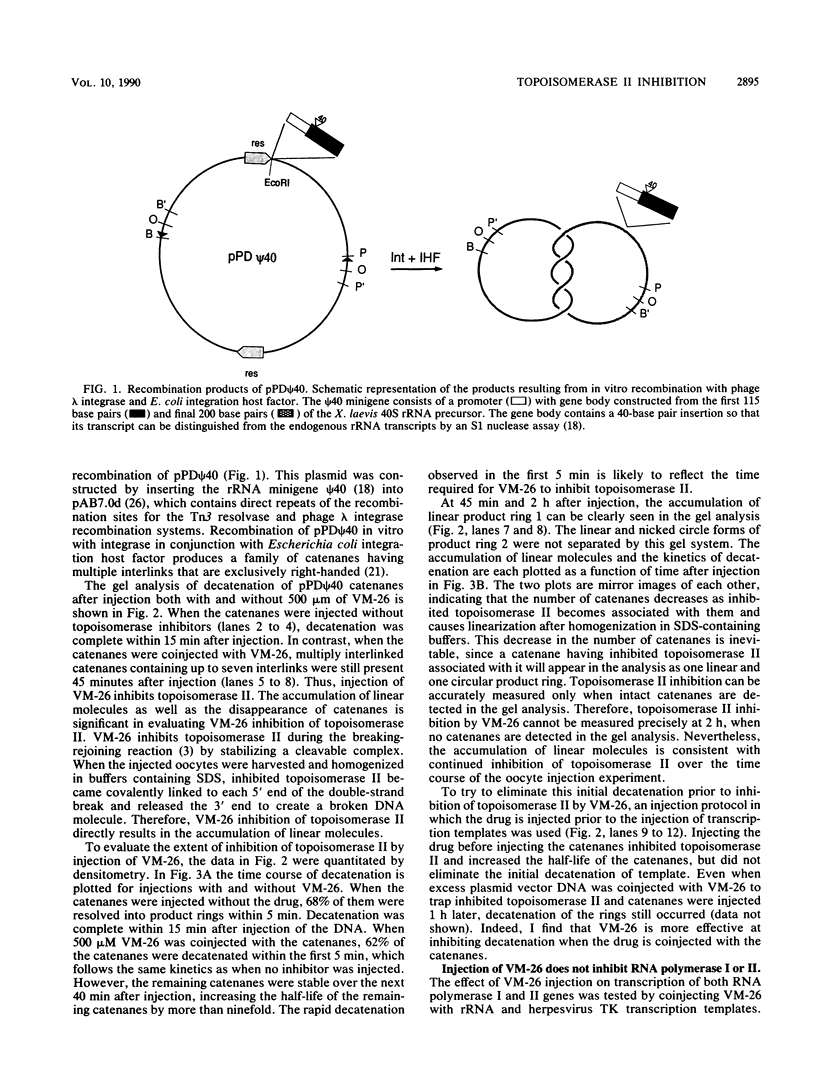
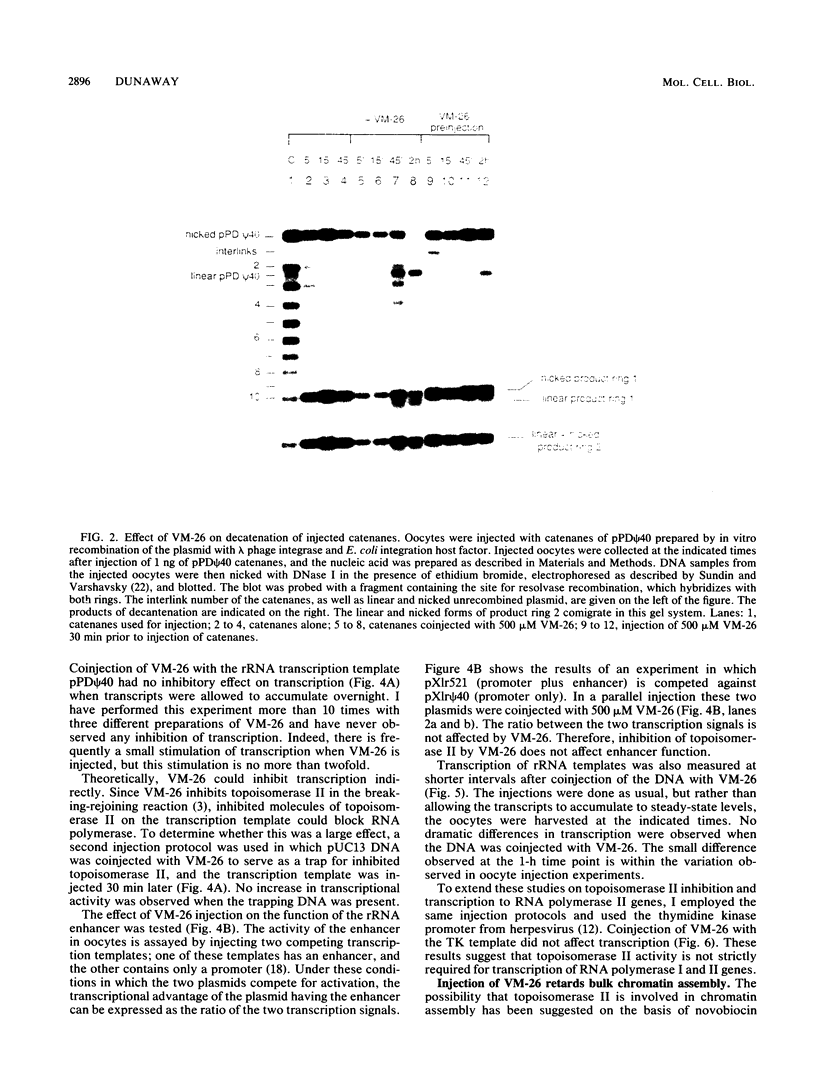
Abstract
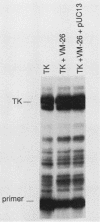
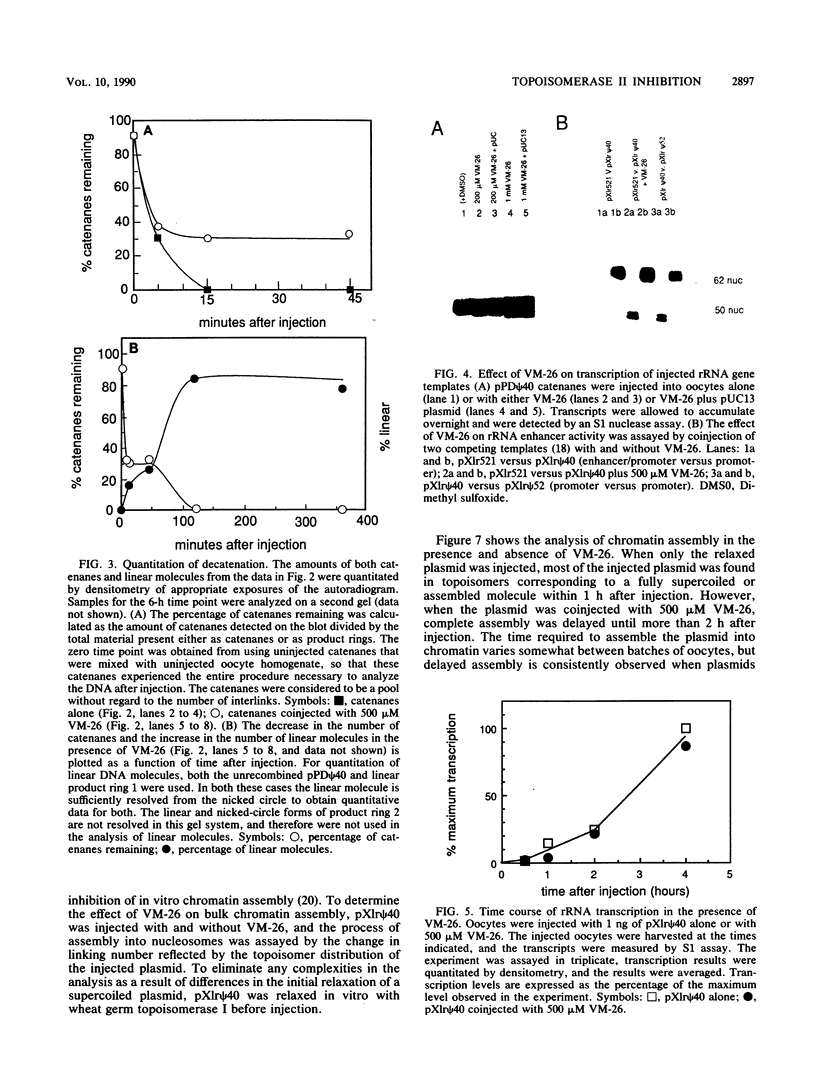
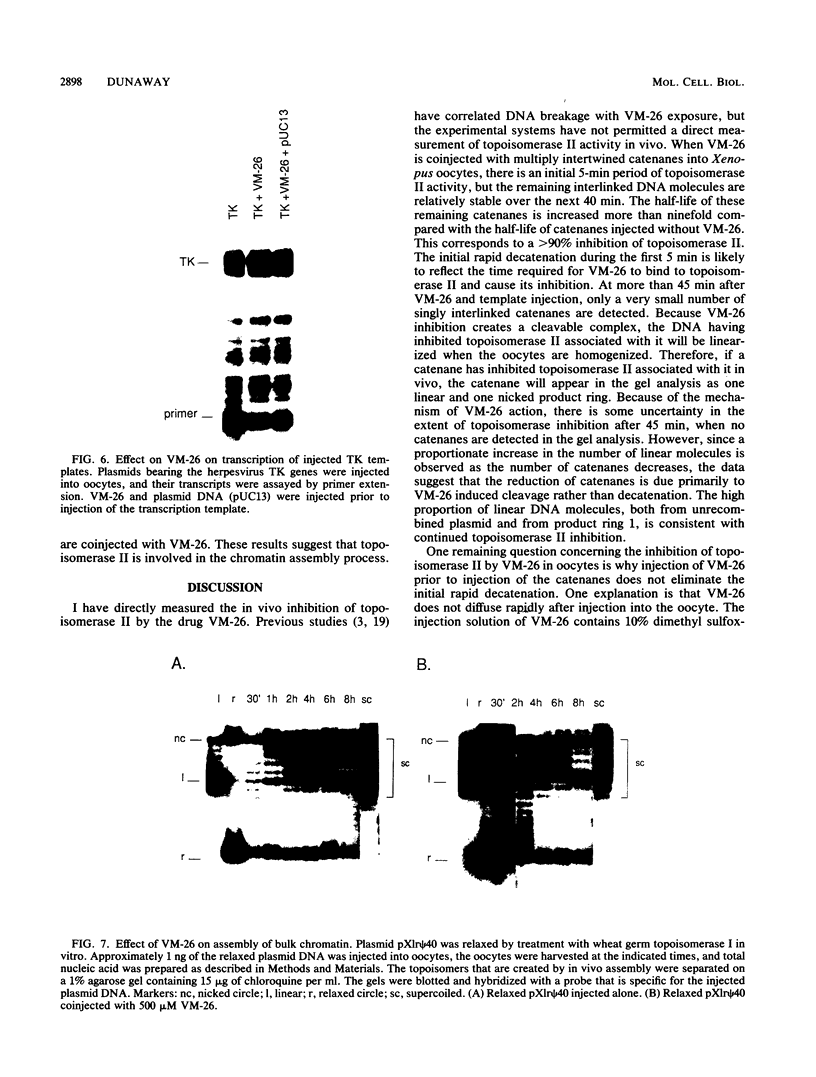
Injection of VM-26 (teniposide) into Xenopus oocytes inhibits the activity of topoisomerase II but does not inhibit transcription by RNA polymerases I and II. A specific assay for topoisomerase II, resolution of catenated DNA molecules into product rings, was used to quantitate VM-26 inhibition in vivo. When catenanes were injected without VM-26, about 60% of them were separated into product rings in the first 5 min after injection, and decatenation of the remainder was complete within 15 min. When VM-26 was coinjected, 60% of the catenanes were separated into product rings in the first 5 min after injection, but the remaining 40% were stable over the next 40 min. At 1 h after injection catenanes were no longer detected in the gel analysis, but the increasing numbers of linear product rings indicated that topoisomerase II continued to be inhibited by VM-26. These results suggest that a short lag of approximately 5 min is required for VM-26 to inhibit topoisomerase II and that after this initial period topoisomerase II is inhibited by more than 90%. There was no detectable decrease in transcription of injected rRNA and thymidine kinase (TK) genes or in the activity of the rRNA enhancer when these transcription templates were coinjected with VM-26. The time required for assembly of injected DNA into chromatin doubled in the presence of VM-26.