Abstract
Purpose
Sclerostin, produced by osteocytes, is a potent inhibitor of Wnt signaling and bone formation. While sclerostin levels increase with age in adults and are higher in men compared to women, there is currently no information on changes in circulating sclerostin levels during growth in humans.
Methods
We measured serum sclerostin levels in 6 to 21 year-old girls (n = 62) and boys (n = 56) and related these to trabecular and cortical bone microarchitectural parameters using high-resolution peripheral quantitative computed tomography and to markers of bone turnover.
Results
Serum sclerostin levels were higher in boys as compared to girls and declined in both sexes following the onset of puberty. There was no consistent relationship between sclerostin levels and trabecular bone parameters in either sex. However, serum sclerostin levels were inversely associated with cortical volumetric bone mineral density and cortical thickness in girls and positively associated with the cortical porosity index in both girls and boys. Bone turnover markers were positively correlated with serum sclerostin levels in both sexes.
Conclusion
The gender difference in serum sclerostin levels appears to be established during puberty, and sclerostin levels tend to decline in late puberty in both girls and boys. Serum sclerostin levels are associated with cortical porosity, suggesting that changes in sclerostin production during growth may play a role in defining cortical structure.
Keywords: sclerostin, puberty, growth
Introduction
The Wnt/β-catenin pathway is a major regulator of bone mass. Increased bone formation through this pathway results from both the expansion of osteoprogenitor cells as well as reduced apoptosis of mature osteoblasts [1, 2]. The effects of Wnt ligands on this pathway are mediated by binding to a seven-transmembrane domain-spanning frizzled receptor and either of two co-receptors, low-density lipoprotein receptor-related protein 5 (LRP5) or 6 (LRP6) [1, 2]. Sclerostin secreted by osteocytes is a potent antagonist of the Wnt/β-catenin pathway and acts by binding to LRP5 and LRP6 [1, 3-6].
Data from both humans and animals clearly illustrate the biological importance of sclerostin as a negative-regulator of bone mass. Loss of function mutations in the sost gene or in its regulatory elements cause two rare human genetic disorders, sclerosteosis [7, 8] and van Buchem disease [9, 10], that result in high bone mass. Sost knockout mice also have high bone mass and increased rates of bone formation [11]. A neutralizing antibody to sclerostin has recently shown to increase bone formation and to also reduce bone resorption both in mice [12] and humans [13]. While the increase in bone formation is likely due to activation of Wnt signaling [1, 3-6], the mechanism(s) for the decrease in bone resorption induced by the sclerostin antibody remains unclear but may be due, at least in part, to increased osteoprotegerin (OPG) production as a result of activation of Wnt signaling [14].
We have recently described age-related changes in circulating sclerostin levels in adult men and women [15]. We observed that serum sclerostin levels increase with age in both sexes, with men having significantly higher sclerostin levels compared to women. However, there is currently no information regarding changes in circulating sclerostin levels during pubertal growth in humans. Since puberty is a time when 25-50% of adult bone mass is accrued [16], it is clearly important to understand the possible role of sclerostin in the regulation of bone mass during this critical period for skeletal development.
We previously defined changes in bone microarchitecture at the distal radius in growing children aged 6 to 21 years using non-invasive high-resolution peripheral quantitative computed tomography (HRpQCT) [17]. Compared to pre-puberty, trabecular parameters (bone volume fraction [BV/TV], trabecular number [TbN] and thickness [TbTh]) did not change in girls, but increased in boys from late puberty onwards. Cortical thickness (CtTh) and volumetric bone mineral density (vBMD) decreased from pre- to mid-puberty in girls, but were unchanged in boys, before rising to higher levels at the end of puberty in both sexes. Interestingly, apparent cortical porosity increased transiently during mid- to late-puberty in both sexes, mirroring the age of peak distal forearm fracture risk in prior studies [18, 19-21] and suggesting that regional deficits in cortical bone may underlie the adolescent peak in forearm fractures. In the present study, we used this well characterized cohort to define changes in circulating sclerostin levels during pubertal growth and to correlate sclerostin levels with bone microarchitecture, bone turnover markers, and serum hormone levels during this phase of growth.
