Abstract
Iron loading is associated with altered lipid metabolism, but underlying mechanisms remain unknown. We compared serum iron and triglycerides (TGs) in Belgrade rats, a genetic model of iron-loading anemia. Homozygous b/b rats had greater serum iron (68 vs. 28 μM; P=0.0004) and TG levels (180 vs. 84 mg/dl; P=0.014) compared to +/b controls. To confirm the association between iron loading and high TGs, Fischer rats were fed chow containing 1% carbonyl iron. Compared to controls pair-fed normal chow, carbonyl iron-fed rats had elevated serum iron (42 vs. 21 μM; P=0.007) and TGs (190 vs. 115 mg/dl; P=0.009). Despite normal hepatic production and secretion, TG clearance was lower in b/b than +/b rats due to reduced serum lipoprotein lipase (LPL) activity (3.1 vs. 5.0 mM/min; P=0.026). Likewise, LPL was lower in carbonyl iron-fed rats compared to controls (2.4 vs. 3.7 mM/min; P=0.017). Direct addition of iron to serum ex vivo or recombinant LPL in vitro decreased enzymatic activity in a dose-dependent manner. Lowering serum iron in Belgrade rats reduced TG levels (274 to 67 mg/dl, P=0.001). This study explains the relationship between iron status and lipid metabolism and provides mechanistic support for interventions that reduce serum iron levels in individuals at risk for hypertriglyceridemia.—Kim, J., Jia, X., Buckett, P. D., Liu, S., Lee, C.-H., Wessling-Resnick, M. Iron loading impairs lipoprotein lipase activity and promotes hypertriglyceridemia.
Keywords: Belgrade rat, iron overload, divalent metal transporter 1
Increased iron stores are associated with well-established risk factors of dyslipidemia and diabetes, including obesity, metabolic syndrome, chronic inflammation, and altered levels of circulating adipokines (1–4). This growing body of evidence is significant, since dietary iron could represent a simple modifiable factor that influences dyslipidemia and diabetes risk, particularly for individuals with genetic susceptibility for primary and secondary iron overload. For example, reduction of iron stores by phlebotomy, iron chelation therapy, or iron-restricted diet improves hypertriglyceridemia and diabetes (4–6). Despite a large body of epidemiological evidence indicating that iron overload alters metabolism (7–11), the mechanistic basis for this association is unclear.
Divalent metal transporter 1 (DMT1) is the major iron transporter required for duodenal absorption of dietary iron (12, 13). A glycine-to-arginine missense mutation (G185R) is found in both mk mice and homozygous Belgrade (b/b) rats, resulting in loss of DMT1 activity and giving rise to microcytic hypochromic anemia (14). DMT1 is also essential for the acquisition of iron required for transferrin-mediated iron delivery to erythroid cells (14). Despite their anemic state, dietary iron promotes extensive tissue iron loading in b/b rats, which have nearly doubled the amount of serum iron compared with heterozygous Belgrade (+/b) controls (15, 16). Similar clinical observations have been reported for patients with disabling mutations in DMT1 (17–19). Thus, a primary defect in erythron iron utilization caused by deficient iron uptake not only gives rise to the Belgrade rat's microcytic anemia but also promotes systemic and liver iron loading.
While mouse models of iron loading are resistant to the development of iron-induced diabetes (20, 21), metabolic regulation can be experimentally altered in iron-loaded rats (22, 23). Injection of rats with iron dextran increases serum triglycerides (TGs), suggesting that iron loading may modify lipid metabolism (23). Our previous studies have shown that iron-loading can be induced by feeding weanling rats a diet modified to contain 1% carbonyl iron (10,000 ppm iron; ref. 24). Here, we characterized the metabolic phenotype of the Belgrade rats and rats fed high-iron diet to better understand the relationships between iron status and lipid metabolism. Our results show that iron loading is associated with hypertriglyceridemia in both models. We establish that a major contributing factor underlying this phenotype is reduced lipoprotein lipase (LPL) activity due to high serum iron.
MATERIALS AND METHODS
Animals and diets
Animal protocols were approved by the Harvard Medical Area Animal Care and Use Committee. Belgrade rats were maintained on a 12-h light-dark cycle and consumed food and water ad libitum. Husbandry, genotyping, and diets for Belgrade rats have been previously described (14, 25). Experimental groups of both b/b and +/b littermates were established at the time of weaning. To induce anemia, a cohort of +/b rats was fed an iron-deficient diet containing 5 mg iron/kg (TD 99397; Harlan Teklad, Madison, WI, USA) for 3 wk to induce iron-deficient anemia (16, 25). To induce dietary iron overload, weanling Fischer F344 rats (Charles River, Boston, MA, USA) were fed diet containing 1% carbonyl iron (TD 09077; Harlan Teklad) for 4 wk (24).
Analysis of iron status
Hematocrits and liver nonheme iron concentrations were measured as described previously (26). Serum iron was measured as described previously (16). Total iron-binding capacity and transferrin saturation of serum samples were determined based on unsaturated iron binding capacity (16, 27).
Analysis of lipids
Serum, plasma, and other tissues, including liver, were collected after food withdrawal for 6 h. TGs, cholesterol, and free fatty acids in serum, plasma, or tissue were determined enzymatically (Thermo DMA, Louisville, CO, USA, and Wako Chemicals USA, Inc., Richmond, VA, USA).
