorpe MS. Non-differential misclassification of exposure always leads to an underestimate of risk: an incorrect conclusion. Occup Environ Med. 1994;51:839–840. doi: 10.1136/oem.51.12.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewers MD, Diaz PT, Wewers ME, Lowe MP, Nagaraja HN, Clanton TL. Cigarette smoking in HIV infection induces a suppressive inflammatory environment in the lung. Am J Respir Crit Care Med. 1998;158:1543–1549. doi: 10.1164/ajrccm.158.5.9802035. [DOI] [PubMed] [Google Scholar]
- Feldman C. Pneumonia associated with HIV infection. Curr Opin Infect Dis. 2005;18:165–170. doi: 10.1097/01.qco.0000160907.79437.5a. [DOI] [PubMed] [Google Scholar]
- Traber MG, van der Vliet A, Reznick AZ, Cross CE. Tobacco-related diseases. Is there a role for antioxidant micronutrient supplementation? Clin Chest Med. 2000;21:173–187. doi: 10.1016/S0272-5231(05)70016-2. [DOI] [PubMed] [Google Scholar]
- Costiniuk CT, Fergusson DA, Doucette S, Angel JB. Discontinuation of Pneumocystis jirovecii pneumonia prophylaxis with CD4 count <200 cells/-ÁL and virologic suppression: a systematic review. PLoS One. 2011;6:e28570. doi: 10.1371/journal.pone.0028570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jit M, Aveyard P, Barton P, Meads C. Predicting the lifetime benefit of school-based smoking prevention programmes. Addiction. 2010;105:1109–1116. doi: 10.1111/j.1360-0443.2010.02924.x. [DOI] [PubMed] [Google Scholar]
- Nahvi S, Cooperman NA. Review: the need for smoking cessation among HIV-positive smokers. AIDS Educ Prev. 2009;21:14–27. doi: 10.1521/aeap.2009.21.3_supp.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidrine DJ. Cigarette smoking and HIV/AIDS: Health implications, smoker characteristics and cessation strategies. AIDS Educ Prev. 2009;21:3–13. doi: 10.1521/aeap.2009.21.3_supp.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Morrow K, Tashima K, Flanigan TP, Abrams DB. Human immunodeficiency virus infection, AIDS, and smoking cessation: the time is now. Clin Infect Dis. 2000;31:808–812. doi: 10.1086/314048. [DOI] [PubMed] [Google Scholar]
- Tonnesen P, Carrozzi L, Fagerstrom KO, Gratziou C, Jimenez-Ruiz C, Nardini S, Viegi G, Lazzaro C, Campell IA, Dagli E, West R. Smoking cessation in patients with respiratory diseases: a high priority, integral component of therapy. Eur Respir J. 2007;29:390–417. doi: 10.1183/09031936.00060806. [DOI] [PubMed] [Google Scholar]
- American Lung Association. Smoking Out a Deadly Threat. Tobacco Use in the LGBT Community. Washington DC: American Lung Association; 2010. pp. 1–20. [Google Scholar]
- Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. J Consult Clin Psychol. 2004;72:1144–1156. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- Stead LF, Bergson G, Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2008;2:CD000165. doi: 10.1002/14651858.CD000165.pub3. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence. Guidance on the use of nicotine replacement therapy (NRT) and bupropion for smoking cessation. 2002. http://www.nice.org.uk/nicemedia/pdf/NiceNRT39GUIDANCE.pdf
- Vidrine DJ, Marks RM, Arduino RC, Gritz ER. Efficacy of cell phone-delivered smoking cessation counseling for persons living with HIV/AIDS: 3-month outcomes. Nicotine Tob Res. 2012;14:106–110. doi: 10.1093/ntr/ntr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzi L, Spoerl D, Voggensperger J, Nicca D, Simcock M, Bucher HC, Spirig R, Battegay M. A smoking cessation programme in HIV-infected individuals: a pilot study. Antiv Ther. 2006;11:787–795. [PubMed] [Google Scholar]
- Ingersoll KS, Cropsey KL, Heckman CJ. Reducing smoking among people with HIV: outcomes and lessons from three pilot projects. Proceedings of the Society for Research on Nicotine and Tobacco 11th Annual Meeting: 2005 March 20-23; Prague, Czech Republic. 2005.
- Vidrine DJ, Arduino RC, Gritz ER. Impact of a cell phone intervention on mediating mechanisms of smoking cessation in individuals living with HIV/AIDS. Nicotine Tob Res. 2006;8(Suppl 1):S103–S108. doi: 10.1080/14622200601039451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy, quality criteria, and funnel plots. A line-by-line search strategy, the criteria used to assign quality points to studies, and the funnel plots of each of Figures 2 to 7.