Abstract
Purpose
We determined the seroprevalence of platelet factor 4 (PF4)/heparin antibodies in healthy subjects.
Methods
A literature search identified studies in which healthy subjects were evaluated using commercial immunoassays for PF4/heparin antibody (IgG/M/A). Proportions of test-positive subjects were calculated, by assay.
Results
Across 11 eligible studies, 860 healthy subjects were tested using the Stago enzyme-linked immunosorbent assay (ELISA) (nine studies), GTI ELISA (three studies), and/or DiaMed particle gel immunoassay (PGIA) (three studies). Seropositivity occurred in 17 of 790 (2.2%, 95% CI, 1.1–3.2%) subjects by Stago ELISA, one of 100 (1.0%, 95% CI, 0–3.0%) subjects by GTI ELISA, and three of 70 (4.3%, 95% CI, 0–9.0%) subjects by PGIA (P > 0.20). Of seven seropositive subjects tested further, none had platelet-activating antibodies.
Conclusion
Commercial immunoassays detect PF4/heparin antibody in 1.0–4.3% of healthy subjects. Because this “background” prevalence overlaps seropositivity rates in heparin-treated patients in various clinical settings, normality cut-offs may require refinement.
Keywords: Immunoassay, Platelet factor 4, Heparin, Antibody, Healthy volunteers, Heparin-induced thrombocytopenia
Introduction
Heparin induces a conformational change in platelet factor 4 (PF4) and exposes epitopes capable of inducing PF4/heparin antibodies [1]. PF4/heparin antibodies, particularly of the IgG isotype, mediate heparin-induced thrombocytopenia (HIT), a serious prothrombotic disorder associated with thrombocytopenia and thrombosis [2, 3]. PF4/heparin antibodies, however, also frequently develop in heparin-exposed patients in the absence of clinical HIT. Asymptomatic seroconversion is greatest in patients administered unfractionated heparin in various clinical settings (~8–50%) and is lesser with exposure to low molecular-weight heparins (~1–8%) or the synthetic pentasaccharide, fondaparinux (~1–3%) [4, 5]. Although recent studies suggest that isolated PF4/heparin antibodies confer an increased risk of thrombosis and/or mortality [6, 7], it remains uncertain if these antibodies serve as nonspecific inflammatory markers or exert pathogenic effects.
PF4/heparin antibodies have been described almost exclusively in the setting of heparin exposure. By possible exception, their presence was reported in a few patients with acute coronary syndrome without documented previous heparin exposure [8]; however, undocumented heparin exposure such as via line flushes could not be excluded. Also preliminary data are available for four patients who, in the absence of preceding heparin therapy, had PF4/heparin antibodies and a HIT-like illness [9]. While a few case reports suggest that crossreactive antibodies may occur in other autoimmune diseases, such as antiphospholipid antibody syndrome [10, 11], to date, there have been no other descriptions of “naturally occurring” PF4/heparin antibodies in the absence of heparin exposure.
The background prevalence of PF4/heparin antibodies in healthy subjects is presumed to be extremely low due to normality ranges established by the manufacturers of the commercial immunoassays. At least four commercial immunoassays have been developed for detecting human antibodies (IgG, IgA or IgM) to PF4/heparin. Two enzyme-linked immunosorbent assays (ELISAs), initially described, respectively, by Amiral and colleagues [12, 13] and by Visentin and colleagues [14, 15], differ most notably in the antigen coated on the microtiter plate, i.e., PF4 with heparin (Asserachrom® HPIA, Diagnostica Stago, Asnieres, France) or PF4 with polyvinyl sulfonate (PVS) (PF4 Enhanced®, GTI, Waukesha, WI, USA). The upper cut-off value for normality, as established by the manufacturer, is an optical density (OD) at 492 nm of ≥0.5 in the PF4/heparin ELISA and an OD at 405 nm of ≥0.4 in the PF4/PVS ELISA. Even with these normal ranges, positivity rates as high as 22% [16] and 30% [17] in healthy subjects have been reported using commercial ELISAs, suggesting that the recommended cut-offs may be too low. Other commercial immunoassays include a particle gel immunoassay (PGIA) (ID-PGIA heparin/PF4, DiaMed, Cressier, Switzerland), initially described by Meyer and colleagues [18], and a particle immunofiltration assay (PIFA) (PIFA Heparin/PF4 Rapid Assay, Akers Bioscience, Thorofare, NJ, USA). These rapid assays employ dyed particles coupled with PF4/heparin or PF4, respectively, and test positivity is based on visual detection of a color pattern consistent with particle agglutination by specific antibodies.
