Abstract
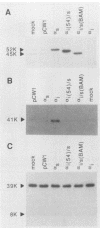
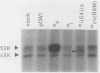
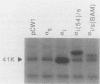
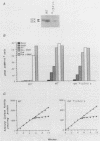
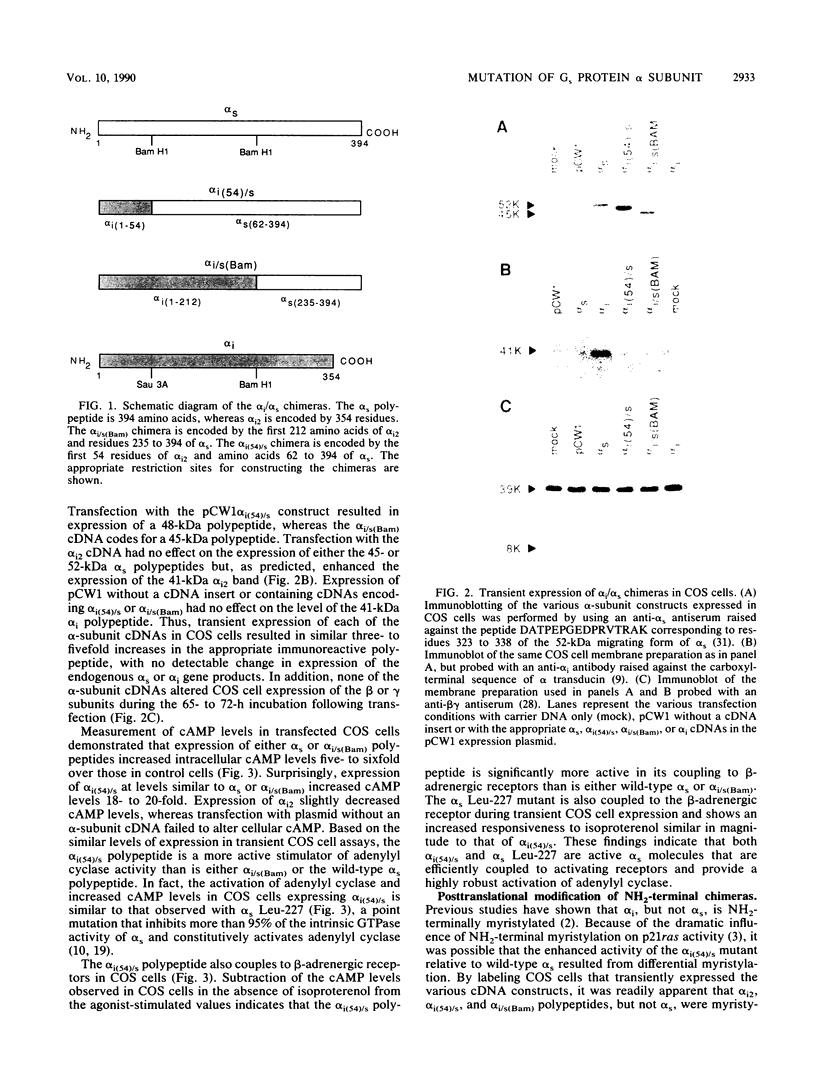
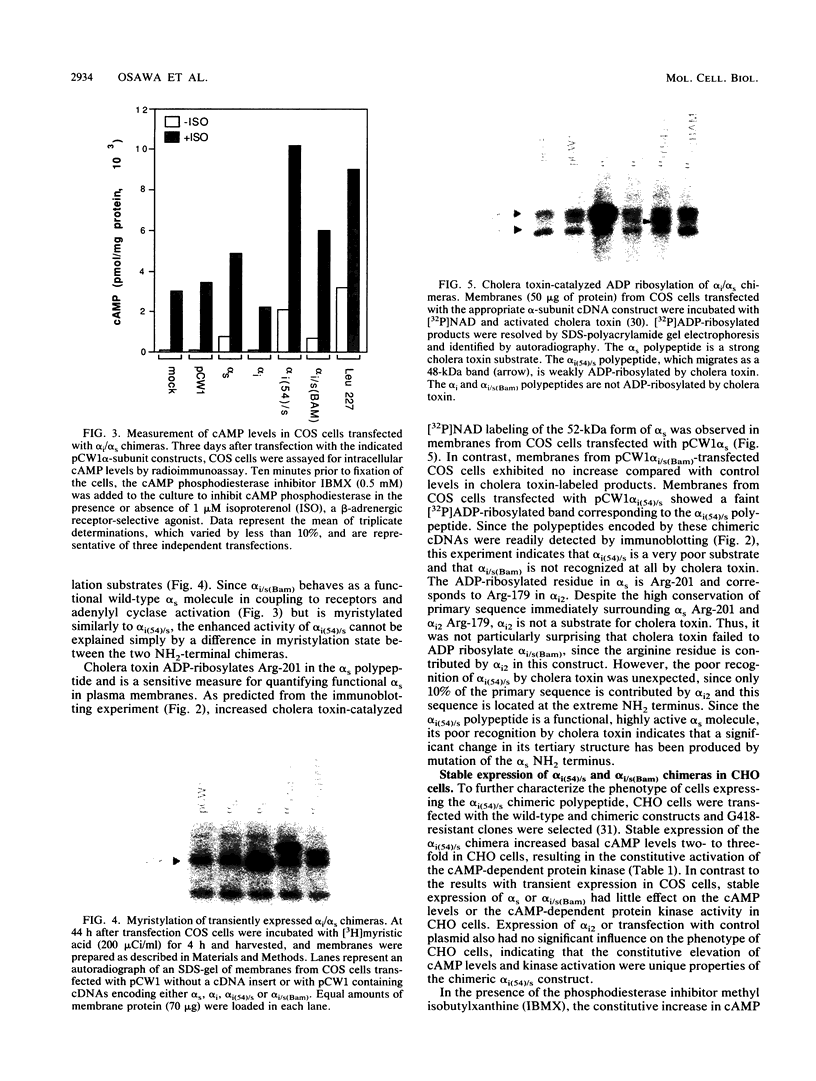
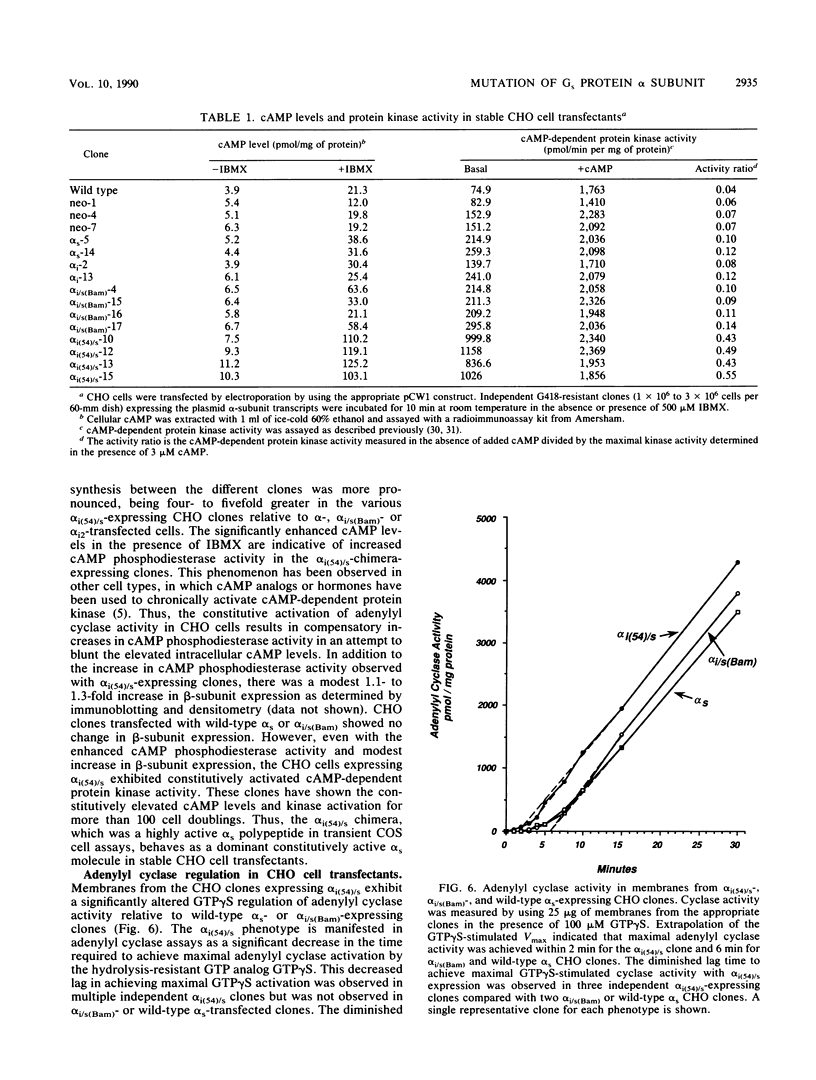
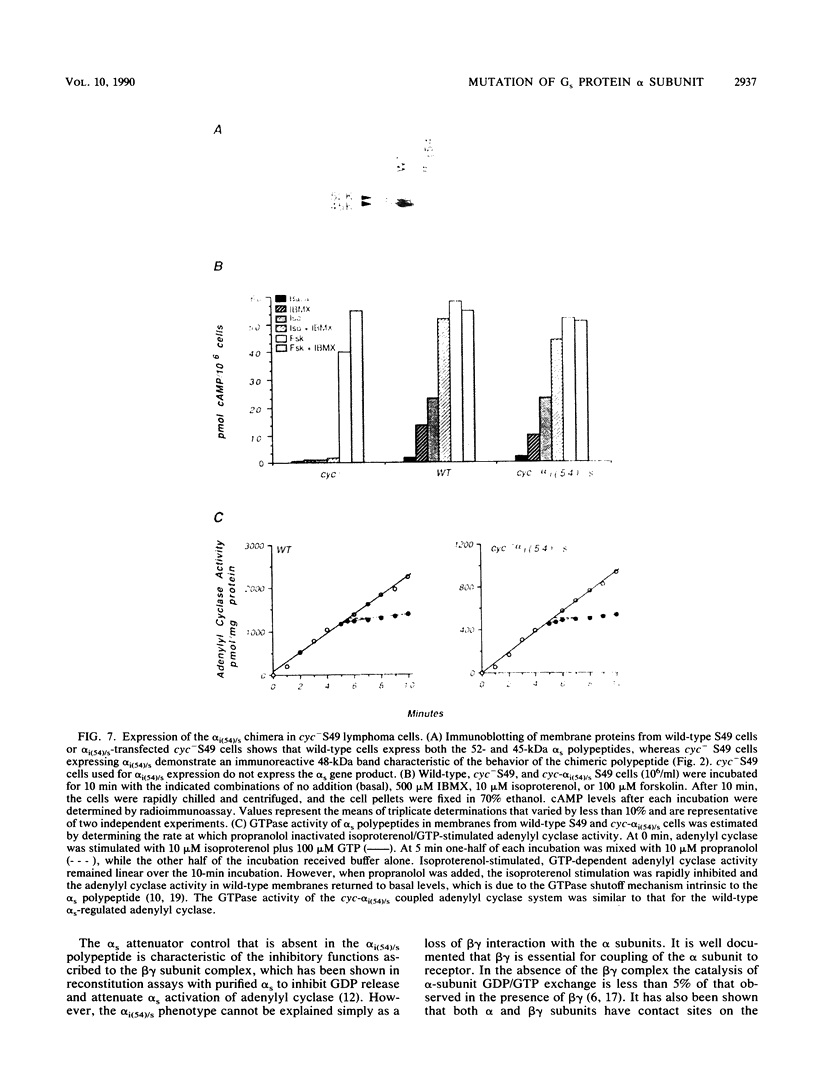
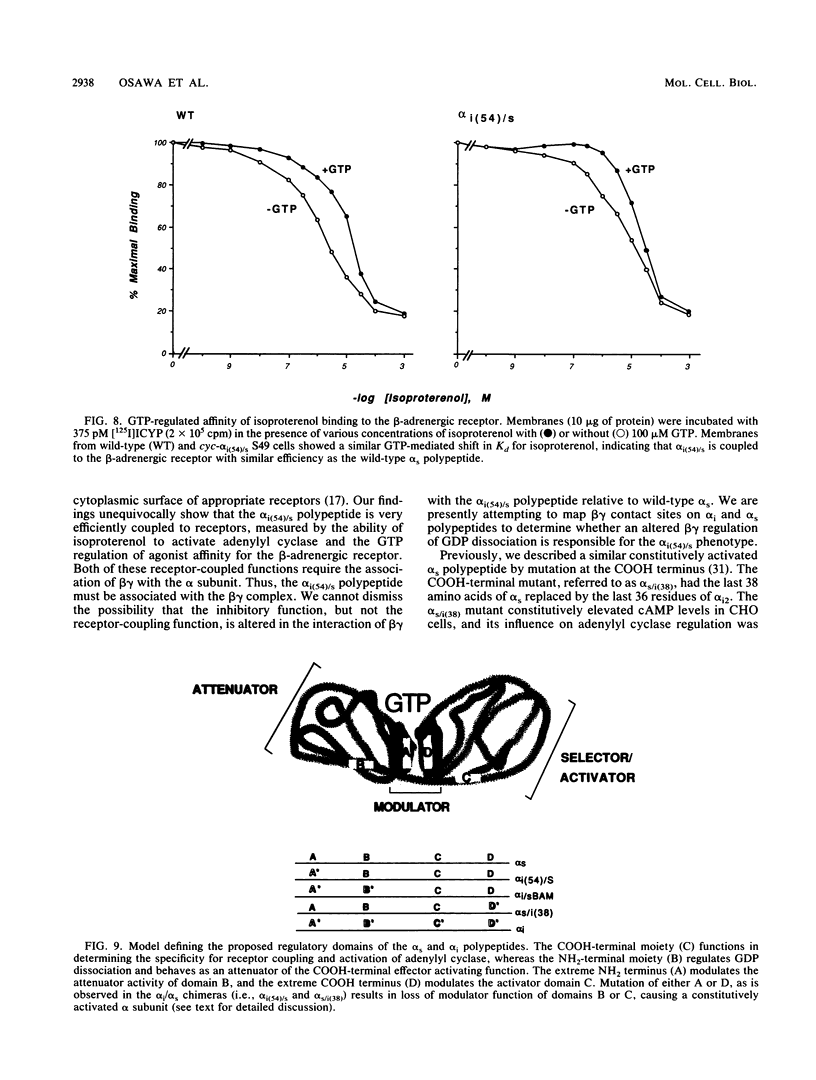
G-proteins couple hormonal activation of receptors to the regulation of specific enzymes and ion channels. Gs and Gi are G-proteins which regulate the stimulation and inhibition, respectively, of adenylyl cyclase. We have constructed two chimeric cDNAs in which different lengths of the alpha subunit of Gs (alpha s) have been replaced with the corresponding sequence of the Gi alpha subunit (alpha i2). One chimera, referred to as alpha i(54)/s' replaces the NH2-terminal 61 amino acids of alpha s with the first 54 residues of alpha i. Within this sequence there are 7 residues unique to alpha s, and 16 of the remaining 54 amino acids are nonhomologous between alpha i and alpha s. The second chimera, referred to as alpha i/s(Bam), replaces the first 234 amino acids of alpha s with the corresponding 212 residues of alpha i. Transient expression of alpha i(54)/s in COS-1 cells resulted in an 18- to 20-fold increase in cyclic AMP (cAMP) levels, whereas expression of either alpha i/s(Bam) or the wild-type alpha s polypeptide resulted in only a 5- to 6-fold increase in cellular cAMP levels. COS-1 cells transfected with alpha i showed a small decrease in cAMP levels. Stable expression