Western blot analysis
For determination of apolipoprotein levels in plasma, FPLC fractions showing the highest TG levels were pooled (3 fractions) and concentrated using an Amicon Ultra-0.5 centrifugal filter (10,000 molecular weight cutoff; EMD Millipore, Billerica, MA, USA). Concentrates were electrophoresed on a 4–15% polyacrylamide gel and transferred to a polyvinylidene difluoride membrane (EMD Millipore). After blocking in 5% nonfat dry milk, the membrane was incubated with rabbit anti-ApoB (1:500; Abcam, Cambridge, MA, USA) or goat anti-ApoE (1:200, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The blot was then incubated with donkey anti-rabbit or donkey anti-goat antibodies conjugated with IRDye800 (1:10,000, Li-Cor Biosciences, Lincoln, NE, USA). Immunoreactivity was detected by infrared imaging (Li-Cor Biosciences), quantified using Odyssey 2.1 software (Li-Cor Biosciences), and expressed as ApoB-100/ApoE or ApoB-48/ApoE ratios.
For expression of lipoprotein receptors and LPL, tissue samples were homogenized in Tris-Nonidet P-40 buffer (50 mM Tris-HCl, 150 mM NaCl, and 0.5% Nonidet P-40, pH 7.5) containing protease inhibitors (Complete Mini, Roche, South San Francisco, CA, USA). For LPL expression, adipose and skeletal muscle tissues were homogenized in Tris-Nonidet P-40 buffer. Samples (50–100 μg proteins) were electrophoresed on a 10% SDS-polyacrylamide gel and transferred to polyvinylidene difluoride membrane. After blocking, blots were incubated with goat anti-LDL receptor antibody (1:200; Santa Cruz Biotechnology), mouse anti-VLDL receptor antibody (1:300; EMD Millipore) or rabbit anti-LPL antibody (1:300; Santa Cruz Biotechnology). Immunoreactivity to mouse anti-actin (1:10,000; MP Biomedicals, Santa Ana, CA, USA) was determined as a loading control. The blots were then incubated with IRDye800/680-conjugated secondary antibody (1:10,000; Li-Cor Biosciences) and scanned using an Odyssey Infrared Imaging System (Li-Cor Biosciences). Relative intensities of protein bands normalized to actin were determined using Odyssey 2.1 software.
Triglyceride production and secretion
The expression of hepatic lipogenic genes was quantified by qPCR, including fatty acid synthase (FAS), and acetyl-CoA carboxylase 1 and 2 (ACC1 and ACC2). Briefly, RNA samples isolated from tissues in Trizol were reverse-transcribed with random hexamer and oligo-dT primers (1:1 ratio). Relative gene expression was determined by SYBR green-based real-time PCR. Transcripts were normalized to 36B4 expression. To determine TG secretion rate, rats were denied access to food overnight and intravenously injected with Triton WR1339 (tyloxapol; 500 mg/kg; Sigma-Aldrich, St. Louis, MO, USA), plasma TG level was measured in samples taken over the time course shown (up to 4 h).
Analysis of LPL activity
Serum samples collected before and after intravenous injection of heparin (100 IU/kg) were used to determine LPL activity by a fluorescence assay (Roar Biomedical, Inc., New York, NY, USA). Briefly, serum samples were mixed in a 96-well microplate with 200 μl of substrate emulsion containing 5% human serum and 0.75% BSA. After 30 min incubation at room temperature, the fluorescent intensity of the reaction mixture was measured at 370 nm excitation/450 nm emission. Prehydrolyzed substrate was used to establish a standard curve, and the relative amounts of LPL activity in sera were calculated and expressed as millimoles per liter per minute. To examine the effect of iron on LPL activity, the serum from +/b rats or purified recombinant human LPL (Sigma-Aldrich) was incubated with different amount of FAC (0–60 μM) for 30 min and activity was determined as described above.
Statistical analyses
Values are reported as means ± sem. Statistical significance was evaluated using the Student's unpaired t test for 2-group comparison (Systat 13; Systat, Chicago, IL, USA) of parameters between b/b and +/b rats. One-way ANOVA was used for parameter comparison among >2 groups (Systat 13). Differences were considered significant at values of P < 0.05.
RESULTS
Belgrade rats display hypertriglyceridemia
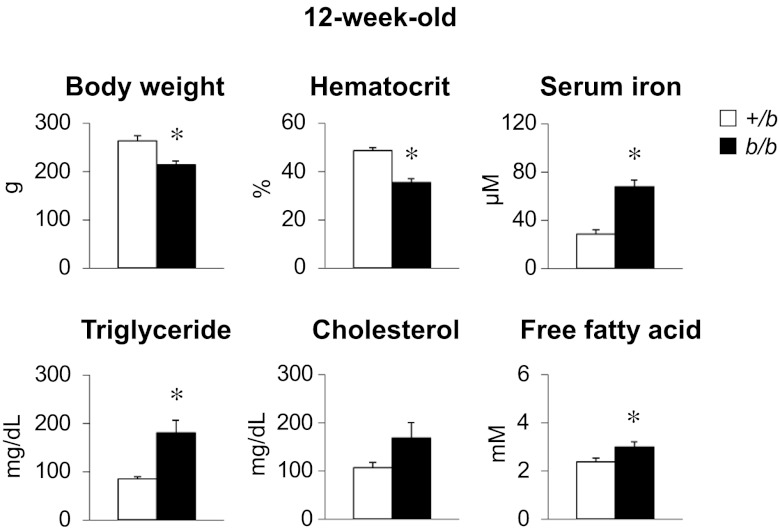
Characteristics of 12-wk-old b/b rats were compared to phenotypically null +/b littermate control rats (Fig. 1). Body weight and hematocrit were significantly lower in b/b rats, but serum iron was higher compared to control +/b rats. These data are consistent with other studies demonstrating iron-loading anemia associated with loss of DMT1 function in b/b rats (15, 16, 28, 29). Further analysis revealed that serum TG level was doubled in b/b rats compared with +/b rats. Free fatty acids were also significantly elevated in b/b rats. Although not statistically significant, serum cholesterol levels tended to be higher in b/b rats, similar to changes in lipid metabolism reported for rats injected with iron dextran (23). Both liver and fat weight were the same for b/b and +/b rats: 3.9 ± 0.2 vs. 3.8 ± 0.2 and 1.5 ± 0.1 vs. 1.6 ± 0.1% body weight, respectively (n=6).