The purpose of this study was to estimate and compare the prevalence of PF4/heparin antibodies in a large sampling of healthy subjects based on literature reports of commercial immunoassays, each used according to its manufacturer’s instructions. Also we sought to characterize, when possible, individual positive responses. We report findings from a total of 11 studies in which a total of 860 healthy subjects were tested for PF4/heparin antibody by immunoassay.
Methods
Identification of literature set
A literature database search was performed in June 2007 using the PubMed database and limited to English language publications and human studies. Search term were “HIT antibodies” or “heparin-platelet factor 4” in combination with “healthy,” “normal,” “volunteers,” “incidence,” “prevalence,” “frequency,” or “laboratory.” The titles and abstracts, when available, of identified publications were screened to determine relevance to our study question. Relevant or possibly relevant articles were retrieved for in-depth review. Articles were eligible if they reported the prevalence in healthy volunteers of PF4/heparin antibodies, as assessed using a commercial immunoassay (ELISA, gel particle, or particle immunofiltration) according to the manufacturer’s instructions. Review articles lacking original data and papers reporting only functional or platelet activation assays were excluded. Additional search strategies included scanning the bibliographies of review articles on HIT or HIT laboratory testing, screening personal files, and querying assay manufacturers. Articles were included in the final literature set upon consensus agreement by the investigators.
Data analysis
From each article in the literature set, pertinent data including the study objective, assay(s) used, description of the healthy volunteers, specimen type, the numbers of tested and test-positive subjects, and details about positive responses were extracted, where available. The proportion (95% confidence interval, CI) of test-positive subjects was calculated by assay type, and comparisons were made using Fisher’s exact test. Statistical analyses were conducted using GraphPad Statistical Software (GraphPad Software, Inc., San Diego, CA), and significance was declared at P < 0.05.
Results
Literature data set
From the search strategies, 254 articles were identified for consideration. Most (n = 236) were excluded because of a lack of original data relevant to our study question. Seven articles were excluded because healthy volunteers were tested using a noncommercial (“in-house”) assay for PF4/heparin antibodies. In three of these seven articles, the described assay ultimately served as basis of a commercial assay [12, 13, 19]. The literature set comprised the remaining 11 articles [10, 16, 17, 20-27], each of which reported the prevalence of PF4/heparin antibodies in healthy subjects, as assessed by a commercial immunoassay according to manufacturer’s directions (Table 1).
Table 1.
Literature set
| First author, year | Study objective | Immunoassay(s) | Specimen | Description of healthy subjects |
|---|---|---|---|---|
| Arepally 1995 [20] | Comparison of PF4/heparin ELISA and serotonin release assay in diagnosis of HIT |
PF4/heparin ELISA | Plasma; stored at −70°C before use |
Healthy volunteers with no history of heparin exposure or thrombocytopenia |
| Bachelot-Loza 1998 [21] |
Evaluation of FcγRIIa polymorphism in HIT |
PF4/heparin ELISA | Plasma, citrated; stored at −80°C before use |
Healthy controls with neither history of heparin exposure or thrombocytopenia |
| Newman 1998 [16] | Evaluation of fluid phase enzyme immunoassay for PF4/heparin antibody |
PF4/heparin ELISA | Serum or plasma, citrated or ACD |