of the chimeric alpha i(54)/s polypeptide in Chinese hamster ovary (CHO) cells constitutively increased both cAMP synthesis and cAMP-dependent protein kinase activity. CHO clones expressing transfected alpha i/s(Bam) or the wild-type alpha s and alpha i cDNAs exhibited cAMP levels and cAMP-dependent protein kinase activities similar to those in control CHO cells. Therefore, the alpha i(54)/s chimera behaves as a constitutively active alpha s polypeptide, whereas the alpha i/s(Bam) polypeptide is regulated similarly to wild-type alpha s. Expression in cyc-S49 cells, which lack expression of wild-type alpha s, confirmed that the alpha i(54)/s polypeptide is a highly active alpha s molecule whose robust activity is independent of any change in intrinsic GTPase activity. The difference in phenotypes observed upon expression of alpha i(54)/s or alpha i/s(Bam) indicates that the NH2-terminal moieties of alpha s and alpha i function as attenuators of the effector enzyme activator domain which is within the COOH-terminal half of the alpha subunit. Mutation at the NH2 terminus of alpha s relieves the attenuator control of the Gs protein and results in a dominant active G-protein mutant.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buss J. E., Mumby S. M., Casey P. J., Gilman A. G., Sefton B. M. Myristoylated alpha subunits of guanine nucleotide-binding regulatory proteins. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7493–7497. doi: 10.1073/pnas.84.21.