Study Subjects and Methods
Study subjects
The study was approved by the Mayo Clinic Institutional Review Board. Informed written consent was obtained from all subjects >12 years of age and from a parent for all subjects <18 year of age. Details regarding recruitment of the study subjects and changes in bone structural parameters during growth have previously been published [17]. Briefly, we recruited 140 healthy girls and boys (n = 70 for each sex) with a chronological age of 6 to 21 years (bone age 4 to 21 years, see below) without a prior history of fracture. Reflecting the ethnic composition of the population of Rochester, MN, all but 6 subjects were white: 4 girls were black, 1 girl was white/Asian, and 1 boy was Asian. None of the subjects had a chronic illness or dietary restrictions. No one was receiving supplements of calcium >1000 mg/d or vitamin D >200 IU/d. No subject had ever used sodium fluoride, calcitonin, bisphosphonates, antiepileptic drugs, or had a history of oral steroid use for >7 days. None of the girls had a history of oral contraceptive use. Skeletal maturity was assessed using plain hand and wrist X-rays, and subjects were divided into groups based on bone-age using the Tanner-Whitehouse III method [22] although subjects who had completed skeletal maturation (bone age >15 years for girls and >16.5 years for boys) were classified according to chronological age. Data on 13 subjects were excluded because of motion artifact in the HRpQCT scans; of the remainder, serum sclerostin levels were available in 62 girls and 56 boys, and the present analysis was done on these 118 subjects.
HRpQCT measurements
Measurements were obtained from the nondominant wrist on all subjects using the XtremeCT system (Scanco Medical, Brüttisellen, Switzerland), as previously described [17]. Using a scout view, a reference line was set at the proximal limit of the epiphyseal growth plate. For subjects whose epiphyseal plates had fused, the remnant of the plate was still visible to set the reference line. The scan was performed on a segment spanning 9.02 mm, starting 1 mm proximal to the reference line, thereby ensuring that, despite differences in arm length, all subjects had the scans performed as close to the identical anatomic site as possible; this is also the site where adolescent fractures most commonly occur [16, 19, 20]. Data were obtained using a 3D stack of 110 high-resolution CT slices with an isotropic voxel size and slice thickness of 82 μm, using an effective energy of 40 keV, field of view of 125.9 mm, and image matrix of 1536 × 1536 pixels. The radiation exposure to the subjects was minimal (local absorbed dose, 0.065 cGy; total radiation exposure, <0.01 mSv). BV/TV (%) was derived from trabecular vBMD, assuming mineral density of fully mineralized bone of 1.2 g hydroxyappatite/cm3. Recognizing that individual trabeculae would not be resolved at their correct thickness (~100 μm) because of partial volume effects, a thickness-independent structure extraction was used to identify 3D ridges (center points of the trabeculae) [23]; TbN (mm−1) was taken as the inverse of the mean spacing of the ridges[24]. Analogous with standard histomorphometry [25], TbTh (μm) was calculated using the formula, TbTh = BV/TV ÷ TbN, and trabecular spacing (TbSp, μm) was calculated as TbSp = (1 - BV/TV) ÷ TbN. While HRpQCT does not provide the same resolution as μCT, validation studies have shown excellent correlation (R ≥ 0.96) for these parameters measured by HRpQCT as compared with the gold standard ex-vivo μCT technique [26]. For the cortical measures, the cortex was segmented from the grayscale image with a Gaussian filter and threshold [24]. Cortical vBMD and area were measured directly, and the periosteal circumference was calculated from the contour. CtTh (μm) was derived using the following formula: CtTh = area/circumference. Again, excellent correlation (R = 0.98) has been shown for CtTh measurements with HRpQCT versus μCT [27]. The automatically generated cortical mask was applied to the whole bone structure to obtain the cortical bone, followed by an inversion resulting in a negative image, including only the “cortical pores.” The cortical porosity index was defined as the ratio of cortical pore volume to cortical bone volume. Contours were defined automatically using the manufacturer’s approach. From these contours, we peeled off two voxels (164 um) at the endocortical surface so as not to include trabecular compartments in the porosity analysis. Due to the relatively low resolution of HRpQCT as compared to μCT, we did not analyze the shapes of the cortical pores as these will be resolution-dependent and would require a validation study. On the other hand, cortical porosity describes a statistical measure of inverse pore density which is much less affected by resolution and can be assessed also in lower resolution images.
Hormone and bone turnover measurements
Serum sclerostin levels were measured using a validated enzyme-linked immunosorbent assay (ELISA, Biomedica, Vienna, Austria; interassay CV < 7%) [15, 28-30]. A serum bone formation marker, amino-terminal propeptide of type I collagen (PINP), and a serum bone resorption marker, C-terminal telopeptide of type I collagen (CTX), were measured using ELISAs (Immunodiagnostic Systems, Fountain Hills, AZ, for PINP [intra-assay CV < 10%] and Nordic Biosciences, Herlev, Denmark, for CTX [CV < 8%]). IGF-1 (CV < 7%) and IGFBP-3 (CV, 3%) were measured by radioimmunoassay (Diagnostic Systems Laboratories, Webster, TX). PTH was measured by automated immunometric assay (Diagnostic Products Corp, Los Angeles, CA; CV, 7%), and 25-hydroxyvitamin-D (25-OHD), total estradiol (E2) and testosterone (T) (intraassay CV’s < 8%) were measured using tandem mass spectroscopy (API 5000, Applied Biosystems-MDS Sciex, Foster City, CA). Details regarding the mass spectroscopy measurements have been previously described [31].
