Abstract
We hypothesized that custom-designed microemulsions would effectively scavenge compounds from bulk media. Pluronic-based oil-in-water microemulsions were synthesized that efficiently reduced the free concentration of the local anesthetic bupivacaine in 0.9% NaCl. Both the molecular nature and concentration of the constituents in the microemulsions significantly affected extraction efficiencies. Pluronic F127-based microemulsions extracted bupivacaine more efficiently than microemulsions synthesized using other Pluronic surfactants (L44, L62, L64, F77, F87, F88, P104). Extraction was markedly increased by addition of fatty acid sodium salts due to greater oil/water interface area, increased columbic interaction between bupivacaine and fatty acids sodium salt, and greater surface activity. These data suggest that oil-in-water microemulsions may be an effective agent to treat cardiotoxicity caused by bupivacaine or other lipophilic drugs.
Introduction
Drug toxicity in humans due to illicit drug use, suicide attempts, or iatrogenic complication represents a major health care problem, both in terms of morbidity and economic cost.1 Unfortunately, most life-threatening drug intoxications do not have specific pharmacological antidotes such as receptor antagonists (e.g., naloxone) or immuno-toxicotherapeutic agents (e.g., fragmented antibody binding digitalis glycosides) to attenuate adverse effects caused by overdose. Therefore, an unmet need exists to develop alternative treatment modalities whereby the acute effects of toxic concentrations of drugs can be reduced. For example, bupivacaine is an amide class local anesthetic commonly used in clinical medicine to provide local or regional anesthesia during surgical procedures. Since this drug’s introduction for human use, many cases of accidental overdose with cardiac arrest and death have been reported.2 Although general supportive therapy (e.g., cardiopulmonary resuscitation, intravenous catecholamines) to support homeostasis can be used to treat bupivacaine intoxication, a specific therapy aimed at reducing free bupivacaine concentrations in the body would be a valuable addition to the clinical armamentarium.
Like most drugs, bupivacaine exists in equilibrium between ionized and un-ionized (i.e., base) forms according to its pKa and environmental pH, as defined by the Henderson–Hasselbalch equation. Approximately 17% of bupivacaine molecules exist in an uncharged lipophilic base form, assuming a physiological pH of 7.40 and a pKa of 8.1.3 This base form is vulnerable to sequestration in lipophilic droplets such as Intralipid, a commercially available macroemulsion employed for intravenous nutrition. In fact, Weinberg and collaborators previously demonstrated that Intralipid treatment after bupivacaine intoxication increased survival in both rats and dogs by segregating bupivacaine molecules from plasma into the macroemulsion lipid droplets with an overall ratio of 1:12.4,5 Given these results and modern advances in nanotechnology, we hypothesized that reductions in particle size to nanometer dimension will provide greater efficacy to this approach for the mass extraction of bupivacaine from bulk media (e.g., saline) due to a substantial increase in the aggregated interfacial area of particles.
One potentially suitable engineered system with nanometer-scale particle size is the microemulsion, a system of water, oil, and amphiphiles that is characterized as an optically isotropic and thermodynamically stable liquid solution.6–8 More specifically, an oil-in-water microemulsion exists if a small amount of oil is dispersed in a large amount of water in the presence of a surfactant. These nanometer dimension droplets have attracted considerable interest as potential delivery vehicles for drugs with poor aqueous solubility.9–11 Several favorable properties of microemulsion such as transparency, straightforward preparation, and easy sterilization lead to continued investigation of pharmaceutical uses.12–18 In addition, the nanometer size of microemulsion droplets provides a relatively high interfacial area and low interfacial energy for drug absorption. In this study, amphiphilic block copolymers (i.e., Pluronic surfactants) were selected to synthesize microemulsions for two reasons. First, these surfactants allow for diverse interfacial properties favorable to our objective, separation of bupivacaine from bulk media.19–21 Second, Pluronic-based microemulsion has been previously administered to human patients in large doses without apparent ill effect. For example, purified poloxamer 188 (Pluronic F-68) was administered to human subjects with sickle cell disease and chest crisis.22 Although not efficacious to reduce the symptoms and duration of chest crisis, the surfactant caused minimal-to-no apparent adverse drug reactions in these patients. In this investigation, we developed several oil-in-water microemulsions using pharmaceutically accepted excipients for the extraction of bupivacaine. We then identified a Pluronic-based oil-in-water microemulsion that was suitable for extraction of bupivacaine from a normal saline medium and optimized its composition for maximal extraction efficiency.