Healthy volunteers, “normals” |
| Walenga 1999 [22] | Evaluation of laboratory tests for diagnosis of HIT |
PF4/heparin ELISA | Serum | Normals who were not receiving heparin and had not received it during at least the past 6 months; platelet counts were normal |
| Woodhams 1999 [23] | Establishment of normality cut-off for PF4/heparin ELISA |
PF4/heparin ELISA | Not specifically stated (presumably serum or plasma) |
Normal volunteers |
| Eichler 2002 [17] | Comparison of PGIA with functional and antigenic tests for PF4/heparin antibody |
PF4/heparin ELISA | Serum; stored in aliquots at −30°C |
Healthy blood donors without any medication; 13 males, seven females; mean ± SD age of 30 ± 10 years |
| PF4/PVS ELISA | ||||
| PF4/heparin PGIA | ||||
| Tazzari 2002 [24] | Comparison of flow cytometry method with commercial assays for PF4/heparin antibody |
PF4/heparin ELISA | Serum | Healthy subjects/donors |
| PF4/PVS ELISA | ||||
| PF4/heparin PGIA | ||||
| Untch 2002 [25] | Determination of isotypes and functionality of PF4/heparin antibody in patients with suspected HIT |
PF4/PVS ELISA | Serum; stored at −80°C before use |
Normal healthy volunteers |
| de Larranaga 2002 [10] |
Evaluation of PF4/heparin-induced antibodies in patients with autoimmune or alloimmune antiphospholipid antibodies and in healthy controls |
PF4/heparin ELISA | Serum | Healthy, normal controls; 25 males, 15 females; mean (range) age of 39.6 (19–57) years |
| Lee 2003 [26] | Determination of prevalence of PF4/heparin antibody in patients on maintenance dialysis |
PF4/heparin ELISA | Serum | Healthy subjects; 20 men, 20 women; mean age 47.9 ± 13.5 years |
| Alberio 2003 [27] | Evaluation of PGIA in diagnosis of HIT |
PF4/heparin PGIA | Plasma, citrated; stored at −70°C before use |
Healthy people |
In the literature set (Table 1), the PF4/heparin ELISA was used in nine studies [10, 16, 17, 20-24, 26], the PF4/PVS ELISA in three studies [17, 24, 25], and the PF4/heparin PGIA in three studies [17, 24, 27]. No study used the PIFA. Two studies [17, 24], one of which was conducted in a blinded fashion [17], used the PGIA and both ELISAs. One article [23] described the process and data used by the manufacturer of the PF4/heparin ELISA to establish the normality cut-off. One study [16] presented results for the PF4/heparin ELISA using both the manufacturer’s cut-off and a different “in-house” cut-off; the results according to the manufacturer’s cut-off are reported herein. The specimen used were sera or plasma in the PF4/heparin ELISA and PGIA, and sera in the PF4/PVS ELISA.
The individual studies evaluated between 20 and 218 healthy subjects (Tables 1 and 2). Across studies, a total of 860 unique, healthy subjects were tested using 1 or more commercial assays, and the majority (n = 790) were tested using the PF4/heparin ELISA.
Table 2.
Test positivity in healthy subjects, by assay
| First author, year | Tested, N | Positive, n | % positive | 95% CI | Comment |
|---|---|---|---|---|---|
| PF4/heparin ELISA by Stago | |||||
| Arepally 1995 [20] | 77 | 1 | 1.3 | Mean OD492 0.19 ± 0.01 | |
| Bachelot-Loza 1998 [21] | 218 | 0 | 0 | Each subject: OD492<0.25 | |
| Newman 1998 [16] | 32 | 7 | 21.9 | Maximum OD490 ~1.1 | |
| Walenga 1999 [22] | 140 | 2 | 1.4 | OD492 not reported | |
| Woodhams 1999 [23] | 193 | 0 | 0 | OD492 0.01–0.38 | |
| Eichler 2002 [17] | 20 | 6 | 30.0 | Maximum OD492 ~0.75 | |
| Tazzari 2002 [24] | 30 | 0 | 0 | OD492 not reported | |
| de Larranaga 2002 [10] | 40 | 1 | 2.5 | OD492 not reported | |
| Lee 2003 [26] | 40 | 0 | 0 | OD492 not reported | |
| Overall | 790 | 17 | 2.2 | 1.1–3.2 | |
| PF4/PVS ELISA by GTI | |||||
| Eichler 2002 [17] | 20 | 1 | 5.0 | OD405 ~0.5 for single positive | |
