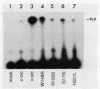
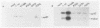Abstract
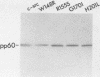
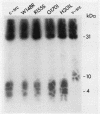
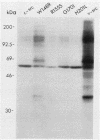
To investigate the importance of a conserved region spanning residues 137 to 241 in the noncatalytic domain of p60c-src (SH2 region), we used oligonucleotide-directed mutagenesis to change residues that are highly conserved in this region. Chicken embryo fibroblasts infected with a p60c-src variant containing arginine instead of tryptophan at residue 148 (W148R) appeared more rounded than cells overexpressing a normal c-src gene, and they formed colonies in soft agar. p60c-src variants containing serine instead of arginine at residue 155 (R155S) or isoleucine instead of glycine at residue 170 (G170I) also appeared transformed and were anchorage independent, but to a lesser extent than W148R. Mutation of residue 201 from histidine to leucine (H201L) had no observable effect. The in vitro kinase activity of cells infected with W148R or G170I was elevated twofold. Expression of p60W148R (or, to a lesser extent, of p60G170I) increased the number of proteins phosphorylated on tyrosine in infected cells. All of the mutants were phosphorylated in vivo on Tyr-527, instead of Tyr-416 as observed for p60v-src. Immunoprecipitated p60W148R and p60G170I were found to be associated with a phosphatidylinositol kinase activity, a factor which appears to be necessary for transformation by tyrosine-specific protein kinases. These results show that a single point mutation in the SH2 region of the cellular src gene can activate its transforming potential. This type of activation is in a new category of alterations at the amino terminus that activate but do not cause a shift in phosphorylation at the carboxy terminus.
Full text
PDF


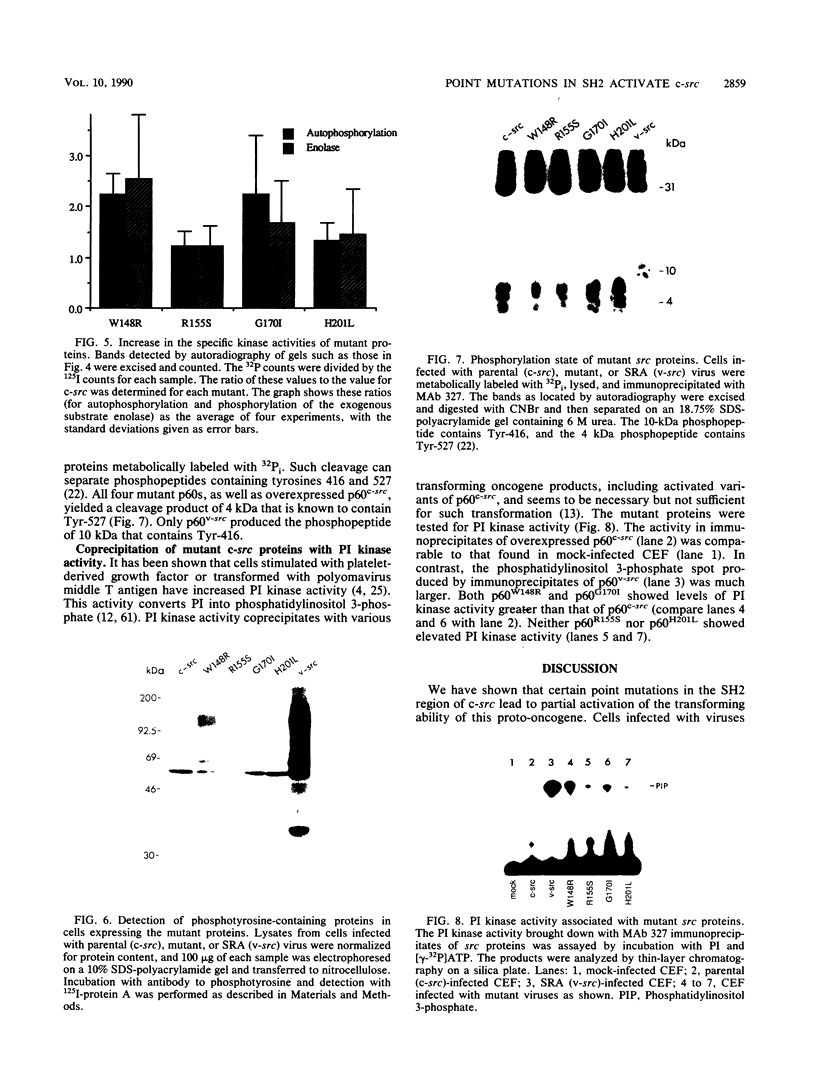



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brugge J. S., Darrow D. Analysis of the catalytic domain of phosphotransferase activity of two avian sarcoma virus-transforming proteins. J Biol Chem. 1984 Apr 10;259(7):4550–4557. [PubMed] [Google Scholar]
- Bryant D., Parsons J. T. Site-directed mutagenesis of the src gene of Rous sarcoma virus: construction and characterization of a deletion mutant temperature sensitive for transformation. J Virol. 1982 Nov;44(2):683–691. doi: 10.1128/jvi.44.2.683-691.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chackalaparampil I., Shalloway D. Altered phosphorylation and activation of pp60c-src during fibroblast mitosis. Cell. 1988 Mar 25;52(6):801–810. doi: 10.1016/0092-8674(88)90422-9. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A., Heber A. An 81 kd protein complexed with middle T antigen and pp60c-src: a possible phosphatidylinositol kinase. Cell. 1987 Sep 25;50(7):1031–1037. doi: 10.1016/0092-8674(87)90169-3. [DOI] [PubMed] [Google Scholar]
- Crawford J. L., Lipscomb W. N., Schellman C. G. The reverse turn as a polypeptide conformation in globular proteins. Proc Natl Acad Sci U S A. 1973 Feb;70(2):538–542. doi: 10.1073/pnas.70.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R., Garber E. A., Hanafusa H. N-terminal deletions in Rous sarcoma virus p60src: effects on tyrosine kinase and biological activities and on recombination in tissue culture with the cellular src gene. Mol Cell Biol. 1985 Oct;5(10):2789–2795. doi: 10.1128/mcb.5.10.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R., Hanafusa H. Local mutagenesis of Rous sarcoma virus: the major sites of tyrosine and serine phosphorylation of pp60src are dispensable for transformation. Cell. 1983 Sep;34(2):597–607. doi: 10.1016/0092-8674(83)90392-6. [DOI] [PubMed] [Google Scholar]