Materials and Methods
Chemicals and Supplies
Pluronic surfactants were obtained from BASF Inc. (Mount Olive, NJ). Pluronic was used as a nonionic surfactant composed of a symmetric triblock copolymer of propylene oxide (PO) and ethylene oxide (EO). The polypropylene oxide block was sandwiched between the more hydrophilic poly(ethylene oxide) blocks. The block copolymer was denoted by (EO)x(PO)y(EO)x, where x and y are the number of units of EO and PO, respectively. Being amphiphilic in nature, Pluronic aggregated in aqueous solution and formed micelles.23–26 Depending on the number of EO and PO units, various types of Pluronics are commercially available with molecular weights ranging between 1100–14 600 and with the weight fraction of the hydrophilic polyethylene block ranging between 0.1 and 0.8.
Bupivacaine hydrochloride (Figure 1), sodium caprylate, sodium laurate, sodium myristate, and sodium stearate were purchased from the Sigma Chemical Co. (St. Louis, MO). Sodium phosphate dibasic, sodium phosphate monobasic, sodium chloride, and perchloric acid was purchased from Fisher Scientific Inc. (Suwanee, GA). Ethyl butyrate and HPLC grade acetonitrile were acquired from Aldrich Chemical Co. (Milwaukee, WI). Deionized water was treated using a water purification system (Nanopure Ultrapure Water, Millipore Corporation, Bedford, MA) to generate pure water with a minimal resistivity of 18.2–18.3 MΔ. Micropartition tubes (Amicon Centrifree) with a 30 000 Da molecular weight cutoff were purchased from the Millipore Corporation.
Figure 1.
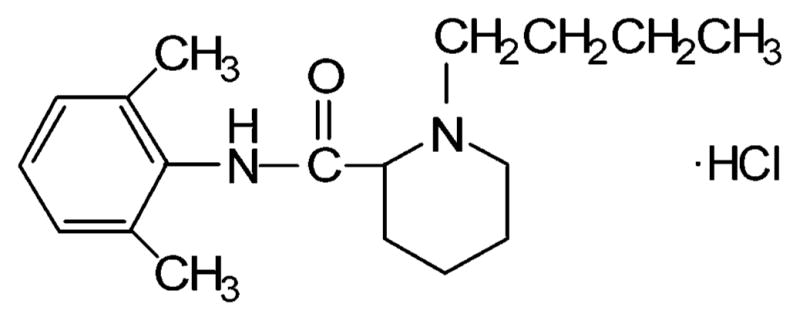
Bupivacaine hydrochloride (M. W. 324.9).
Syntheses of Oil-in-Water Pluronic Microemulsions
Oil-in-water microemulsions were prepared as 1% w/w solutions of ethyl butyrate by dissolving appropriate amounts of Pluronic, sodium salt of fatty acids, and ethyl butyrate into normal saline. The addition of ethyl butyrate caused slight turbidity in the solution that was resolved with magnetic stirring to form a microemulsion. The sodium salt of fatty acids was used to facilitate the microemulsion formation. It should be noted that, in the present study, no distinction was made between a microemulsion and a swollen micelle. The amount of oil (i.e., ethyl butyrate) was expressed as a molar ratio of the oil to the Pluronic content (i.e., Ow = [ethyl butyrate]/[Pluronic]).
Particle Size Measurement
Particle size distribution of the microemulsion was measured by the dynamic light scattering method using a submicrometer particle size analyzer (90Plus, Brookhaven Instruments Corp., Holtsville, NY). This instrument measures particle sizes ranging from approximately 2–3000 nm in any liquid. In these measurements, random intensity fluctuations arising from the Brownian motion of particles were analyzed using photon correlation spectroscopy (PCS), at a 90° scattering angle. The associated software performs statistical analysis of data and calculates mean particle size (e.g., effective diameter). After microemulsion synthesis as described above, samples were filtered through a 0.22-μm syringe filter before measurement of particle size for each sample. Measurements were conducted at room temperature (23 °C) in triplicate.
Bupivacaine Extraction and Ultrafiltration
To determine bupivacaine extraction, concentrated microemulsion was added to 1 mL normal saline containing 1 mM bupivacaine. These samples were transferred to ultrafiltration devices (Centricon YM-30, Millipore Corp.), placed in a tube rack, and shaken for 15 min at room temperature (22–24 °C). Subsequently, samples were capped and immediately transferred to a centrifuge (Marathon 8K, Fisher Scientific, Pittsburgh, PA). The ultrafiltration devices were centrifuged at 1800g for 30 min at room temperature (22–24 °C, pH = 7.4). Thereafter, the ultrafiltrate from each collection tube was transferred to autosampler vials for high performance liquid chromatography (HPLC) analysis.