| Untch 2002 [25] | 50 | 0 | 0 | Mean ± SEM OD410 ~0.28 ± 0.05 | |
| Tazzari 2002 [24] | 30 | 0 | 0 | OD405 not reported | |
| Overall | 100 | 1 | 1.0 | 0–3.0 | |
| PF4/heparin PGIA by DiaMed | |||||
| Eichler 2002 [17] | 20 | 0 | 0 | ||
| Tazzari 2002 [24] | 30 | 0 | 0 | ||
| Alberio 2003 [25] | 20 | 3 | 15.0 | Antibody titer of 1 for positives | |
| Overall | 70 | 3 | 4.3 | 0–9.0 |
Antibody prevalence and characterization of positive results
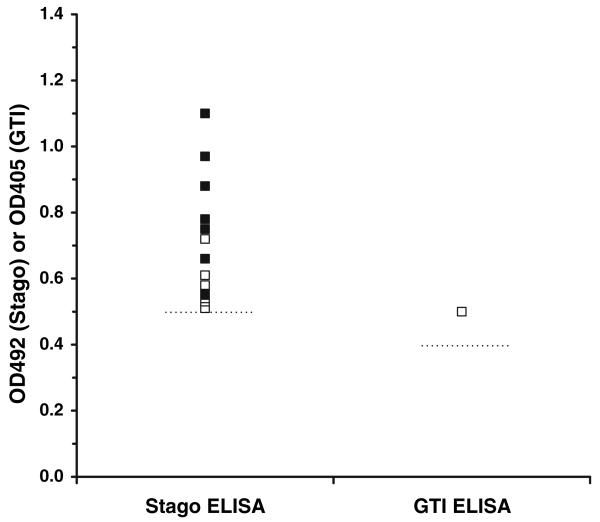
The PF4/heparin ELISA was positive, by separate study (nine studies), in 0–30% of healthy subjects, and overall in 17 of 790 (2.2%, 95% CI, 1.1–3.2%) subjects (Table 2). In 14 of 17 test-positive subjects, OD492 results were available and ranged from approximately 0.51–1.1, with a median value of 0.63 (Fig. 1). The platelet-activating ability of the antibody was reported in six test-positive subjects: none had a positive serotonin release or platelet aggregation test [17].
Fig. 1.
ELISA results for 15 seropositive healthy subjects with available data, as determined by the Stago PF4/heparin ELISA (n = 14) and GTI PF4/PVS ELISA (n = 1). Normality cut-off values for the respective ELISAs (OD492 ≥ 0.5 and OD405 ≥ 0.4) are shown as horizontal, dashed lines. OD values were extracted from published figures [16, 17, 20]. For the seven ELISA-positive subjects further tested for platelet-activating antibody (eachfound to be negative), OD values are shown as open squares
The PF4/PVS ELISA was positive by study (three studies) in 0–5% of healthy subjects, and overall in one of 100 (1.0%, 95% CI, 0–3.0%) subjects (Table 2). The single test-positive subject had an OD405 of approximately 0.5 (Fig. 1) and had negative serotonin release and platelet aggregation tests [17]. In one of the three studies, none of 50 subjects had a positive PF4/PVS ELISA or serotonin release assay, although in-house assays detected IgG antibody in two subjects and IgM antibody in 33 subjects [25].
The PF4/heparin PGIA was positive by study (three studies) in 0–15% of healthy subjects, and overall in three of 70 (4.3, 95% CI, 0–9.0%) subjects (Table 2). The three test-positive subjects each had an antibody titer (defined as the last positive detection followed by either borderline or negative results for undiluted and serially diluted plasma) of one [27]. The platelet-activating ability of these antibodies was not reported.
No difference in seropositivity among the methods was detected (P > 0.20).
Discussion
In this study, we estimated in a large sampling of healthy subjects (n = 860, across 11 studies) the prevalence of PF4/heparin antibody by three commercial immunoassays, i.e., the Stago PF4/heparin ELISA, GTI PF4/PVS ELISA, and DiaMed PGIA, and characterized, as possible, the positive responses. Each assay was performed according to the manufacturer’s instructions, including the use of the recommended specimen. Test results, at least for the PF4/PVS ELISA, are not appreciably affected by sample preparation or storage [28]. Our literature search identified no study that evaluated healthy subjects using the Akers PIFA. After our analysis was completed, however, a study was published that described “numerous” false-positive reactions with this assay in normal blood donors; the actual data were not reported [29]. Other study limitations include the substantially smaller numbers of subjects tested by the PF4/PVS ELISA (n = 100) and the PGIA (n = 70) as compared with the PF4/heparin ELISA (n = 790), and the inconsistent availability of certain data such as the platelet-activating, functional abilities of detected antibodies, the optical density values by ELISA, imprecision for the testing, and the age, sex, or other factors describing the study subjects.