Statistical analysis
Serum sclerostin levels and bone structural parameters were summarized using medians and interquartile ranges. Comparisons between the girls and boys were made using the Wilcoxon rank-sum test. The relationship between bone age and serum sclerostin levels was explored using a loess smoothing function. Linear regression models were used to establish the point at which the relationship between bone age and serum sclerostin changed, i.e. the split point. The split point was chosen such that it maximized the model R2 value. Unadjusted and bone age-adjusted Spearman correlations were used to describe the relationships between serum sclerostin levels and bone structural variables, bone turnover markers, and hormone measurements. P < 0.05 was considered significant. Analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC) and R (R Foundation for Statistical Computing, Vienna, Austria).
Results
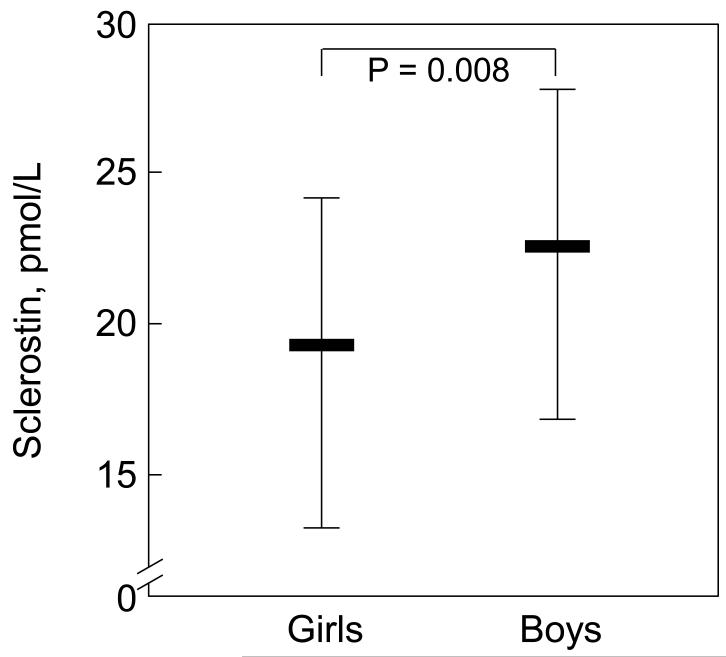
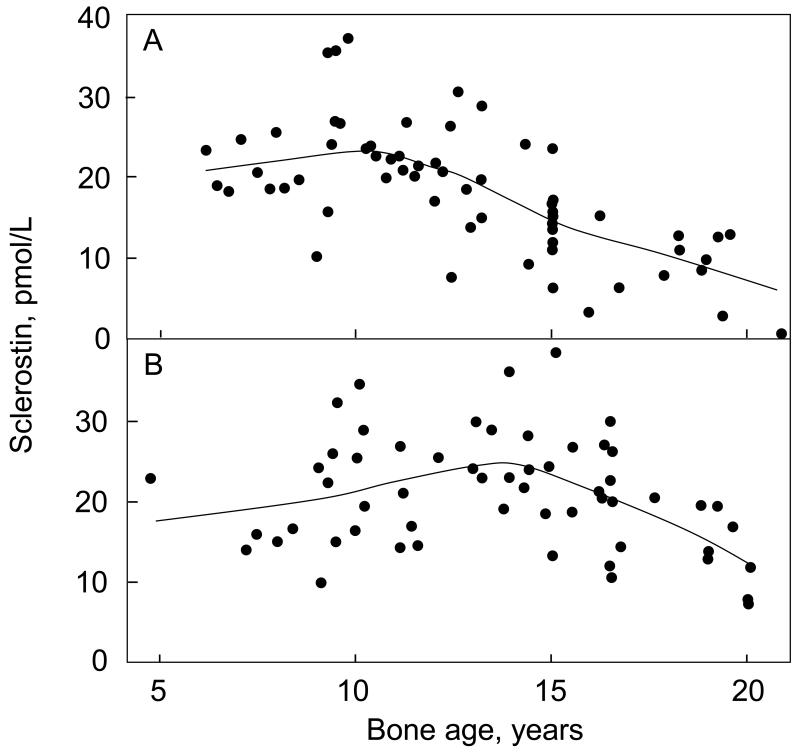
Serum sclerostin levels were significantly higher in boys as compared to girls (Figure 1). In exploratory analyses, the relationship between sclerostin levels and bone age was found to be non-linear. Specifically, there appeared to be a split point in this relationship (see Methods) in girls at bone age 10 years (Figure 2A) and in boys at bone age 14 years (Figure 2B). Table 1 shows the correlation coefficients between serum sclerostin levels and bone age in the girls and boys based on these split points. For both sexes, sclerostin levels were positively associated with bone age below the respective split points and negatively associated with bone age above the split points.