Figure 1.
Belgrade b/b rats display elevated serum TGs. After denying 12-wk old rats access to food for 6 h, blood and serum samples were analyzed for the measurement of hematocrit, serum iron, TGs, cholesterol, and free fatty acids. Data are presented as means ± sem for control +/b (open bars) and b/b (solid bars) rats. *P < 0.05 vs. control; 2-sample t test (n=6).
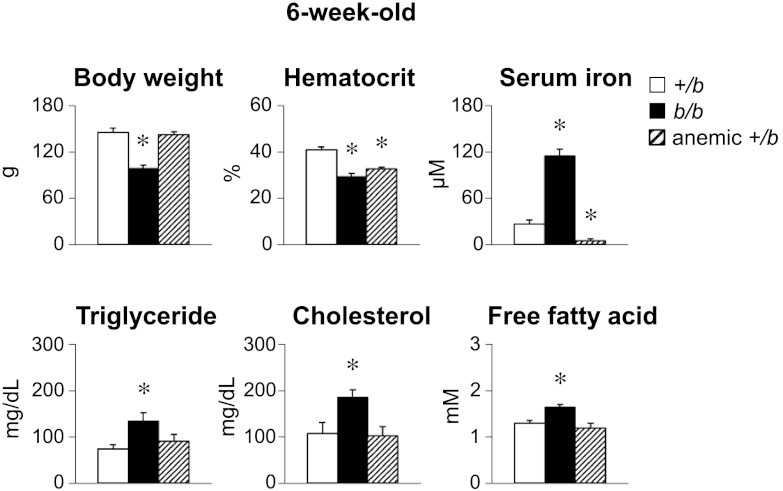
Some studies have suggested that iron deficiency anemia is associated with dyslipidemia, although reports are inconsistent (30–37). Since b/b rats not only are iron loaded but also are anemic, a matched cohort of +/b rats was made iron deficient by feeding a low-iron diet (5 ppm iron). Characteristics of the 6-wk-old groups showed that body weights of the iron-deficient +/b rats were similar to control +/b rats (Fig. 2). Hematocrit values of b/b and iron-deficient +/b rats were similar, 29.2 ± 1.4% (n=5) and 32.7 ± 0.8% (n=8), respectively, and both were significantly lower than control +/b rats (41.1±1.2%, n=8; P<0.001). While serum iron was higher in the Belgrade rat group, the iron-deficient +/b rats had significantly lower serum iron compared to both b/b and control +/b rats. Notably, serum lipids of iron-deficient +/b rats were similar to +/b controls, while levels of TGs, cholesterol, and free fatty acids in b/b rats were all significantly elevated by comparison to both groups (Fig. 2). Collectively, these metabolic data support the notion that iron loading impairs lipid metabolism in Belgrade rats.
Figure 2.
Iron-deficient +/b rats do not display hypertriglyceridemia. Experimental procedure was identical to conditions as described in Fig. 1, except that rats were 6 wk old. To induce iron deficiency, +/b rats were fed a diet containing 5 mg/kg iron. Data are presented as means ± sem for control +/b (open bars, n=6-11), b/b (solid bars, n=5–12), and iron-deficient anemic +/b (hatched bars, n=4–10) rats. *P < 0.05 vs. control; 1-way ANOVA.
Rats fed high-iron diet display hypertriglyceridemia
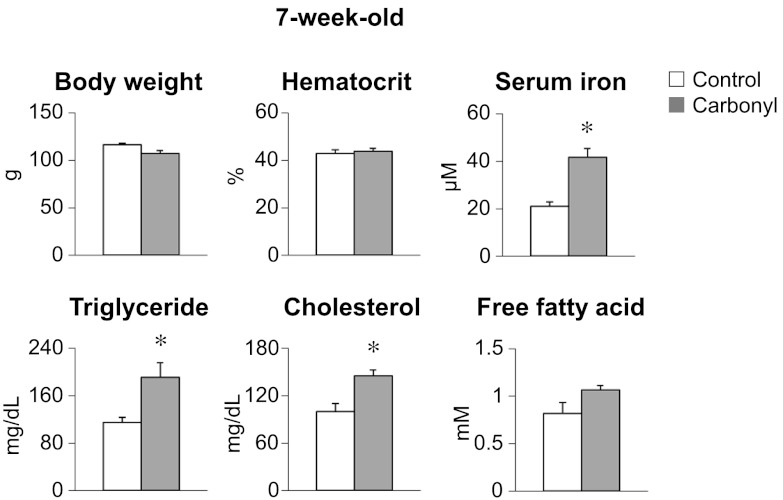
To determine more directly the effects of iron loading on lipid metabolism, weanling rats were fed a diet containing 1% carbonyl iron for 4 wk. This cohort was matched to a control group that was pair-fed normal chow (Fig. 3). Body weight and hematocrit were similar for both groups, but serum iron was greater in rats fed carbonyl iron. Notably, serum TG and cholesterol levels were also higher. The marked increase of serum TG levels upon iron loading is consistent with observations in 6- and 12-wk-old Belgrade rats (Figs. 1 and 2), supporting the conclusion that high serum iron plays a causal role.
Figure 3.
Iron-loaded rats have high serum TGs. Fischer F344 rats were fed a diet containing 1% carbonyl iron for 4 wk. Serum samples were analyzed for hematological and metabolic parameters as described in Fig. 1, except that rats were 7 wk old. Data are presented as means ± sem for control (open bars) and carbonyl iron-fed (shaded bars) rats. *P < 0.05 vs. control; 2-sample t test (n=6).