We found that the seropositivity rate in healthy subjects was 2.2% (95% CI, 1.1–3.2%) by the PF4/heparin ELISA, 1.0% (95% CI, 0–3.0%) by the PF4/PVS ELISA, and 4.3% (95% CI, 0–9.0%) by the PGIA, without significant differences by method (P > 0.20). Positive results were typically, but not always, of low OD and close to the cut-off by ELISA or of the lowest titer (titer of 1) by PGIA, that is, they were “weak” positives. However, three of the 14 seropositive subjects by PF4/heparin ELISA with available data had OD492 values exceeding 0.8 (including one value > 1.0), higher positive values that are often seen in patients with HIT [13, 16, 17, 20]. An OD405 > 1.0 in the PF4/PVS ELISA has been associated with thrombotic predisposition [30], although the specific relationship between OD values from the different ELISAs is unclear. To what extent these high false-positive values are from crossreactive antibodies with other antigen specificities [10, 11] or nonspecific binding of IgM isotype is unknown, as these samples were not further characterized for antigen specificity or isotype. Of the seven seropositive subjects who also were tested using a functional assay, each had an ELISA OD value <0.8 and each lacked platelet-activating antibodies.
For the PF4/heparin ELISA, the normality cut-off was set at the 99th percentile of the log transformed OD data of 193 normal volunteers and 95 non-HIT patients [23]. Using this dataset, the manufacturer noted that the two highest OD values occurred in individuals with autoimmune disease (OD492 of 0.48) and thrombotic thrombocytopenic purpura (OD492 of 0.44), respectively. The data used to set the normality cut-off for the PF4/PVS ELISA are not published. However, according to GTI technical support (Michele Westlake, MT, ASCP, Technical Support Specialist, personal communication), results from 120 healthy individuals were analyzed for normality of the distribution of the OD values, and the cut-off was determined using calculations based on a nonparametric 95% reference interval with a 90% confidence. The modeling used for establishing these cut-offs would suggest that approximately 1% (PF4/heparin ELISA) to 5% (PF4/PVS ELISA) of non-HIT individuals, including healthy subjects, would be predicted to have a positive ELISA result. For the PGIA, the minimum detection limit is not reported. However, preliminary data (Dr. Ronald Orynich, DiaMed-North America, personal communication) suggest that the assay detects antibody when present at a level that would yield an OD405 of approximately 0.7 in the PF4/PVS ELISA.
Our analysis considered data from commercial immunoassays for PF4/heparin antibodies that detect IgG, IgM, and IgA classes. The clinical specificity of laboratory testing for HIT is enhanced by measurement of only the IgG class [31], and immunoassays specific for IgG antibodies have recently become commercially available. Overdiagnosis of HIT may occur if laboratory testing is done only using commercial immunoassays, without complementary assays for antibody function [32]. With the immunoassays, the actual OD or antibody titer is often more informative than a simple positive or negative result [33]. Patients, if tested over several days, often seroconvert from borderline negative results to borderline positive results [34].
Higher ELISA OD values are associated with increased thrombotic risk in patients with isolated HIT, with the risk being sixfold greater when the OD405 (PF4/PVS ELISA) is ≥1.0 versus 0.4–0.99 [30]. In the test-positive, healthy subjects in our study, antibodies of higher OD or titer were relatively infrequent (one of 15 seropositive subjects with available data had an OD > 1.0), and no functional antibodies were reported. This remains to be further investigated using commercial Ig class-specific assays. Also, the extent to which, if at all, seropositivity in healthy subjects with a normal platelet count is associated with increased thrombotic events or mortality, as has been previously demonstrated in certain patient populations [6, 7], remains to be determined.