HPLC
Bupivacaine concentration was measured using a modern HPLC system (Alliance, Waters Inc., Milliford, MA) consisting of a 2695 separation module with online degassing, automated injection, and a sampling system. This device was coupled to a photodiode array detector (996, Waters Inc.). Samples were run on a prepacked C18 column, 4.6 mm × 250 mm (5-μm particle size, Waters Inc.). A 10-min isocratic elution with a mobile phase consisting of acetonitrile and phosphate buffer (pH 3.5, 50 mM) in a ratio of 70:30 v/v was employed. The flow rate was 1.5 mL/min with UV detection at 210 nm. Chromatographic data were processed using commercially available software (Millennium 32 v3.2, Waters Inc.) installed on an IBM-compatible personal computer (Dell Computer Corporation, Round Rock, TX). All data points were determined in quadruplicate.
Results and Discussion
Microemulsion Synthesis and Bupivacaine Extraction
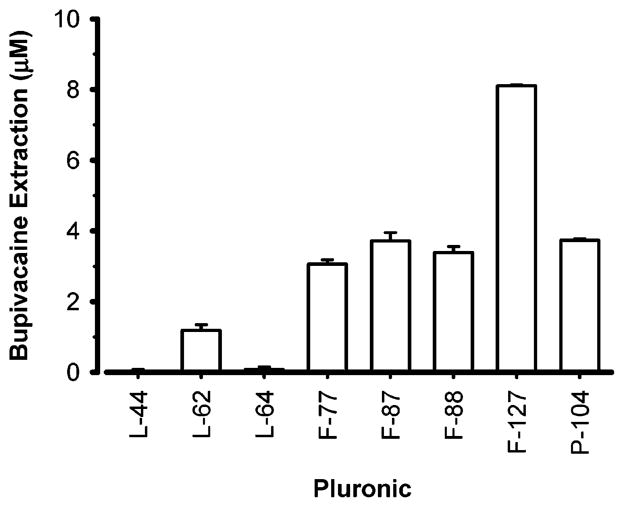
Several microemulsions were developed by using different types and concentrations of Pluronic surfactants and by varying the sodium caprylate surfactant concentration, as shown in Table 1. The concentration of ethyl butyrate remained constant at 189 mM so that the extraction efficiency of bupivacaine caused by each microemulsion could be subsequently compared without the confounding effect of fluctuating oil concentrations. These syntheses yielded microemulsions with particle sizes that ranged between 11.2 and 38.9 nm. These sizes were probably dependent on both the aggregation pattern of the micelles and the quantity of ethylene oxide (EO) block units found in a given Pluronic molecule. The particle size less than 60 nm of these microemulsions will be an advantage in pharmaceutical applications as it will reduce uptake by the reticuloendothelial system in vivo and thereby extend the life of such particles in the circulation.27 After synthesis, the ability of these microemulsions to extract bupivacaine from normal saline bulk media was determined (Figure 2). The Pluronic F127 microemulsion caused significantly greater bupivacaine extraction from the bulk media than all other microemulsions. In addition, the greater extraction of bupivacaine caused by the F127 microemulsion occurred even though the F127 microemulsion had an approximately 3- to 5-fold lower concentration of the Pluronic surfactant than the other microemulsions (Table 1). Most likely, this greater extraction of bupivacaine by the Pluronic F127 microemulsion can be attributed to the higher molecular weight of the hydrophobe (PO) as well as a larger number of EO units compared to the other Pluronic microemulsions. The differential extraction of bupivacaine by these various microemulsions despite a constant concentration of oil, points to an important role of the water/oil interface and its constituents in drug extraction, whereas the role of the oil core appears limited.
Table 1.
Mean Particle Diameter (Mean ± Standard Error of the Mean) and Composition of Various Oil-in-Water Microemulsions at Room Temperaturea
| Pluronic type | structure | Pluronic (M) | sodium caprylate (M) | particle size (nm) | polydispersity index |
|---|---|---|---|---|---|
| L44 | EO11PO20EO11 | 0.046 | 0.075 | 38.9 ± 0.94 | 0.124 |
| L62 | EO6PO35EO6 | 0.023 | 0.076 | 19.1 ± 1.2 | 0.133 |
| L64 | EO13PO30EO13 | 0.023 | 0.075 | 20.2 ± 1.5 | 0.139 |
| F77 | EO53PO38EO53 | 0.023 | 0.105 | 27.0 ± 6.7 | 0.367 |
| F87 | EO59PO43EO59 | 0.023 | 0.151 | 15.2 ± 1.3 | 0.322 |
| F88 | EO104PO39EO104 | 0.023 | 0.135 | 11.2 ± 3.8 | 0.355 |
| F127 | EO100PO64EO100 | 0.009 | 0.018 | 23.6 ± 2.5 | 0.362 |
| P104 | EO27PO61EO27 | 0.022 | 0.045 | 17.7 ± 1.1 | 0.205 |
The concentration of ethyl butyrate was held constant at 0.189 M. The bulk media was normal saline (0.9% NaCl).