Figure 1.
Serum sclerostin levels (median, 25th-75th percentile, IQR) in the girls versus the boys.
Figure 2.
Smoother plots (see Methods) showing the relationship between serum sclerostin levels and bone age in (A) girls and (B) boys. See Table 1 for correlation coefficients based on the split point analysis.
Table 1.
Spearman correlation coefficients (rp-value) between serum sclerostin levels and bone age based on the respective split points for these relationships in the girls and the boys.
| Girls | Boys | ||
|---|---|---|---|
| Bone age 4-9 yrs (n = 17) |
Bone age 10-21 yrs (n = 45) |
Bone age 4-13 yrs (n = 29) |
Bone age 14-21 yrs (n = 27) |
| 0.530.029 | −0.74<0.001 | 0.380.042 | −0.590.001 |
Since we have previously described in detail the changes in the bone structural parameters as a function of age in these subjects [17], Table 2 summarizes the bone structural parameters used in the subsequent analyses in all subjects combined as well as in the girls and boys separately, based on the split points as defined above. We next examined the relationship between serum sclerostin levels and the bone structural parameters in the girls and boys. For this analysis, we looked at correlations in all subjects combined and then at the correlations after separating the girls and boys based on the split points defined above in order to define which sub-group may be driving the observed correlations. As shown in Table 3, in all girls combined, cortical vBMD and CtTh were inversely associated with serum sclerostin, and the cortical porosity index was positively associated with serum sclerostin, unadjusted and following adjustment for bone age. Moreover, these correlations were driven by the associations in the girls aged 10-21 years, when serum sclerostin levels were declining (Figure 2A). Similar associations between cortical vBMD and CtTh in the older boys did not remain after age-adjustment; and, overall, cortical vBMD and CtTh were not associated with sclerostin levels in the boys. However, as seen in the girls, the cortical porosity index was positively associated with sclerostin levels, unadjusted and following adjustment for bone age (Table 3). Again, this relationship was driven principally by the boys aged 14-21 years, when sclerostin levels were declining (Figure 2B). By contrast, in all subjects combined, trabecular structural parameters did not show any clear associations with serum sclerostin levels in either sex. However, TbN (positive) and TbSp (negative) were associated with sclerostin levels in the older girls, and BV/TV and TbTh were both positively associated with sclerostin levels in the younger boys. Further adjustment of the association of serum sclerostin levels with the bone structural parameters for body weight did not significantly alter any of the correlation coefficients (data not shown).
Table 2.
Trabecular and cortical structural parameters in the study subjects. Data (median, IQR) are shown for all subjects combined, as well as separated based on the split points for the relationships between bone age and serum sclerostin levels in the girls and boys.
| Girls | |||
|---|---|---|---|
| All | Bone age 4-9 | Bone age 10-21 | |
| N | 62 | 17 | 45 |
| Weight, kg | 46.6 (33.7-58.3) | 29.4 (24.6-33.1) | 51.3 (43.4-61.6) |
| Trabecular parameters | |||
| BV/TV | 0.14 (0.12-0.16) | 0.14 (0.13-0.16) | 0.14 (0.12-0.16) |
| TbN | 1.96 (1.79-2.11) | 2.02 (1.76-2.11) | 1.95 (1.80-2.11) |
| TbTh | 0.072 (0.067-0.076) | 0.073 (0.069-0.075) | 0.071 (0.066-0.078) |
| TbSp | 0.44 (0.39-0.49) | 0.43 (0.39-0.49) | 0.45 (0.40-0.49) |
| Cortical parameters | |||
| Cortical vBMD | 651 (572-786) | 617 (592-677) | 654 (568-798) |
| CtTh | 0.37 (0.21-0.72) | 0.29 (0.24-0.46) | 0.42 (0.20-0.83) |
| Cortical porosity index | 0.60 (0.38-0.85) | 0.51 (0.35-0.65) | 0.73 (0.40-0.88) |
| Boys | |||
| All | Bone age 4-13 | Bone age 14-21 | |
| N | 56 | 29 | 27 |
| Weight, kg | 54.8 (39.4-68.2) | 39.8 (31.6-46.2) | 68.7 (60.1-79.3) |
| Trabecular parameters | |||
| BV/TV | 0.16 (0.14-0.18) | 0.14 (0.13-0.16) | 0.18 (0.16-0.19) |
| TbN | 2.03 (1.88-2.18) | 1.98 (1.82-2.05) | 2.12 (1.98-2.21) |
| TbTh | 0.078 (0.072-0.084) | 0.073 (0.069-0.078) | 0.083 (0.078-0.092) |
| TbSp | 0.41 (0.37-0.45) | 0.43 (0.41-0.48) | 0.39 (0.36-0.41) |
| Cortical parameters | |||
| Cortical vBMD | 676 (624-718) | 651 (597-681) | 712 (650-835) |
| CtTh | 0.50 (0.33-0.74) | 0.42 (0.26-0.53) | 0.80 (0.44-1.06) |
| Cortical porosity index | 0.85 (0.61-1.10) | 0.77 (0.51-0.89) | 0.98 (0.77-1.54) |
Table 3.