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brill S. J., DiNardo S., Voelkel-Meiman K., Sternglanz R. Need for DNA topoisomerase activity as a swivel for DNA replication for transcription of ribosomal RNA. 1987 Mar 26-Apr 1Nature. 326(6111):414–416. doi: 10.1038/326414a0. [DOI] [PubMed] [Google Scholar]
- Brill S. J., Sternglanz R. Transcription-dependent DNA supercoiling in yeast DNA topoisomerase mutants. Cell. 1988 Jul 29;54(3):403–411. doi: 10.1016/0092-8674(88)90203-6. [DOI] [PubMed] [Google Scholar]
- Chen G. L., Yang L., Rowe T. C., Halligan B. D., Tewey K. M., Liu L. F. Nonintercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Nov 10;259(21):13560–13566. [PubMed] [Google Scholar]
- Cockerill P. N., Garrard W. T. Chromosomal loop anchorage of the kappa immunoglobulin gene occurs next to the enhancer in a region containing topoisomerase II sites. Cell. 1986 Jan 31;44(2):273–282. doi: 10.1016/0092-8674(86)90761-0. [DOI] [PubMed] [Google Scholar]
- Culotta V., Sollner-Webb B. Sites of topoisomerase I action on X. laevis ribosomal chromatin: transcriptionally active rDNA has an approximately 200 bp repeating structure. Cell. 1988 Feb 26;52(4):585–597. doi: 10.1016/0092-8674(88)90471-0. [DOI] [PubMed] [Google Scholar]
- Giaever G. N., Wang J. C. Supercoiling of intracellular DNA can occur in eukaryotic cells. Cell. 1988 Dec 2;55(5):849–856. doi: 10.1016/0092-8674(88)90140-7. [DOI] [PubMed] [Google Scholar]