One article in our literature set reported that in 50 healthy subjects who were seronegative by both PF4/PVS ELISA and serotonin release assay, in-house, Ig class-specific immunoassays detected IgG PF4/heparin antibodies in two subjects and IgM antibodies in 33 [25]. The authors speculated that this reflected a possible humoral response in the donors to the PF4/heparin complex, prior to exogenous heparin exposure. It is possible that individuals with chronic platelet activation and PF4 release, in the absence of heparin, could become sensitized and manifest naturally occurring PF4/heparin antibodies. Whether PF4/heparin antibodies can develop in such individuals or healthy subjects, as naturally occurring antibodies, remains to be shown. Normal human sera contains naturally occurring antibodies to a variety of self-antigens and are thought to contribute to self-tolerance through functional roles in clearance of antigens from damaged tissues or through neutralizing activities [35]. The relationship of naturally occurring antibodies to autoimmune disease is not clear and how naturally occurring antibodies influence the host response to environmental injury [36] remains to be defined.
Commercial immunoassays detect PF4/heparin antibody in 1.0–4.3% of healthy subjects. Because this “background” prevalence overlaps seropositivity rates with heparin-treated patients in various clinical settings, the normality cut-offs may require further refinement. A prospective study is warranted to better estimate seropositivity in a larger healthy population and help refine normality thresholds.
Acknowledgments
The authors have full control of all primary data and agree to allow the journal to review their data if requested. Supported by the National Institutes of Health HL081395 (GMA).
Footnotes
Disclosure GMA has received research support from Amgen, GlaxoSmithKline, and Progen Industries; Marcie J. Hursting has received consultancy fees from GlaxoSmithKline.
Frederick Spencer, MD served as the guest editor for the manuscript.
Contributor Information
Gowthami M. Arepally, Division of Hematology, Duke University Medical Center, DUMC Box 3486, Room 304 Sands Building, Research Drive, Durham, NC 27710, USA
Marcie J. Hursting, Clinical Science Consulting, Austin, TX, USA
References
- 1.Horsewood P, Warkentin TE, Hayward CPM, et al. The epitope specificity of heparin-induced thrombocytopenia. Br J Haematol. 1996;95:161–167. doi: 10.1046/j.1365-2141.1996.d01-1876.x. [DOI] [PubMed] [Google Scholar]
- 2.Jang IK, Hursting MJ. When heparins promote thrombosis: review of heparin-induced thrombocytopenia. Circulation. 2005;111:2671–2683. doi: 10.1161/CIRCULATIONAHA.104.518563. [DOI] [PubMed] [Google Scholar]
- 3.Arepally GM, Ortel TL. Heparin-induced thrombocytopenia. N Engl J Med. 2006;355:809–817. doi: 10.1056/NEJMcp052967. [DOI] [PubMed] [Google Scholar]
- 4.Warkentin TE, Sheppard JA, Horsewood P, et al. Impact of the patient population on the risk for heparin-induced thrombocytopenia. Blood. 2000;96:1703–1708. [PubMed] [Google Scholar]
- 5.Warkentin TE, Cook RJ, Marder VJ, et al. Anti-platelet factor 4/heparin antibodies in orthopedic surgery patients receiving antithrombotic prophylaxis with fondaparinux or enoxaparin. Blood. 2005;106:3791–3796. doi: 10.1182/blood-2005-05-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy JH, Hursting MJ. Heparin-induced thrombocytopenia, a prothrombotic disease. Hematol Oncol Clin N Am. 2007;21:65–88. doi: 10.1016/j.hoc.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Stribling WK, Slaughter TF, Houle TT, et al. Beyond the platelet count: heparin antibodies as independent risk predictors. Am Heart J. 2007;153:900–906. doi: 10.1016/j.ahj.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Matsuo T, Suzuki S, Matsuo M, et al. Preexisting antibodies to platelet factor 4-heparin complexes in patients with acute coronary syndrome who have no history of heparin exposure. Pathophysiol Haemost Thromb. 2005;34:18–22. doi: 10.1159/000088543. [DOI] [PubMed] [Google Scholar]