Spearman Correlation coefficients (Rp-value) for unadjusted/bone-age adjusted relationships of serum sclerostin levels with bone structural parameters in the girls and boys.
| Girls | |||
|---|---|---|---|
| All | Bone age 4-9 | Bone age 10-21 | |
| Trabecular parameters | |||
| BV/TV | 0.090.509/0.140.291 | −0.020.937/0.130.618 | 0.060.710/0.220.144 |
| TbN | 0.270.035/0.250.054 | 0.060.823/0.210.436 | 0.350.017/0.340.023 |
| TbTh | −0.090.470/−0.020.851 | −0.010.981/0.230.390 | −0.160.299/0.090.576 |
| TbSp | −0.250.054/−0.240.057 | −0.070.801/−0.210.427 | −0.320.034/−0.330.026 |
| Cortical parameters | |||
| Cortical vBMD | −0.60<0.001/−0.340.007 | 0.160.544/0.190.472 | −0.73<0.001/−0.310.039 |
| CtTh | −0.60<0.001/−0.340.007 | 0.090.718/0.180.501 | −0.7l<0.001/−0 280.066 |
| Cortical porosity index | 0.370.003/0.300.018 | −0.210.417/−0.070.798 | 0.66<0.001/0.340.022 |
| Boys | |||
| All | Bone age 4-13 | Bone age 14-21 | |
| Trabecular parameters | |||
| BV/TV | 0.050.722/0.170.222 | O.360.054/0.390.040 | 0.030.900/−0.030.879 |
| TbN | 0.130.329/0.170.205 | 0.310.102/0.290.135 | 0.120.535/−0.260.201 |
| TbTh | 0.010.942/0.140.324 | 0.310.103/0.420.027 | −0.070.711/0.100.624 |
| TbSp | −0.090.519/−0.150.276 | −0.300.113/−0.300.126 | −0.130.511/0.200.317 |
| Cortical parameters | |||
| Cortical vBMD | −0.220.107/−0.150.270 | 0.270.152/0.430.021 | −0.550.003/−0.080.714 |
| CtTh | −0.150.260/−0.070.618 | 0.310.098/0.480.009 | −0.480.011/−0.010.978 |
| Cortical porosity index | 0.340.009/0.410.002 | 0.320.088/0.300.120 | 0.580.001/0.360.074 |
We have previously described the changes in bone turnover markers and hormonal variables in this cohort [17], and Table 4 shows the analogous correlation coefficients between serum sclerostin levels and these parameters. In both sexes, bone turnover markers (PINP and CTX) were positively associated with sclerostin levels in unadjusted and bone age-adjusted analyses. In the girls, these associations were driven principally by girls in the 10-21 year bone age group, but in the boys, the younger and older boys tended to show similar associations. In the boys, but not the girls, serum sclerostin levels were also positively associated with serum IGF-I and IGFBP-3 levels in unadjusted and bone age-adjusted analyses. Serum E2 levels were inversely associated with sclerostin levels in all girls combined as well as in the older girls, but these associations were no longer present following adjustment for bone age.
Table 4.