- Gilmour D. S., Elgin S. C. Localization of specific topoisomerase I interactions within the transcribed region of active heat shock genes by using the inhibitor camptothecin. Mol Cell Biol. 1987 Jan;7(1):141–148. doi: 10.1128/mcb.7.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesfeld J. M. Novobiocin inhibits RNA polymerase III transcription in vitro by a mechanism distinct from DNA topoisomerase II. Nucleic Acids Res. 1986 Mar 11;14(5):2075–2088. doi: 10.1093/nar/14.5.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland R. M., Weintraub H., McKnight S. L. Transcription of DNA injected into Xenopus oocytes is influenced by template topology. Nature. 1983 Mar 3;302(5903):38–43. doi: 10.1038/302038a0. [DOI] [PubMed] [Google Scholar]
- Holm C., Stearns T., Botstein D. DNA topoisomerase II must act at mitosis to prevent nondisjunction and chromosome breakage. Mol Cell Biol. 1989 Jan;9(1):159–168. doi: 10.1128/mcb.9.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kressmann A., Clarkson S. G., Telford J. L., Birnstiel M. L. Transcription of xenopus tDNAmet1 and sea urchin histone DNA injected into the Xenopus oocyte nucleus. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1077–1082. doi: 10.1101/sqb.1978.042.01.108. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Gavis E. R., Kingsbury R., Axel R. Analysis of transcriptional regulatory signals of the HSV thymidine kinase gene: identification of an upstream control region. Cell. 1981 Aug;25(2):385–398. doi: 10.1016/0092-8674(81)90057-x. [DOI] [PubMed] [Google Scholar]