Figure 2.
Varying the type of Pluronic changes the extraction of bupivacaine from normal saline caused by microemulsions of equal oil content. Concentrated microemulsion (10 μL) was added to 1 mL of 1 mM bupivacaine as indicated in the Materials and Methods section. Details on the composition of the microemulsions are noted in Table 1. Data expressed as mean ± standard error of the mean of four experiments per Pluronic surfactant.
Optimization of Microemulsion Constituents
Because the F127 microemulsion demonstrated superior bupivacaine extraction compared to other microemulsions, we selected it for further study. Specifically, the modulating effect of varying the concentration and/or nature of three microemulsion components on bupivacaine extraction efficiency were studied: (1) Pluronic surfactant, (2) ethyl butyrate oil core, and (3) fatty acid chain length and concentration. In doing so, we hoped to determine the optimal composition of a Pluronic microemulsion for maximal extraction of bupivacaine from normal saline media.
Pluronic Concentration
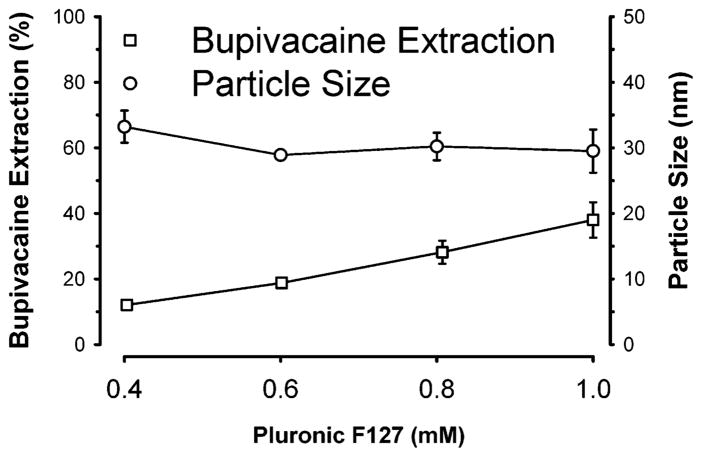
As illustrated in Figure 3, the extraction of bupivacaine from normal saline increased linearly with greater concentrations of surfactant (0.4–1.0 mM) in a two-component microemulsion that consist of F127 and ethyl butyrate. In contrast, the mean particle size (28.9–33.2 nm) did not significantly change over the same concentration range of surfactant. This observation suggests that the microemulsion droplets became more numerous but remained a constant size. In contrast, if greater Pluronic concentrations caused micelles to further swell without becoming more populous, then one would expect a progressively greater mean particle size with increasing Pluronic concentrations. These data suggest that increased Pluronic concentrations generated greater populations of swollen micelles with a resultant increase in the total interfacial area of the microemulsion and a superior bupivacaine extraction profile. Concentrations of Pluronic F127 surfactant in excess of 12 mM at room temperature caused the concentrated microemulsion to form a gel, a physical state unsuitable for intravenous injection as a therapeutic agent to treat in vivo drug intoxication. Therefore, 10 mM likely represents an upper limit for the Pluronic F127 concentration in this preparation.
Figure 3.
Effects of increasing the Pluronic F127 concentration in a microemulsion on bupivacaine extraction and particle size. The oil-in-water microemulsions were composed of a variable Pluronic F127 concentration as noted with a final concentration of 16 mM ethyl butyrate in a normal saline medium. Concentrated microemulsion (100 μL) was added to 1 mL of 1 mM bupivacaine in normal saline. Data expressed as mean ± standard error of the mean of four observations per Pluronic concentration. The initial free concentration of bupivacaine was 1 mM.