- DeClue J. E., Martin G. S. Linker insertion-deletion mutagenesis of the v-src gene: isolation of host- and temperature-dependent mutants. J Virol. 1989 Feb;63(2):542–554. doi: 10.1128/jvi.63.2.542-554.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeClue J. E., Sadowski I., Martin G. S., Pawson T. A conserved domain regulates interactions of the v-fps protein-tyrosine kinase with the host cell. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9064–9068. doi: 10.1073/pnas.84.24.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis C., Moran M., McCormick F., Pawson T. Phosphorylation of GAP and GAP-associated proteins by transforming and mitogenic tyrosine kinases. Nature. 1990 Jan 25;343(6256):377–381. doi: 10.1038/343377a0. [DOI] [PubMed] [Google Scholar]
- Fukui Y., Hanafusa H. Phosphatidylinositol kinase activity associates with viral p60src protein. Mol Cell Biol. 1989 Apr;9(4):1651–1658. doi: 10.1128/mcb.9.4.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., Kornbluth S., Jong S. M., Wang L. H., Hanafusa H. Phosphatidylinositol kinase type I activity associates with various oncogene products. Oncogene Res. 1989;4(4):283–292. [PubMed] [Google Scholar]
- Gould K. L., Hunter T. Platelet-derived growth factor induces multisite phosphorylation of pp60c-src and increases its protein-tyrosine kinase activity. Mol Cell Biol. 1988 Aug;8(8):3345–3356. doi: 10.1128/mcb.8.8.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H. Rapid transformation of cells by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1969 Jun;63(2):318–325. doi: 10.1073/pnas.63.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. A tail of two src's: mutatis mutandis. Cell. 1987 Apr 10;49(1):1–4. doi: 10.1016/0092-8674(87)90745-8. [DOI] [PubMed] [Google Scholar]
- Iba H., Takeya T., Cross F. R., Hanafusa T., Hanafusa H. Rous sarcoma virus variants that carry the cellular src gene instead of the viral src gene cannot transform chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4424–4428. doi: 10.1073/pnas.81.14.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jove R., Hanafusa H. Cell transformation by the viral src oncogene. Annu Rev Cell Biol. 1987;3:31–56. doi: 10.1146/annurev.cb.03.110187.000335. [DOI] [PubMed] [Google Scholar]
- Jove R., Hanafusa T., Hamaguchi M., Hanafusa H. In vivo phosphorylation states and kinase activities of transforming p60c-src mutants. Oncogene Res. 1989;5(1):49–60. [PubMed] [Google Scholar]
- Jove R., Kornbluth S., Hanafusa H. Enzymatically inactive p60c-src mutant with altered ATP-binding site is fully phosphorylated in its carboxy-terminal regulatory region. Cell. 1987 Sep 11;50(6):937–943. doi: 10.1016/0092-8674(87)90520-4. [DOI] [PubMed] [Google Scholar]
- Jove R., Mayer B. J., Iba H., Laugier D., Poirier F., Calothy G., Hanafusa T., Hanafusa H. Genetic analysis of p60v-src domains involved in the induction of different cell transformation parameters. J Virol. 1986 Dec;60(3):840–848. doi: 10.1128/jvi.60.3.840-848.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Identification of multiple novel polypeptide substrates of the v-src, v-yes, v-fps, v-ros, and v-erb-B oncogenic tyrosine protein kinases utilizing antisera against phosphotyrosine. Oncogene. 1988 Apr;2(4):305–315. [PubMed] [Google Scholar]
- Kaplan D. R., Whitman M., Schaffhausen B., Pallas D. C., White M., Cantley L., Roberts T. M. Common elements in growth factor stimulation and oncogenic transformation: 85 kd phosphoprotein and phosphatidylinositol kinase activity. Cell. 1987 Sep 25;50(7):1021–1029. doi: 10.1016/0092-8674(87)90168-1. [DOI] [PubMed] [Google Scholar]
- Kaplan J. M., Mardon G., Bishop J. M., Varmus H. E. The first seven amino acids encoded by the v-src oncogene act as a myristylation signal: lysine 7 is a critical determinant. Mol Cell Biol. 1988 Jun;8(6):2435–2441. doi: 10.1128/mcb.8.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J. Y., Takeya T., Grandori C., Iba H., Levy J. B., Hanafusa H. Amino acid substitutions sufficient to convert the nontransforming p60c-src protein to a transforming protein. Mol Cell Biol. 1986 Dec;6(12):4155–4160. doi: 10.1128/mcb.6.12.