- Mizuuchi K., Gellert M., Weisberg R. A., Nash H. A. Catenation and supercoiling in the products of bacteriophage lambda integrative recombination in vitro. J Mol Biol. 1980 Aug 25;141(4):485–494. doi: 10.1016/0022-2836(80)90256-9. [DOI] [PubMed] [Google Scholar]
- Petryniak B., Lutter L. C. Topological characterization of the simian virus 40 transcription complex. Cell. 1987 Jan 30;48(2):289–295. doi: 10.1016/0092-8674(87)90432-6. [DOI] [PubMed] [Google Scholar]
- Pruitt S. C., Reeder R. H. Effect of intercalating agents on RNA polymerase I promoter selection in Xenopus laevis. Mol Cell Biol. 1984 Dec;4(12):2851–2857. doi: 10.1128/mcb.4.12.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt S. C., Reeder R. H. Effect of topological constraint on transcription of ribosomal DNA in Xenopus oocytes. Comparison of plasmid and endogenous genes. J Mol Biol. 1984 Mar 25;174(1):121–139. doi: 10.1016/0022-2836(84)90368-1. [DOI] [PubMed] [Google Scholar]
- Reeder R. H., Roan J. G., Dunaway M. Spacer regulation of Xenopus ribosomal gene transcription: competition in oocytes. Cell. 1983 Dec;35(2 Pt 1):449–456. doi: 10.1016/0092-8674(83)90178-2. [DOI] [PubMed] [Google Scholar]
- Rowe T. C., Wang J. C., Liu L. F. In vivo localization of DNA topoisomerase II cleavage sites on Drosophila heat shock chromatin. Mol Cell Biol. 1986 Apr;6(4):985–992. doi: 10.1128/mcb.6.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoji M., Worcel A. Chromatin assembly in Xenopus oocytes: in vivo studies. Cell. 1984 May;37(1):21–32. doi: 10.1016/0092-8674(84)90297-6. [DOI] [PubMed] [Google Scholar]
