Peptidyl-tRNA hydrolase from T. thermophilus has been expressed, purified and crystallized. The crystals diffracted X-rays to atomic resolution (beyond 1.0 Å resolution).
Keywords: peptidyl-tRNA hydrolase, Thermus thermophilus
Abstract
Peptidyl-tRNA is produced from the ribosome as a result of aborted translation. Peptidyl-tRNA hydrolase cleaves the ester bond between the peptide and the tRNA of peptidyl-tRNA molecules, to recycle tRNA for further rounds of protein synthesis. In this study, peptidyl-tRNA hydrolase from Thermus thermophilus HB8 (TthPth) was crystallized using 2-methyl-2,4-pentanediol as a precipitant. The crystals belonged to the orthorhombic space group P212121, with unit-cell parameters a = 47.45, b = 53.92, c = 58.67 Å, and diffracted X-rays to atomic resolution (beyond 1.0 Å resolution). The asymmetric unit is expected to contain one TthPth molecule, with a solvent content of 27.13% (V M = 1.69 Å3 Da−1). The structure is being solved by molecular replacement.
1. Introduction
In cells, translating ribosomes often stall for various reasons (Caplan & Menninger, 1979 ▶; Cruz-Vera et al., 2004 ▶; Singh & Varshney, 2004 ▶). Such stalled ribosomes release peptidyl-tRNAs with the aid of ribosomal recycling factor, elongation factor G and initiation factor 3, as well as other factors, to prepare for further translation cycles (Heurgué-Hamard et al., 1998 ▶; Karimi et al., 1998 ▶; Gong et al., 2007 ▶; Singh et al., 2008 ▶). The accumulation of peptidyl-tRNAs, however, halts protein synthesis because of the depletion of free tRNA (Heurgué-Hamard et al., 1996 ▶; Menez et al., 2000 ▶). In order to prevent this situation, peptidyl-tRNA hydrolase releases tRNA from peptidyl-tRNA by cleaving the ester bond between the C-terminal end of the peptide and the 3′-end ribose of the tRNA (Cuzin et al., 1967 ▶; Kössel & RajBhandary, 1968 ▶; Das & Varshney, 2006 ▶). The peptidyl-tRNA hydrolase activity is essential for bacterial viability (Atherly & Menninger, 1972 ▶; Menez et al., 2002 ▶; Sassetti et al., 2003 ▶).
Peptidyl-tRNA hydrolase genes have been identified in organisms belonging to all three kingdoms of life. The peptidyl-tRNA hydrolases (Pth) can be classified into two types, which are sometimes referred to as Pth1 and Pth2. The Pth1 gene exists in bacteria and Eukarya, while the Pth2 gene is present in Eukarya and Archaea. Although there is no significant sequence or structural similarity between Pth1 and Pth2, the two enzymes possess similar biological activities (Schmitt et al., 1997 ▶; Rosas-Sandoval et al., 2002 ▶; Fromant et al., 2003 ▶; De Pereda et al., 2004 ▶).
The features of the Escherichia coli Pth enzyme have been well characterized. Biochemical analyses revealed that Pth cleaves N-blocked aminoacyl-tRNAs (Cuzin et al., 1967 ▶; Kössel & RajBhandary, 1968 ▶). However, N-formyl-methionyl-tRNAf Met is resistant to hydrolysis by Pth (Kössel & RajBhandary, 1968 ▶). Indeed, N-formyl-methionyl-tRNAf Met remains intact in the cell and can associate with the initiation complex. This resistance is due to the unpaired 1–72 nucleotides, which are a unique characteristic of the prokaryotic initiators, since N-formyl-methionyl-tRNAf Met becomes hydrolysed by Pth upon the introduction of the 1–72 base pair (Schulman & Pelka, 1975 ▶; Dutka et al., 1993 ▶). Biochemical studies also revealed that the enzyme can accept peptidyl-tRNAHis as a substrate, which has a unique extra base pair between G−1 and C73 (Menninger, 1978 ▶; Fromant et al., 2000 ▶).