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss J. E., Solski P. A., Schaeffer J. P., MacDonald M. J., Der C. J. Activation of the cellular proto-oncogene product p21Ras by addition of a myristylation signal. Science. 1989 Mar 24;243(4898):1600–1603. doi: 10.1126/science.2648572. [DOI] [PubMed] [Google Scholar]
- Cerione R. A., Staniszewski C., Gierschik P., Codina J., Somers R. L., Birnbaumer L., Spiegel A. M., Caron M. G., Lefkowitz R. J. Mechanism of guanine nucleotide regulatory protein-mediated inhibition of adenylate cyclase. Studies with isolated subunits of transducin in a reconstituted system. J Biol Chem. 1986 Jul 15;261(20):9514–9520. [PubMed] [Google Scholar]
- Coffino P., Bourne H. R., Insel P. A., Melmon K. L., Johnson G., Vigne J. Studies of cyclic AMP action using mutant tissue culture cells. In Vitro. 1978 Jan;14(1):140–145. doi: 10.1007/BF02618180. [DOI] [PubMed] [Google Scholar]
- Fung B. K. Characterization of transducin from bovine retinal rod outer segments. I. Separation and reconstitution of the subunits. J Biol Chem. 1983 Sep 10;258(17):10495–10502. [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Goldsmith P., Gierschik P., Milligan G., Unson C. G., Vinitsky R., Malech H. L., Spiegel A. M. Antibodies directed against synthetic peptides distinguish between GTP-binding proteins in neutrophil and brain. J Biol Chem. 1987 Oct 25;262(30):14683–14688. [PubMed] [Google Scholar]
- Graziano M. P., Gilman A. G. Synthesis in Escherichia coli of GTPase-deficient mutants of Gs alpha. J Biol Chem. 1989 Sep 15;264(26):15475–15482. [PubMed] [Google Scholar]