- Sundin O., Varshavsky A. Terminal stages of SV40 DNA replication proceed via multiply intertwined catenated dimers. Cell. 1980 Aug;21(1):103–114. doi: 10.1016/0092-8674(80)90118-x. [DOI] [PubMed] [Google Scholar]
- Uemura T., Tanagida M. Mitotic spindle pulls but fails to separate chromosomes in type II DNA topoisomerase mutants: uncoordinated mitosis. EMBO J. 1986 May;5(5):1003–1010. doi: 10.1002/j.1460-2075.1986.tb04315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T., Yanagida M. Isolation of type I and II DNA topoisomerase mutants from fission yeast: single and double mutants show different phenotypes in cell growth and chromatin organization. EMBO J. 1984 Aug;3(8):1737–1744. doi: 10.1002/j.1460-2075.1984.tb02040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Wasserman S. A., White J. H., Cozzarelli N. R. The helical repeat of double-stranded DNA varies as a function of catenation and supercoiling. Nature. 1988 Aug 4;334(6181):448–450. doi: 10.1038/334448a0. [DOI] [PubMed] [Google Scholar]
- Wu H. Y., Shyy S. H., Wang J. C., Liu L. F. Transcription generates positively and negatively supercoiled domains in the template. Cell. 1988 May 6;53(3):433–440. doi: 10.1016/0092-8674(88)90163-8. [DOI] [PubMed] [Google Scholar]
- Yang L., Rowe T. C., Nelson E. M., Liu L. F. In vivo mapping of DNA topoisomerase II-specific cleavage sites on SV40 chromatin. Cell. 1985 May;41(1):127–132. doi: 10.1016/0092-8674(85)90067-4. [DOI] [PubMed] [Google Scholar]