X-ray crystallography, NMR spectroscopy and detailed mutational analyses have shown that, in addition to the active-site cavity, the positively charged patch around the active site, the helix loop covering the active site and the C-terminal α-helix of Pth participate in substrate binding (Schmitt et al., 1997 ▶; Fromant et al., 1999 ▶; Goodall et al., 2004 ▶; Giorgi, Plateau et al., 2011 ▶; Giorgi, Bontems et al., 2011 ▶). Recently, we determined the structure of E. coli Pth in complex with the CCA-acceptor-TΨC domain of tRNA (Ito et al., 2011 ▶, 2012 ▶). This structure revealed the mechanism by which Pth accepts the diverse sequences of the elongator-tRNAs as substrate components. Specifically, the structure showed that Pth interacts with the tRNA moiety through the backbone phosphates and riboses, and no base-specific interactions are present, except for the highly conserved base G53.
Enzymes from thermophiles possess various factors to perform their functions at high temperature. Comparisons of the structures of enzymes from thermophiles and mesophiles will provide valuable insights into these factors. The Pth structures from some mesophilic bacteria have been determined (Schmitt et al., 1997 ▶; Selvaraj et al., 2007 ▶; Pulavarti et al., 2008 ▶; Clarke et al., 2011 ▶; Kumar et al., 2012 ▶); however, no structure from a thermophilic bacterium has been determined. Thus, we attempted to determine the structure of Pth from Thermus thermophilus HB8 (TthPth). T. thermophilus is a Gram-negative thermophilic bacterium and can grow at temperatures from 323 to 355 K (Oshima & Imahori, 1974 ▶). We now report the cloning, expression, purification and preliminary X-ray crystallographic studies of TthPth. This is the first report of the crystallization of Pth from a thermophilic bacterium.
2. Materials and methods
2.1. Protein expression and purification
The plasmid containing the T. thermophilus HB8 (JCM 10941T) Pth gene (TTHA1588) was provided by RIKEN BRC, which is participating in the National Bio-Resources Project of the MEXT Japan (Yokoyama et al., 2000 ▶). The gene of interest was PCR-amplified using the above vector as the template, with the oligonucleotides 5′-AAAGGGAATTCCATATGATGTTCCTGGTGGTGGGCCA-3′, bearing an NdeI site (bold), and 5′-AAACCCAAGCTTTTACCCCAAGGAGAGGTCCA-3′, bearing a HindIII site (bold). The fragment was cloned into the NdeI and HindIII sites of the pET-28c vector (Novagen).
The recombinant TthPth protein was overexpressed in E. coli strain BL21 (DE3) (Novagen) with an N-terminal His tag and a thrombin cleavage site (MGSSHHHHHHSSGLVPR/GSH, where ‘/’ represents the thrombin cleavage site). Protein expression was induced by adding 1.0 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) to early exponential-phase cultures (OD600 ≃ 0.6) and continuing the culture for 3 h at 310 K in LB medium. The cells were harvested by centrifugation, suspended in 20 mM HEPES–KOH buffer pH 7.6, containing 150 mM KCl and 7 mM β-mercaptoethanol, and then disrupted by sonication. After centrifugation at 277 K and 13 2000g for 30 min, the supernatant was incubated at 343 K for 20 min to denature the E. coli proteins, and the precipitate was removed by centrifugation at 277 K and 13 2000g for 30 min. Subsequently, the sample was loaded onto a HisTrap HP column (GE Healthcare) equilibrated with buffer A [20 mM HEPES–KOH pH 7.6, 1 M ammonium chloride, 5%(v/v) glycerol, 7 mM β-mercaptoethanol] and TthPth was eluted with buffer A containing a linear gradient up to 250 mM imidazole. To digest the N-terminal His tag, 12.5 units of thrombin (GE Healthcare) were added to the eluate containing 1 mg of TthPth. After incubation at 289 K for 48 h, the solution was dialysed against buffer B (20 mM HEPES–KOH pH 7.6, 150 mM NaCl, 7 mM β-mercaptoethanol) and the protease was removed by adding