Spearman Correlation coefficients (Rp-value) for unadjusted/bone-age adjusted of serum sclerostin levels with bone turnover markers and hormonal variables in the girls and boys.
| Girls | |||
|---|---|---|---|
| All | Bone age 4-9 | Bone age 10-21 | |
| PINP | 0.63<0.001/0.290.022 | −0.080.765/0.090.746 | 0.72<0.001/0.260.092 |
| CTX | 0.65<0.001/0.390.002 | 0.020.933/0.130.635 | 0.67<0.001/0.240.113 |
| 25(OH)D | 0.170.180/0.050.709 | 0.550.021/0.540.031 | −0.070.671/−0.150.347 |
| PTH | 0.080.562/0.120.360 | 0.240.355/0.440.087 | 0.040.795/0.050.752 |
| E2 | −0.51<0.001/0.070.607 | 0.170.510/−0.230.398 | −0.380.010/0.170.272 |
| T | −0.53<0.001/0.060.659 | 0.310.228/−0.080.764 | −0.460.002/0.120.424 |
| IGF-I | −0.320.012/0.070.589 | 0.130.616/−0.100.717 | −0.150.310/−0.130.396 |
| IGFBP-3 | −0.130.322/0.160.208 | 0.430.084/0.120.654 | −0.010.969/−0.130.412 |
| Boys | |||
| All | Bone age 4-13 | Bone age 14-21 | |
| PINP | 0.400.002/0.380.004 | 0.330.077/0.260.173 | 0.570.002/0.070.725 |
| CTX | 0.420.001/0.390.003 | 0.370.048/0.350.071 | 0.410.031/0.150.450 |
| 25(OH)D | 0.020.863/0.000.999 | −0.060.773/0.130.502 | 0.090.657/0.240.231 |
| PTH | 0.070.623/0.050.731 | −0.130.495/−0.180.370 | 0.290.145/−0.170.397 |
| E2 | −0.050.737/0.270.043 | 0.390.038/0.120.550 | 0.010.956/0.230.255 |
| T | −0.090.486/0.140.305 | 0.220.248/−0.240.215 | −0.080.698/0.060.776 |
| IGF-I | 0.250.066/0.410.002 | 0.400.033/0.170.389 | 0.390.046/0.010.974 |
| IGFBP-3 | 0.200.138/0.320.018 | 0.420.022/0.250.206 | 0.130.531/0.060.763 |
Discussion
In this study examining sclerostin levels during growth, we found that, similar to our recent findings in adults [15], serum sclerostin levels are higher in boys as compared to girls. Thus, the gender difference in sclerostin levels appears to be established during puberty. We have also shown previously that estrogen, but not testosterone, reduces circulating sclerostin levels in adults [29]; thus, it is plausible that the higher estrogen levels present in girls following the onset of puberty lead to their lower sclerostin levels that persist during adult life. Consistent with this, we did observe an inverse association between serum sclerostin and E2 levels in the all girls combined as well as in the older girls, but these associations were no longer present following adjustment for bone age. Overall, our study was well powered, as with the sample size of this analysis, we would have had 90% power to detect a difference of 1.8 pmol/L or greater in sclerostin levels between sexes and a correlation of 0.41 or higher with age.
Our analysis also demonstrated that between the chronological ages of 6 and 21 years (bone ages of 4 to 21 years), the relationship of serum sclerostin levels with bone age was not linear, but rather was best described by a split point. This split point occurred earlier in girls (bone age 10 years) than in boys (bone age 14 years), perhaps related to the earlier onset of puberty in girls. Subsequent to the age of the split point, serum sclerostin levels declined as a function of bone age in both sexes. The possible explanation for a split point for the relationship of serum sclerostin levels with age in both sexes is unclear, but may be related to hormonal factors (e.g., sex steroids) with the onset of puberty that cause sclerostin levels to decline later in puberty. In addition, we have previously shown that starting around age 20 years, serum sclerostin levels increase over life in both sexes [15]. Combining these previous findings with the results of the current study, it would appear that serum sclerostin levels peak early in life (~age 10 years in girls and 14 years in boys), decline during the later stages of puberty towards a nadir at the end of puberty, and then increase over the remainder of adult life.
In assessing the relationship of serum sclerostin levels to bone microarchitectural parameters using HRpQCT, we did not observe a consistent relationship between sclerostin levels and trabecular bone parameters in either sex. However, we did find that serum sclerostin levels were inversely associated with cortical vBMD and CtTh in girls and positively associated with apparent cortical porosity in both girls and boys. These findings raise the possibility that, during growth, changes in sclerostin production regulate cortical structure. Since we have previously demonstrated in this cohort that the cortical porosity index also peaks around the age when the incidence of distal forearm fractures is maximal [17], further studies testing whether sclerostin levels are higher in children with adolescent forearm fractures as compared to non-fracture controls are warranted. In addition, the role of changes in sclerostin production during growth in defining the ultimate porosity and strength of cortical bone needs further investigation. Moreover, the mechanism for the transient increase in cortical porosity in both sexes during maximal growth remains unclear. For example, during growth concomitant periosteal bone formation and endocortical bone resorption may lead to some trabecularization of endocortical bone, contributing to the observed increase in cortical porosity. It is also possible that adolescents with larger increases in cortical porosity during growth have an increased risk of forearm fractures, although further studies are needed to address this issue.