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Yoshida M. Small deletion in src of Rous sarcoma virus modifying transformation phenotypes: identification of 207-nucleotide deletion and its smaller product with protein kinase activity. J Virol. 1983 Jun;46(3):985–992. doi: 10.1128/jvi.46.3.985-992.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C. A., Moran M., Sadowski I., Pawson T. The common src homology region 2 domain of cytoplasmic signaling proteins is a positive effector of v-fps tyrosine kinase function. Mol Cell Biol. 1989 Oct;9(10):4131–4140. doi: 10.1128/mcb.9.10.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Levinson A. D., Courtneidge S. A., Bishop J. M. Structural and functional domains of the Rous sarcoma virus transforming protein (pp60src). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1624–1628. doi: 10.1073/pnas.78.3.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. B., Brugge J. S. Biological and biochemical properties of the c-src+ gene product overexpressed in chicken embryo fibroblasts. Mol Cell Biol. 1989 Aug;9(8):3332–3341. doi: 10.1128/mcb.9.8.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. B., Dorai T., Wang L. H., Brugge J. S. The structurally distinct form of pp60c-src detected in neuronal cells is encoded by a unique c-src mRNA. Mol Cell Biol. 1987 Nov;7(11):4142–4145. doi: 10.1128/mcb.7.11.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. B., Iba H., Hanafusa H. Activation of the transforming potential of p60c-src by a single amino acid change. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4228–4232. doi: 10.1073/pnas.83.12.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder M. E., Burr J. G. Immunological characterization of proteins detected by phosphotyrosine antibodies in cells transformed by Rous sarcoma virus. J Virol. 1988 Aug;62(8):2665–2673. doi: 10.1128/jvi.62.8.2665-2673.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsich L. A., Lewis A. J., Brugge J. S. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983 Nov;48(2):352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez R., Mathey-Prevot B., Bernards A., Baltimore D. Neuronal pp60c-src contains a six-amino acid insertion relative to its non-neuronal counterpart. Science. 1987 Jul 24;237(4813):411–415. doi: 10.1126/science.2440106. [DOI] [PubMed] [Google Scholar]
- Mayer B. J., Hamaguchi M., Hanafusa H. A novel viral oncogene with structural similarity to phospholipase C. Nature. 1988 Mar 17;332(6161):272–275. doi: 10.1038/332272a0. [DOI] [PubMed] [Google Scholar]
- Morgan D. O., Kaplan J. M., Bishop J. M., Varmus H. E. Mitosis-specific phosphorylation of p60c-src by p34cdc2-associated protein kinase. Cell. 1989 Jun 2;57(5):775–786. doi: 10.1016/0092-8674(89)90792-7. [DOI] [PubMed] [Google Scholar]
- Nemeth S. P., Fox L. G., DeMarco M., Brugge J. S. Deletions within the amino-terminal half of the c-src gene product that alter the functional activity of the protein. Mol Cell Biol. 1989 Mar;9(3):1109–1119. doi: 10.1128/mcb.9.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R. C., Varmus H. E., Bishop J. M. Expression of v-src and chicken c-src in rat cells demonstrates qualitative differences between pp60v-src and pp60c-src. Cell. 1984 May;37(1):131–139. doi: 10.1016/0092-8674(84)90308-8. [DOI] [PubMed] [Google Scholar]
- Pawson T. Non-catalytic domains of cytoplasmic protein-tyrosine kinases: regulatory elements in signal transduction. Oncogene. 1988 Nov;3(5):491–495. [PubMed] [Google Scholar]
- Potts W. M., Reynolds A. B., Lansing T. J., Parsons J. T. Activation of pp60c-src transforming potential by mutations altering the structure of an amino terminal domain containing residues 90-95. Oncogene Res. 1988;3(4):343–355. [PubMed] [Google Scholar]
- Raymond V. W., Parsons J. T. Identification of an amino terminal domain required for the transforming activity of the Rous sarcoma virus src protein. Virology. 1987 Oct;160(2):400–410. doi: 10.1016/0042-6822(87)90011-0. [DOI] [PubMed] [Google Scholar]