Benzamidine Sepharose 4 Fast Flow (High Sub) (GE Healthcare) equilibrated with buffer B, followed by filtration. Subsequently, to remove the isolated His tag and the undigested His-tagged TthPth, the protein solution was diluted threefold with 20 mM HEPES–KOH pH 7.6, containing 3 M ammonium chloride, 7 mM β-mercaptoethanol, and loaded onto a Ni–NTA Agarose (Qiagen) column equilibrated with 20 mM HEPES–KOH pH 7.6, containing 1 M ammonium chloride, 7 mM β-mercaptoethanol. The flow-through fraction was collected and dialysed against buffer C (10 mM HEPES–KOH pH 7.6, 7 mM β-mercaptoethanol). Finally, to absorb the residual contaminant proteins, the sample was loaded onto a Q Sepharose Fast Flow (GE Healthcare) column equilibrated with buffer C. The flow-through fraction was collected and concentrated using a Vivaspin centrifugal concentrator (3 kDa cutoff size, Sartorius). The protein concentration was determined by the Bradford method (Bio-Rad Protein Assay), using BSA as the standard protein. The purified sample was stored at 193 K prior to use.
2.2. Crystallization
Initial crystallization trials were performed by the sitting-drop vapour-diffusion method using Crystal Screen, Crystal Screen 2 (Hampton Research), Wizard I and II, and Cryo I and II (Emerald BioSystems) kits. Drops were prepared by mixing 1 µl of sample solution (40.0 mg ml−1 protein in buffer C) with an equal volume of reservoir solution, and were equilibrated against 50 µl of reservoir solution in a 96-well plate at 293 K. Trials to improve the crystallization conditions were performed by varying the pH, precipitant concentration and protein concentration. All of these trials were performed by the sitting-drop vapour-diffusion method using 24-well plates. The drops were prepared by mixing 1 µl of sample solution with an equal volume of reservoir solution and were equilibrated against 450 µl of reservoir solution.
2.3. Data collection and processing
The crystal was picked up in a nylon loop (Hampton Research) and flash-cooled in a stream of nitrogen. All diffraction data were collected with a wavelength of 1.000 Å at 100 K on the NW12A beamline of the Photon Factory Advanced Ring (PF-AR) (Tsukuba, Japan), using an ADSC Quantum 210 CCD detector. The indexing, integration, scaling and merging of the diffraction data were performed with the HKL-2000 program suite (Otwinowski & Minor, 1997 ▶). Other crystallographic calculations were performed with the CCP4 package (Winn et al., 2011 ▶).
3. Results and discussion
TthPth was overexpressed and purified, with a yield of 5.5 mg protein from 1 l of culture. The purity of the protein was estimated to be greater than 95% by SDS–PAGE. After the initial crystallization trials, two morphologically different crystals were observed within 3 d. Form I grew as needle-shaped crystals from conditions No. 1 [100 mM phosphate–citrate buffer pH 4.2, 40%(v/v) 2-methyl-2,4-pentanediol] and No. 9 [100 mM phosphate–citrate buffer pH 4.2, 35%(v/v) 2-propanol] of Cryo I at 293 K. Form II grew as rhombic shaped crystals from condition No. 15 of Crystal Screen 2 (0.5 M ammonium sulfate, 100 mM sodium citrate tribasic dihydrate buffer pH 5.6, 1.0 M lithium sulfate monohydrate) at 293 K. As a result of refining these crystallization conditions, crystals suitable for X-ray analysis were obtained by the sitting-drop vapour-diffusion method, by mixing 1 µl of sample solution (60 mg ml−1 protein in buffer C) and 1 µl of reservoir solution consisting of 100 mM phosphate–citrate buffer pH 4.2, 50%(v/v) 2-methyl-2,4-pentanediol. The crystals grew within a week to maximum dimensions of approximately 800 × 100 × 100 µm at 293 K (Fig. 1 ▶). On the other hand, the crystals from conditions No. 9 of Cryo I and No. 15 of Crystal Screen 2 could not be improved to a level suitable for X-ray analysis.