TG distribution in serum lipoproteins
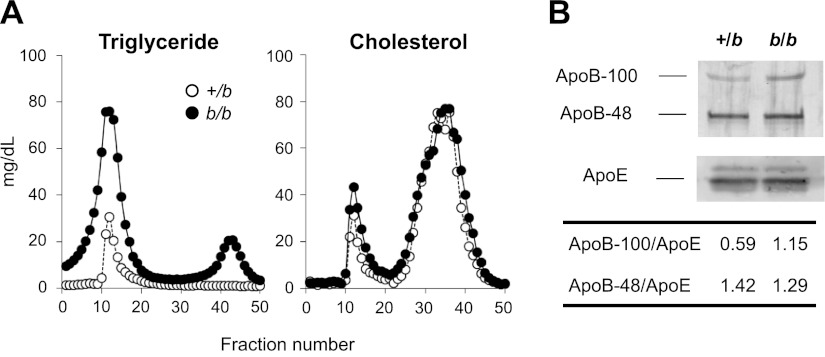
Plasma samples from unfed 12-wk-old b/b and +/b rats were collected, then pooled and separated by FPLC. In both groups (n=4), plasma TG was associated with VLDL fraction; as expected, b/b rats showed a higher TG level compared with +/b rats (Fig. 4A). The amount of cholesterol did not significantly differ and was found to be associated with HDL and VLDL in both groups. The pooled VLDL fractions were further analyzed to determine ApoB and ApoE protein content. Both ApoB-100 and ApoB-48 may be produced in rat liver, although ApoB-48 is predominantly synthesized by the intestine and is a component of chylomicrons (38). The ratio of ApoB-100/ApoE was doubled in b/b rats, while ApoB-48/ApoE was similar to heterozygous littermates (Fig. 4B). These data exclude postprandial effects on the lipoprotein profile and indicate that liver-derived VLDL is either produced in greater amounts or removed from the circulation more slowly under iron-loading conditions.
Figure 4.
Enhanced TG levels in b/b rats are associated with VLDL. A) Plasma samples were collected from 12-wk-old +/b and b/b rats denied access to food for 6 h (n=4/group). After pooling, distribution of TGs and cholesterol was determined by FPLC fractionation. Open and solid circles indicate +/b and b/b rats, respectively. B) Western blot analysis was performed using the VLDL fractions in A to quantify the expression levels of ApoB-100, ApoB-48, and ApoE proteins.
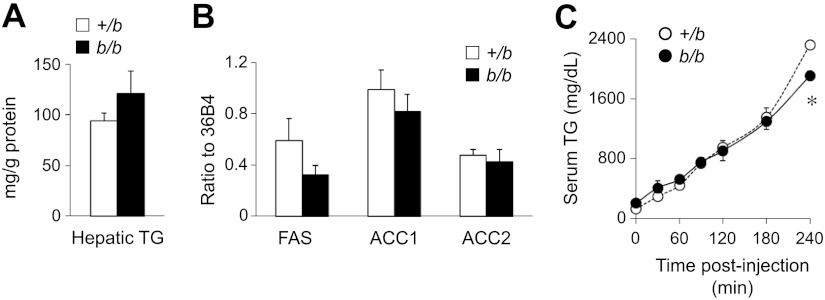
Liver TG production and secretion
To evaluate whether elevated serum TGs reflected increased hepatic production, b/b liver TG levels were first determined. Belgrade rat hepatic TG levels were similar to +/b rats (Fig. 5A). In addition, hepatic lipogenic gene expression did not differ between b/b and +/b rats, as reflected by transcript levels for FAS, ACC1, and ACC2 (Fig. 5B).
Figure 5.
Elevated serum TG levels do not result from increases in lipoprotein production or secretion in b/b rats. A) Hepatic TG levels from unfed 12-wk-old rats were determined. B) qPCR was performed to examine the hepatic expression levels of lipogenic genes, including FAS, ACC1, and ACC2. Shown are relative ratios of expression levels of genes of interest to level of 36B4 gene. C) Rats were denied access to food and injected with Triton WR1339 (tyloxapol), and serum TG levels were determined at different time points. Data are presented as means ± sem for +/b (open bars and circles, n=4–6) and b/b (solid bars and circles, n=4–6) rats. *P < 0.05 vs. control; 2-sample t test.
Next, TG secretion was directly examined by determining serum levels following intravenous administration of Triton WR1339 (tyloxapol). This inhibitor blocks activity of LPL, a key enzyme necessary for hydrolysis of TGs from VLDL and the subsequent uptake of fatty acids. In the presence of Triton WR1339 and under nonfeeding conditions, the amount of serum TG over time proportionally reflects its rate of secretion. Similar rates of TG secretion are observed in b/b and +/b rats in the presence of Triton WR1339, with Belgrade rats showing a slight decrease in the amount of TGs secreted 4 h postinjection (Fig. 5C). These results indicate that the elevated TG levels observed under basal conditions do not reflect enhanced secretion of TGs into circulation.
Clearance of serum TGs
In addition to peripheral VLDL receptors, hepatic LDL receptors play a role in VLDL clearance in rats (39). To examine whether altered lipoprotein receptor levels contribute to reduced TG clearance, Western blot analysis was performed. Levels of VLDL receptors in skeletal muscle and LDL receptor levels in liver were the same in b/b and +/b rats (Supplemental Fig. S1). Another critical step associated with TG uptake is LPL-mediated lipolysis, which produces fatty acids and monoacylglycerol for transport into cells. Western blotting also indicated that levels of LPL associated with adipose and skeletal muscle were similar in b/b and +/b rats. Although little difference in TG secretion between groups was observed in the presence of Triton WR1339, this experiment does not discriminate whether LPL catalytic activity differed in the absence of the inhibitor. Therefore, serum LPL activity was measured using a fluorescence-based assay. These results showed that the activity of serum LPL was significantly lower in b/b rats, and this pattern persisted after injection of heparin to release tissue-associated LPL (Fig. 6A). Moreover, serum LPL activity was also reduced in rats fed high-iron diet, and was significantly lower after heparin injection compared to control serum levels (Fig. 6B). The observations made in both genetic and dietary iron loading models bolster the idea that high serum iron promotes reduced TG clearance due to LPL inhibition.