We also found that in both sexes, bone turnover markers were positively correlated with serum sclerostin levels. This differs from our recent findings in adults [15], where serum sclerostin levels were inversely associated with bone turnover markers, at least in women. Whether the observed association in children reflects a role for sclerostin in regulating bone turnover or is a secondary response to the changes in bone turnover remains to be determined.
Given that sclerostin is produced almost exclusively by osteocytes [1, 3, 5, 6], changes in circulating sclerostin levels, which we recently found to be significantly correlated with bone marrow plasma sclerostin levels [32], should reflect changes in skeletal sclerostin production. However, we cannot exclude the possibility that altered clearance or metabolism of sclerostin during growth is contributing to the changes we observed. In addition, we acknowledge that our study was cross-sectional, and the associations observed here need to be replicated in a longitudinal study for further validation. We also recognize several limitations of our cortical porosity analysis. First, while recent work by Burghardt et al. [33] has indicated the need for a manual review of bone contours in assessing cortical porosity, we relied on our cortical porosity analysis [17] done prior to the paper of Burghardt et al. [33]; thus, our analysis followed the approach of the manufacturer to segment the cortex without any corrections. Second, although we recognize that during certain growth phases cortical trabecularization happens at the endocortical surface, the resolution of HRpQCT did not allow for us to distinguish cortical trabecularization from “regular” trabecular bone.
In summary, the gender difference in serum sclerostin levels appears to be established during puberty, and sclerostin levels tend to decline in late puberty in girls and in boys. Serum sclerostin levels are associated with cortical porosity, suggesting that changes in sclerostin production during growth may play a role in defining cortical structure.
Acknowledgments
Funding: This work was supported by NIH Grants AR027065 and UL1-RR24150 (Center for Translational Science Activities).
Footnotes
Conflict of interest: None of the authors has a conflict to disclose.
References
- 1.Baron R, Rawadi G. Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology. 2007;148:2635–2643. doi: 10.1210/en.2007-0270. [DOI] [PubMed] [Google Scholar]
- 2.Krishnan V, Bryant HU, MacDougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116:1202–1209. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Bezooijen RL, Roelen BAJ, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE, ten Dijke P, Lowik CWGM. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199:805–814. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semenov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005;280:26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- 5.Poole KES, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, Reeve J. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19:1842–1844. doi: 10.1096/fj.05-4221fje. [DOI] [PubMed] [Google Scholar]
- 6.van Bezooijen RL, ten Dijke P, Papapoulos SE, Lowik CW. SOST/sclerostin, an osteocyte-derived negative regulator of bone formation. Cytokine Growth Factor Rev. 2005;16:319–327. doi: 10.1016/j.cytogfr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Brunkow ME, Gardner JC, Van Ness J, et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001;68:577–589. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balemans W, Ebeling M, Patel N, et al. Increase bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10:537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 9.Staehling-Hampton K, Proll S, Paeper BW, et al. A 52-kb deletion in the SOST-MEOX1 intergenic region on 17q12-q21 is associated with van Buchem disease in the Dutch population. Am J Med Genet. 2002;110:144–152. doi: 10.1002/ajmg.10401. [DOI] [PubMed] [Google Scholar]
- 10.Balemans W, Patel N, Ebeling M, et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002;39:91–97. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Ominsky MS, Niu Q-T, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23:860–869. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Ominsky MS, Warmington KS, et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res. 2009;24:578–588. doi: 10.1359/jbmr.081206. [DOI] [PubMed] [Google Scholar]
- 13.Padhi D, Jang G, Stouch B, Fang L, Posvar E. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res. 2011;26:19–26. doi: 10.1002/jbmr.173. [DOI] [PubMed] [Google Scholar]