Figure 1.

Crystal of T. thermophilus Pth.
A single crystal of TthPth was obtained by breaking apart the cluster. The crystal diffracted X-rays beyond 1.0 Å resolution (Fig. 2 ▶), which are the highest-resolution data for any bacterial Pth reported to date. The concentration of 2-methyl-2,4-pentanediol in the drop was sufficient to prevent ice-ring formation. Since many low-resolution diffraction spots were saturated, we collected two data sets using the same crystal. For the first data set (long-exposure data set), the exposure time was 5 s, the crystal-to-detector distance was 60.5 mm, the oscillation angle was 1° and the total oscillation range was 180°. For the second data set (short-exposure data set), the exposure time was 0.5 s and the crystal-to-detector distance was 117.1 mm. The oscillation angle and the total oscillation range were the same as in the long-exposure data set. These two data sets were indexed, scaled and merged. As a result, the crystals were found to belong to the orthorhombic space group P212121, with unit-cell parameters a = 47.45, b = 53.92 and c = 58.67 Å. The asymmetric unit is expected to contain one TthPth molecule, with a solvent content of 27.13% and a Matthews coefficient V M (Matthews, 1968 ▶) of 1.69 Å3 Da−1 (the molecular weight of TthPth is 20.18 kDa). The data-collection statistics are summarized in Table 1 ▶. In order to solve the structure, molecular-replacement calculations were performed using the program BALBES (Long et al., 2008 ▶). The best solution was found using the chloroplast group II intron splicing factor CRS2 from Z. mays (PDB entry 1ryb; Ostheimer et al., 2005 ▶) as the search model. The final R factor, R free (5% of the total reflections) and Q factor parameter values, after the REFMAC5 processing at the end of the BALBES run, were 43.5%, 46.6%, and 0.604, respectively. The structure refinement is now in progress.
Figure 2.
X-ray diffraction image of a crystal of T. thermophilus Pth. This is an image from the long-exposure data set. The contrasts were varied at the central and outer parts. The edge of the detector corresponds to a resolution of 1.0 Å.
Table 1. Data-collection and processing statistics.
Values in parentheses are for the highest-resolution shell.
| Beamline | NW12A, PF-AR |
| Wavelength (Å) | 1.000 |
| Space group | P212121 |
| Unit-cell parameters (Å) | a = 47.45, b = 53.92, c = 58.67 |
| Resolution range (Å) | 50.0–1.00 (1.04–1.00) |
| No. of measured reflections | 598481 |
| No. of unique reflections | 75984 |
| Completeness (%) | 92.6 (95.7) |
| Redundancy | 7.9 (5.8) |
| Average I/σ(I) | 57.2 (5.2) |
| R merge † (%) | 4.5 (46.0) |
R
merge = 
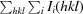 , where Ii(hkl) is the ith intensity measurement of reflection hkl, including symmetry-related reflections, and 〈I(hkl)〉 is its average.
, where Ii(hkl) is the ith intensity measurement of reflection hkl, including symmetry-related reflections, and 〈I(hkl)〉 is its average.