- 9.Warkentin TE, Jay RM, Makris M, et al. Platelet-activating anti-platelet factor 4/polyanion antibodies without preceding heparin therapy: a transient autoimmune disorder resembling heparin-induced thrombocytopenia (“spontaneous HIT”) Blood. 2006;108:311a–312a. [Google Scholar]
- 10.de Larranaga G, Martinuzzo M, Bocassi A, et al. Heparin-platelet factor 4 induced antibodies in patients with either autoimmune or alloimmune antiphospholipid antibodies. Thromb Haemost. 2002;88:371–373. [PubMed] [Google Scholar]
- 11.Martin-Toutain I, Piette JC, Diemert MC, et al. High prevalence of antibodies to platelet factor 4 heparin in patients with antiphospholipid antibodies in absence of heparin-induced thrombocytopenia. Lupus. 2007;16:79–83. doi: 10.1177/0961203306075562. [DOI] [PubMed] [Google Scholar]
- 12.Amiral J, Bridey F, Dreyfus M, et al. Platelet factor 4 complexed to heparin is the target for antibodies generated in heparin-induced thrombocytopenia. Thromb Haemost. 1992;68:95–96. [PubMed] [Google Scholar]
- 13.Amiral J, Bridey F, Wolf M, et al. Antibodies to macro-molecular platelet factor 4-heparin complexes in heparin-induced thrombocytopenia: a study of 44 cases. Thromb Haemost. 1995;73:21–28. [PubMed] [Google Scholar]
- 14.Visentin GP, Ford SE, Scott JP, et al. Antibodies from patients with heparin-induced thrombocytopenia/thrombosis are specific for platelet factor 4 complexed with heparin or bound to endothelial cells. J Clin Invest. 1994;93:81–88. doi: 10.1172/JCI116987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Visentin GP, Moghaddam M, Beery SE, et al. Heparin is not required for detection of antibodies associated with heparin-induced thrombocytopenia/thrombosis. J Lab Clin Med. 2001;138:22–31. doi: 10.1067/mlc.2001.115525. [DOI] [PubMed] [Google Scholar]
- 16.Newman PM, Swanson RL, Chong BH. Heparin-induced thrombocytopenia: IgG binding to PF4-heparin complexes in the fluid phase and cross-reactivity with low molecular weight heparin and heparinoid. Thromb Haemost. 1998;80:2892–2897. [PubMed] [Google Scholar]
- 17.Eichler P, Raschke R, Lubenow N, et al. The new ID-heparin/PF4 antibody test for rapid detection of heparin-induced antibodies in comparison with functional and antigenic assays. Br J Haematol. 2002;116:887–891. doi: 10.1046/j.0007-1048.2002.03363.x. [DOI] [PubMed] [Google Scholar]
- 18.Meyer O, Salama A, Pittet N, et al. Rapid detection of heparin-induced platelet antibodies with particle gel immunoassay (ID-HPF4) Lancet. 1999;354:1525–1526. doi: 10.1016/S0140-6736(99)03625-9. [DOI] [PubMed] [Google Scholar]
- 19.Amiral J, Marfaing-Koka A, Wolf M, et al. Presence of autoantibodies to interleukin-8 or neutrophil-activating peptide-2 in patients with heparin-associated thrombocytopenia. Blood. 1996;88:410–416. [PubMed] [Google Scholar]
- 20.Arepally G, Reynolds C, Tomaski A, et al. Comparison of PF4/heparin ELISA assay with the 14C-serotonin release assay in the diagnosis of heparin-induced thrombocytopenia. Am J Clin Pathol. 1995;104:648–654. doi: 10.1093/ajcp/104.6.648. [DOI] [PubMed] [Google Scholar]
- 21.Bachelot-Loza C, Saffroy R, Lasne D, et al. Importance of the FccRIIa-Arg/His-131 polymorphism in heparin-induced thrombocytopenia diagnosis. Thromb Haemost. 1998;79:523–528. [PubMed] [Google Scholar]