- Reddy E. P., Smith M. J., Srinivasan A. Nucleotide sequence of Abelson murine leukemia virus genome: structural similarity of its transforming gene product to other onc gene products with tyrosine-specific kinase activity. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3623–3627. doi: 10.1073/pnas.80.12.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A. B., Roesel D. J., Kanner S. B., Parsons J. T. Transformation-specific tyrosine phosphorylation of a novel cellular protein in chicken cells expressing oncogenic variants of the avian cellular src gene. Mol Cell Biol. 1989 Feb;9(2):629–638. doi: 10.1128/mcb.9.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. G., Suh P. G., Ryu S. H., Lee S. Y. Studies of inositol phospholipid-specific phospholipase C. Science. 1989 May 5;244(4904):546–550. doi: 10.1126/science.2541501. [DOI] [PubMed] [Google Scholar]
- Sadowski I., Stone J. C., Pawson T. A noncatalytic domain conserved among cytoplasmic protein-tyrosine kinases modifies the kinase function and transforming activity of Fujinami sarcoma virus P130gag-fps. Mol Cell Biol. 1986 Dec;6(12):4396–4408. doi: 10.1128/mcb.6.12.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Kato J., Takeya T. Characterization of partially activated p60c-src in chicken embryo fibroblasts. J Virol. 1989 Feb;63(2):683–688. doi: 10.1128/jvi.63.2.683-688.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalloway D., Coussens P. M., Yaciuk P. Overexpression of the c-src protein does not induce transformation of NIH 3T3 cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7071–7075. doi: 10.1073/pnas.81.22.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy S., Choi J. K., Bagrodia S., Copeland T. D., Maller J. L., Shalloway D. Purified maturation promoting factor phosphorylates pp60c-src at the sites phosphorylated during fibroblast mitosis. Cell. 1989 Jun 2;57(5):763–774. doi: 10.1016/0092-8674(89)90791-5. [DOI] [PubMed] [Google Scholar]
- Shibuya M., Hanafusa H. Nucleotide sequence of Fujinami sarcoma virus: evolutionary relationship of its transforming gene with transforming genes of other sarcoma viruses. Cell. 1982 Oct;30(3):787–795. doi: 10.1016/0092-8674(82)90283-5. [DOI] [PubMed] [Google Scholar]
- Stahl M. L., Ferenz C. R., Kelleher K. L., Kriz R. W., Knopf J. L. Sequence similarity of phospholipase C with the non-catalytic region of src. Nature. 1988 Mar 17;332(6161):269–272. doi: 10.1038/332269a0. [DOI] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983 Mar;32(3):881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Verderame M. F., Kaplan J. M., Varmus H. E. A mutation in v-src that removes a single conserved residue in the SH-2 domain of pp60v-src restricts transformation in a host-dependent manner. J Virol. 1989 Jan;63(1):338–348. doi: 10.1128/jvi.63.1.338-348.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel U. S., Dixon R. A., Schaber M. D., Diehl R. E., Marshall M. S., Scolnick E. M., Sigal I. S., Gibbs J. B. Cloning of bovine GAP and its interaction with oncogenic ras p21. Nature. 1988 Sep 1;335(6185):90–93. doi: 10.1038/335090a0. [DOI] [PubMed] [Google Scholar]
- Wang H. C., Parsons J. T. Deletions and insertions within an amino-terminal domain of pp60v-src inactivate transformation and modulate membrane stability. J Virol. 1989 Jan;63(1):291–302. doi: 10.1128/jvi.63.1.291-302.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Y. Isolation of antibodies for phosphotyrosine by immunization with a v-abl oncogene-encoded protein. Mol Cell Biol. 1985 Dec;5(12):3640–3643. doi: 10.1128/mcb.5.12.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler P. A., Boschelli F. Src homology 2 domain deletion mutants of p60v-src do not phosphorylate cellular proteins of 120-150 kDa. Oncogene. 1989 Feb;4(2):231–236. [PubMed] [Google Scholar]
- Whitman M., Downes C. P., Keeler M., Keller T., Cantley L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature. 1988 Apr 14;332(6165):644–646. doi: 10.1038/332644a0. [DOI] [PubMed] [Google Scholar]