Figure 6.
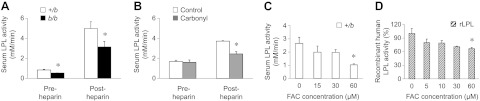
LPL activity is reduced by high iron. A) LPL activity in serum was determined before and after intravenous injection of heparin (100 IU/kg). Data are presented as means ± sem for +/b (open bars, n=6) and b/b (solid bars, n=4) rats. B) Serum LPL activity was also determined in control and carbonyl-iron-fed rats before and after intravenous injection of heparin. Data are presented as means ± sem for control (open bars, n=3) and carbonyl-iron fed (shaded bars, n=3) rats. C) LPL activity was measured with +/b sera 30 min after addition of increasing amounts of ferric ammonium citrate (FAC; 0, 15, 30, and 60 μM). Data are presented as means ± sem (n=4). *P < 0.05 vs. 0 μM FAC; 1-way ANOVA. D) LPL activity was measured in vitro using purified recombinant LPL 30 min after addition of FAC. Data are presented as means ± sem (n=3). Data are from a single experiment performed with triplicate samples, with similar results observed on several occasions. *P < 0.05 vs. 0 μM FAC; 1-way ANOVA.
Inhibition of LPL activity by iron
To examine directly the influence of high serum iron on LPL catalytic activity, increasing amounts of ferric ammonium citrate (FAC) were added to control rat serum up to the level of iron found in b/b rat serum (∼60 μM). LPL activity was inhibited by FAC in a dose-dependent manner ex vivo (P=0.017; Fig. 6C). To confirm that iron inhibits LPL activity, FAC was also added to in vitro assays with recombinant human LPL. Again, an inverse relationship between the activity of purified LPL and iron concentration was observed (P=0.031; Fig. 6D).
Lowering serum iron reduces TGs
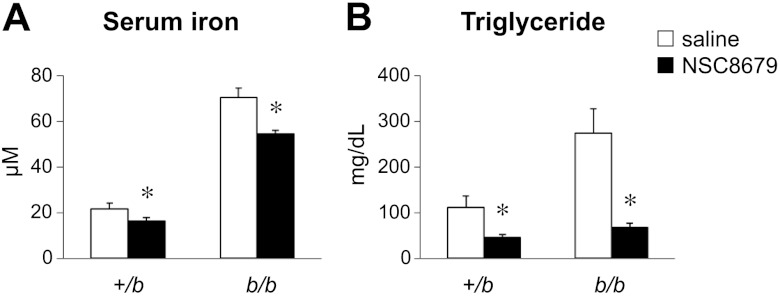
To evaluate whether reducing serum iron would lower TG levels in Belgrade rats, an ortholog of the iron transport inhibitor ferristatin was used. Like ferristatin, NSC8679 has been shown to induce degradation of transferrin receptors (40, 41) but is not known to chelate iron. Treatment with the small molecule inhibitor NSC8679 significantly reduced serum iron (Fig. 7A) as well as TGs (Fig. 7B) in both b/b and control +/b rats.
Figure 7.
Iron transport inhibitor reduces both serum iron and TGs. Rats were injected twice daily for 3 d with 40 mg/kg NSC8679 or saline as a vehicle control. On d 4, rats were injected once and denied access to food for 6 h, followed by serum collection for the measurements of serum iron and TGs. Data are presented as means ± sem for control (open bars) and treated (solid bars) rats (n=5); +/b and b/b groups are indicated. *P < 0.05 vs. control; 2-way ANOVA.
DISCUSSION
While accumulating evidence in humans indicates a strong association between iron loading and dysregulated metabolic homeostasis (7–9), the role of iron in lipid metabolism has remained ambiguous. One goal of the present study was to define the metabolic phenotype of the Belgrade rat as a model of iron-loading anemia. Hypertriglyceridemia is a clear feature of the b/b rat phenotype. Studies of dyslipidemia under anemic conditions have been inconsistent (11, 30–37). In this study, we observed that TG levels in +/b rats fed a low-iron diet to induce iron deficiency anemia are similar to controls. This evidence suggests that iron loading in the Belgrade rat is the primary underlying condition that promotes high TGs. One caveat is that it is possible that other metabolic changes linked to the DMT1(G185R) mutation might also contribute to the observed dyslipidemia.
Our conclusion that iron loading gives rise to hypertriglyceridemia is further supported by the rat model of dietary iron overload, which displays increased serum TGs. The fact that rats injected with iron dextran also have increased serum TGs (23) further confirms the close association between high serum iron and TG levels. It should be noted that cholesterol and free fatty acid levels tended to be higher in both the genetic and dietary iron-loading models, although differences between groups did not always achieve statistical significance.
Hypertriglyceridemia was observed under nonfeeding conditions in both models. The observation that ApoB-48 and ApoE levels are similar in b/b and +/b rats further excludes postprandial effects. In fact, the ratio of ApoB-100/ApoE is greater in Belgrade rats, supporting the idea that TG-rich liver-derived VLDL is removed from circulation more slowly. Several other findings indicate that hypertriglyceridemia arises due to decreased clearance rather than increased production. For example, tissue TG levels were similar in b/b and +/b rat livers. Hepatic lipogenic gene expression was also the same. Finally, comparable rates of TG secretion in the presence of Triton WR1339 were observed for b/b and +/b rats, providing further support for the idea that TG production is not enhanced.