Acknowledgments
The synchrotron radiation experiments were performed on the NW12A beamline of PF-AR with the approval of KEK (Tsukuba, Japan) (proposal No. 2009G178). This work was supported in part by a Grant-in-Aid for Young Scientists (Start-up) (20870018 to KI) and a Grant-in-Aid for Young Scientists (B) (21770108 to KI) from the Japan Society for the Promotion of Science (JSPS), by a grant from the Uchida Energy Science Promotion Foundation (22-1-10 to KI), by a grant from UNION TOOL CO (to KI), by a Grant for the Promotion of Niigata University Research Projects (22C017 to KI) and by Grants-in-Aid for Education and Research at the Institute of Science and Technology from Niigata University (to KI).
References
- Atherly, A. G. & Menninger, J. R. (1972). Nature New Biol. 240, 245–246. [DOI] [PubMed]
- Caplan, A. B. & Menninger, J. R. (1979). J. Mol. Biol. 134, 621–637. [DOI] [PubMed]
- Clarke, T. E., Romanov, V., Lam, R., Gothe, S. A., Peddi, S. R., Razumova, E. B., Lipman, R. S., Branstrom, A. A. & Chirgadze, N. Y. (2011). Acta Cryst. F67, 446–449. [DOI] [PMC free article] [PubMed]
- Cruz-Vera, L. R., Magos-Castro, M. A., Zamora-Romo, E. & Guarneros, G. (2004). Nucleic Acids Res. 32, 4462–4468. [DOI] [PMC free article] [PubMed]
- Cuzin, F., Kretchmer, N., Greenberg, R. E., Hurwitz, R. & Chapeville, F. (1967). Proc. Natl Acad. Sci. USA, 58, 2079–2086. [DOI] [PMC free article] [PubMed]
- Das, G. & Varshney, U. (2006). Microbiology, 152, 2191–2195. [DOI] [PubMed]
- De Pereda, J. M., Waas, W. F., Jan, Y., Ruoslahti, E., Schimmel, P. & Pascual, J. (2004). J. Biol. Chem. 279, 8111–8115. [DOI] [PubMed]
- Dutka, S., Meinnel, T., Lazennec, C., Mechulam, Y. & Blanquet, S. (1993). Nucleic Acids Res. 21, 4025–4030. [DOI] [PMC free article] [PubMed]
- Fromant, M., Ferri-Fioni, M. L., Plateau, P. & Blanquet, S. (2003). Nucleic Acids Res. 31, 3227–3235. [DOI] [PMC free article] [PubMed]
- Fromant, M., Plateau, P. & Blanquet, S. (2000). Biochemistry, 39, 4062–4067. [DOI] [PubMed]
- Fromant, M., Plateau, P., Schmitt, E., Mechulam, Y. & Blanquet, S. (1999). Biochemistry, 38, 4982–4987. [DOI] [PubMed]
- Giorgi, L., Bontems, F., Fromant, M., Aubard, C., Blanquet, S. & Plateau, P. (2011). J. Biol. Chem. 286, 39585–39594. [DOI] [PMC free article] [PubMed]
- Giorgi, L., Plateau, P., O’Mahony, G., Aubard, C., Fromant, M., Thureau, A., Grøtli, M., Blanquet, S. & Bontems, F. (2011). J. Mol. Biol. 412, 619–633. [DOI] [PubMed]
- Gong, M., Cruz-Vera, L. R. & Yanofsky, C. (2007). J. Bacteriol. 189, 3147–3155. [DOI] [PMC free article] [PubMed]
- Goodall, J. J., Chen, G. J. & Page, M. G. (2004). Biochemistry, 43, 4583–4591. [DOI] [PubMed]
- Heurgué-Hamard, V., Karimi, R., Mora, L., MacDougall, J., Leboeuf, C., Grentzmann, G., Ehrenberg, M. & Buckingham, R. H. (1998). EMBO J. 17, 808–816. [DOI] [PMC free article] [PubMed]