Oil Content
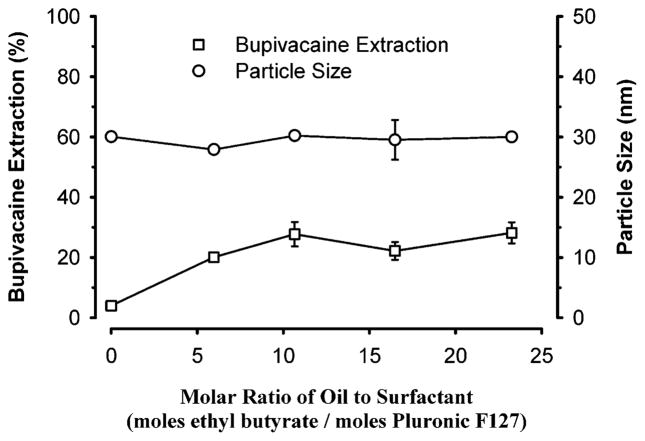
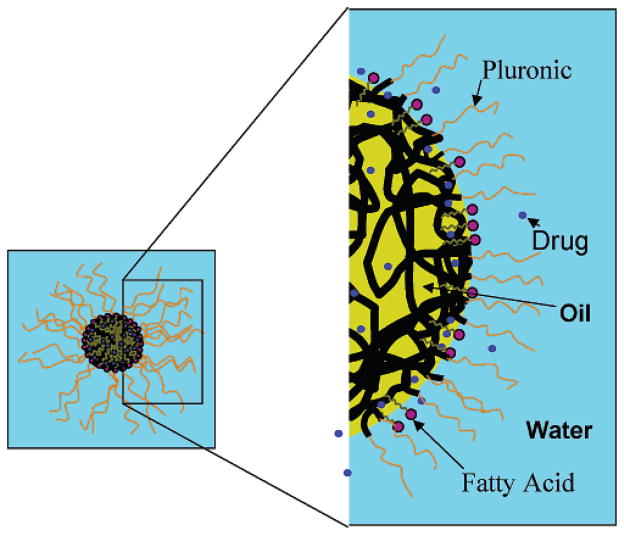
Increasing the molar ratio of oil to Pluronic surfactant concentration in the microemulsion from 0 to 23 increased bupivacaine extraction by approximately 6-fold beyond that of Pluronic micelles (Figure 4). In addition, plateau values of approximately 25% bupivacaine extraction were noted for ratios greater than 10. These observations indicate that in the absence of oil, Pluronic monomers were in equilibrium with micelles. Consequently, the resultant interfacial area for partitioning of drugs was considerably less than might be expected if oil were present. However, addition of oil (i.e., ethyl butyrate) to the micellar solution caused a stable oil/water interface to be created in the form of an oil-in-water microemulsion or swollen micelles but did not significantly change particle size. This observation indicates that the addition of oil facilitates aggregation of Pluronic in bulk medium and thereby increases the number of microemulsion droplets up to Ow = 8, which provides an increase in total surface area. Once all Pluronic molecules aggregate, oil molecules are embedded in lipophilic regions of the aggregated structure without significantly increasing the particle size of the microemulsion. Therefore, the total interfacial area increased up to a limit of the oil/Pluronic ratio. These results suggest that extraction of bupivacaine primarily occurs at the interface of the microemulsion with its aqueous bulk media. On the other hand, if extraction of bupivacaine increased linearly with the concentration of oil in the microemulsion, then the mechanism of extraction would likely be due to the partitioning of the drug into the oil core of the microemulsion. However, because the fractional extraction of bupivacaine achieved plateau values at higher oil to Pluronic molar ratios, the partitioning of the drug is primarily an adsorption phenomenon, as schematically illustrated in Figure 5.
Figure 4.
Effects of varying the molar ratio of oil to surfactant on bupivacaine extraction and particle size. The concentrated micelles or oil-in-water microemulsions were composed of a variable concentration of ethyl butyrate with a constant concentration of Pluronic F127 (8 mM). Concentrated microemulsion (100 μL) was added to 1 mL of 1 mM bupivacaine in normal saline. Data expressed as mean ± standard error of the mean of four experiments.
Figure 5.
Schematic illustration of extraction of bupivacaine by adsorption at the interface of (oil-in-water) microemulsion droplets. Shown is an oil-in-water microemulsion composed of an oil (yellow), a Pluronic surfactant, and a fatty acid (purple polar head and gray aliphatic tail) in a normal saline bulk medium (light blue background). The ethylene oxide (black threads) and propylene oxide (orange threads) are components of the Pluronic surfactant. Drug molecules (e.g., bupivacaine) targeted for extraction are represented as blue circles.
Fatty Acid Chain Length and Concentration
The effects of alkyl chain lengthening of fatty acid sodium salts on the uptake of bupivacaine were investigated (Figure 6). Extraction of bupivacaine increased approximately 4-fold as the alkyl chain was lengthened from 0 to 18 carbon atoms, although plateau values were observed when the alkyl chain length reached 14 carbon atoms. In addition, the particle size decreased approximately 40% over the same range of fatty acid alkyl chain lengths. This increase in bupivacaine extraction can be attributed to a greater oil/water interface area with reductions in particle size and to columbic interactions of the drug with anionic group of fatty acids. Because sodium caprylate, a fatty acid with 10 carbon alkyl chains, is currently used to stabilize intravenously administered pharmaceuticals (e.g., albumin) for human use, we selected this ionic surfactant for further study in Pluronic F127-based microemulsions.28
Figure 6.