It is possible that the observed hypertriglyceridemia is due to decreased clearance. Lipoprotein receptors do not appear to be affected, since liver LDL receptors and skeletal muscle VLDL receptor protein were the same in b/b and +/b rats. Impaired endocytosis of lipoprotein receptors and their ligands and/or defective fatty acid uptake and transport could contribute to elevated TG levels. These possibilities are not mutually exclusive and other mechanisms may potentially contribute to hypertriglyceridemia. The observation that cholesterol and free fatty acid levels also trended higher is consistent with defective lipid import processes in general.
In this study, we focused on one major determinant of TG uptake, LPL-mediated lipolysis. The reduced postheparin serum LPL activities observed in b/b rats and rats fed a high-iron diet support our conclusion that systemic lipolysis is significantly impaired by iron loading (42). Based on Western blot analysis, it is unlikely that loss of LPL activity results from reduced levels of the protein. To test directly the hypothesis that high serum iron affected the enzyme's activity, control rat serum was incubated with iron ex vivo. Addition of FAC reduced lipolytic activity in a dose-dependent manner and over a concentration range that reflects serum iron levels in b/b rats and rats fed carbonyl iron. Moreover, in vitro experiments with recombinant LPL confirmed that the presence of iron suppresses the enzyme's activity. Finally, reducing serum iron in the Belgrade rat by pharmacological intervention significantly lowered TG levels, suggesting an inverse relationship between iron and LPL activity.
Note that early studies of the Belgrade rat compared its features of iron-loading anemia to characteristics associated with β-thalassemia (15, 29). Thalassemias (11, 43–47) and other iron-loading disorders (4, 10, 48) are frequently associated with high serum TG levels. In fact, a preliminary study of 8 patients with thalassemia reported reduced pre- and postheparin serum LPL activity (43). More generally, the reduction of iron loading by phlebotomy (4), iron chelation (49), iron-restricted diet (35), or pharmacological use of iron inhibitors (50) could provide therapeutic advantages to resolve lipid dysregulation. Further study will be necessary to determine the molecular basis for iron-mediated regulation of LPL activity. We speculate that oxidative modification of the enzyme, its substrates, and/or reaction products may interfere with lipolysis. Our study provides at least one explanation for the relationship between iron status and lipid metabolism, as well as mechanistic support for interventions that reduce serum iron levels in individuals at risk for hypertriglyceridemia.
Acknowledgments
This work was supported by grants from the U.S. National Institutes of Health (NIH) to M.W.-R. (R01 ES014638, R01 DK064750, and RC1 DK086774) and to C.-H.L. (R01 DK075046). J.K. was supported by an NIH Roadmap Fellowship (R90 DK071507) and the Yerby Postdoctoral Fellowship at the Harvard School of Public Health.
The authors thank Dr. David E. Cohen and Ms. Yingxia Li (Brigham and Women's Hospital and the Molecular and Cellular Biochemistry Core at the Harvard Digestive Diseases Center, Boston, MA, USA; P30 DK34854) for their help in separating lipid fractions by FPLC for analysis, and Dr. Jorge Plutsky (Joslin Diabetes Center, Boston, MA, USA) for helpful discussion and advice. The authors declare no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- ACC1/2
- acetyl-CoA carboxylase 1/2
- +/b
- heterozygous Belgrade
- b/b
- homozygous Belgrade
- DMT1
- divalent metal transporter 1
- FAC
- ferric ammonium citrate
- FAS
- fatty acid synthase
- LPL
- lipoprotein lipase
- TG
- triglyceride
REFERENCES
- 1. Fumeron F., Pean F., Driss F., Balkau B., Tichet J., Marre M., Grandchamp B. (2006) Ferritin and transferrin are both predictive of the onset of hyperglycemia in men and women over 3 years: the data from an epidemiological study on the Insulin Resistance Syndrome (DESIR) study. Diabetes Care 29, 2090–2094 [DOI] [PubMed] [Google Scholar]
- 2. Sun L., Franco O. H., Hu F. B., Cai L., Yu Z., Li H., Ye X., Qi Q., Wang J., Pan A., Liu Y., Lin X. (2008) Ferritin concentrations, metabolic syndrome, and type 2 diabetes in middle-aged and elderly Chinese. J. Clin. Endocrinol. Metab. 93, 4690–4696 [DOI] [PubMed] [Google Scholar]
- 3. Vari I. S., Balkau B., Kettaneh A., Andre P., Tichet J., Fumeron F., Caces E., Marre M., Grandchamp B., Ducimetiere P. (2007) Ferritin and transferrin are associated with metabolic syndrome abnormalities and their change over time in a general population: Data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR). Diabetes Care 30, 1795–1801 [DOI] [PubMed] [Google Scholar]
- 4. Casanova-Esteban P., Guiral N., Andres E., Gonzalvo C., Mateo-Gallego R., Giraldo P., Paramo J. A., Civeira F. (2011) Effect of phlebotomy on lipid metabolism in subjects with hereditary hemochromatosis. Metabolism 60, 830–836 [DOI] [PubMed] [Google Scholar]
- 5. Bofill C., Joven J., Bages J., Vilella E., Sans T., Cavalle P., Miralles R., Llobet J., Camps J. (1994) Response to repeated phlebotomies in patients with non-insulin-dependent diabetes mellitus. Metabolism 43, 614–620 [DOI] [PubMed] [Google Scholar]