- Heurgué-Hamard, V., Mora, L., Guarneros, G. & Buckingham, R. H. (1996). EMBO J. 15, 2826–2833. [PMC free article] [PubMed]
- Ito, K., Murakami, R., Mochizuki, M., Qi, H., Shimizu, Y., Miura, K., Ueda, T. & Uchiumi, T. (2012). Nucleic Acids Res. 40, 10521–10531. [DOI] [PMC free article] [PubMed]
- Ito, K., Qi, H., Shimizu, Y., Murakami, R., Miura, K., Ueda, T. & Uchiumi, T. (2011). Acta Cryst. F67, 1566–1569. [DOI] [PMC free article] [PubMed]
- Karimi, R., Pavlov, M. Y., Heurgué-Hamard, V., Buckingham, R. H. & Ehrenberg, M. (1998). J. Mol. Biol. 281, 241–252. [DOI] [PubMed]
- Kössel, H. & RajBhandary, U. L. (1968). J. Mol. Biol. 35, 539–560. [DOI] [PubMed]
- Kumar, A., Singh, N., Yadav, R., Kumar, R. P., Sharma, S., Arora, A. & Singh, T. P. (2012). Int. J. Biochem. Mol. Biol. 3, 58–69. [PMC free article] [PubMed]
- Long, F., Vagin, A. A., Young, P. & Murshudov, G. N. (2008). Acta Cryst. D64, 125–132. [DOI] [PMC free article] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- Menez, J., Buckingham, R. H., de Zamaroczy, M. & Campelli, C. K. (2002). Mol. Microbiol. 45, 123–129. [DOI] [PubMed]
- Menez, J., Heurgué-Hamard, V. & Buckingham, R. H. (2000). Nucleic Acids Res. 28, 4725–4732. [DOI] [PMC free article] [PubMed]
- Menninger, J. R. (1978). J. Biol. Chem. 253, 6808–6813. [PubMed]
- Oshima, T. & Imahori, K. (1974). Int. J. Syst. Bacteriol. 24, 102–112.
- Ostheimer, G. J., Hadjivassiliou, H., Kloer, D. P., Barkan, A. & Matthews, B. W. (2005). J. Mol. Biol. 345, 51–68. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Pulavarti, S. V., Jain, A., Pathak, P. P., Mahmood, A. & Arora, A. (2008). J. Mol. Biol. 378, 165–177. [DOI] [PubMed]
- Rosas-Sandoval, G., Ambrogelly, A., Rinehart, J., Wei, D., Cruz-Vera, L. R., Graham, D. E., Stetter, K. O., Guarneros, G. & Söll, D. (2002). Proc. Natl Acad. Sci. USA, 99, 16707–16712. [DOI] [PMC free article] [PubMed]
- Sassetti, C. M., Boyd, D. H. & Rubin, E. J. (2003). Mol. Microbiol. 48, 77–84. [DOI] [PubMed]
- Schmitt, E., Mechulam, Y., Fromant, M., Plateau, P. & Blanquet, S. (1997). EMBO J. 16, 4760–4769. [DOI] [PMC free article] [PubMed]
- Schulman, L. H. & Pelka, H. (1975). J. Biol. Chem. 250, 542–547. [PubMed]
- Selvaraj, M., Roy, S., Singh, N. S., Sangeetha, R., Varshney, U. & Vijayan, M. (2007). J. Mol. Biol. 372, 186–193. [DOI] [PubMed]
- Singh, N. S., Ahmad, R., Sangeetha, R. & Varshney, U. (2008). J. Mol. Biol. 380, 451–464. [DOI] [PubMed]
- Singh, N. S. & Varshney, U. (2004). Nucleic Acids Res. 32, 6028–6037. [DOI] [PMC free article] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.
- Yokoyama, S., Hirota, H., Kigawa, T., Yabuki, T., Shirouzu, M., Terada, T., Ito, Y., Matsuo, Y., Kuroda, Y., Nishimura, Y., Kyogoku, Y., Miki, K., Masui, R. & Kuramitsu, S. (2000). Nature Struct. Biol. 7, 943–945. [DOI] [PubMed]