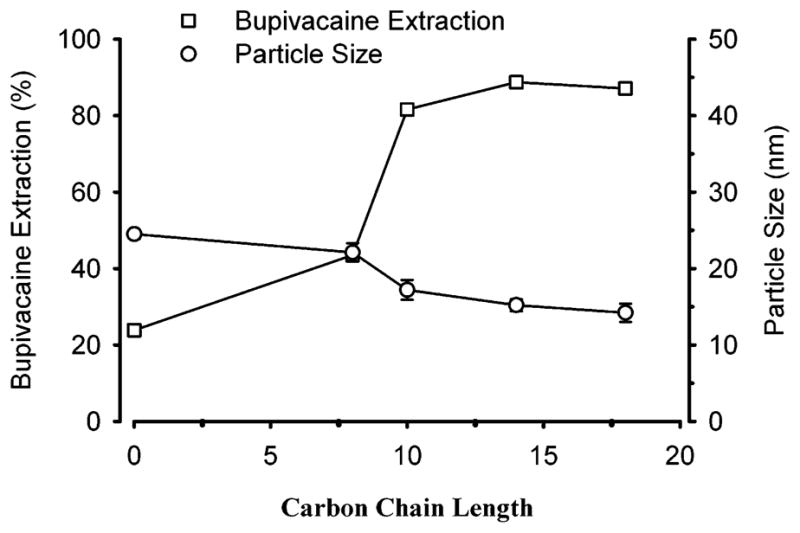
Effect of carbon chain length of sodium salt of fatty acid on the extraction of bupivacaine from normal saline by a concentrated microemulsion composed of Pluronic (8 mM), sodium salt of fatty acid (8 mM), and ethyl butyrate (160 mM). Concentrated microemulsion (100 μL) was added to 1 mL of 1 mM bupivacaine in normal saline. Data expressed as mean ± standard error of the mean of four experiments.
The addition of sodium caprylate to a Pluronic F127 microemulsion enhanced the uptake by 2-fold but then plateaued (Figure 7). As described above, sodium caprylate molecules can initially reach the microemulsion interface as long as they satisfy the packing parameters for the oil-in-water microemulsion and enhance the lipophilicity at the interface. Both of these properties increased the bupivacaine extraction. Further addition of sodium caprylate molecules would form soap micelles that would not significantly contribute to increased extraction efficiency.
Figure 7.
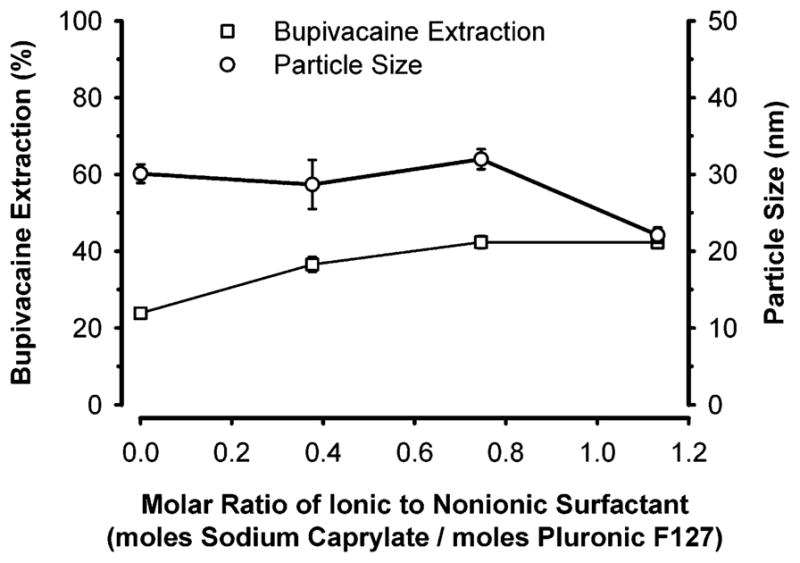
Effect of the ratio of ionic to nonionic surfactant on the extraction of bupivacaine from normal saline by a microemulsion composed of Pluronic (8 mM), sodium caprylate (as indicated), and ethyl butyrate (160 mM). Concentrated microemulsion (100 μL) was added to 1 mL of 1 mM bupivacaine in normal saline. Data expressed as mean ± standard error of the mean of four experiments.