- 6. Fernandez-Real J. M., Penarroja G., Castro A., Garcia-Bragado F., Hernandez-Aguado I., Ricart W. (2002) Blood letting in high-ferritin type 2 diabetes: effects on insulin sensitivity and beta-cell function. Diabetes 51, 1000–1004 [DOI] [PubMed] [Google Scholar]
- 7. Ford E. S., Cogswell M. E. (1999) Diabetes and serum ferritin concentration among U.S. adults. Diabetes Care 22, 1978–1983 [DOI] [PubMed] [Google Scholar]
- 8. Jiang R., Manson J. E., Meigs J. B., Ma J., Rifai N., Hu F. B. (2004) Body iron stores in relation to risk of type 2 diabetes in apparently healthy women. JAMA 291, 711–717 [DOI] [PubMed] [Google Scholar]
- 9. Salonen J. T., Tuomainen T. P., Nyyssonen K., Lakka H. M., Punnonen K. (1998) Relation between iron stores and non-insulin dependent diabetes in men: case-control study. BMJ 317, 727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Solanas-Barca M., Mateo-Gallego R., Calmarza P., Jarauta E., Bea A. M., Cenarro A., Civeira F. (2009) Mutations in HFE causing hemochromatosis are associated with primary hypertriglyceridemia. J. Clin. Endocrinol. Metab. 94, 4391–4397 [DOI] [PubMed] [Google Scholar]
- 11. Al-Quobaili F. A., Abou Asali I. E. (2004) Serum levels of lipids and lipoproteins in Syrian patients with beta-thalassemia major. Saudi Med. J. 25, 871–875 [PubMed] [Google Scholar]
- 12. Fleming M. D., Trenor C. C., 3rd, Su M. A., Foernzler D., Beier D. R., Dietrich W. F., Andrews N. C. (1997) Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat. Genet. 16, 383–386 [DOI] [PubMed] [Google Scholar]
- 13. Gunshin H., Mackenzie B., Berger U. V., Gunshin Y., Romero M. F., Boron W. F., Nussberger S., Gollan J. L., Hediger M. A. (1997) Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388, 482–488 [DOI] [PubMed] [Google Scholar]
- 14. Fleming M. D., Romano M. A., Su M. A., Garrick L. M., Garrick M. D., Andrews N. C. (1998) Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc. Natl. Acad. Sci. U. S. A. 95, 1148–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thompson K., Molina R. M., Brain J. D., Wessling-Resnick M. (2006) Belgrade rats display liver iron loading. J. Nutr. 136, 3010–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim J., Molina R. M., Donaghey T. C., Buckett P. D., Brain J. D., Wessling-Resnick M. (2011) Influence of DMT1 and iron status on inflammatory responses in the lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 300, L659–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mims M. P., Guan Y., Pospisilova D., Priwitzerova M., Indrak K., Ponka P., Divoky V., Prchal J. T. (2005) Identification of a human mutation of DMT1 in a patient with microcytic anemia and iron overload. Blood 105, 1337–1342 [DOI] [PubMed] [Google Scholar]
- 18. Iolascon A., d'Apolito M., Servedio V., Cimmino F., Piga A., Camaschella C. (2006) Microcytic anemia and hepatic iron overload in a child with compound heterozygous mutations in DMT1 (SCL11A2). Blood 107, 349–354 [DOI] [PubMed] [Google Scholar]
- 19. Beaumont C., Delaunay J., Hetet G., Grandchamp B., de Montalembert M., Tchernia G. (2006) Two new human DMT1 gene mutations in a patient with microcytic anemia, low ferritinemia, and liver iron overload. Blood 107, 4168–4170 [DOI] [PubMed] [Google Scholar]
- 20. Cooksey R. C., Jouihan H. A., Ajioka R. S., Hazel M. W., Jones D. L., Kushner J. P., McClain D. A. (2004) Oxidative stress, beta-cell apoptosis, and decreased insulin secretory capacity in mouse models of hemochromatosis. Endocrinology 145, 5305–5312 [DOI] [PubMed] [Google Scholar]
- 21. Ramey G., Faye A., Durel B., Viollet B., Vaulont S. (2007) Iron overload in Hepc1(−/−) mice is not impairing glucose homeostasis. FEBS Lett. 581, 1053–1057 [DOI] [PubMed] [Google Scholar]
- 22. Brunet S., Thibault L., Delvin E., Yotov W., Bendayan M., Levy E. (1999) Dietary iron overload and induced lipid peroxidation are associated with impaired plasma lipid transport and hepatic sterol metabolism in rats. Hepatology 29, 1809–1817 [DOI] [PubMed] [Google Scholar]
- 23. Silva M., Silva M. E., de Paula H., Carneiro C. M., Pedrosa M. L. (2008) Iron overload alters glucose homeostasis, causes liver steatosis, and increases serum triacylglycerols in rats. Nutr. Res. 28, 391–398 [DOI] [PubMed] [Google Scholar]
- 24. Thompson K., Molina R., Donaghey T., Brain J. D., Wessling-Resnick M. (2006) The influence of high iron diet on rat lung manganese absorption. Toxicol. Appl. Pharmacol. 210, 17–23 [DOI] [PubMed] [Google Scholar]
- 25. Thompson K., Molina R. M., Donaghey T., Schwob J. E., Brain J. D., Wessling-Resnick M. (2007) Olfactory uptake of manganese requires DMT1 and is enhanced by anemia. FASEB J. 21, 223–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heilig E., Molina R., Donaghey T., Brain J. D., Wessling-Resnick M. (2005) Pharmacokinetics of pulmonary manganese absorption: evidence for increased susceptibility to manganese loading in iron-deficient rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 288, L887–893 [DOI] [PubMed] [Google Scholar]