- Harris B. A., Robishaw J. D., Mumby S. M., Gilman A. G. Molecular cloning of complementary DNA for the alpha subunit of the G protein that stimulates adenylate cyclase. Science. 1985 Sep 20;229(4719):1274–1277. doi: 10.1126/science.3839937. [DOI] [PubMed] [Google Scholar]
- Higashijima T., Ferguson K. M., Sternweis P. C., Smigel M. D., Gilman A. G. Effects of Mg2+ and the beta gamma-subunit complex on the interactions of guanine nucleotides with G proteins. J Biol Chem. 1987 Jan 15;262(2):762–766. [PubMed] [Google Scholar]
- Hingorani V. N., Ho Y. K. Fluorescent labeling of signal-transducing G-proteins. Pertussis toxin-catalyzed etheno-ADP ribosylation of transducin. J Biol Chem. 1988 Dec 25;263(36):19804–19808. [PubMed] [Google Scholar]
- Holbrook S. R., Kim S. H. Molecular model of the G protein alpha subunit based on the crystal structure of the HRAS protein. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1751–1755. doi: 10.1073/pnas.86.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House C., Kemp B. E. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987 Dec 18;238(4834):1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- Jones D. T., Reed R. R. Molecular cloning of five GTP-binding protein cDNA species from rat olfactory neuroepithelium. J Biol Chem. 1987 Oct 15;262(29):14241–14249. [PubMed] [Google Scholar]
- Kelleher D. J., Johnson G. L. Transducin inhibition of light-dependent rhodopsin phosphorylation: evidence for beta gamma subunit interaction with rhodopsin. Mol Pharmacol. 1988 Oct;34(4):452–460. [PubMed] [Google Scholar]