- 14.Glass DA, II, Bialek P, Ahn JD, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 15.Modder UI, Hoey KA, Amin S, McCready LK, Achenbach SJ, Riggs BL, Melton LJI, Khosla S. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res. 2011;26:373–379. doi: 10.1002/jbmr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey DA, Martin AD, McKay HA, Whiting S, Mirwald R. Calcium accretion in girls and boys during puberty: a longitudinal analysis. J Bone Miner Res. 2000;15 doi: 10.1359/jbmr.2000.15.11.2245. [DOI] [PubMed] [Google Scholar]
- 17.Kirmani S, Christen D, van Lenthe GH, et al. Bone structure at the distal radius during adolescent growth. J Bone Miner Res. 2009;24:1033–1042. doi: 10.1359/JBMR.081255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khosla S, Melton LJ, III, Dekutoski MB, Achenbach SJ, Oberg AL, Riggs BL. Incidence of childhood distal forearm fractures over 30 years: A population-based study. JAMA. 2003;290:1479–1485. doi: 10.1001/jama.290.11.1479. [DOI] [PubMed] [Google Scholar]
- 19.Landin LA. Fracture patterns in children. Analysis of 8,682 fractures with special reference to incidence, etiology and secular changes in a Swedish urban population 1950-1979. Acta Orthop Scand Suppl. 1983;202:1–109. [PubMed] [Google Scholar]
- 20.Kramhoft M, Bodtker S. Epidemiology of distal forearm fractures in Danish children. Acta Orthop Scand. 1988;59:557–559. doi: 10.3109/17453678809148784. [DOI] [PubMed] [Google Scholar]
- 21.Bailey DA, Wedge JH, McCulloch RG, Martin AD, Bernhardson SC. Epidemiology of fractures of the distal end of the radius in children as associated with growth. J Bone Joint Surg. 1989;71-A:1225–1231. [PubMed] [Google Scholar]
- 22.Tanner JM, Healy MJR, Goldstein H, Cameron N. Assessment of skeletal maturity and prediction of adult height: TW3 Method Saunders. Philadelphia: 2001. [Google Scholar]
- 23.Laib A, Hildebrand T, Hauselmann HJ, Ruegsegger P. Ridge number density: a new parameter for in vivo bone structure analysis. Bone. 1997;21:541–546. doi: 10.1016/s8756-3282(97)00205-6. [DOI] [PubMed] [Google Scholar]
- 24.Laib A, Hauselmann HJ, Ruegsegger P. In vivo high resolution 3D-QCT of the human forearm. Technol Health Care. 1998;6:329–337. [PubMed] [Google Scholar]
- 25.Parfitt AM. Stereologic basis of bone histomorphometry: Theory of quantitative microscopy and reconstruction of the third dimension. CRC Press; Boca Raton: 1983. [Google Scholar]
- 26.Laib A, Ruegsegger P. Calibration of trabecular bone structure measurements of in vivo three-dimensional peripheral quantitative computed tomography with 28-microm-resolution microcomputed tomography. Bone. 1999;24:35–39. doi: 10.1016/s8756-3282(98)00159-8. [DOI] [PubMed] [Google Scholar]
- 27.MacNeil JA, Boyd SK. Accuracy of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med Eng Phys. 2007;29:1096–1105. doi: 10.1016/j.medengphy.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Terpos E, Christoulas D, Katodritou E, et al. High serum sclerostin correlates with advanced stage, increased bone resorption, reducted osteoblast function, and poor survival in newly-diagnosed patients with multiple myeloma. Blood. 2009;114 Abstract 425. [Google Scholar]
- 29.Modder UIL, Clowes JA, Hoey K, Peterson JM, McCready L, Oursler MJ, Riggs BL, Khosla S. Regulation of circulating sclerostin levels by sex steroids in women and men. J Bone Miner Res. 2011;26:27–34. doi: 10.1002/jbmr.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaudio A, Pennisi P, Bratengeier C, Torrisi V, Lindner B, Mangiafico RA, Pulvirenti I, Hawa G, Tringali G, Fiore CE. Increased sclerostin serum levels associated with bone formation and resorption markers in patients with immoilization-induced bone loss. J Clin Endocrinol Metab. 2010;95:2248–2253. doi: 10.1210/jc.2010-0067. [DOI] [PubMed] [Google Scholar]
- 31.Khosla S, Amin S, Singh RJ, Atkinson EJ, Melton LJ, Riggs BL. Comparison of sex steroid measurements in men by immunoassay versus mass spectroscopy and relationships with cortical and trabecular volumetric bone mineral density. Osteoporos Int. 2008;19:1465–1471. doi: 10.1007/s00198-008-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drake MT, Srinivasan B, Modder UI, Peterson JM, McCready LK, Riggs BL, Dwyer D, Stolina M, Kostenuik P, Khosla S. Effects of parathyroid hormone treatment on ciculating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab. 2010;95:5056–5062. doi: 10.1210/jc.2010-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burghardt AJ, Buie HR, Laib A, Majumdar S, Boyd SK. Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT. Bone. 2010;47:519–528. doi: 10.1016/j.bone.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]