- 27. Chaudhury C., Kim J., Mehnaz S., Wani M. A., Oberyszyn T. M., Bronson C. L., Mohanty S., Hayton W. L., Robinson J. M., Anderson C. L. (2006) Accelerated transferrin degradation in HFE-deficient mice is associated with increased transferrin saturation. J. Nutr. 136, 2993–2998 [DOI] [PubMed] [Google Scholar]
- 28. Garrick M., Scott D., Walpole S., Finkelstein E., Whitbred J., Chopra S., Trivikram L., Mayes D., Rhodes D., Cabbagestalk K., Oklu R., Sadiq A., Mascia B., Hoke J., Garrick L. (1997) Iron supplementation moderates but does not cure the Belgrade anemia. Biometals 10, 65–76 [DOI] [PubMed] [Google Scholar]
- 29. Sladic-Simic D., Martinovitch P. N., Zivkovic N., Pavic D., Martinovic J., Kahn M., Ranney H. M. (1969) A thalassemia-like disorder in Belgrade laboratory rats. Ann. N. Y. Acad. Sci. 165, 93–99 [DOI] [PubMed] [Google Scholar]
- 30. Lewis M., Iammarino R. M. (1971) Lipemia in rodent iron-deficiency anemia. J. Lab. Clin. Med. 78, 546–554 [PubMed] [Google Scholar]
- 31. Stangl G. I., Kirchgessner M. (1998) Different degrees of moderate iron deficiency modulate lipid metabolism of rats. Lipids 33, 889–895 [DOI] [PubMed] [Google Scholar]
- 32. Galan P., Hercberg S., Touitou Y. (1984) The activity of tissue enzymes in iron-deficient rat and man: an overview. Comp. Biochem. Physiol. B. 77, 647–653 [DOI] [PubMed] [Google Scholar]
- 33. Davis M. R., Rendina E., Peterson S. K., Lucas E. A., Smith B. J., Clarke S. L. (2012) Enhanced expression of lipogenic genes may contribute to hyperglycemia and alterations in plasma lipids in response to dietary iron deficiency. Genes Nutr. 7, 415–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamagishi H., Okazaki H., Shimizu M., Izawa T., Komabayashi T. (2000) Relationships among serum triacylglycerol, fat pad weight, and lipolysis in iron-deficient rats. J. Nutrit. Biochem. 11, 455–460 [DOI] [PubMed] [Google Scholar]
- 35. Cooksey R. C., Jones D., Gabrielsen S., Huang J., Simcox J. A., Luo B., Soesanto Y., Rienhoff H., Abel E. D., McClain D. A. (2010) Dietary iron restriction or iron chelation protects from diabetes and loss of beta-cell function in the obese (ob/ob lep−/−) mouse. Am. J. Physiol. Endocrin. Metabol. 298, E1236–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Casanova-Esteban P., Guiral N., Andres E., Gonzalvo C., Mateo-Gallego R., Giraldo P., Paramo J. A., Civeira F. (2011) Effect of phlebotomy on lipid metabolism in subjects with hereditary hemochromatosis. Metabolism 60, 830–834 [DOI] [PubMed] [Google Scholar]
- 37. Wlazlo N., Greevenbroek M. M. (2012) Lipid metabolism: a role for iron? Curr. Opin. Lipid. 23, 258–259 [DOI] [PubMed] [Google Scholar]
- 38. Bell-Quint J., Forte T., Graham P. (1981) Synthesis of two forms of apolipoprotein B by cultured rat hepatocytes. Biochem. Biophys. Res. Commun. 99, 700–706 [DOI] [PubMed] [Google Scholar]
- 39. Jones A. L., Hradek G. T., Hornick C., Renaud G., Windler E. E., Havel R. J. (1984) Uptake and processing of remnants of chylomicrons and very low density lipoproteins by rat liver. J. Lipid Res. 25, 1151–1158 [PubMed] [Google Scholar]
- 40. Horonchik L., Wessling-Resnick M. (2008) The small-molecule iron transport inhibitor ferristatin/NSC306711 promotes degradation of the transferrin receptor. Chem. Biol. 15, 647–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Buckett P. D., Wessling-Resnick M. (2009) Small molecule inhibitors of divalent metal transporter-1. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G798–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Holm C., Olivecrona G., Ottosson M. (2001) Assays of lipolytic enzymes. Methods Mol. Biol. 155, 97–119 [DOI] [PubMed] [Google Scholar]
- 43. Cherchi G. M., Baggi M. A., Coinu R., Deiana M. G., Pacifico A., Maioli M. (1983) [Post-heparin lipase activity in beta-thalassemia major: preliminary data]. Boll. Soc. Ital. Biol. Sper. 59, 1739–1743 [PubMed] [Google Scholar]
- 44. Hartman C., Tamary H., Tamir A., Shabad E., Levine C., Koren A., Shamir R. (2002) Hypocholesterolemia in children and adolescents with beta-thalassemia intermedia. J. Pediatr. 141, 543–547 [DOI] [PubMed] [Google Scholar]
- 45. Tantawy A. A., Adly A. A., El Maaty M. G., Amin S. A. (2009) Subclinical atherosclerosis in young beta-thalassemia major patients. Hemoglobin 33, 463–474 [DOI] [PubMed] [Google Scholar]
- 46. Papanastasiou D. A., Siorokou T., Haliotis F. A. (1996) beta-Thalassaemia and factors affecting the metabolism of lipids and lipoproteins. Haematologia (Budapest) 27, 143–153 [PubMed] [Google Scholar]
- 47. Nasr M. R., Abdelmaksoud A. M., Abd El-Aal K. S., Mabrouk N. A., Ismael W. M. (2008) Plasma lipid profile and lipid peroxidation in beta-thalassemic children. J. Clin. Lipidol. 2, 405–409 [DOI] [PubMed] [Google Scholar]
- 48. Mateo-Gallego R., Calmarza P., Jarauta E., Burillo E., Cenarro A., Civeira F. (2010) Serum ferritin is a major determinant of lipid phenotype in familial combined hyperlipidemia and familial hypertriglyceridemia. Metabolism 59, 154–158 [DOI] [PubMed] [Google Scholar]
- 49. Cutler P. (1989) Deferoxamine therapy in high-ferritin diabetes. Diabetes 38, 1207–1210 [DOI] [PubMed] [Google Scholar]
- 50. Byrne S. L., Krishnamurthy D., Wessling-Resnick M. (2013) Pharmacology of iron transport. Ann. Rev. Pharmacol. Toxicol. 53 In press [DOI] [PMC free article] [PubMed] [Google Scholar]