- Kelleher D. J., Rashidbaigi A., Ruoho A. E., Johnson G. L. Rapid vesicle reconstitution of alprenolol-Sepharose-purified beta 1-adrenergic receptors. Interaction of the purified receptor with N. J Biol Chem. 1983 Nov 10;258(21):12881–12885. [PubMed] [Google Scholar]
- Masters S. B., Miller R. T., Chi M. H., Chang F. H., Beiderman B., Lopez N. G., Bourne H. R. Mutations in the GTP-binding site of GS alpha alter stimulation of adenylyl cyclase. J Biol Chem. 1989 Sep 15;264(26):15467–15474. [PubMed] [Google Scholar]
- Masters S. B., Sullivan K. A., Miller R. T., Beiderman B., Lopez N. G., Ramachandran J., Bourne H. R. Carboxyl terminal domain of Gs alpha specifies coupling of receptors to stimulation of adenylyl cyclase. Science. 1988 Jul 22;241(4864):448–451. doi: 10.1126/science.2899356. [DOI] [PubMed] [Google Scholar]
- Mattera R., Graziano M. P., Yatani A., Zhou Z., Graf R., Codina J., Birnbaumer L., Gilman A. G., Brown A. M. Splice variants of the alpha subunit of the G protein Gs activate both adenylyl cyclase and calcium channels. Science. 1989 Feb 10;243(4892):804–807. doi: 10.1126/science.2536957. [DOI] [PubMed] [Google Scholar]
- Miller R. T., Masters S. B., Sullivan K. A., Beiderman B., Bourne H. R. A mutation that prevents GTP-dependent activation of the alpha chain of Gs. Nature. 1988 Aug 25;334(6184):712–715. doi: 10.1038/334712a0. [DOI] [PubMed] [Google Scholar]
- Pai E. F., Kabsch W., Krengel U., Holmes K. C., John J., Wittinghofer A. Structure of the guanine-nucleotide-binding domain of the Ha-ras oncogene product p21 in the triphosphate conformation. Nature. 1989 Sep 21;341(6239):209–214. doi: 10.1038/341209a0. [DOI] [PubMed] [Google Scholar]