- 22.Walenga JM, Jeske WP, Fasanella AR, et al. Laboratory tests for the diagnosis of heparin-induced thrombocytopenia. Semin Thromb Hemost. 1999;25(Suppl 1):43–49. [PubMed] [Google Scholar]
- 23.Woodhams BJ, Grimaux M. Detection of PF4-heparin auto-antibodies by ELISA. Thromb Haemost. 1999;82:157–158. [PubMed] [Google Scholar]
- 24.Tazzari PL, Ricci F, Vitale M, et al. Heparin-induced thrombocytopenia: detection of antiheparin/PF4 antibodies by means of heparin/PF4-coated beads and flow cytometry. Transfus Med. 2002;12:193–198. doi: 10.1046/j.1365-3148.2002.00376.x. [DOI] [PubMed] [Google Scholar]
- 25.Untch B, Ahmad S, Jeske WP, et al. Prevalence, isotype, and functionality of antiheparin-platelet factor 4 antibodies in patients treated with heparin and clinically suspected for heparin-induced thrombocytopenia. The pathogenic role of IgG. Thromb Res. 2002;105:117–123. doi: 10.1016/s0049-3848(02)00004-x. [DOI] [PubMed] [Google Scholar]
- 26.Lee EY, Hwang KY, Yang JO, et al. Anti-heparin platelet factor 4 antibody is a risk factor for vascular access obstruction in patients undergoing hemodialysis. J Korean Med Sci. 2003;18:69–72. doi: 10.3346/jkms.2003.18.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alberio L, Kimmerle S, Baumann A, et al. Rapid determination of anti-heparin/platelet factor 4 antibody titers in the diagnosis of heparin-induced thrombocytopenia. Am J Med. 2003;114:528–536. doi: 10.1016/s0002-9343(03)00080-9. [DOI] [PubMed] [Google Scholar]
- 28.Krakow EF, Goudar R, Petzold E, et al. Influence of sample collection and storage on the detection of platelet factor 4-heparin antibodies. Am J Clin Pathol. 2007;128:150–156. doi: 10.1309/RFQK57F5QMURQ1HY. [DOI] [PubMed] [Google Scholar]
- 29.Warkentin TE, Sheppard JI, Raschke R, et al. Performance characteristics of a rapid assay for anti-PF4/heparin antibodies, the particle immunofiltration assay. J Thromb Haemost. 2007;5:2308–2310. doi: 10.1111/j.1538-7836.2007.02723.x. [DOI] [PubMed] [Google Scholar]
- 30.Zwicker JI, Uhl L, Huang WY, Shaz BH, et al. Thrombosis and ELISA optical density values in hospitalized patients with heparin-induced thrombocytopenia. J Thromb Haemost. 2004;2:2133–2137. doi: 10.1111/j.1538-7836.2004.01039.x. [DOI] [PubMed] [Google Scholar]
- 31.Warkentin TE, Sheppard JA, Moore JC, et al. Laboratory testing for the antibodies that cause heparin-induced thrombocytopenia: how much class do we need? J Lab Clin Med. 2005;146:341–346. doi: 10.1016/j.lab.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Lo GK, Sigouin CS, Warkentin TE. What is the potential for overdiagnosis of heparin-induced thrombocytopenia? Am J Hematol. 2007;82:1037–1043. doi: 10.1002/ajh.21032. [DOI] [PubMed] [Google Scholar]
- 33.Keeling D, Davidson S, Watson H. The management of heparin-induced thrombocytopenia. Br J Haematol. 2006;133:259–269. doi: 10.1111/j.1365-2141.2006.06018.x. [DOI] [PubMed] [Google Scholar]
- 34.Refaai MA, Laposata M, Van Cott EM. Clinical significance of a borderline titer in a negative ELISA for heparin-induced thrombocytopenia. Am J Clin Pathol. 2003;119:61–65. doi: 10.1309/6922-EWGP-HVDX-9EQJ. [DOI] [PubMed] [Google Scholar]
- 35.Guilbert B, Dighiero G, Avrameas S. Naturally occurring antibodies against nine common antigens in human sera. I. Detection, isolation and characterization. J Immunol. 1982;128:2779–2787. [PubMed] [Google Scholar]
- 36.Palinski W, Witztum JL. Immune responses to oxidative neoepitopes on LDL and phospholipids modulate the development of atherosclerosis. J Intern Med. 2000;247:371–380. doi: 10.1046/j.1365-2796.2000.00656.x. [DOI] [PubMed] [Google Scholar]