Bupivacaine Extraction by Microemulsions and Macroemulsion
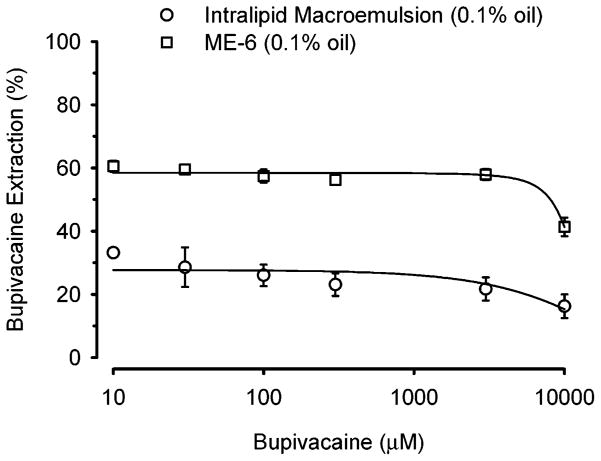
On the basis of the results presented earlier, an optimized microemulsion composition (ME-6) was synthesized with these constituents: Pluronic F127 (9 mM), sodium laurate (36 mM), ethyl butyrate (155 mM), and phosphate-buffered saline (pH 7.4) bulk medium. Extraction of bupivacaine (10–10 000 μM) caused by this microemulsion was compared to that due to Intralipid, a commercially available macroemulsion for intravenous injection in humans that is composed of soybean oil (21.4%), phospholipids (1.2%), glycerin (2.2%), and water. Both the ME-6 and Intralipid were prepared so that the final oil concentration was kept constant (0.1% w/v) to directly compare extraction efficiencies. As illustrated in Figure 8, bupivacaine extraction by ME-6 was 2-fold greater than that of Intralipid. In addition, exaction fraction of bupivacaine from the bulk medium was relatively constant until extremely high bupivacaine concentrations (3000–10 000 μM) were employed. Also, it is clear from the figure that the extraction efficiency of ME-6 begins to fall at a bupivacaine concentration greater than that of Intralipid, which is due to the larger number of ME-6 particles per unit volume compared to Intralipid, as shown in Table 2. Tabulated data based on measured particle size and geometrical calculations demonstrate that the overall interfacial area per unit volume for the ME-6 was significantly greater than that for Intralipid (Table 2). Presumably, the decrease in the fractional drug extraction is caused by saturation of drug binding mechanism(s). It is also evident from the table that the partition coefficient of the bupivacaine into ME-6 per unit volume is 18-fold higher than the Intralipid emulsion; however, the number of drug molecules per Intralipid particle is higher than the ME-6 particle, which is evident due to the larger particle size of Intralipid emulsion than that of the ME-6. Further, the percentage of ME-6 volume occupied by the bupivacaine is about 40%, whereas it is 2% in case of Intralipid. These results suggest that the nano size and composition of Pluronic aggregates makes ME-6 a highly efficient extraction system compared to an emulsion system that is composed of larger sized particles.
Figure 8.
Comparison of the extraction efficiency of ME-6 with Intralipid macroemulsion for bupivacaine. In this experiment, the total oil content of the microemulsion and macroemulsion was 0.1% w/v. Data expressed as mean ± standard error of the mean of four experiments.
Table 2.
Several Parameters of Particles Properties and Binding Characteristics with Bupivacainea
| parameter | microemulsion (ME-6) | macroemulsion (intralipid) |
|---|---|---|
| particle diameter (nm) | 29 ± 2 | 430 ± 10 |
| surface area of a particle (nm2) | 2.697 × 103 | 5.81 × 105 |
| particle density (particle/mL) | 6.95 × 1014 | 1.08 × 1012 |
| total interfacial area (m2/mL) | 1.87 | 0.628 |
| partition coefficient of drug | 146.56 | 8.41 |
| percentage volume of particle occupied by drug | 40.27 | 2.049 |
To calculate number of droplets and interfacial area, per milliliter, it was assumed that particles are closely uniformly packed. Summary data is mean ± standard error of the mean. See Supporting Information for methods of calculation.
Conclusions
Pluronic microemulsions are an efficient system for the extraction of bupivacaine from normal saline. Up to 90% of bupivacaine can be extracted from the bulk medium by using a microemulsion composed of Pluronic F127, sodium caprylate, and ethyl butyrate. The extraction efficiency was dependent on the type and concentration of Pluronic surfactant and amount of fatty acid ionic surfactant. The extraction process may be due to adsorption at the interface of microemulsion droplet. Due to the nanometer dimension of the ME-6 microemulsion droplets and resultant increase in interfacial surface area, it is a better system for uptake of bupivacaine compared to the Intralipid macroemulsion. Further investigations to develop microemulsions with superior bupivacaine extraction and to determine their efficacy to attenuate cardiotoxicity in living organisms are warranted.
Supplementary Material
Acknowledgments
Financial support was provided by the Engineering Research Center for Particle Science & Technology at the University of Florida, Gainesville, FL via National Science Foundation Grant EEC-94-02989 and by the National Institute of General Medical Sciences via Grant RO1 GM63679-01A1. Additional funding was provided by the Department of Anesthesiology, University of Florida, Gainesville, FL.