- Picard D., Salser S. J., Yamamoto K. R. A movable and regulable inactivation function within the steroid binding domain of the glucocorticoid receptor. Cell. 1988 Sep 23;54(7):1073–1080. doi: 10.1016/0092-8674(88)90122-5. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Maguire M. E., Sturgill T. W., Biltonen R. L., Gilman A. G. Relationship between the beta-adrenergic receptor and adenylate cyclase. J Biol Chem. 1977 Aug 25;252(16):5761–5775. [PubMed] [Google Scholar]
- Spandidos D. A., Wilkie N. M. Malignant transformation of early passage rodent cells by a single mutated human oncogene. Nature. 1984 Aug 9;310(5977):469–475. doi: 10.1038/310469a0. [DOI] [PubMed] [Google Scholar]
- Wessling-Resnick M., Johnson G. L. Evidence for oligomeric forms of transducins alpha subunit: formation of intermolecular alpha-alpha disulfide linkages. Biochem Biophys Res Commun. 1989 Mar 15;159(2):651–657. doi: 10.1016/0006-291x(89)90044-2. [DOI] [PubMed] [Google Scholar]
- Woon C. W., Heasley L., Osawa S., Johnson G. L. Mutation of glycine 49 to valine in the alpha subunit of GS results in the constitutive elevation of cyclic AMP synthesis. Biochemistry. 1989 May 30;28(11):4547–4551. doi: 10.1021/bi00437a006. [DOI] [PubMed] [Google Scholar]
- Woon C. W., Soparkar S., Heasley L., Johnson G. L. Expression of a G alpha s/G alpha i chimera that constitutively activates cyclic AMP synthesis. J Biol Chem. 1989 Apr 5;264(10):5687–5693. [PubMed] [Google Scholar]