Footnotes
Supporting Information Available: Mathematical derivations were used to calculate values in Table 2 (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Litovitz T. Drug Saf. 1998;18:9. doi: 10.2165/00002018-199818010-00002. [DOI] [PubMed] [Google Scholar]
- 2.Richman PB, Nashed AH. Am J Emerg Med. 1999;17:264. doi: 10.1016/s0735-6757(99)90122-5. [DOI] [PubMed] [Google Scholar]
- 3.Strichartz GR, Sanchez V, Arthur GR, Chafetz R, Martin D. Anesth Analg. 1990;71:158. doi: 10.1213/00000539-199008000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Weinberg GL, VadeBoncouer T, Ramaraju GA, Garcia-Amaro MF, Cwik MJ. Anesthesiol. 1998;88:1071. doi: 10.1097/00000542-199804000-00028. [DOI] [PubMed] [Google Scholar]
- 5.Weinberg G, Ripper R, Feinstein DL, Hoffman W. Reg Anesth Pain Med. 2003;28:198. doi: 10.1053/rapm.2003.50041. [DOI] [PubMed] [Google Scholar]
- 6.Shinoda K, Lindman B. Langmuir. 1987;3:135. [Google Scholar]
- 7.Sjoblom J, Lindberg R, Friberg SE. Adv Colloid Interface Sci. 1996;65:125. [Google Scholar]
- 8.Shah DO, editor. Micelles, Microemulsions, and Monolayers: Science and Technology. Marcel Dekker; New York: 1998. [Google Scholar]
- 9.Malcolmson C, Satra C, Kantaria S, Sidhu A, Lawrence MJ. J Pharm Sci. 1998;87:109. doi: 10.1021/js9700863. [DOI] [PubMed] [Google Scholar]
- 10.Kawakami K, Yoshikawa T, Moroto Y, Kanaoka E, Takahashi K, Nishihara Y, Masuda K. J Controlled Release. 2002;81:65. doi: 10.1016/s0168-3659(02)00049-4. [DOI] [PubMed] [Google Scholar]
- 11.von Corswant C, Thoren P, Engstrom S. J Pharm Sci. 1998;87:200. doi: 10.1021/js970258w. [DOI] [PubMed] [Google Scholar]
- 12.Florence AT, Halbert GW. Targeted Diagn Ther. 1991;5:141. doi: 10.1201/9780203748831-5. [DOI] [PubMed] [Google Scholar]
- 13.Bagwe RP, Kanicky JR, Palla BJ, Patanjali PK, Shah DO. Crit Rev Ther Drug Carrier Syst. 2001;18:77. [PubMed] [Google Scholar]
- 14.Tenjarla S. Crit Rev Ther Drug Carrier Syst. 1999;16:461. [PubMed] [Google Scholar]
- 15.Lawrence MJ, Rees GD. Adv Drug Delivery Rev. 2000;45:89. doi: 10.1016/s0169-409x(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 16.Osborne DW, Ward AJ, O’Neill KJ. J Pharm Pharmacol. 1991;43:450. [PubMed] [Google Scholar]
- 17.Varshney M, Khanna T, Changez M. Colloids Surf, B. 1999;13:1. [Google Scholar]
- 18.Changez M, Varshney M. Drug Dev Ind Pharm. 2000;26:507. doi: 10.1081/ddc-100101261. [DOI] [PubMed] [Google Scholar]
- 19.Sottmann T. Curr Opin Colloid Interface Sci. 2002;7:57. [Google Scholar]
- 20.Jakobs B, Sottmann T, Strey R, Allgaier J, Willner L, Richter D. Langmuir. 1999;15:6707. [Google Scholar]
- 21.Friberg SE, Mortensen M, Neogi P. Sep Sci Technol. 1985;20:285. [Google Scholar]
- 22.Orringer EP, Casella JF, Ataga KI, Koshy M, Adams-Graves P, Luchtman-Jones L, Wun T, Watanabe M, Shafer F, Kutlar A, Abboud M, Steinberg M, Adler B, Swerdlow P, Terregino C, Saccente S, Files B, Ballas S, Brown R, Wojtowicz-Praga S, Grindel JM. JAMA. 2001;286:2099. doi: 10.1001/jama.286.17.2099. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Z, Chu B. Macromolecules. 1994;27:2025. [Google Scholar]
- 24.Nagarajan R. Polym Adv Technol. 2001;12:23. [Google Scholar]
- 25.Nagarajan R. Colloids Surf, B. 1999;16:55. [Google Scholar]
- 26.Jain NJ, Aswal VK, Goyal PS, Bahadur P. Colloids Surf, A. 2000;173:85. [Google Scholar]
- 27.Allemann E, Gurny R, Delker E. Eur J Pharm Biopharm. 1993;39:173. [Google Scholar]
- 28.Johnston A, Uren E, Johnstone D, Wu J. Biologicals. 2003;31:213. doi: 10.1016/s1045-1056(03)00062-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.